Abstract
Notch genes encode a family of transmembrane proteins that are involved in many cellular processes such as differentiation, proliferation, and apoptosis. Although it is well established that all four Notch genes can act as oncogenes, the mechanism by which Notch proteins transform cells remains unknown. Previously, we have shown that transformation of RKE cells can be conditionally induced by hormone activation of Notchic-estrogen receptor (ER) chimeras. Using this inducible system, we show that Notchic activates transcription of the cyclin D1 gene with rapid kinetics. Transcriptional activation of cyclin D1 is independent from serum-derived growth factors and de novo synthesis of secondary transcriptional activators. Moreover, hormone activation of Notchic-ER proteins induces CDK2 activity in the absence of serum. Upregulation of cyclin D1 and activation of CDK2 by Notchic result in the promotion of S-phase entry. These data demonstrate the first evidence that Notchic proteins can directly regulate factors involved in cell cycle control and affect cellular proliferation. Furthermore, nontransforming Notchic proteins do not induce cyclin D1 expression, indicating that the mechanism of transformation involves cell cycle deregulation through constitutive expression of cyclin D1. Finally, we have identified a CSL [stands for CBF1, Su(H), and Lag-1] binding site within the human and rat cyclin D1 promoters, suggesting that Notchic proteins activate cyclin D1 transcription through a CSL-dependent pathway.
There is now significant evidence that constitutively active alleles of all four Notch genes are involved in oncogenesis (2, 3, 14, 37, 42, 48). Furthermore, the importance of Notch mutations in the development of neoplastic diseases is underscored by studies of proviral-insertional mutagenesis. Infection of mice carrying a c-myc, E2A-PBX1, or erbB2 transgene with replication-competent retroviruses results in proviral integrations into the Notch1 locus, generating constitutively active alleles of Notch1 (9, 11, 15). In T-cell acute lymphoblastic leukemia, the chromosomal translocation t(7;9)(q34;q34.3) joins a portion of the T-cell receptor gene to the Notch1 gene, resulting in the aberrant expression of truncated Notch proteins (10). Although this is the only known genetic alteration involving the Notch1 locus in a human cancer, aberrant expression of Notch1, Notch2, and Notch3 has been observed in human cancers of different origins (1b, 6, 40, 56). Truncation of the extracellular sequences from Notch proteins results in constitutively active forms (termed Notchic) that are no longer tethered to the plasma membrane and localize primarily in the nucleus. Mutant forms of Notch1 and Notch2 analogous to those found in cells harboring a t(7;9)(q34;q34.3) translocation (Notch1ic and Notch2ic, respectively) transform an E1A-immortalized baby rat kidney cell line (RKE). These transformed cells form colonies in semisolid media and are tumorigenic in nude mice (3). The molecular mechanism by which Notch induces neoplastic transformation is not known. However, we have recently shown that both nuclear localization and transcriptional activation are requirements for Notchic-induced transformation (23, 43).
The current model for Notch signaling proposes that, following ligand binding, Notch is proteolytically processed, releasing the intracellular domain (Notchic) from the plasma membrane, which subsequently translocates to the nucleus. Once in the nucleus, Notchic interacts with CSL [acronym for CBF1, Su(H) and Lag-1] (22, 26, 29, 44, 49). It has been shown that CSL interacts with SMRT and CIR, which form complexes with histone deacetylases, suggesting a role for CSL in the repression of transcription (20, 25, 57). It has been proposed that Notchic binds to CSL, displacing the repressor complex and forming an activating complex, thereby converting CSL into an activator of transcription. It has been shown that Notch is able to interact with the histone acetylases PCAF and GCN5 by two-hybrid analysis, suggesting that a possible mechanism of induction of transcription of CSL target genes may involve chromatin remodeling (13, 19, 26, 30, 33).
A hallmark of neoplastic transformation is uncontrolled cellular proliferation. Deregulation of components of the cell cycle machinery that control the transition from the G1 to the S phase of the cell cycle is frequently involved in tumorigenesis. As a consequence, cells proliferate independently of extracellular queues. For example, tumor cells generally do not respond to growth-inhibitory signals induced by contact with neighboring cells and grow with a reduced dependence on serum in media (45, 52). Cyclin D1 has been shown to play a critical role in the control of the G1/S transition of the cell cycle. The level of expression of cyclin D1 is crucial for its function, and it is finely regulated. Cyclin D1 accumulation depends on the activation of transcription of the gene and is induced by many mitogenic signals. Cyclin D1 downregulation is, instead, mediated by a rapid degradation of the protein following ubiquitination (7, 8, 27, 46, 47). Overexpression of cyclin D1 has been shown to be associated with alterations of cell cycle kinetics and the acceleration of G1 phase, suggesting that cyclin D1 can directly induce cellular proliferation. Cells overexpressing cyclin D1 show a reduced dependence on serum and are, therefore, able to proliferate in lower concentrations of serum. However, cells overexpressing cyclin D1 grow in a reduced amount of serum with lower kinetics than those observed in cells in the presence of normal levels of serum, indicating that cyclin D1 is not able to fully compensate for the absence of serum-derived mitogens (36, 39, 58). Cyclin D1 alone is not able to induce complete cellular transformation. Expression of cyclin D1 in both primary and immortalized rodent cells did not result in the formation of foci but produced morphological transformation accompanied by acceleration of growth (18, 24). However, in several human tumors aberrant expression of cyclin D1 has been detected as a consequence of amplification or rearrangement of the gene and it has recently been suggested that cyclin D1 is likely to play an important role in transformation mediated by several other oncogenes, indicating a key role for cyclin D1 in tumorigenesis (16, 34, 41, 45, 50).
Previously, we demonstrated that Notchic proteins transform RKE cells and that transformation can be finely modulated by hormone activation of inducible Notchic-estrogen receptor (ER) chimeras (3, 23, 43). Here we use this inducible system in order to define the mechanism of transformation mediated by Notchic. We provide the first evidence that Notchic proteins can affect factors that play a critical role in cell cycle control. Following hormone induction, Notchic-ER chimeras activate cyclin D1 mRNA transcription, independently from serum-derived growth factors and de novo synthesis of secondary transcription factors. Furthermore, hormone induction of Notchic-ER chimeras leads to activation of CDK2. As a consequence, cells expressing Notchic display an increase in the number of cells undergoing DNA synthesis. Nontransforming Notchic mutant (mut) proteins fail to activate cyclin D1 transcription, indicating that Notchic proteins induce the transformation of RKE cells, in part, by deregulating the cell cycle through overexpression of cyclin D1. Finally, we demonstrate that CSL can bind to a CSL binding site identified in the cyclin D1 promoter, suggesting that activation of cyclin D1 transcription by Notchic proteins is CSL dependent.
MATERIALS AND METHODS
Cells.
RKE and 293T cells were propagated in culture in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS). Clonal RKE cell lines expressing the Notchic-ER chimeras Nic, Nic with RAM deleted (NicΔR), and Nic with amino acids 2105 to 2114 deleted (NicΔ2105–2114) were previously described (23, 43). In order to obtain the clonal RKE cell line expressing RasV12 (RKE-Ras), RKE cells were transfected with ZipNeo Kras4BV12 (kindly provided by C. J. Der) using lipofectamine (Gibco-BRL). Isolated neomycin-resistant colonies were selected, and cell lines were propagated in Dulbecco's modified Eagle's medium supplemented with 10% FBS and 400 μg of neomycin per ml.
RT-PCR analysis.
Total RNA was extracted from confluent cultures using Trizol Reagent (Life Technologies) according the manufacturer's specifications. cDNA was synthesized from 2 μg of RNA using Moloney murine leukemia virus reverse transcriptase (RT; Promega) according to the manufacturer's protocol. One microliter of the 20-μl cDNA sample was used for PCR amplification. PCRs were performed in a 50-μl mixture containing a 0.5 μM concentration of each gene-specific primer, 1.5 mM MgCl2, 200 μM each deoxynucleoside triphosphate, 1× PCR buffer (Hybaid), 0.2 U of DNA polymerase (Hybaid), and 0.2 μCi of [α-32P]dCTP. Sequences of primers used to specifically amplify endogenous rat mRNA for cyclin D1, cyclin E, E2F-1, and Cdc25A are as described in the work of Vigo et al. (51). The sequences of rat β-actin primers used for PCR are 5′-CGATATCGCTGCGCTCGTCGTCGAC-3′ and 5′-GGCCAGGATAGAGCCACCAATCCAC-3′. In order to perform semiquantitative PCR, we used cycling parameters that displayed a linear range of amplification for each set of primers, determined by amplifications of serial dilutions of cDNAs. Amplified PCR products were separated on a 4% polyacrylamide gel in 1× Tris-acetate-EDTA buffer. Quantification of the mRNA levels was performed using a Storm PhosphorImager (Molecular Dynamics) and Image Quant analysis software.
Northern blot analysis.
Total RNA was isolated as described for RT-PCR. Thirty micrograms of RNA per sample was electrophoresed on a 1% agarose–6.5% formaldehyde gel and transferred overnight onto a nylon filter. The hybridization was performed overnight at 65°C using cyclin D1 and β-actin cDNAs radiolabeled with [α-32P] dCTP using a randomly primed labeling kit (New England Biological Laboratories). The hybridization mixture contained 6× SSC (1× SSC is 0.15 μ NaCl plus 0.015 μ sodium citrate), 5× Denhardt's solution, 0.5% sodium dodecyl sulfate (SDS), and 0.1 mg of salmon sperm DNA per ml. The blots were washed under high-stringency conditions, and messages were visualized by autoradiography.
CDK2 assay.
To measure CDK2 activity, clonal RKE cell lines were grown to confluence in 100-mm-diameter plates. Following serum starvation in medium containing 0.1% FBS for 48 h, cells were incubated overnight in medium containing either 0.1 or 10% FBS in the presence or absence of 1 μM 4-hydroxytamoxifen (OHT). Samples not treated with hormone received an equal volume of a 100% ethanol vehicle. Cells were lysed as described in the work of Matsushime et al. (35). CDK2 was immunoprecipitated from precleared lysates with an anti-CDK2 polyclonal antibody (kindly provided by K. Keyomarsi). The immunocomplexes were collected and assayed for kinase activity as previously described (4). Labeled proteins were separated by SDS-polyacrylamide gel electrophoresis and visualized by autoradiography. Quantitative analysis was performed as described for RT-PCR.
Thymidine incorporation.
Clonal RKE cell lines were seeded in 24-well plates at 80% confluence. Following serum deprivation for 48 h, cells were induced in the presence or absence of 1 μM OHT in 0.1, 5, or 10% FBS for the times indicated in Fig. 4B. Measurement of [3H]thymidine incorporation into DNA was performed as previously described by incubating cells with 1 μCi of [3H]thymidine per ml for 2 h at 37°C (55).
FIG. 4.
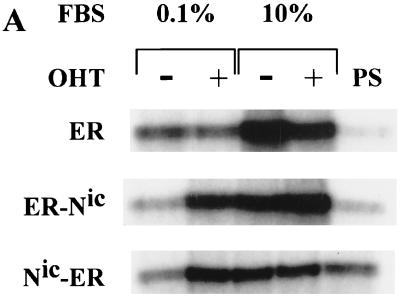
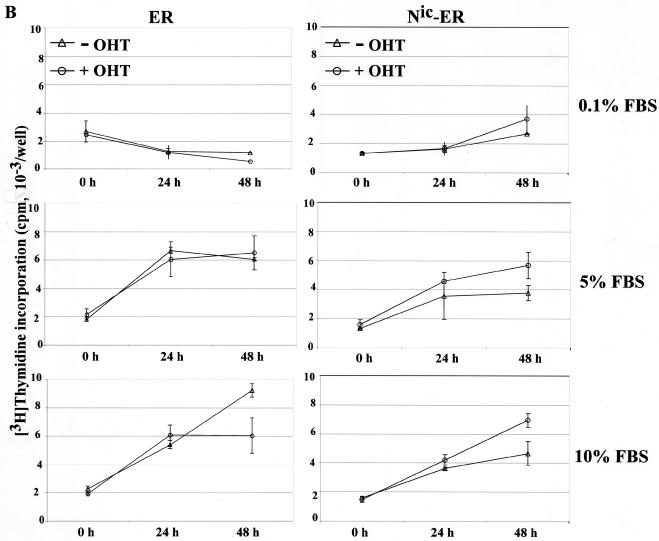
Hormone-dependent induction of CDK2 activity and DNA synthesis by Notchic-ER chimeras. (A) CDK2 assay. Clonal RKE cell lines were starved in medium containing 0.1% serum for 48 h and induced with (+) or without (−) 1 μM OHT in medium with a low (0.1%) or a full (10%) concentration of FBS for 12 h. Cell lysates were prepared from RKE clones expressing ER alone, ER-Nic, and Nic-ER chimeras. Proteins were immunoprecipitated with anti-CDK2 antibody or preimmune rabbit serum (PS). Immunocomplexes were assayed for kinase activity using histone H1 as a substrate. Labeled proteins were separated by SDS-polyacrylamide gel electrophoresis and visualized by autoradiography. (B) DNA synthesis was assayed by measuring [3H]thymidine incorporation. RKE clonal lines expressing ER alone or Nic-ER chimeras were serum deprived for 48 h and, subsequently, induced with 0.1, 5, or 10% FBS in the presence (+ OHT) or absence (− OHT) of 1 μM OHT. [3H]thymidine incorporation was measured as described in Materials and Methods. Data are expressed as counts per minute per well and are the means ± standard errors for quadruplicate measurements. The results are representative of three independent experiments.
EMSA.
293T cells were transiently transfected, using BES-modified calcium phosphate precipitation, with 5 μg of either green fluorescent protein (GFP) or a hemagglutinin (HA)-tagged CSL expression plasmid (CBF1-HA; kindly provided by P. D. Ling). (To be consistent with current literature, we refer to this protein as CSL throughout this paper.) After 48 h, cells were lysed and protein expression was assayed by Western immunoblotting as previously described (43), using an anti-CSL antibody (kindly provided by E. Bresnick) or an anti-HA tag antibody (HA-probe, Y-11; Santa Cruz). DNA binding reactions were performed according to the method of Lam and Bresnick (32) by incubating 20 μg of total cell extract with 60 fmol of 32P-labeled duplex oligonucleotides containing CSL binding sites. DNA-protein complexes were resolved on a 6.5% nondenaturing polyacrylamide gel in 0.75× Tris-acetate-EDTA buffer. The complete sequence of the 5′-3′ strand of the oligonucleotides used for electrophoretic mobility shift assays (EMSA) is shown in Fig. 5A. The putative CSL binding site, GCTGAGAT, is in the center of the oligonucleotide and is flanked at both the 5′ and 3′ ends by 10 residues of a sequence derived from the human or rat cyclin D1 promoter. The mutant site used in our experiments, GCCTGCAG, was previously shown not to bind CSL (31). As a control for DNA binding experiments, we also used an oligonucleotide that contains three copies of a high-affinity CSL binding sequence, GTGGGAA, spanned by 10 random residues (JK3X). The complete sequence of the wild-type (wt) JK3X oligonucleotide is 5′-CGTGGGAAATTTGACTTCGTGGGAAATTTGACTTCGTGGGAA-3′, and that of the mut JK3X is 5′-CTCTGGAAATTTGACTTCTCTGGAAATTTGACTTCTCTGGAA-3′, where the CSL binding site is underlined and the mutated residues are shown in boldface.
FIG. 5.
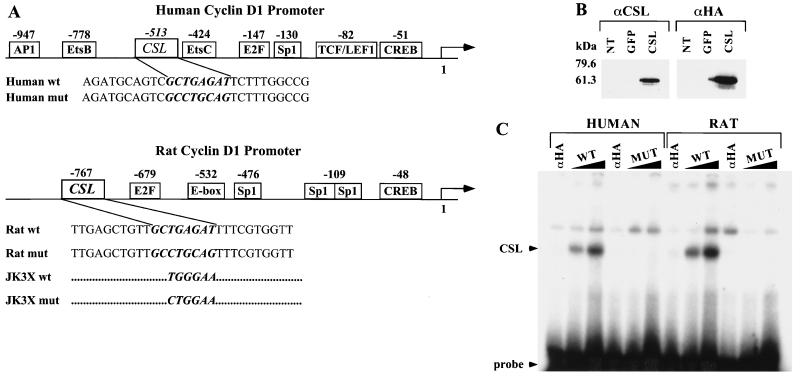
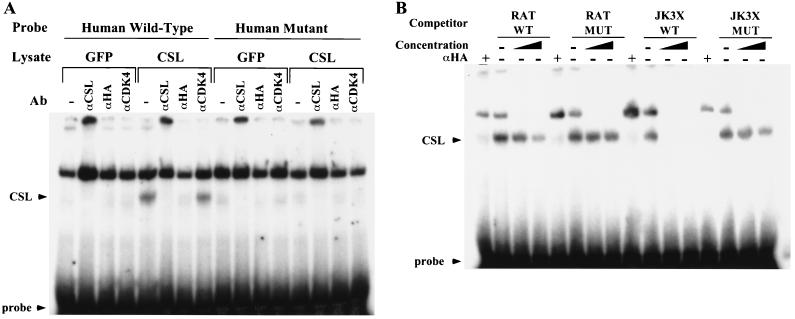
Identification of a CSL binding site in the human and rat cyclin D1 promoters. (A) Schematic representation of the human and rat cyclin D1 promoter. The binding sites for transcription factors identified in the cyclin D1 promoter are indicated with their positions relative to the starting site of transcription. The sequences of coding strands of the oligonucleotides used in the EMSA are reported under the schematic representation. In italic and bold is the wt or mut sequence for the CSL binding site identified in the human or the rat cyclin D1 promoter. The JK3X oligonucleotides contain three repeats of the indicated wt or mut consensus sequence for the CSL binding site. The complete sequences for the JK3X oligonucleotides are described in Materials and Methods. (B) Expression of HA-tagged CSL in 293T cells. Cell lysates were prepared from 293T cells not transfected (NT) or transiently transfected with an expression vector encoding GFP (lanes or GFP) the CSL HA-tagged protein (lanes CSL). Protein expression was analyzed by Western immunoblotting using an anti-CSL antibody (αCSL) and an anti-HA tag antibody (αHA). Standard molecular markers are indicated to the left. (C) CSL binds to the sequence identified in the human and rat cyclin D1 promoter. EMSA analysis was performed by incubating increasing amounts (60 and 120 fmol) of the indicated radiolabeled oligonucleotide duplex with 20 μg of lysate extracted from 293T cells expressing HA-tagged CSL. Sixty femtomoles of each oligonucleotide duplex was also incubated with lysates preincubated with the anti-HA probe antibody (lanes αHA). The positions of the CSL-DNA complex and of the free probe are indicated.
RESULTS
Hormone-dependent activation of Notchic-ER chimeras induces cyclin D1 mRNA transcription.
Recently we created inducible Notchic chimeric proteins by fusing the intracellular portion of Notch to the estrogen binding domain of the human ER. We reported that the activation of Notchic-ER chimeras can be finely modulated by treatment with OHT and that hormone activation of Notchic-ER proteins induces transformation of RKE cells in a dose-dependent manner (43). Transformed cells are characterized by uncontrolled cellular proliferation. An important checkpoint of cellular proliferation is the transition from the G1 to the S phase of the cell cycle. A fundamental mechanism to control the G1/S transition is the regulation of expression of cell cycle machinery components. In order to determine if Notchic-induced transformation involves transcriptional activation of genes encoding factors known to play a role in this transition, we analyzed the mRNA levels of the cyclin D1, cyclin E, Cdc25A, and E2F-1 genes by RT-PCR.
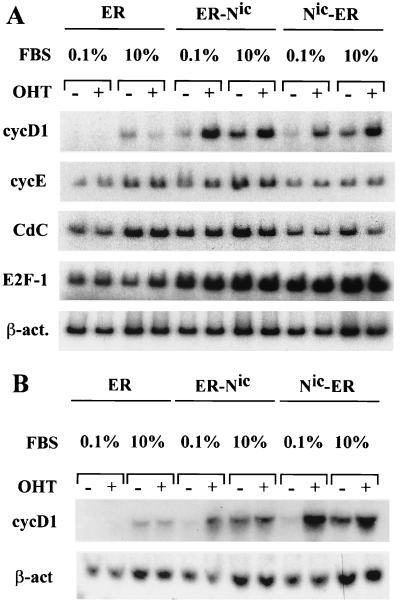
Total RNA was extracted from clonal RKE cell lines expressing either Notchic-ER chimeras (ER-Nic and Nic-ER) or the estrogen binding domain (ER) alone (43). Following serum deprivation for 48 h, cells were treated for 5 h with or without 1 μM OHT in medium containing either a low (0.1%) or a full (10%) concentration of serum. Hormone activation of Notchic-ER chimeras induced the accumulation of cyclin D1 mRNA in a low concentration of serum (Fig. 1A). In cells expressing the ER-Nic chimera, the level of cyclin D1 mRNA detected in samples treated in 0.1% serum plus 1 μM OHT was approximately sevenfold higher than the level detected in a low concentration of serum in the absence of OHT. Under the same experimental conditions, cells expressing the Nic-ER chimeric protein displayed a four-fold increase in the induction of cyclin D1 transcription. The level of cyclin D1 induced by serum alone was approximately threefold higher than the basal level detected in 0.1% serum in the absence of OHT. However, the induction of cyclin D1 transcription by serum in the presence of OHT was twofold higher than the induction by serum alone (Fig. 1). In control cells expressing ER alone, cyclin D1 mRNA was detectable only upon stimulation with 10% serum. OHT had no effect on cyclin D1 mRNA expression (Fig. 1A). Northern blot analysis of total RNA extracted from samples treated under the experimental conditions described above confirmed the induction of cyclin D1 transcription by hormone-activated Notchic-ER chimeras, validating the results observed by RT-PCR (Fig. 1B). We did not observe an increase in mRNA levels for cyclin E, E2F-1, and Cdc25A upon hormone activation of Notchic-ER (Fig. 1A), indicating that hormone-activated Notchic-ER chimeras specifically induced transcription of cyclin D1. Furthermore, our data show that the activation of cyclin D1 induced by Notchic-ER proteins is independent of the induction by other serum growth factors.
FIG. 1.
Hormone-dependent upregulation of cyclin D1 mRNA level by Notchic-ER chimeras. (A) Following starvation for 48 h in medium containing 0.1% serum, cells were cultured for 5 h in low-concentration serum (FBS at 0.1%) or full-concentration serum (FBS at 10%) in the presence (+) or absence (−) of 1 μM OHT. Total RNA was extracted from the clonal RKE cell line expressing the ER hormone binding domain (lanes ER), the clonal RKE cell line expressing the ER-Nic chimera (lanes ER-Nic), and the clonal RKE cell line expressing the Nic-ER chimera (lanes Nic-ER). The levels of cyclin D1 mRNA (cycD1), cyclin E (cycE), Cdc25A (CdC), and E2F-1 were examined by RT-PCR as described in Materials and Methods. The level of β-actin mRNA (β-act.) is shown for normalization. (B) Northern blot analysis was performed on total RNA extracted from the clonal RKE cell lines treated as described for panel A.
Induction of cyclin D1 expression by Notchic-ER proteins is direct.
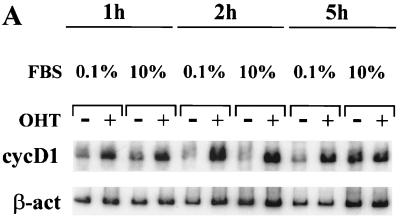
It has been reported that the induction of cyclin D1 transcription by serum typically requires from 3 to 4 h (5, 21, 53, 54). In RKE cells, induction of cyclin D1 mRNA by serum is detectable in approximately 5 h (Fig. 2A). In contrast, cyclin D1 mRNA induced by hormone activation of Notchic-ER proteins can be detected in 1 h (Fig. 2). The rapid induction of cyclin D1 mRNA suggests that Notchic can activate cyclin D1 transcription through a direct mechanism.
FIG. 2.
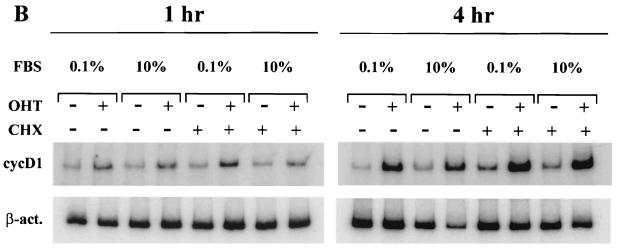
The induction of cyclin D1 transcription by Notchic-ER proteins is direct. (A) Hormone activation of Notchic-ER proteins induces cyclin D1 upregulation in 1 h both in low- and in full-concentration serum. RT-PCR was performed on RNA samples prepared from a clonal RKE cell line expressing the ER-Nic chimera. Cells were cultured in medium containing 0.1% serum for 48 h and subsequently induced for the indicated times by addition of medium containing 0.1% or 10% FBS and 1 μM OHT (+) or ethanol vehicle (−). cycD1, cyclin D1-amplified mRNA samples; β-act; β-actin-amplified mRNA samples for a normalization control. (B) Cyclin D1 induction by Notchic-ER chimeras does not require de novo protein synthesis. Levels of cyclin D1 mRNA were analyzed by RT-PCR. A clonal RKE cell line expressing Nic-ER protein was treated as described for panel A for 1 or 4 h in the absence (−) or presence (+) of 15 μg of CHX per ml.
In order to determine if the accumulation of cyclin D1 mRNA induced by hormone-activated Notchic-ER chimeras was independent of de novo synthesis of secondary transcription factors, we analyzed cyclin D1 expression in cells treated with the protein synthesis inhibitor cycloheximide (CHX). RT-PCR was performed on RNA samples extracted from cells expressing Notchic-ER proteins induced for 1 or 4 h under conditions similar to those described above but in the presence or absence of 15 μg of CHX per ml. CHX had no effect on the induction of cyclin D1 transcription by Notchic-ER proteins. The levels of cyclin D1 mRNA detected were similar in the presence and absence of the inhibitor (Fig. 2B). Under these experimental conditions 99% of protein synthesis was inhibited, as determined by [35S]methionine incorporation (data not shown). These data indicate that the induction of cyclin D1 transcription mediated by hormone-activated Notchic-ER proteins is direct and does not require de novo synthesis of secondary transcription factors.
Nontransforming Notchic proteins fail to activate cyclin D1 transcription.
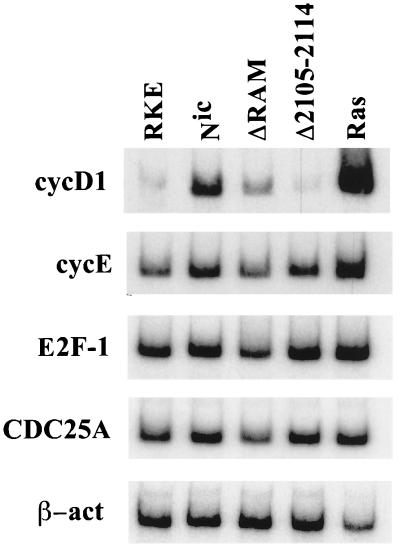
We have previously described mutations that affect the ability of Notchic proteins to induce the transformation of RKE cells (23). In order to determine if the transforming ability of Notchic proteins depends on their ability to activate cyclin D1 transcription, we extracted total RNA from clonal RKE cell lines expressing Notchic and two different Notchic mut proteins and analyzed cyclin D1 mRNA by RT-PCR. Clonal RKE cells expressing Notchic displayed a level of cyclin D1 mRNA sevenfold higher than the level detected for the parental RKE cell line. In contrast, the level of cyclin D1 mRNA detected in the RKE clonal line expressing NicΔR was only threefold higher than the level in RKE cells (Fig. 3). NicΔR lacks the primary binding domain for CSL (RAM domain). We have previously shown that NicΔR displays a weaker ability to form foci than that of Notchic, likely due to a diminished ability to interact with CSL and activate transcription (23). In contrast, NicΔ2105–2114 failed to induce cyclin D1 mRNA expression. The level of cyclin D1 mRNA detected in cells expressing NicΔ2105–2114 was similar to the basal level detected in RKE cells (Fig. 3). NicΔ2105–2114 has a 10-amino-acid deletion that abolishes its ability to transform cells and to activate transcription from a reporter construct containing CSL high-affinity binding sites, although it is still able to bind CSL (23). As described for Notchic-ER chimeras (Fig. 1A), transcriptional activation of cyclin D1 by Notchic was specific, since all clonal RKE cell lines express similar constitutive levels of cyclin E, E2F-1, and Cdc25A mRNA (Fig. 3). For comparison, we determined the level of cyclin D1 mRNA in RKE cells transformed by RasV12 (RKE-Ras). RKE-Ras expressed cyclin D1 to a level 20-fold higher than the level observed in RKE cells. Here we show that Notchic proteins that are incapable of activating cyclin D1 transcription fail to transform cells, suggesting that the mechanism of transformation mediated by Notchic requires the induction of cyclin D1 expression.
FIG. 3.
The level of cyclin D1 expression correlates with the transforming ability of Notchic proteins. RT-PCR was performed on total RNA extracted from confluent RKE clonal cell lines cultured in medium containing 10% FBS. mRNA levels of cyclin D1 (cycD1), cyclin E (cycE), E2F-1, Cdc25A, and β-actin (β-act) were analyzed by RT-PCR. RKE, parental RKE cell line; Nic, clonal RKE cell line expressing Notchic; ΔRAM, clonal RKE cell line expressing NicΔR; Δ2105-2114, clonal RKE cell line expressing NicΔ2105–2114; Ras, clonal RKE cell line expressing Rasv12.
Hormone activation of Notchic-ER chimeras induces CDK2 activity and promotes entry into the S phase of the cell cycle.
We demonstrated that Notchic activates cyclin D1 transcription. We wanted to determine if Notchic is able to affect components of the cell cycle machinery that act downstream of cyclin D1 and, specifically, if it is able to induce cells to progress into the cell cycle. We measured CDK2 activity, which is induced in late G1 and commits cells to entry into the S phase. Following serum deprivation for 48 h, cells were treated in 0.1 or 10% serum in the presence or absence of 1 μM OHT for 12 h. CDK2 was immunoprecipitated from precleared lysates with an anti-CDK2 antibody. Immunocomplexes were assayed for kinase activity using histone H1 as an exogenous substrate for CDK2. Hormone activation of both ER-Nic and Nic-ER chimeras resulted in induction of CDK2 activity in the absence of other serum growth factors (0.1% FBS plus OHT) (Fig. 4A). The level of CDK2 activity detected in samples incubated in low-concentration serum in the presence of OHT was approximately threefold higher than the basal level (Fig. 4A). In contrast, treatment of cells expressing ER with OHT in 0.1% serum did not result in CDK2 activation above the basal level (Fig. 4A). Treatment with serum resulted in CDK2 activity approximately threefold above background in all the RKE cell lines. However, no significant difference was detected in samples incubated in the presence or absence of OHT in 10% serum (Fig. 4A). These results indicate that Notchic proteins can play a critical role in the regulation of the cell cycle through induction of CDK2 activity, independently of other serum-derived mitogenic signals.
In order to determine if Notchic promotes entry into S phase, we determined the effect of hormone activation of Notchic-ER on DNA synthesis. The fraction of cells synthesizing DNA under different culture conditions was determined by [3H]thymidine incorporation. Cells were synchronized by serum deprivation and, subsequently, induced with medium containing 0.1, 5, or 10% FBS in the presence or absence of 1 μM OHT. Following induction for 0, 24, and 48 h, cells were pulse-labeled with [3H]thymidine and the incorporation of [3H]thymidine was measured as described in Materials and Methods. Hormone activation of Notchic-ER chimeras increased DNA synthesis in the presence of serum. In the clonal line expressing the Nic-ER chimera, the level of DNA synthesis in the presence of OHT was approximately 50% higher than the level detected in the absence of hormone, in both 5 and 10% FBS (Fig. 4B, right panels). In low-concentration serum (0.1% FBS), OHT had a modest effect on [3H]thymidine incorporation but the level of DNA synthesis was not significantly higher than the level detected in the absence of hormone (Fig. 4B, top right panel). In the control clonal line expressing ER alone, the addition of hormone had no effect on DNA synthesis. The levels of [3H]thymidine incorporation were similar in the presence and in the absence of OHT at each time point in all concentrations of serum (Fig. 4B, left panels). Since the cells were seeded at 80% confluence, at about 24 h postinduction the cells expressing ER alone reached a plateau in the level of [3H]thymidine incorporation, indicating that they were contact inhibited in 5% FBS with and without OHT and in 10% FBS with OHT (Fig. 4B, left panels). In contrast, cells expressing the Nic-ER chimera were not contact inhibited and incorporated an increasing amount of [3H]thymidine over time in 5 and 10% FBS (Fig. 4B, right panels). Our data indicate that the activation of Notchic-ER promotes the entry into the S phase of the cell cycle. Furthermore, cells expressing the ER were quiescent in the absence of serum, as they did not incorporate [3H]thymidine above the basal level observed for synchronized cells in 0.1% FBS (Fig. 4B, left panel), indicating that E1A, in our experimental system, is not sufficient to drive cells into the cell cycle.
CSL binds to the cyclin D1 promoter.
In order to define the mechanism of induction of cyclin D1 mRNA mediated by Notchic, we searched for possible regulatory elements related to the Notch signal transduction pathway in the cyclin D1 promoter. We identified a putative CSL binding site located at position −513 in the human cyclin D1 promoter (GenBank accession no. Z29078) (17) and at position −767 in the rat cyclin D1 promoter (GenBank accession no. AF148946) (28). A schematic representation of the human and rat cyclin D1 promoter with binding sites for transcription factors as described in the literature is shown in Fig. 5A. The sequence of the putative CSL binding site is GCTGAGAT; this sequence is different from the consensus sequence for the high-affinity CSL binding site (GTGGGAA) previously described. However, a similar sequence, GCTGAGAA, has been identified in the human β-globin locus control region, which has been demonstrated to be an authentic CSL binding site (31).
We examined the ability of CSL to bind the identified sequences by EMSA. The sequences of the wt and mut oligonucleotides used in the EMSA analysis are shown in Fig. 5A and are described in Materials and Methods. Total cell lysates were prepared from 293T cells transiently transfected with an expression vector encoding HA-tagged CSL (CSL-HA). Immunoblotting was used to show specific expression of CSL in 293T cells transfected with the vector encoding CSL-HA (Fig. 5B). A protein-DNA complex containing CSL was identified when the radiolabeled wt D1 oligonucleotides were incubated with lysates from CSL-HA-transfected 293T cells. The complex was specifically supershifted by preincubation of the cell lysates with an anti-HA tag-specific antibody (Fig. 5C) and was formed by both the human and the rat wt D1 oligonucleotides. In contrast, no bands corresponding to the CSL-DNA complex were detected in samples incubated with the mut sequences (Fig. 5C).
In order to demonstrate the specificity of CSL binding to the human and rat cyclin D1 promoter, we preincubated lysates from 293T cells expressing GFP or CSL-HA with different antibodies prior to incubating them with the wt or mut labeled oligonucleotides. The CSL-DNA complex was detectable only in lysates from cells expressing CSL-HA and was specifically supershifted only by anti-CSL and anti-HA tag antibodies (Fig. 6A), and an anti-CDK4 antibody did not supershift the CSL-DNA complex (Fig. 6A). Furthermore, the complex was not detected in lysates from cells expressing GFP or when cells were incubated with the mut oligonucleotides. For competition analysis, 60 fmol of the radiolabeled wt D1 oligonucleotides was incubated in the presence of increasing amounts (50- and 200-fold excesses) of the unlabeled competitor oligonucleotides indicated in Fig. 6B. Increasing concentrations of the unlabeled wt D1 oligonucleotide proportionally decreased the formation of the complex, and a 50-fold molar excess of the wt JK3X oligonucleotide completely competed its formation. In contrast, the mut oligonucleotides did not display any competitive effect on the binding of CSL to the wt D1 sequence (Fig. 6B). Similar results were obtained for both the human and the rat D1 oligonucleotides, demonstrating that CSL binds with similar affinities to both the human and the rat sequences of the cyclin D1 promoter. The conservation of the CSL site between species indicates that CSL might play an important role in the regulation of cyclin D1 transcription.
FIG. 6.
Binding of CSL to the cyclin D1 promoter is sequence specific. (A) DNA binding activity was analyzed by EMSA. Lysates from 293T cells expressing GFP (lanes marked GFP) or HA-tagged CSL (lanes marked CSL) were preincubated with no antibody (Ab) (−), an anti-CSL antibody (αCSL), an anti-HA probe antibody (αHA) or an anti-CDK4 antibody (αCDK4), used as an unrelated control antibody. Sixty femtomoles of the human wt or mut radiolabeled D1 probe was then added to the lysates for the binding reaction. DNA-protein complexes are indicated as for Fig. 5C. (B) Competition analysis was performed by incubating lysates of 293T cells expressing HA-tagged CSL with increasing concentrations (50- and 200-fold molar excesses) of the indicated unlabeled competitor duplex and with 60 fmol of the radiolabeled rat wt oligonucleotide. DNA-protein complexes were resolved by EMSA. The DNA-protein complex, free probe, and supershift with the anti-HA tag antibody are indicated as for Fig. 5C.
DISCUSSION
Hormone-induced Notchic-ER chimeras activate key components of the cell cycle machinery that regulate the G1/S transition.
A hallmark of neoplastic transformation is the deregulation of cellular proliferation through the disruption of key components of the cell cycle machinery that control the G1/S transition of the cell cycle (45, 52). Activation of transcription is an important mechanism in the regulation of several of these key factors. By examining the mRNA level of several genes that encode factors known to regulate the transition from the G1 to the S phase, we determined that hormone-activated Notchic-ER chimeras specifically induce cyclin D1 transcription. Our data demonstrate that hormone activation of Notchic-ER induces cyclin D1 transcription with rapid kinetics and independently of de novo synthesis of secondary transcription factors. Moreover, serum-derived mitogens are not required for Notchic to induce cyclin D1 mRNA expression. However, induction of cyclin D1 mRNA by serum was approximately twofold higher in the presence of OHT than in serum alone in the absence of hormone (Fig. 1 and 2B), indicating that Notchic can act synergistically with serum factors to induce cyclin D1 transcription. Indeed, the regulation of cyclin D1 transcription is complex and tightly modulated. Binding sites for numerous transcription factors have been identified in the promoter of the cyclin D1 gene, and several signal transduction pathways are involved in the activation of cyclin D1 mRNA transcription (1a, 2a, 34a, 34c). For example, transcriptional activation of cyclin D1 through mitogenic signals primarily involves the Ras-dependent pathway through MEK1-ERK and the AP1 binding site present in the cyclin D1 promoter (1, 1a, 4a, 27a).
Cyclins control cell cycle progression by regulating the activity of cyclin-dependent kinases (CDK). Association of cyclins with specific CDK partners activates the catalytic activities of these kinases, which then phosphorylate target proteins, such as Rb, to regulate the cell cycle. Specifically, the cyclin E-CDK2 complex is active in late G1 and is required to commit cells to S-phase entry by phosphorylating Rb and thereby, causing the release of E2F (27, 27b, 34b, 36a). We demonstrate that hormone-activated Notchic-ER chimeras induce CDK2 activity in the absence of serum. These data suggest that Notchic can stimulate cells to progress into the cell cycle through the induction of cyclin D1 transcription and activation of CDK2 activity, providing the first evidence that Notchic can affect key components of the cell cycle machinery and indicating a direct involvement of Notch proteins in the regulation of cellular proliferation. By analyzing the incorporation of [3H]thymidine, we determined that the activation of Notchic-ER increases the number of cells that initiate DNA synthesis both in full (10%) and in reduced (5%) concentrations of serum, indicating that Notchic is able to promote cells to enter into the S phase of the cell cycle. However, hormone activation of Notchic -ER proteins did not significantly increase DNA synthesis in 0.1% serum, suggesting that induction of cyclin D1 expression and activation of CDK2 by Notchic in RKE cells are not sufficient to induce proliferation. RKE cells are immortalized by the adenoviral oncoprotein E1A. E1A is thought to immortalize cells by inactivating Rb and thereby causing the release of E2F, which in turn induces genes involved in the G1-to-S transition (38). However, cells expressing ER alone were quiescent in the absence of serum, indicating that E1A, in our experimental system, is not sufficient to override normal cell cycle controls and induce a transition from G1 to S. Furthermore, in 10% serum RKE cells density arrest, indicating that, although they are immortal and express E1A, RKE cells retain tight regulation of the cell cycle. Activation of Notchic leads to a constitutive level of cyclin D1 and CDK2 activity and thereby contributes to the disruption of cell cycle control, resulting in the failure of RKE cells to withdraw from the cell cycle due to contact inhibition. However, the requirement for serum in order to promote S-phase entry by Notchic suggests that constitutive activation of cyclin D1 and CDK2 (in addition to E1A) is not sufficient to transform cells and that other factors in serum are likely to contribute to oncogenic transformation by Notchic. Interestingly, cells derived from mammary carcinomas induced in Notch4ic transgenic mice require MEK and P13K activity, as indicated by the failure of these cells to form colonies in semisolid media in the presence of the inhibitors PD098059 and LY294002, respectively (12). These results might provide a clue to the identities of the missing components required in our study. We therefore propose that to drive RKE cells into S phase and for these to become transformed, it is necessary to have cooperation among Notchic, E1A, and the Ras pathways.
The mechanism of transformation induced by Notchic proteins involves the activation of cyclin D1 transcription through a CSL-dependent pathway.
CSL is a sequence-specific DNA-binding transcriptional regulator and is one of the principal effectors of Notch signaling. CSL acts in the nuclei of cells as a transcriptional repressor, inhibiting the transcription of genes containing CSL binding sites. It has been proposed that, following ligand activation, Notchic translocates to the nucleus and associates with CSL, converting CSL to a transcriptional activator by displacing the corepressor complex containing SMRT, HDAC1, NcoR or CIR and HDAC2. Therefore, the formation of the Notchic-CSL complex results in the induction of transcription of CSL target genes (35a). We have identified a CSL binding site in the cyclin D1 promoter and showed that CSL specifically binds to this sequence. Previously, we reported that transformation mediated by Notchic proteins requires transcriptional activation (23). Here we show that a 10-amino-acid deletion in NicΔ2105–2114 results in the complete loss of the transcriptional activation of cyclin D1 by Notchic. NicΔ2105–2114 is nontransforming, and although it is able to bind CSL as well as Notchic, it fails to activate transcription from a reporter construct containing eight copies of a CSL consensus sequence (23). Therefore, it is likely that NicΔ2105–2114 is missing a domain responsible for transcriptional activation. In contrast, deletion of the RAM domain in Notchic (NicΔR) results in a decreased ability of NicΔR to induce cyclin D1 transcription compared to that of Notchic proteins. NicΔR displays a weaker ability to form foci and to activate CSL-dependent transcription than that of Notchic (23). In glutathione S-transferase pull-down experiments, deletion of the RAM domain is sufficient to abolish the binding of Notchic to CSL. However, in cells transformed by versions of NotchicΔR we find that small fractions of Notch and CSL exist in a stable complex (S. Jeffries and A. J. Capobianco, unpublished data). Therefore, the reduced level of cyclin D1 transcription is consistent with a reduced level of binding between Notchic and CSL. Our results suggest that, in order to induce cyclin D1 expression, Notchic proteins must be able to bind to CSL and to activate gene transcription. We have previously shown that Notchic-ER chimeras are able to activate transcription of a reporter construct containing eight CSL binding sites only in the presence of OHT. However, Notchic-ER chimeras bind to CSL both in the presence and in the absence of OHT (43). These data suggest that, in the absence of OHT, Notchic-ER and CSL form a complex that is not transcriptionally active. Our hypothesis is that OHT may induce a conformational change in Notchic that favors the binding to the complex of factors required for activation of transcription.
Aberrant expression of cyclin D1 has been observed in numerous human tumors, and it has recently been proposed that cyclin D1 is involved in the mechanism of transformation mediated by several different oncogenes (16, 34, 41, 45, 50). Here we show that nontransforming Notchic proteins fail to activate cyclin D1 transcription and that a diminished ability of NicΔR to transform cells is related to its diminished ability to induce cyclin D1 mRNA expression. Therefore, we propose that the oncogenic mechanism driven by Notchic involves the disruption of the normal cell cycle control and that the induction of cyclin D1 and the activation of CDK2 play a critical role in the acquisition of the transformed state induced by Notchic proteins.
ACKNOWLEDGMENTS
We thank members of the Capobianco laboratory for support and technical assistance. We thank David Robbins and Yolanda Sanchez for insightful comments on our work. We are grateful to C. J. Der (University of North Carolina), K. Keyomarsy (M. D. Anderson Cancer Center), P. D. Ling (Baylor College of Medicine), E. Bresnick (University of Winsconsin), and J. A. Diehl (University of Nebraska) for kindly providing reagents used in this study.
This work was funded in part by grants from the American Cancer Society (RPG LBC-99465 to A. J. Capobianco) and the National Cancer Institute (ROI CA 83736 to A. J. Capobianco).
REFERENCES
- 1.Aktas H, Cai H, Cooper G M. Ras links growth factor signaling to the cell cycle machinery via regulation of cyclin D1 and the Cdk inhibitor p27KIP1. Mol Cell Biol. 1997;17:3850–3857. doi: 10.1128/mcb.17.7.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell R G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 1b.Aster J, Pear W, Hasserjian R, Erba H, Davi F, Luo B, Scott M, Baltimore D, Sklar J. Functional analysis of the TAN-1 gene, a human homolog of Drosophila notch. Cold Spring Harbor Symp Quant Biol. 1994;59:125–136. doi: 10.1101/sqb.1994.059.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Bellavia D, Campese A F, Alesse E, Vacca A, Felli M P, Balestri A, Stoppacciaro A, Tiveron C, Tatangelo L, Giovarelli M, Gaetano C, Ruco L, Hoffman E S, Hayday A C, Lendahl U, Frati L, Gulino A, Screpanti I. Constitutive activation of NF-kappaB and T-cell leukemia/lymphoma in Notch3 transgenic mice. EMBO J. 2000;19:3337–3348. doi: 10.1093/emboj/19.13.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Bromberg J F, Wrzeszczynska M H, Devgan G, Zhao Y, Pestell R G, Albanese C, Darnell J E., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. . (Erratum, 99:239.) [DOI] [PubMed] [Google Scholar]
- 3.Capobianco A J, Zagouras P, Blaumueller C M, Artavanis-Tsakonas S, Bishop J M. Neoplastic transformation by truncated alleles of human NOTCH1/TAN1 and NOTCH2. Mol Cell Biol. 1997;17:6265–6273. doi: 10.1128/mcb.17.11.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng M, Olivier P, Diehl J A, Fero M, Roussel M F, Roberts J M, Sherr C J. The p21 (Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Cheng M, Sexl V, Sherr C J, Roussel M F. Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1) Proc Natl Acad Sci USA. 1998;95:1091–1096. doi: 10.1073/pnas.95.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daksis J I, Lu R Y, Facchini L M, Marhin W W, Penn L J. Myc induces cyclin D1 expression in the absence of de novo protein synthesis and links mitogen-stimulated signal transduction to the cell cycle. Oncogene. 1994;9:3635–3645. [PubMed] [Google Scholar]
- 6.Daniel B, Rangarajan A, Mukherjee G, Vallikad E, Krishna S. The link between integration and expression of human papillomavirus type 16 genomes and cellular changes in the evolution of cervical intraepithelial neoplastic lesions. J Gen Virol. 1997;78:1095–1101. doi: 10.1099/0022-1317-78-5-1095. [DOI] [PubMed] [Google Scholar]
- 7.Diehl J A, Cheng M, Roussel M F, Sherr C J. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diehl J A, Zindy F, Sherr C J. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1977;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- 9.Dievart A, Beaulieu N, Jolicoeur P. Involvement of Notch1 in the development of mouse mammary tumors. Oncogene. 1999;18:5973–5981. doi: 10.1038/sj.onc.1202991. [DOI] [PubMed] [Google Scholar]
- 10.Ellisen L W, Bird J, West D C, Soreng A L, Reynolds T C, Smith S D, Sklar J. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 11.Feldman B J, Hampton T, Cleary M L. A carboxy-terminal deletion mutant of Notch1 accelerates lymphoid oncogenesis in E2A-PBX1 transgenic mice. Blood. 2000;96:1906–1913. [PubMed] [Google Scholar]
- 12.Fitzgerald K, Harrington A, Leder P. Ras pathway signals are required for notch-mediated oncogenesis. Oncogene. 2000;19:4191–4198. doi: 10.1038/sj.onc.1203766. [DOI] [PubMed] [Google Scholar]
- 13.Fortini M E, Artavanis-Tsakonas S. The suppressor of hairless protein participates in notch receptor signaling. Cell. 1994;79:273–282. doi: 10.1016/0092-8674(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 14.Gallahan D, Kozak C, Callahan R. A new common integration region (int-3) for mouse mammary tumor virus on mouse chromosome 17. J Virol. 1987;61:218–220. doi: 10.1128/jvi.61.1.218-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard L, Hanna Z, Beaulieu N, Hoemann C D, Simard C, Kozak C A, Jolicoeur P. Frequent provirus insertional mutagenesis of Notch1 in thymomas of MMTVD/myc transgenic mice suggests a collaboration of c-myc and Notch1 for oncogenesis. Genes Dev. 1996;10:1930–1944. doi: 10.1101/gad.10.15.1930. [DOI] [PubMed] [Google Scholar]
- 16.Hall M, Peters G. Genetic alterations of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human cancer. Adv Cancer Res. 1996;68:67–108. doi: 10.1016/s0065-230x(08)60352-8. [DOI] [PubMed] [Google Scholar]
- 17.Herber B, Truss M, Beato M, Muller R. Inducible regulatory elements in the human cyclin D1 promoter. Oncogene. 1994;9:1295–1304. . (Erratum, 9:2105–2107.) [PubMed] [Google Scholar]
- 18.Hinds P W, Dowdy S F, Eaton E N, Arnold A, Weinberg R A. Function of a human cyclin gene as an oncogene. Proc Natl Acad Sci USA. 1994;91:709–713. doi: 10.1073/pnas.91.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh J J, Henkel T, Salmon P, Robey E, Peterson M G, Hayward S D. Truncated mammalian Notch1 activates CBF1/RBPJk-repressed genes by a mechanism resembling that of Epstein-Barr virus EBNA2. Mol Cell Biol. 1996;16:952–959. doi: 10.1128/mcb.16.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh J J, Zhou S, Chen L, Young D B, Hayward S D. CIR, a corepressor linking the DNA binding factor CBF1 to the histone deacetylase complex. Proc Natl Acad Sci USA. 1999;96:23–28. doi: 10.1073/pnas.96.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishii T, Shimizu M, Kanayama Y, Nakada S, Nojima H, Oda K. Differential activation of cyclin and cyclin-dependent kinase genes by adenovirus E1A12S cDNA product. Exp Cell Res. 1993;208:407–414. doi: 10.1006/excr.1993.1262. [DOI] [PubMed] [Google Scholar]
- 22.Jarriault S, Brou C, Logeat F, Schroeter E H, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 23.Jeffries S, Capobianco A J. Neoplastic transformation by Notch requires nuclear localization. Mol Cell Biol. 2000;20:3928–3941. doi: 10.1128/mcb.20.11.3928-3941.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang W, Kahn S M, Zhou P, Zhang Y J, Cacace A M, Infante A S, Doi S, Santella R M, Weinstein I B. Overexpression of cyclin D1 in rat fibroblasts causes abnormalities in growth control, cell cycle progression and gene expression. Oncogene. 1993;8:3447–3457. [PubMed] [Google Scholar]
- 25.Kao H Y, Ordentlich P, Koyano-Nakagawa N, Tang Z, Downes M, Kintner C R, Evans R M, Kadesch T. A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes Dev. 1998;12:2269–2277. doi: 10.1101/gad.12.15.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato H, Taniguchi Y, Kurooka H, Minoguchi S, Sakai T, Nomura-Okazaki S, Tamura K, Honjo T. Involvement of RBP-J in biological functions of mouse Notch1 and its derivatives. Development. 1997;124:4133–4141. doi: 10.1242/dev.124.20.4133. [DOI] [PubMed] [Google Scholar]
- 27.Kato J. Induction of S phase by G1 regulatory factors. Front Biosci. 1999;4:D787–D792. doi: 10.2741/kato. [DOI] [PubMed] [Google Scholar]
- 27a.Kerkhoff E, Rapp U R. Induction of cell proliferation in quiescent NIH 3T3 cells by oncogenic c-Raf-1. Mol Cell Biol. 1997;17:2576–2586. doi: 10.1128/mcb.17.5.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27b.Keyomarsi K, Herliczek T W. The role of cyclin E in cell proliferation, development and cancer. Prog Cell Cycle Res. 1997;3:171–191. doi: 10.1007/978-1-4615-5371-7_14. [DOI] [PubMed] [Google Scholar]
- 28.Kitazawa S, Kitazawa R, Maeda S. Transcriptional regulation of rat cyclin D1 gene by CpG methylation status in promoter region. J Biol Chem. 1999;274:28787–28793. doi: 10.1074/jbc.274.40.28787. [DOI] [PubMed] [Google Scholar]
- 29.Kopan R, Schroeter E H, Weintraub H, Nye J S. Signal transduction by activated mNotch: importance of proteolytic processing and its regulation by the extracellular domain. Proc Natl Acad Sci USA. 1996;93:1683–1688. doi: 10.1073/pnas.93.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurooka H, Honjo T. Functional interaction between the mouse notch1 intracellular region and histone acetyltransferases PCAF and GCN5. J Biol Chem. 2000;275:17211–17220. doi: 10.1074/jbc.M000909200. [DOI] [PubMed] [Google Scholar]
- 31.Lam L T, Bresnick E H. Identity of the beta-globin locus control region binding protein HS2NF5 as the mammalian homolog of the notch-regulated transcription factor suppressor of hairless. J Biol Chem. 1998;273:24223–24231. doi: 10.1074/jbc.273.37.24223. [DOI] [PubMed] [Google Scholar]
- 32.Lam L T, Bresnick E H. A novel DNA-binding protein, HS2NF5, interacts with a functionally important sequence of the human beta-globin locus control region. J Biol Chem. 1996;271:32421–32429. doi: 10.1074/jbc.271.50.32421. [DOI] [PubMed] [Google Scholar]
- 33.Lecourtois M, Schweisguth F. The neurogenic suppressor of hairless DNA-binding protein mediates the transcriptional activation of the enhancer of split complex genes triggered by Notch signaling. Genes Dev. 1995;9:2598–2608. doi: 10.1101/gad.9.21.2598. [DOI] [PubMed] [Google Scholar]
- 34.Lee R J, Albanese C, Fu M, D'Amico M, Lin B, Watanabe G, Haines III G K, Siegel P M, Hung M C, Yarden Y, Horowitz J M, Muller W J, Pestell R G. Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol Cell Biol. 2000;20:672–683. doi: 10.1128/mcb.20.2.672-683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34a.Lee R J, Albanese C, Stenger R J, Watanabe G, Inghirami G, Haines III G K, Webster M, Muller W J, Brugge J S, Davis R J, Pestell R G. pp60(v-src) induction of cyclin D1 requires collaborative interactions between the extracellular signal-regulated kinase, p38, and Jun kinase pathways. A role for cAMP response element-binding protein and activating transcription factor-2 in pp60(v-src) signaling in breast cancer cells. J Biol Chem. 1999;274:7341–7350. doi: 10.1074/jbc.274.11.7341. [DOI] [PubMed] [Google Scholar]
- 34b.Lukas J, Herzinger T, Hansen K, Moroni M C, Resnitzky D, Helin K, Reed S I, Bartek J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997;11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- 34c.Matsumura I, Kitamura T, Wakao H, Tanaka H, Hashimoto K, Albanese C, Downward J, Pestell R G, Kanakura Y. Transcriptional regulation of the cyclin D1 promoter by STAT5: its involvement in cytokine-dependent growth of hematopoietic cells. EMBO J. 1999;18:1367–1377. doi: 10.1093/emboj/18.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35a.Mumm J S, Kopan R. Notch signaling: from the outside in. Dev Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- 36.Musgrove E A, Lee C S, Buckley M F, Sutherland R L. Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle. Proc Natl Acad Sci USA. 1994;91:8022–8026. doi: 10.1073/pnas.91.17.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pear W S, Aster J C, Scott M L, Hasserjian R P, Soffer B, Sklar J, Baltimore D. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J Exp Med. 1996;183:2283–2291. doi: 10.1084/jem.183.5.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peeper D S, Zantema A. Adenovirus-E1A proteins transform cells by sequestering regulatory proteins. Mol Biol Rep. 1993;17:197–207. doi: 10.1007/BF00986728. [DOI] [PubMed] [Google Scholar]
- 39.Quelle D E, Ashmun R A, Shurtleff S A, Kato J Y, Bar-Sagi D, Roussel M F, Sherr C J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 40.Rae F K, Stephenson S A, Nicol D L, Clements J A. Novel association of a diverse range of genes with renal cell carcinoma as identified by differential display. Int J Cancer. 2000;88:726–732. doi: 10.1002/1097-0215(20001201)88:5<726::aid-ijc7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 41.Rimerman R A, Gellert-Randleman A, Diehl J A. Wnt1 and MEK1 cooperate to promote cyclin D1 accumulation and cellular transformation. J Biol Chem. 2000;275:14736–14742. doi: 10.1074/jbc.m910241199. [DOI] [PubMed] [Google Scholar]
- 42.Rohn J L, Lauring A S, Linenberger M L, Overbaugh J. Transduction of Notch2 in feline leukemia virus-induced thymic lymphoma. J Virol. 1996;70:8071–8080. doi: 10.1128/jvi.70.11.8071-8080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ronchini C, Capobianco A J. Notch(ic)-ER chimeras display hormone-dependent transformation, nuclear accumulation, phosphorylation and CBF1 activation. Oncogene. 2000;19:3914–3924. doi: 10.1038/sj.onc.1203719. [DOI] [PubMed] [Google Scholar]
- 44.Schroeter E H, Kisslinger J A, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 45.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 46.Sherr C J. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 47.Sherr C J. Mammalian G1 cyclins and cell cycle progression. Proc Assoc Am Physicians. 1995;107:181–186. [PubMed] [Google Scholar]
- 48.Soriano J V, Uyttendaele H, Kitajewski J, Montesano R. Expression of an activated Notch4(int-3) oncoprotein disrupts morphogenesis and induces an invasive phenotype in mammary epithelial cells in vitro. Int J Cancer. 2000;86:652–659. doi: 10.1002/(sici)1097-0215(20000601)86:5<652::aid-ijc8>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 49.Struhl G, Adachi A. Nuclear access and action of notch in vivo. Cell. 1998;93:649–660. doi: 10.1016/s0092-8674(00)81193-9. [DOI] [PubMed] [Google Scholar]
- 50.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 51.Vigo E, Muller H, Prosperini E, Hateboer G, Cartwright P, Moroni M C, Helin K. CDC25A phosphatase is a target of E2F and is required for efficient E2F-induced S phase. Mol Cell Biol. 1999;19:6379–6395. doi: 10.1128/mcb.19.9.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 53.Winston J T, Pledger W J. Growth factor regulation of cyclin D1 mRNA expression through protein synthesis-dependent and -independent mechanisms. Mol Biol Cell. 1993;4:1133–1144. doi: 10.1091/mbc.4.11.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Won K A, Xiong Y, Beach D, Gilman M Z. Growth-regulated expression of D-type cyclin genes in human diploid fibroblasts. Proc Natl Acad Sci USA. 1992;89:9910–9914. doi: 10.1073/pnas.89.20.9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto M, Acevedo-Duncan M, Chalfant C E, Patel N A, Watson J E, Cooper D R. Acute glucose-induced downregulation of PKC-betaII accelerates cultured VSMC proliferation. Am J Physiol Cell Physiol. 2000;279:C587–C595. doi: 10.1152/ajpcell.2000.279.3.C587. [DOI] [PubMed] [Google Scholar]
- 56.Zagouras P, Stifani S, Blaumueller C M, Carcangiu M L, Artavanis-Tsakonas S. Alterations in Notch signaling in neoplastic lesions of the human cervix. Proc Natl Acad Sci USA. 1995;92:6414–6418. doi: 10.1073/pnas.92.14.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou S, Fujimuro M, Hsieh J J, Chen L, Miyamoto A, Weinmaster G, Hayward S D. SKIP, a CBF1-associated protein, interacts with the ankyrin repeat domain of Notch1C to facilitate Notch1C function. Mol Cell Biol. 2000;20:2400–2410. doi: 10.1128/mcb.20.7.2400-2410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zwijsen R M, Klompmaker R, Wientjens E B, Kristel P M, van der Burg B, Michalides R J. Cyclin D1 triggers autonomous growth of breast cancer cells by governing cell cycle exit. Mol Cell Biol. 1996;16:2554–2560. doi: 10.1128/mcb.16.6.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]