Dear editor,
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) appeared in late 2019. Since then, SARS-CoV-2 spread worldwide and led to a health crisis due to coronavirus disease (COVID-19). To fight against this pandemic, several vaccines against SARS-CoV-2 were developed [1]. Two mRNA vaccines, BNT162b2 from Pfizer-BioNTech and mRNA-1273 from Moderna, were the first to be approved and administered since December 2020. Pivotal studies reported high seroconversion rates after mRNA SARS-CoV-2 vaccination [2].
A prior exposure to SARS-CoV-2 was associated with higher antibody titers after vaccination ,[3] although the variation of the antibody titers over time and the persistence of the leverage effects of prior exposure are poorly known. In addition, persons who have had COVID-19 are thought to have protective immunity and memory responses. The appearance of new cases in vaccinated patients or patients with prior COVID-19 infection have raised concerns about the effectiveness of vaccines over time, and, in many countries, a third-dose vaccine administration, used as a booster, is being implemented for all patients according to age and risk.
Using data from a cohort of vaccinated health care professionals (HCP) VACCICOVAO study, we examined the relationship between previous COVID-19 infection and vaccine protection over time and compared the evolution of the neutralizing antibody titers against SARS-CoV-2 after 2 doses of the BNT162b2 or mRNA-1273 vaccines. Serologic testing was performed 2 and 8 months after the first dose (March-April and September-October 2021, respectively) (Fig. 1S supplementary material). The SARS-CoV-2 IgG II Quant assay (Abbott) was used for the determination of IgG antibodies to the receptor binding domain (RBD) of the S1 subunit of the spike protein of SARS-CoV-2 (S1 IgG). Data on age, sex, hospital job post, comorbidity, COVID-19 previous infection, chronic treatment, vaccination schedule, specific vaccine and adverse events after vaccination were collected from each participant. The study was performed in accordance with the institutional review board of HURH (21-EO031). Written informed consent was obtained from all participants. Prior infection was defined a previous positive polymerase chain reaction (PCR) or positive antigen test on nasopharyngeal swab, and/or IgG antibodies to the anti-nuclei capsid (anti N) or anti S1 positivity at any point. New infection after vaccination was defined as a new positive PCR or antigen test. Date of infection was defined as the date of the first positive test.
A total of 680 volunteers (87% women, 45 ± 11 years, 0.8 ± 1 point on Charlson comorbidity index) were enrolled in this study (21% of the 3204 vaccinated HCP in HURH). A total of 125 (18%) was previously infected with SARS-CoV-2 (95 had symptomatic COVID-19 and 4 needed to be hospitalized). Five hundred and sixty-one were vaccinated with BNT162b2 (83%) and 119 with mRNA-1273 (18%) (baseline characteristics of participants, Table S1, vaccine side-effects, Table S2, supplementary material).
Eight vaccinated HCP (1.2%) had a SARS-CoV-2 infection during the 8 months period after the first dose of vaccine. From these eight new COVID-19 cases, 1 had a prior COVID-19 infection (0.8%) vs. 7 in the group of uninfected patients (1.3%) (log Rank Test, P = x003D0.670). Seven received BNT162b2 vaccine (1.3%) and 1 mRNA-1273 vaccine (0.9%) (log Rank Test, P = 0.733) (Fig. S2, supplementary material). All the new cases were female (1.4%) (log Rank Test, P = 0.263). Seven new cases were in the 41 to 60 years old group (1.8%), 1 case in the group of under 40 and no case in the oldest participants. All the new cases were mild or asymptomatic.
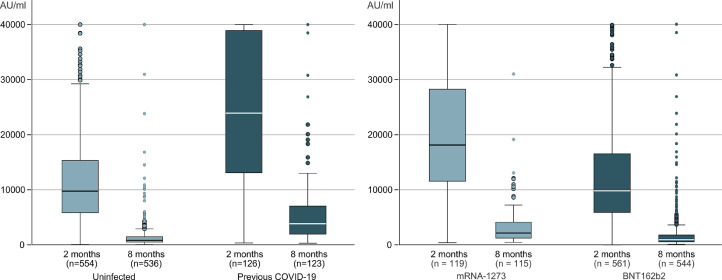
S1 IgG titers after eight months (mean 2309 U/ml [95% CI, 1956 to 2653]) were clearly reduced compared with titers 2 months after vaccination (mean 14,132 U/ml [95% CI, 13,342 to 14,922]) with a mean 75% reduction [95% CI, −63 to −87], p < 0.001 (Table S3, Supplementary material). At eight months, only 1 volunteer become seronegative. On multiple lineal regression, the higher titers of antibodies were associated with previous COVID-19 infection and type of mRNA vaccine at 2 and 8 month (Fig. 1 ). Age, gender, and comorbidity were not associated with antibodies titers in our series.
Fig. 1.
Antibodies titers S1 SARS CoV-2, 2 and 8 months after first dose of vaccine of COVID-19 according to previous infection with SARS-CoV-2 or mRNA vaccine.
Our study confirmed the significantly humoral immunogenicity reduction in patients vaccinated with mRNA vaccines with the passing of the months. However, a high level of protection against new infection, and especially against severe COVID-19 infection remains. The correlation of neutralizing antibodies and the protection against COVID-19 has been showed [4] although the ability to predict protection against the virus based on antibodies has not been proven.
The incidence of new cases of SARS-CoV-2 infection in vaccinated HCP in our series is similar to previously published series [5] accordingly with the reduction with the decline of new cases expected after vaccination. The new cases were mild with no severe presentation. There were no differences between the two mRNA vaccines at preventing symptomatic COVID-19.
All the participants develop immune response and only one patient (previously uninfected) tested negative for S1 IgG at eight months. The lack of non-respondent participants may be explained because the sample consisted of HCP, most of them healthy, under the age of 60 years and with a very small percentage of participants on immunosuppressive therapies.
The waning of immune humoral response to vaccines over months in our series is similar to recently published series [6]. In our series there were no differences between gender, even there was a trend to lowest waning in Ac titers in men, contrary to what Levin's study show [6]. More studies are needed to confirm or exclude this association.
As might be expected, previous infection was the main factor related with increased immunogenicity against the virus [7] and with the persistence of the level of antibodies in vaccinated patients [8]. On the other hand, in our cohort, as in previous studies [9,10], vaccination with mRNA-1273 elicited somewhat stronger immune responses than BNT162b2. The longer interval between doses and the higher mRNA doses of the mRNA-1273 vaccine are proposed as likely explanations. The clinical relevance of these difference in antibodies titers between the two mRNA vaccines, and whether this translates to better clinical protection are unknown.
Finally, the data on side-effects after vaccination in our cohort were similar to other real-world studies. Local side effects were reported for most participants. Systemic reactions following the second dose were higher than after the first dose, especially for those without previous SARS-CoV-2 infection. Side-effects were more common among individuals with previous SARS-CoV-2 infection than among those without known past infection after the first dose. The rate of side effects equalized following the second dose. There were no severe side effects.
The study has several limitations. First, the use of a convenience sample of HCP, which means results may not be generalizable. Second, cellular immunity was not tested. Third, prior infection was identified based on record of PCR or Ag positive results and the presence of positive Antibodies test. Cases that were unaware of their infection and did not seek testing to confirm the disease were missing. Fourth, due to the low number of new COVID cases during follow-up, it is not possible to detect differences regarding the ability to protect against new infections.
Prior infection with SARS-CoV-2 was associated with higher humoral immunogenicity. The greatest reduction of antibodies titers in patients without previous infection could be considered to guide the strategy to prioritize the administration of booster doses of vaccines.
Additional contributions
We thank Sandra Pérez Fernández for her work with data collection. We thank Dr. Ruíz-Albi, Tomás y Dr. López-Izquierdo, Raúl for critically reviewed the study proposal and manuscript. We acknowledge the blood collection area and the laboratory staff at HURH for their efforts and contributions to make this study possible.
Role of the funder/sponsor
None.
CRediT authorship contribution statement
Jesús Fernando García-Cruces-Méndez: Data curation, Formal analysis, Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing, Project administration. Luis Corral-Gudino: Conceptualization, Funding acquisition, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Project administration. María Piedad Del-Amo-Merino: Funding acquisition, Formal analysis, Data curation, Writing – review & editing. José María Eiros-Bouza: Funding acquisition, Formal analysis, Data curation, Writing – review & editing, Supervision. Marta Domínguez-Gil González: Conceptualization, Funding acquisition, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Project administration.
Declaration of Competing Interest
The authors declare they have no conflict of interest.
Funding/support
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ejim.2022.01.011.
Appendix. Supplementary materials
References
- 1.Angeli F., Spanevello A., Reboldi G., Visca D., Verdecchia P. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1–8. doi: 10.1016/j.ejim.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steensels D., Pierlet N., Penders J., Mesotten D., Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021 doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari D., Clementi N., Mancini N., Locatelli M. SARS-CoV-2 infection despite high levels of vaccine-induced anti-RBD antibodies: a study on 1110 healthcare professionals from a northern Italian university hospital. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.10.010. S1198-743X(21)00611-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021 doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassaniti I., Bergami F., Percivalle E., Gabanti E., Sammartino J.C., Ferrari A., et al. Humoral and cell-mediated response against SARS-CoV-2 variants elicited by mRNA vaccine BNT162b2 in healthcare workers: a longitudinal observational study. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.09.016. S1198-743X(21)00536-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egbert E.R., Xiao S., Colantuoni E., Caturegli P., Gadala A., Milstone A.M., et al. Durability of spike immunoglobin G antibodies to SARS-CoV-2 among health care workers with prior infection. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.23256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markewitz R., Pauli D., Dargvainiene J., Steinhagen K., Engel S., Herbst V., et al. The temporal course of T- and B-cell responses to vaccination with BNT162b2 and mRNA-1273. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.09.006. S1198-743X(21)00496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu-Raddad L.J., Chemaitelly H., Ayoub H.H., Yassine H.M., Benslimane F.M., Al Khatib H.A., et al. Association of prior SARS-CoV-2 infection with risk of breakthrough infection following mRNA vaccination in Qatar. JAMA. 2021 doi: 10.1001/jama.2021.19623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.