Abstract
Objective:
Following traumatic brain injury (TBI), depressive symptoms are common and may influence recovery. We performed a meta-analysis to estimate the benefit of antidepressants following TBI and compare the estimated effects between antidepressants and placebo
Participants:
Multiple databases were searched to find prospective pharmacological treatment studies of major depressive disorder (MDD) in adults following TBI.
Main Measures:
Effect sizes for antidepressant medications in patients with TBI were calculated for within-subjects designs that examined change from baseline after receiving medical treatment and treatment/placebo designs that examined the differences between the antidepressants and placebo groups.
Design:
A random-effects model was used for both analyses.
Results:
Of 1028 titles screened, 11 were included. Pooled estimates showed nonsignificant difference in reduction of depression scores between medications and placebo (standardized mean difference of 5 trials = −0.3; 95% CI, −0.6 to 0.0; I2 = 17%), and a significant reduction in depression scores for individuals after pharmacotherapy (mean change = −11.2; 95% CI, −14.7 to −7.6 on the Hamilton Depression Scale; I2 = 87%).
Conclusions:
This meta-analysis found no significant benefit of antidepressant over placebo in the treatment of MDD following TBI. Pooled estimates showed a high degree of bias and heterogeneity. Prospective studies on the impact of antidepressants in well-defined cohorts of TBI patients are warranted
Keywords: antidepressant, depression, traumatic brain injury
Traumatic Brain Injury (TBI) is an important public health problem in the United States. There were approximately 2.8 million emergency department visits in 2013, with nearly 50 000 of those resulting in deaths.1 There is a prevalence of 3.32 million to 5.3 million people in the United States with long-term deficits and disabilities from TBI.2,3
Depressive disorders are one of the most common long-term effects of TBI of all severities, with an estimated prevalence of 20% to 45% postinjury.4–8 Major depressive disorder (MDD) is associated with an increased risk of suicide following TBI,9 and there is an increased prevalence of MDD over a lifetime, even when the injury was 50 years prior.10 Furthermore, TBI is frequently comorbid with other conditions such as seizures, chronic pain, or posttraumatic stress disorder (PTSD), all of which have an increased risk of MDD.11–13 Despite this heavy burden of MDD in TBI survivors, clinical best practice guidelines for treatment have not been developed.
MDD can be challenging to diagnose in neurologic disorders, as it may have a more complicated presentation, and it may be a specific sequela of the brain injury itself.14 In addition, patients with MDD after TBI do not recover as well as those without. Decreased executive function5 and poorer functional outcome, as measured by the Glasgow Outcome Scale Extended,15 are associated with MDD after TBI. Thus, it is critical to determine whether conventional methods of treating depression, such as pharmacological antidepressants, are effective following TBI.
Understanding research to date on treatments of depressive disorders post-TBI is a crucial step in developing evidence-based clinical guidelines. The most common treatment reported for depressive disorders is pharmacological.16–20 The aim of this meta-analysis was to evaluate research on pharmacological treatments of post-TBI depressive disorders by (1) estimating within-subjects effect size of pharmacological treatment, (2) estimating difference in effect size when comparing pharmacological treatment with a placebo, (3) describing limitations of research conducted to date, and (4) recommending potential next steps given our findings.
METHODS
This meta-analysis evaluated all prospective studies in which any antidepressant drug was used to treat MDD diagnosed following TBI. We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.21 Two authors (N.K. and R.A.) independently reviewed all articles. We considered published and unpublished studies. Only English language articles were considered. PubMed, ClinicalTrials.gov, and the Cochrane database were searched using the terms “anti-depressant traumatic brain injury,” “SSRI traumatic brain injury,” “tri-cyclic anti-depressant traumatic brain injury,” and “depression treatment traumatic brain injury.” The original search was conducted on February 1, 2017. A second search was conducted on September 20, 2017, to determine whether new studies had been added to the literature. We also searched articles that were referenced within other studies, as well as articles that were referenced in previous systematic reviews.
Prospective studies evaluating use of an antidepressant for MDD following TBI were selected. Studies were excluded if they specifically evaluated the use of antidepressants for MDD refractory to first-line agents or if they utilized the same patient cohort from a prior study. The first and second authors dual abstracted from articles using the Cochrane Collaboration Data Collection Form for Intervention Review—RCTs and non-RCTs. This tool was also used to evaluate bias in each study including selection, blind assignment, performance, detection, attrition, or other biases.22 Each extracted approximately half of the included studies and then reviewed extractions and bias assessments done by the other author to confirm. In cases of conflict, the authors would review the discordant component together. If the first and second authors could not mutually agree on a value or bias value, the last author on the manuscript was the arbiter to determine final value. All data from the forms were then exported into an Excel file. These data were checked by the first and second authors and confirmed by an outside third-party research assistant. If data were not included in the original report, we attempted to contact the investigators to obtain it. Depression scores not reported as mean and standard deviation were transformed if possible. Primary outcomes were calculated for the following: (1) mean change (MC) in effect size of pharmacological treatment within subjects sharing a common depression outcome measure; and (2) standardized mean difference (SMD) to compare effect of pharmacological treatments within subjects sharing a common depression outcome measure. Random-effects models were used because of expected random differences between the studies. A sensitivity analysis was also conducted to examine the influence of each study on the combined estimate. All analyses and plots were completed using R (version 3.4.0)23 in R Studio (version 1.0.143) interface,24 including the R package metaphor.25
RESULTS
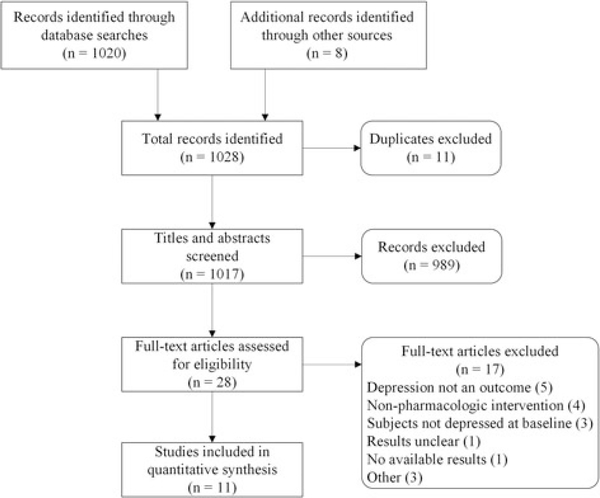
Our search resulted in 1020 total titles. The search terms “traumatic brain injury” and “depression” resulted in 12 studies from ClinicalTrials.gov. In addition, we reviewed the references in the studies selected, which resulted in 8 additional studies to review (see Figure 1). From this, we removed 11 duplicates, excluded 989 titles/abstracts, and 28 studies were chosen for full review. Of these manuscripts, 11 met with inclusion/exclusion criteria and were included in the analysis (see Table 1).
Figure 1.
Exclusions for prior antidepressant use were defined as excluding for any prior use of antidepressants. Note that the following studies were defined as not excluding for prior antidepressant use for the following reasons: Lee et al,26 Newburn et al,27 and Rapoport et al28 excluded patients who had taken antidepressants recently or were currently taking antidepressants. Rao29 excluded patients with good response to other antidepressants in the past as well as patients with poor response to escitalopram in the past and patients currently taking antidepressants. Saran30 had a washout period of 1 week prior to study onset.
TABLE 1.
Descriptive information about included studies
| First author (Year) | Country | Antidepressant(s) | Weeks Treated | N | Time since injury | Severity of injury |
|---|---|---|---|---|---|---|
| Ansari31 (2014) | India | Sertraline | 24 | 80 | >2 wk | Mild and moderate |
| Ashman32 (2009) | USA | Sertraline | 10 | 41 | 17 y | Mild to severe |
| Dinan30 (1992) | Ireland | Amitriptyline | 6 | 13 | 4.8 wk | Mild |
| Fann33 (2000) | USA | Sertraline | 8 | 15 | 10.6 mo | Mild |
| Fann34 (2017) | USA | Sertraline | 12 | 53 | 4.6 mo | Mild to severe |
| Horsfield35 (2002) | USA | Fluoxetine | 35 | 5 | Not reported | Mild |
| Lee26 (2005) | Korea | Methylphenidate and sertraline | 4 | 30 | 32.2 d | Mild to moderate |
| Newburn27 (1999) | New Zealand | Moclobemide | 3 | 26 | 4.67 y | Not reported |
| Rao29 (2013) | USA | Escitalopram | 12 | 14 | Not reported | Not reported |
| Rapoport28 (2008) | Canada | Citalopram | 10 | 54 | <1 y | Mild to severe |
| Saran30 (1985) | USA | Amitriptyline and phenelzine | 156 | 10 | <1 y | Mild |
Pharmacological interventions included selective serotonin reuptake inhibitors (SSRIs) in 8 studies,26,28,29,31–35 monoamine oxidase inhibitors (MAOIs) in 1 study,27 tricyclic antidepressants (TCAs) in 1 study,36 methylphenidate for 1 study,26 and both MAOIs and TCAs in 1 study.30 Five studies were randomized controlled trials (RCTs).26,29,31,32,34 Nine measured depression with the Hamilton Depression Scale (HAM-D),26–28,30,32–35 1 study used the Montgomery-Asberg Depression Scale (MADRS),29 and 1 study used the Patient Health Questionnaire-9 (PHQ-9).31 Duration of treatment differed significantly, with the longest being 34 weeks of treatment35 and the shortest being 4 weeks of treatment (interquartile range = 7–18 weeks).30 The length of time since TBI differed significantly as well, with the longest being 243.7 weeks27 and the shortest being 4.8 weeks.36 All but 4 of the included studies had a high risk of bias (see Table 2), as demonstrated by the Cochrane Risk of Bias tool. In one instance, the posttreatment standard deviation was not included and could not be calculated, and the pretreatment standard deviation was imputed.27 One study had 2 pharmacological treatment groups that were combined by calculating the combined mean and standard deviations.26
TABLE 2.
Risk of bias for each included study
| First Author (Year) | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | |
|---|---|---|---|---|---|---|
|
|
|
|||||
| Random assignment | Allocation concealment | Blinding: subject/personnel | Blinding: Outcome assessor | Complete data | Complete reporting | |
| Ansari31 (2014) | ✓ | ✕ | ✕ | ✕ | ✕ | ✓ |
| Ashman32 (2009) | ✓ | ✓ | ✓ | ✓ | ✕ | ✓ |
| Dinan36 (1992) | ✕ | ✕ | ✕ | ✕ | ✓ | ✓ |
| Fann33 (2000) | ✕ | ✓ | ✕ | ✕ | ✓ | ✓ |
| Fann34 (2017) | ✓ | ✓ | ✓ | ✓ | ✕ | ✓ |
| Horsfield35 (2002) | ✕ | ✕ | ✕ | ✕ | ✓ | ✓ |
| Lee26 (2005) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Newburn27 (1999) | ✕ | ✕ | ✕ | ✕ | ✕ | ✓ |
| Rao29 (2013) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Rapoport28 (2008) | ✕ | ✕ | ✕ | ✕ | ✕ | ✓ |
| Saran30 (1985) | ✕ | ✕ | ✕ | ✕ | ✓ | ✓ |
Severity of TBI preceding initiation of pharmacological treatment of depression was available for 9 studies.26,28,30–36 Of these, 4 studies included only mild TBI patients,30,33,35,36 3 included mild to severe TBI patients,28,32,34 and 2 included mild to moderate TBI patients.26,31 Pre-TBI history of any depressive disorder or prior use of medications for depression was an exclusion criterion for 5 of the included studies.28,31,33,35,36 Only one study reported whether the patients enrolled had a history of TBI.33
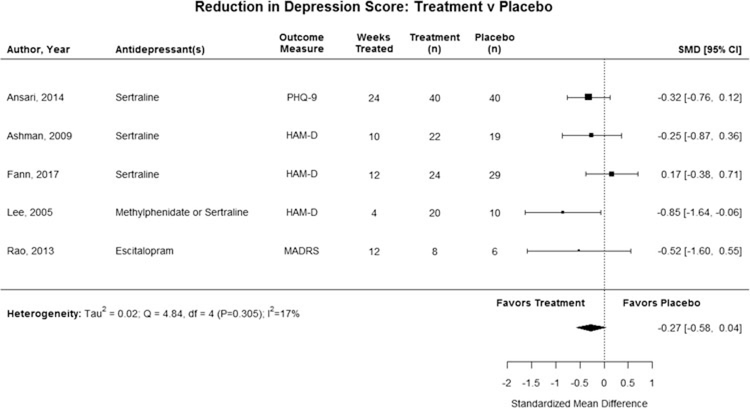
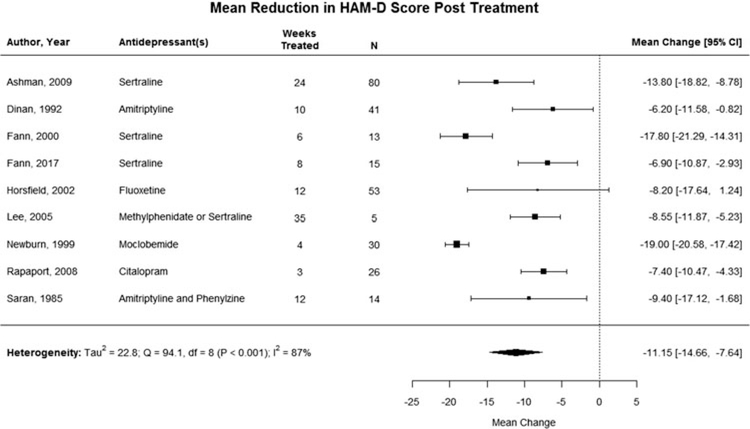
Depressive symptoms were not significantly reduced after pharmacological treatment in post-TBI patients when compared with placebo (SMD = −0.3; 95% CI, −0.6 to 0.0), with low statistical heterogeneity (I2 = 17%), for the 5 RCTs that included a control group (see Figure 2).26,29,31,32,34 Nine studies reported the HAM-D as an outcome measure, and we were able to pool the pre-and posttreatment depression scores following pharmacological treatment.26–28,30,32–36 In this pooled analysis, depression score was significantly reduced after pharmacological treatment post-TBI (MC = −11.2; 95% CI, −14.7 to −7.6) (see Figure 3) with high statistical heterogeneity (I2 = 87%).26–28,30,32–36 Two studies were excluded from the pooled analysis because they reported the MADRS or PHQ-9 rather than the HAM-D.29,31
Figure 2.
Standardized mean difference in reduction of self-reported depression after treatment with antidepressant versus treatment with placebo. The combined estimate was not significant, and 80% of the studies individually did not find significant improvement in depression scores in patients who received a pharmacological treatment over patients who received a placebo.
Figure 3.
Mean change in HAM-D scores, baseline to posttreatment. Depression score was significantly reduced after pharmacological treatment post-TBI for the 8 studies reporting HAM-D score as their outcome measure, however, with high heterogeneity. HAM-D indicates Hamilton Depression Scale.
A sensitivity analysis was performed by removing each study from the combined estimate. This demonstrated that the 2017 study by Fann et al34 was the only study to influence the results of the RCT meta-analysis. Removing it changed the combined estimate from a nonsignificant finding to a significant improvement for those in the treatment group compared with placebo. Publication bias was not examined because of the lack of power for adequate asymmetry assessment.
DISCUSSION
In our meta-analysis of 5 RCTs, we did not find a significant difference in depression severity between patients treated with pharmacological antidepressants compared with those treated with placebo. There was 1 prior meta-analysis on this topic prior to the publication of a large RCT, and that publication suggested that antidepressants after TBI may be associated with reduced depressive symptoms.37 Our meta-analysis added a single, large RCT and found no effect of antidepressants compared with placebo.34 Inclusion of this large RCT in our meta-analysis places the current state of knowledge in context. The change in results from the evidence when pooled together with all other studies underscores the impact of this single, large RCT. Our findings demonstrate that additional studies are needed to ascertain which post-TBI patients with depression are likely to respond to antidepressants.
Historically, there have been a number of challenges in clinical trials for TBI including retention, heterogeneity, and small sample sizes.38 Heterogeneity of treatment, depression severity, and TBI severity influenced the results of this meta-analysis. Future studies should consider severity of depression, severity of TBI, genetics, biomarkers, and injury characteristics to elucidate the interrelatedness of TBI and post-TBI depression. For instance, there is no definitive scale to accurately distinguish depressive symptoms from other effects of TBI, such as slow processing speed and apathy. Interestingly, the one study that included stimulants has one of the highest effect sizes of the studies reviewed, with both improvement in cognitive function and depressive symptoms.26 This is hypothesis generating, and it is possible that in some cases a stimulant may be a better first-line choice depending on the contribution of the underlying TBI to the mood disorder.
We included studies of adult patients with TBI and MDD of any severity. There is evidence that MDD and TBI severity are associated, although the association is not linear. When patients with TBI of all severities (mild, moderate, and severe) are considered, the prevalence of depression changes on the basis of the tool used for diagnosis, self-reporting, and type of interview conducted, thus making the diagnosis of MDD following TBI challenging.39 Many depressive symptoms overlap with those that are naturally present following TBI.40 Furthermore, the extent to which antidepressants are more effective than placebo depends on the severity of depression at baseline such that those with mild to moderate MDD have similar effects to individuals treated with placebo.41
Although the heterogeneity of pooled analysis of the pre/post-TBI studies was high, it did demonstrate that there was a significant improvement in HAM-D depression scores before and after treatment with an antidepressant, which was not confirmed by our meta-analysis of only placebo-controlled trials. There are several plausible explanations for these different findings. In studies without a placebo group, it is impossible to know whether depression scores improved because of drug treatment or for other reasons, such as normal variation in symptoms over time or spontaneous improvement. It is also plausible that those in the placebo group have reduced depressive symptoms because of study participation, as placebo rates for depression in general show improvement when patients are enrolled in a study.42 This is further supported by the fact that in one RCT, both the placebo and experimental groups demonstrated significant reduction in depressive symptoms over the course of the study.34 Given the findings of this meta-analysis, it is worthwhile to mention that there have been recent promising studies describing the role of psychotherapy, in particular cognitive behavioral therapy (CBT).43–45 Multimodal approaches, such as the combination of CBT and pharmacotherapy, may also be effective and are worthy of future study.
Another potential reason that there was no significant difference in the treatment/placebo analysis is that the HAM-D score alone may have not been the best outcome measure. Individuals with TBI may have depressive symptoms, as shown on depression scales that will not change over time, due to TBI sequelae. Instead of demonstrating antidepressants do not work over time, it could be possible that our scales either (a) are inadequate to diagnose MDD in this population or (b) do not change sufficiently with time due to factors related to the underlying TBI. The HAM-D includes somatic questions and in treatment/placebo trials of medically ill patient populations, a revised subscale, such as the Maier subscale excluding somatic questions, may be more appropriate, cost-effective, and informative than the full HAM-D.46 Interestingly, the 2017 study by Fann et al34 also included the Maier subscale, which did not produce any additional separation between treatment and controls compared with the full HAM-D. A recent secondary analysis of a randomized controlled study demonstrated that the PHQ-9 requires fewer modifications when used in persons with TBI than using Symptom Checklist-20 (SCL-20), the HAM-D, or subscales derived from these tests.47 More research is needed to delineate which scales maximize the sensitivity to change of depression in individuals with TBI.
Previous studies have described both imaging and blood-based biomarkers to identify comorbid MDD in individuals with TBI, but none are currently used in practice.48–57 Finding such a marker may help diagnose depressive symptoms, differentiate depressive symptoms post-TBI from TBI sequelae, and evaluate responses to treatment of post-TBI MDD. For instance, inhibitory deficits may account for memory impairments in individuals with concomitant MDD and TBI.58 In addition, there may be a higher genetic risk for post-TBI depression in certain individuals.59 This is contrasted with other mechanisms known to influence the development of MDD, such as early adversity or substance abuse.60,61 Some literature suggests that reduced neurotrophin support, impaired monoamine neurotransmission, and inflammation could contribute to both MDD and PTSD.62–64 For example, brain-derived neurotrophic factor (BDNF) levels may be a biomarker for identifying those at risk of post-TBI depression.56 A prospective cohort study also implicates inflammation as a contributor to post-TBI depression, with individuals with higher levels of acute CSF (cerebrospinal fluid) surface cytokine markers having a 3.92 times increased risk of depression at 6 months postinjury.57 Incorporating these modalities to differentiate depressive symptoms from TBI sequelae is required to move the treatment of post-TBI depression forward.
LIMITATIONS
There are several limitations to this meta-analysis. Studies were inconsistent in their reporting of important descriptive and demographic information about the patients, so whether or not the difference was due to differences in the study populations could not be assessed. The lack of statistical reporting prevented standardization of studies in the pre/posttreatment analysis, and the lack of reporting on potentially significant covariates prevented use of controlling for differences through a regression model, resulting in the high heterogeneity.26–28,30,32–36 We included the analysis of difference in patients before and after treatment as a visual and numerical description of what has been reported to date and also to provide context for the comparison of patients treated with an antidepressant versus placebo. Clinically, there were a number of differences to explain heterogeneity within the pre/posttreatment studies. The most important clinical factor was likely injury severity, which may have yielded a heterogeneous mix of individuals with varied contributors to the development of depressive symptoms. Many studies either provided little information about injury severity27,29 or included individuals with a wide range of severity.26,28,31,32,34 Furthermore, some studies did not exclude patients with serious comorbidities,33,35,36 some studies did not exclude those with substance abuse,26,28,30,33,35 one study did not exclude patients with non-TBI neurologic disorders,35 and one study did not exclude patients with previous mental health disorders.29 It is reasonable to assume that in the studies where older patients were included, there might have also been additional health comorbidities that impacted the diagnosis of MDD. The heterogeneity between these studies provides evidence for questioning the estimated treatment effect when considering each study individually. This was confirmed when we examined pharmacological treatment versus placebo, which showed an insignificant effect size.
Although we included all types of antidepressants in this study to provide a complete description of existing practice, results of the pre/posttreatment analysis may have been affected by this decision, as non-SSRI drugs were studied in 33% of the studies included in this analysis. Conversely, of the 218 individuals enrolled in the RCTs, only the 10 individuals who received methylphenidate were not treated with an SSRI or placebo, making these results more reflective of SSRI treatment rather than any antidepressant.26
CONCLUSIONS
This meta-analysis found no benefit of antidepressant over placebo in the treatment of MDD following TBI. However, there were promising findings in a small group of studies, indicating that there may be a subset of individuals likely to respond better. Overall, there was a high degree of bias and heterogeneity regarding TBI severity, time since injury, depression severity, and demographics among studies. Larger prospective studies on the impact of antidepressants in well-defined cohorts of TBI patients are warranted to better understand treatment effects and the relationship of post-TBI MDD and functional outcome.
Acknowledgments
The authors thank Dr Uwe Stolz for assistance with methodology expertise and comments that greatly improved the manuscript.
Dr Foreman receives speaking fees from UCB Pharma Inc and research funding through the Department of Defense and the National Institutes of Health/National Institutes of Neurological Disorders and Stroke.
Footnotes
The authors declare no conflicts of interest.
Contributor Information
Natalie Kreitzer, Division of Neurocritical Care, University of Cincinnati, Cincinnati, Ohio; Department of Emergency Medicine, University of Cincinnati, Cincinnati, Ohio.
Rachel Ancona, Department of Emergency Medicine, University of Cincinnati, Cincinnati, Ohio.
Cheryl McCullumsmith, Department of Psychiatry, University of Cincinnati, Cincinnati, Ohio.
Brad G. Kurowski, Department of Pediatrics, University of Cincinnati, Cincinnati, Ohio; Department of Physical Medicine and Rehabilitation, University of Cincinnati, Cincinnati, Ohio.
Brandon Foreman, Division of Neurocritical Care, University of Cincinnati, Cincinnati, Ohio; Department of Neurology and Rehabilitation Medicine, University of Cincinnati, Cincinnati, Ohio.
Laura B. Ngwenya, Department of Neurology and Rehabilitation Medicine, University of Cincinnati, Cincinnati, Ohio.
Opeolu Adeoye, Division of Neurocritical Care, University of Cincinnati, Cincinnati, Ohio; Department of Emergency Medicine, University of Cincinnati, Cincinnati, Ohio.
REFERENCES
- 1.Taylor CA. Traumatic brain injury–related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selassie AW, Zaloshnja E, Langlois JA, Miller T, Jones P, Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil. 2008;23(2):123–131. [DOI] [PubMed] [Google Scholar]
- 3.Zaloshnja E, Miller T, Langlois JA, Selassie AW. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil. 2008;23(6):394–400. [DOI] [PubMed] [Google Scholar]
- 4.Pagulayan KF, Hoffman JM, Temkin NR, Machamer JE, Dikmen SS. Functional limitations and depression after traumatic brain injury: examination of the temporal relationship. Arch Phys Med Rehabil. 2008;89(10):1887–1892. [DOI] [PubMed] [Google Scholar]
- 5.Jorge RE, Robinson RG, Moser D, Tateno A, Crespo-Facorro B, Arndt S. Major depression following traumatic brain injury. Arch Gen Psychiatry. 2004;61(1):42–50. [DOI] [PubMed] [Google Scholar]
- 6.Kreutzer JS, Seel RT, Gourley E. The prevalence and symptom rates of depression after traumatic brain injury: a comprehensive examination. Brain Inj. 2001;15(7):563–576. [DOI] [PubMed] [Google Scholar]
- 7.Hibbard MR, Uysal S, Kepler K, Bogdany J, Silver J. Axis I psychopathology in individuals with traumatic brain injury. J Head Trauma Rehabil. 1998;13(4):24–39. [DOI] [PubMed] [Google Scholar]
- 8.Jorge RE, Robinson RG, Arndt SV, Starkstein SE, Forrester AW, Geisler F. Depression following traumatic brain injury: a 1 year longitudinal study. J Affect Disord. 1993;27(4):233–243. [DOI] [PubMed] [Google Scholar]
- 9.Brooks N, Campsie L, Symington C, Beattie A, McKinlay W. The five year outcome of severe blunt head injury: a relative’s view. J Neurol Psychiatry. 1986;49(7):764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holsinger T, Steffens DC, Phillips C, et al. Head injury in early adulthood and the lifetime risk of depression. Arch Gen Psychiatry. 2002;59(1):17–22. [DOI] [PubMed] [Google Scholar]
- 11.Juengst SB, Wagner AK, Ritter AC, et al. Post-traumatic epilepsy associations with mental health outcomes in the first two years after moderate to severe TBI: a TBI Model Systems analysis. Epilepsy Behav. 2017;73:240–246. [DOI] [PubMed] [Google Scholar]
- 12.Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: a systematic review. JAMA. 2008;300(6):711–719. [DOI] [PubMed] [Google Scholar]
- 13.Blakey SM, Wagner HR, Naylor J, et al. Chronic pain, TBI, and PTSD in military veterans: a link to suicidal ideation and violent impulses? JPain. 2018;19(7):797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silver JM, Hales RE, Yudofsky SC. Psychopharmacology of depression in neurologic disorders. J Clin Psychiatry. 1990;51 (suppl):33–39. [PubMed] [Google Scholar]
- 15.Haagsma JA, Scholten AC, Andriessen TM, Vos PE, Van Beeck EF, Polinder S. Impact of depression and posttraumatic stress disorder on functional outcome and health-related quality of life of patients with mild traumatic brain injury. J Neurotrauma. 2015;32(11):853–862. [DOI] [PubMed] [Google Scholar]
- 16.Sepehry AA, Lee PE, Hsiung GY, Beattie BL, Jacova C. Effect of selective serotonin reuptake inhibitors in Alzheimer’s disease with comorbid depression: a meta-analysis of depression and cognitive outcomes. Drugs Aging. 2012;29(10):793–806. [DOI] [PubMed] [Google Scholar]
- 17.Fann JR, Bombardier CH, Richards JS, et al. Venlafaxine extended-release for depression following spinal cord injury: a randomized clinical trial. JAMA Psychiatry. 2015;72(3):247–258. [DOI] [PubMed] [Google Scholar]
- 18.Marquez-Romero JM, Arauz A, Ruiz-Sandoval JL, et al. Fluoxetine for motor recovery after acute intracerebral hemorrhage (FMRICH): study protocol for a randomized, double-blind, placebo-controlled, multicenter trial. Trials. 2013; 14:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bremner JD. A double-blind comparison of Org 3770, amitriptyline, and placebo in major depression. J Clin Psychiatry. 1995; 56(11):519–525. [PubMed] [Google Scholar]
- 20.Lydiard RB, Stahl SM, Hertzman M, Harrison WM. A double-blind, placebo-controlled study comparing the effects of sertraline versus amitriptyline in the treatment of major depression. JClin Psychiatry. 1997;58(11):484–491. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Vol 4. Chichester, UK: John Wiley & Sons; 2011. [Google Scholar]
- 23.Kurowski BG, Hugentobler J, Quatman-Yates C, et al. Aerobic exercise for adolescents with prolonged symptoms after mild traumatic brain injury: an exploratory randomized clinical trial. JHead Trauma Rehabil. 2017;32(2):79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Team RStudio. RStudio: Integrated Development for R. Boston, MA: RStudio I; 2015. http://www.rstudio.com. Accessed November 28, 2017. [Google Scholar]
- 25.Viechtbauer W. Conducting meta-analyses in R with the metaphor Package. J Stat Software. 2010;36(3):1–48. http://www.jstatsoft.org/v36/i03. fAccessed November 28, 2017. [Google Scholar]
- 26.Lee H, Kim SW, Kim JM, Shin IS, Yang SJ, Yoon JS. Comparing effects of methylphenidate, sertraline and placebo on neuropsychiatric sequelae in patients with traumatic brain injury. Hum Psychopharmacol. 2005;20(2):97–104. [DOI] [PubMed] [Google Scholar]
- 27.Newburn G, Edwards R, Thomas H, Collier J, Fox K, Collins C. Moclobemide in the treatment of major depressive disorder (DSM-3) following traumatic brain injury. Brain Inj. 1999;13(8): 637–642. [DOI] [PubMed] [Google Scholar]
- 28.Rapoport MJ, Chan F, Lanctot K, Herrmann N, McCullagh S, Feinstein A. An open-label study of citalopram for major depression following traumatic brain injury. J Psychopharmacol. 2008; 22(8):860–864. [DOI] [PubMed] [Google Scholar]
- 29.Rao V; Forest Laboratories. Lexapro for the treatment of traumatic brain injury (TBI) depression & other psychiatric conditions. https://www.clinicaltrials.gov/ct2/show/NCT01368432. Accessed November 28, 2017.
- 30.Saran AS. Depression after minor closed-head injury: role of dexamethasone suppression test and antidepressants. J Clin Psychiatry. 1985;46(8):335–338. [PubMed] [Google Scholar]
- 31.Ansari A, Jain A, Sharma A, Mittal RS, Gupta ID. Role of sertraline in posttraumatic brain injury depression and quality of life in TBI. Asian J Neurosurg. 2014;9(4):182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashman TA, Cantor JB, Gordon WA, et al. A randomized controlled trial of sertraline for the treatment of depression in persons with traumatic brain injury. Arch Phys Med Rehabil. 2009; 90(5):733–740. [DOI] [PubMed] [Google Scholar]
- 33.Fann JR, Uomoto JM, Katon WJ. Sertraline in the treatment of major depression following mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2000;12(2):226–232. [DOI] [PubMed] [Google Scholar]
- 34.Fann JR, Bombardier CH, Temkin N, et al. Sertraline for major depression during the year following traumatic brain injury: a randomized controlled trial. J Head Trauma Rehabil. 2017;32(5):332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horsfield SA, Rosse RB, Tomasino V, Schwartz BL, Mastropaolo J, Deutsch SI. Fluoxetine’s effects on cognitive performance in patients with traumatic brain injury. Int J Psychiatry Med. 2002; 32(4):337–344. [DOI] [PubMed] [Google Scholar]
- 36.Dinan TG, Mobayed M. Treatment resistance of depression after head injury: a preliminary study of amitriptyline response. Acta Psychiatr Scand. 1992;85(4):292–294. [DOI] [PubMed] [Google Scholar]
- 37.Salter KL, McClure JA, Foley NC, Sequeira K, Teasell RW. Pharmacotherapy for depression posttraumatic brain injury: a meta-analysis. J Head Trauma Rehabil. 2016;31(4):E21–E32. [DOI] [PubMed] [Google Scholar]
- 38.Maas AI, Lingsma HF; IMPACT Study Group. New approaches to increase statistical power in TBI trials: insights from the IMPACT study. Acta Neurochir Suppl. 2008;101:119–124. [DOI] [PubMed] [Google Scholar]
- 39.Osborn AJ, Mathias JL, Fairweather-Schmidt AK. Depression following adult, non-penetrating traumatic brain injury: a meta-analysis examining methodological variables and sample characteristics. Neurosci Biobehav Rev. 2014;47:1–15. [DOI] [PubMed] [Google Scholar]
- 40.Seel RT, Macciocchi S, Kreutzer JS. Clinical considerations for the diagnosis of major depression after moderate to severe TBI. J Head Trauma Rehabil. 2010;25(2):99–112. [DOI] [PubMed] [Google Scholar]
- 41.Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutherford BR, Mori S, Sneed JR, Pimontel MA, Roose SP. Contribution of spontaneous improvement to placebo response in depression: a meta-analytic review. J Psychiatr Res. 2012;46(6):697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fann JR, Bombardier CH, Vannoy S, et al. Telephone and in-person cognitive behavioral therapy for major depression after traumatic brain injury: a randomized controlled trial. J Neurotrauma. 2015;32(1):45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponsford J, Lee NK, Wong D, et al. Efficacy of motivational interviewing and cognitive behavioral therapy for anxiety and depression symptoms following traumatic brain injury. Psychol Med. 2016;46(5):1079–1090. [DOI] [PubMed] [Google Scholar]
- 45.Bedard M, Felteau M, Marshall S, et al. Mindfulness-based cognitive therapy reduces symptoms of depression in people with a traumatic brain injury: results from a randomized controlled trial. J Head Trauma Rehabil. 2014;29(4):E13–E22. [DOI] [PubMed] [Google Scholar]
- 46.Entsuah R, Shaffer M, Zhang J. A critical examination of the sensitivity of unidimensional subscales derived from the Hamilton Depression Rating Scale to antidepressant drug effects. J Psychiatr Res. 2002;36(6):437–448. [DOI] [PubMed] [Google Scholar]
- 47.Dyer JR, Williams R, Bombardier CH, Vannoy S, Fann JR. Evaluating the psychometric properties of 3 depression measures in a sample of persons with traumatic brain injury and major depressive disorder. JHeadTraumaRehabil. 2016;31(3):225–232. [DOI] [PubMed] [Google Scholar]
- 48.Han K, Chapman SB, Krawczyk DC. Altered amygdala connectivity in individuals with chronic traumatic brain injury and comorbid depressive symptoms. Front Neurol. 2015;6:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hudak A, Warner M, Marquez de la Plata C, Moore C, Harper C, Diaz-Arrastia R. Brain morphometry changes and depressive symptoms after traumatic brain injury. Psychiatry Res. 2011;191(3):160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jorge RE, Acion L, Starkstein SE, Magnotta V. Hippocampal volume and mood disorders after traumatic brain injury. Biol Psychiatry. 2007;62(4):332–338. [DOI] [PubMed] [Google Scholar]
- 51.Maller JJ, Thomson RH, Pannek K, Bailey N, Lewis PM, Fitzgerald PB. Volumetrics relate to the development of depression after traumatic brain injury. Behav Brain Res. 2014;271:147–153. [DOI] [PubMed] [Google Scholar]
- 52.Schonberger M, Ponsford J, Reutens D, Beare R, Clarke D, O’Sullivan R. The relationship between mood disorders and MRI findings following traumatic brain injury. Brain Inj. 2011; 25(6):543–550. [DOI] [PubMed] [Google Scholar]
- 53.Maller JJ, Thomson RH, Pannek K, et al. The (Eigen)value of diffusion tensor imaging to investigate depression after traumatic brain injury. Hum Brain Mapp. 2014;35(1):227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao V, Mielke M, Xu X, et al. Diffusion tensor imaging atlas-based analyses in major depression after mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2012;24(3):309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen CH, Suckling J, Ooi C, et al. Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology. 2008;33(8):1909–1918. [DOI] [PubMed] [Google Scholar]
- 56.Failla MD, Juengst SB, Arenth PM, Wagner AK. Preliminary associations between brain-derived neurotrophic factor, memory impairment, functional cognition, and depressive symptoms following severe TBI. Neurorehabil Neural Repair. 2016;30(5):419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Juengst SB, Kumar RG, Failla MD, Goyal A, Wagner AK. Acute inflammatory biomarker profiles predict depression risk following moderate to severe traumatic brain injury. J Head Trauma Rehabil. 2015;30(3):207–218. [DOI] [PubMed] [Google Scholar]
- 58.Bailey NW, Segrave RA, Hoy KE, Maller JJ, Fitzgerald PB. Impaired upper alpha synchronisation during working memory retention in depression and depression following traumatic brain injury. Biol Psychol. 2014;99:115–124. [DOI] [PubMed] [Google Scholar]
- 59.Failla MD, Burkhardt JN, Miller MA, et al. Variants of SLC6A4 in depression risk following severe TBI. Brain Inj. 2013;27(6):696–706. [DOI] [PubMed] [Google Scholar]
- 60.Luby JL, Barch D, Whalen D, Tillman R, Belden A. Association between early life adversity and risk for poor emotional and physical health in adolescence: a putative mechanistic neurodevelopmental pathway. JAMA Pediatr. 2017;171(12):1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pettinati HM O´Brien CP, Dundon WD. Current status of cooccurring mood and substance use disorders: a new therapeutic target. Focus. 2015;13(3):356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10(9):1089–1093. [DOI] [PubMed] [Google Scholar]
- 63.Vecht CJ, van Woerkom CA, Teelken AW, Minderhoud JM. Homovanillic acid and 5-hydroxyindoleacetic acid cerebrospinal fluid levels. A study with and without probenecid administration of their relationship to the state of consciousness after head injury. Arch Neurol. 1975;32(12):792–797. [DOI] [PubMed] [Google Scholar]
- 64.Kaplan GB, Vasterling JJ, Vedak PC. Brain-derived neurotrophic factor in traumatic brain injury, posttraumatic stress disorder, and their comorbid conditions: role in pathogenesis and treatment. Behav Pharmacol. 2010;21(5/6):427–437. [DOI] [PubMed] [Google Scholar]