Abstract
Background
Prone positioning (PP) reduces mortality of patients with acute respiratory distress syndrome (ARDS). The potential benefit of prone positioning maneuvers during venovenous extracorporeal membrane oxygenation (ECMO) is unknown. The aim of this study was to evaluate the association between the use of prone positioning during extracorporeal support and ICU mortality in a pooled population of patients from previous European cohort studies.
Methods
We performed a pooled individual patient data analysis of European cohort studies which compared patients treated with prone positioning during ECMO (Prone group) to “conventional” ECMO management (Supine group) in patients with severe ARDS.
Results
889 patients from five studies were included. Unadjusted ICU mortality was 52.8% in the Supine Group and 40.8% in the Prone group. At a Cox multiple regression analysis PP during ECMO was not significantly associated with a reduction of ICU mortality (HR 0.67 95% CI: 0.42–1.06). Propensity score matching identified 227 patients in each group. ICU mortality of the matched samples was 48.0% and 39.6% for patients in the Supine and Prone group, respectively (p = 0.072).
Conclusions
In a large population of ARDS patients receiving venovenous extracorporeal support, the use of prone positioning during ECMO was not significantly associated with reduced ICU mortality. The impact of this procedure will have to be definitively assessed by prospective randomized controlled trials.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-021-03879-w.
Keywords: Acute respiratory distress syndrome, Extracorporeal membrane oxygenation, Prone positioning, Pooled data analysis, Mortality
Introduction
Prone positioning (PP) has proven to reduce mortality of patients with moderate to severe acute respiratory distress syndrome (ARDS) [1]. Previous studies suggested that the survival benefit of prone positioning is greater in the most hypoxemic patients [2]. Among these patients, a further worsening of respiratory failure or the impossibility of maintaining protective ventilation may require extracorporeal gas exchange, specifically venovenous extracorporeal membrane oxygenation (V-V ECMO). However, patients on V-V ECMO support have historically been managed in supine position, because of the fear of life-threatening complications associated with prone positioning during extracorporeal support and lack of experience. Recently, numerous teams have reported feasibility of PP during V-V ECMO, which might be associated with an improvement of oxygenation [3], CO2 clearance and respiratory system compliance [3, 4]. Several European cohort studies [3–7] evaluated the association between PP during ECMO and outcome, with conflicting results.
Two randomized controlled studies are currently undergoing in China (NCT04139733) and France (NCT04607551) to prospectively assess the impact of this procedure on duration of ECMO support and time to successful weaning from ECMO. Both studies will assess mortality as a secondary endpoint. However, since these two studies recently started patient recruitment, results will not be available soon. Until then, physicians may wonder if PP should be considered a reasonable treatment adjunct for ARDS patients on venovenous extracorporeal support. The expanding use of ECMO during the ongoing COVID-19 pandemic [8, 9] also urge to get a better knowledge of the effect of this procedure on outcomes.
We performed a pooled individual patient data analysis of five European cohort studies comparing severe ARDS patients treated with PP during V-V ECMO to “conventional” ECMO management in the supine position. The primary aim of the study was to evaluate the effect of PP during ECMO on ICU mortality.
Methods
We pooled individual adult patient data from studies evaluating the association between the use of PP during V-V ECMO and clinical outcome, by comparing this population (Prone group) with patients managed in the supine position (Supine group). We restricted our analysis to studies published in the last 10 years (July 2011—June 2021) because of the improvements in ECMO management achieved in the last decade and the recent evidence of a proven benefit on survival of PP and ECMO in severe ARDS patients [1, 10–12].
Search strategy
A PubMed search was done on January 15th 2021 and July 31st 2021 to identify cohort studies which fulfilled the eligibility criteria. “Extracorporeal membrane oxygenation” and “prone positioning” were used as key terms. Manuscripts were then reviewed to assess the eligibility. For each eligible study, we asked the corresponding author to provide fully anonymized individual patient data together with variable definitions.
Ethics approval
All studies have been independently reviewed and approved by the local Institutional Review Boards (see Electronic Supplementary Material for approval numbers/ID).
Data collection
We extracted from the single study databases the following parameters at baseline (i.e., before the ECMO start): age, sex, body mass index, comorbidities (see Electronic Supplementary Material for definitions), etiology of ARDS, ratio of arterial oxygen tension to inspiratory oxygen fraction (PaO2 to FiO2), Sequential Organ Failure Assessment (SOFA) score[13] at the ECMO start, and therapies before ECMO start (i.e. nitric oxide, PP, renal replacement therapy, and duration of mechanical ventilation). The ECMO configuration was described according to the ELSO Maastricht treaty [14]. Complications of PP maneuvers were recorded. We also collected duration of ECMO support, ICU length of stay and outcome at hospital discharge.
For patients who underwent PP during ECMO, we also collected the ECMO day when PP was started, and the number of PP cycles.
Endpoints
The primary endpoint was to assess the association between the use of PP during ECMO and ICU mortality in patients with ARDS. The secondary endpoint included the assessment of the association of PP during ECMO with hospital mortality, rate of successful ECMO weaning, ICU length of stay and duration of time to successful ECMO weaning within 60 days from ECMO start. Moreover, as an explorative endpoint, we aimed to assess if the use of PP during ECMO was associated with improved outcome in specific pre-defined patient subgroups.
All studies have been independently reviewed and approved by each local Institutional Review Boards.
Statistical analysis
Continuous data were reported as mean ± standard deviation or by median (interquartile range), according to the data distribution. Data normality was assessed by Shapiro–Wilk test and by visual inspection using histograms of distribution. Categorical data were reported as count (proportion). There was no imputation for missing data. To assess differences between patients who underwent prone positioning during venovenous ECMO (Prone group) or who were managed in supine position (Supine group) we performed unpaired Student’s T-test (or Wilcoxon–Mann–Whitney test) and Chi-square test (or Fisher’s exact test) in continuous and categorical variables, respectively.
ICU survival was described between Prone and Supine group by the Kaplan–Meier approach. The date of hospital discharge (or death after ICU discharge) was not available in all patients. For this reason, patients discharged alive from ICU before 60-days were right-censored. Statistical difference between the survival curves was assessed by log-rank test.
We explored the independent association with 60-day ICU mortality by a COX-proportional regression model. We included in this model a set of clinically meaningful variables which were selected a-priori: age; sex; obesity; chronic respiratory disease, chronic heart failure, malignancy, immunodeficiency, chronic liver disease; PaO2/FiO2; SOFA score; mobile ECMO transfer; duration mechanical ventilation days before ECMO; use of PP before ECMO and use of PP during ECMO. The comorbidities included in the model were chosen based on previous literature suggesting their independent role on outcome in critically ill patients with ARDS [15, 16]. Independent predictors of hospital mortality were assessed by a multiple logistic regression model using the same abovementioned clinically meaningful variables.
Results of multivariable models were reported as odds ratio (OR) or hazard ratio (HR) with 95% confidence interval (95% CI) for logistic and COX-proportional regression models, respectively, all adjusted for robust clustering by using the original study as cluster variable.
The propensity score matching method was applied to estimate the effect of PP during ECMO on main outcomes. Details on matching technique are provided in the Supplemental Methods (see Electronic Supplementary Material). Difference in 60-day survival probability was estimated between matched Prone and Supine groups by the Kaplan–Meier approach and compared by the log-rank test. For the matched cohorts we also described ICU and hospital mortality, rate of successful ECMO weaning, ICU length of stay and duration of time to successful ECMO weaning within 60 days from ECMO start [4].
To explore the potential role of prone positioning in specific subgroups of ECMO patients, we evaluated the association between Prone and Supine groups with hospital mortality in pre-defined clinically relevant subgroups. Patients were then classified according to age (age > 50); obesity (cutoff: BMI > 30 kg/m2); SOFA score (SOFA > 12); baseline PaO2/FiO2 > 60; use of prone position before ECMO; duration of mechanical ventilation before ECMO (> to 7 days).
Statistical significance was reported with a p-value < 0.05 (two-sided). Statistical analyses were performed using STATA/MP 16.0 for Windows (StataCorp LLC, College Statio, TX 77,845, USA) and GraphPad Prism 8 for Windows (version 8.0.2, GraphPad Software, Inc.).
Results
From the 151 references identified by the search strategy, five manuscripts fulfilled the eligibility criteria and were included in the analysis (see Table 1).
Table 1.
Characteristics of the studies included in the analysis
| Study | Guervilly et al. [6] | Garcia et al. [5] | Rilinger et al. [7] | Giani et al. [3] | Petit et al. [4] |
|---|---|---|---|---|---|
| Design | Monocentric retrospective cohort study | Monocentric retrospective cohort study | Monocentric retrospective cohort study | Multicentric retrospective cohort study | Monocentric retrospective cohort study |
| Country | France | France | Germany | Italy | France |
| Case volume# of the ECMO centers, runs/year (V-V runs) | 55 (50) | 45 (25) | 125 (35) | 35–55 (25–33)& | 450 (60) |
| Total number of patients (Prone group) | 168 (91) | 25 (14) | 158 (38) | 240 (107) | 298 (64) |
| Population | ARDS patients on | COVID-19 ARDS patients on V-V ECMO | ARDS patients on V-V ECMO | ARDS patients on V-V ECMO | ARDS patients on V-V ECMO |
| Prone positioning duration | 16 (12–16) hours | 16 (15–17) hours | 20 (17–21) hours | 15 (12–18) hours | 16 (16–16) hours |
| Statistics | Univariable analysis, individual matching | Univariable analysis | Multivariable analysis, propensity score matching | Multivariable analysis, propensity score matching | Propensity score matching |
| Outcome | |||||
| Last follow-up | 90 days | 28 days | Hospital discharge | Hospital discharge | 90 days |
| Mortality, Prone vs Supine group | 36% vs 58%* | 79% vs 27%° | 63% vs 63%* | 30% vs 53%* | 25% vs 46%* |
&Annual case volume before the COVID-19 pandemic
%Case volume range of the centers participating to the multicentric study
*Mortality rates of matched patient cohorts
°Unadjusted mortality
Unadjusted ICU and hospital mortality of the 5 included study are presented as a Forest plot graph in the see Additional file 1 (Fig. E1).
A total of 889 patients were included, 575 in the Supine group and 314 in the Prone group. The more frequent ECMO configuration was the femoro-jugular approach (66%), followed by femoro-femoral (18%) and jugular dual-lumen (16%) cannulation.
Patients in the Prone group underwent a median of 2 (1–3) PP session while on V-V ECMO, for a total of 824 PP sessions during ECMO support. Median ECMO duration before the first prone positioning was 5 (2–6) days. Complications were reported in 53 (6.4%) PP sessions. 9 (1.1%) PP cycles were aborted due to complications. We did not record any accidental extubation or dislodgement of the ECMO cannulae. One patient suffered an episode of ventricular arrhythmia while on prone position. Bleeding from cannula insertion site and drop of the extracorporeal blood flow occurred in 16 (1.9%) and 15 (1.8%) cases, respectively, and were the most frequent complications. Other reported complications were vomiting (13, 1.6%) and hemodynamic instability (8, 1%).
Table 2 describes the study population according to the study group. Age, PaO2 to FiO2 ratio and duration of mechanical ventilation before ECMO were similar in the two study groups. Median BMI was higher in the Prone group. Immunodeficiency was more frequent in the Supine group, whereas the SOFA score was higher compared to the Prone group. PP before ECMO was used more frequently in the Prone group.
Table 2.
Patient baseline characteristics, pre-ECMO parameters and outcomes in the Supine and Prone group
| Baseline characteristics | Supine group (n = 575) | Prone group (n = 314) | p-value |
|---|---|---|---|
| Age, years | 52 (41–61) | 51 (39–61) | 0.506 |
| Sex, female | 191 (33.2) | 91 (29.0) | 0.195 |
| BMI, kg/m2 | 27 (23–32) | 29 (25–34) | 0.003 |
| Pre-existing conditions at baseline | |||
| Obesity (BMI ≥ 30) | 180 (35.7) | 113 (42.3) | 0.072 |
| Hypertension | 105 (32.1) | 58 (23.9) | 0.031 |
| Diabetes | 88 (15.7) | 53 (17.3) | 0.547 |
| Chronic respiratory disease | 94 (16.8) | 37 (12.1) | 0.064 |
| Malignancy | 68 (12.1) | 28 (9.1) | 0.178 |
| Vascular disease | 57 (10.2) | 27 (8.8) | 0.515 |
| Chronic heart failure | 54 (9.6) | 30 (9.8) | 0.944 |
| Chronic renal disease | 22 (3.9) | 17 (5.5) | 0.272 |
| Chronic liver disease | 34 (6.1) | 10 (3.2) | 0.072 |
| Immunodeficiency | 101 (18.0) | 36 (11.7) | 0.015 |
| ARDS etiology | < 0.001 | ||
| Pulmonary ARDS | 405 (70.4) | 234 (74.5) | |
| Extrapulmonary ARDS | 58 (10.1) | 52 (16.6) | |
| Other/unknown | 112 (19.5) | 28 (8.9) | |
| COVID-19 ARDS | 11 (1.9) | 14 (4.5) | 0.028 |
| Mobile ECMO transfer | 336 (59.5) | 215 (68.5) | 0.008 |
| Pre-ECMO variables | |||
| PaO2/FiO2, mmHg | 67 (56–80) | 66 (55–80) | 0.568 |
| SOFA score | 12 (9–16) | 10 (8–13) | < 0.001 |
| Use of nitric oxide | 189 (33.3) | 91 (29.0) | 0.184 |
| Use of prone positioning | 248 (45.1) | 180 (57.3) | 0.001 |
| Use of renal replacement therapy | 61 (13.7) | 31 (11.3) | 0.362 |
| Days of mechanical ventilation before ECMO | 3 (1–8) | 3 (1–7) | 0.632 |
| Outcomes | |||
| ECMO duration, days | 8 (4–17) | 15 (10–26) | < 0.001 |
| ECMO successful weaning | 326 (57.0) | 211 (67.2) | 0.003 |
| ICU LOS, days | 20 (10–36) | 31 (19–48) | < 0.001 |
| Survivors, n = 449 | 27 (15–45) | 34 (23–49) | < 0.001 |
| Non-survivors, n = 397 | 13 (4–26) | 23 (16–41) | < 0.001 |
| Time to successful ECMO weaning within 60 days from ECMO start, days | 60 (8–60) | 28 (12–60) | 0.906 |
| ICU mortality, n (%) | 298 (52.8) | 128 (40.8) | 0.001 |
| Hospital mortality, n (%) | 306 (54.4) | 136 (43.5) | 0.002 |
Data are presented as count (percentage) or median (25th-75th percentile). BMI, body mass index; LOS, length of stay
Unadjusted ICU, 60 days (see Kaplan–Meier analysis, Fig. E2, Additional file 1) and hospital mortality were lower in the Prone group.
Independent predictors of 60-day ICU mortality are displayed in Table E1 (see Additional file 1). Malignancy, chronic liver disease and SOFA score were independently associated with higher mortality. The reduction of ICU mortality in patients who underwent PP during ECMO was not statistically significant (HR 0.67 95% IC: 0.42–1.06).
Predictors of hospital mortality are displayed in Table E2 (see Additional file 1). Age, chronic heart failure, malignancy, immunodeficiency, chronic liver disease and SOFA score were independently associated with higher hospital mortality. The use of PP during ECMO was not significantly associated with mortality reduction (HR 0.79 95% IC: 0.46–1.35).
A total of 227 propensity score-matched patients were identified in each group. Characteristics and outcomes of the matched populations are provided in Table 3. In the “matched” Prone group, patients underwent a median of 2 (1–3) prone positioning maneuvers, which started after 5 (2–5) days of ECMO support.
Table 3.
Characteristics and clinical outcomes of matched sample of patients in the Supine vs Prone group
| Variable | Supine group, n = 227 | Prone group, n = 227 | Standardized difference |
|---|---|---|---|
| Baseline characteristics | |||
| Age, years | 53 (40–62) | 51 (40–61) | − 0.08 |
| Sex, males | 158 (69.6) | 159 (70.0) | 0.01 |
| Obesity (BMI ≥ 30) | 93 (41.0) | 93 (41.0) | 0 |
| Chronic respiratory disease | 30 (13.2) | 27 (11.9) | − 0.04 |
| Chronic heart failure | 21 (9.3) | 21 (9.3) | 0 |
| Chronic liver disease | 6 (2.6) | 8 (3.5) | 0.05 |
| Malignancy | 24 (10.6) | 25 (11.0) | 0.01 |
| Immunodeficiency | 35 (15.4) | 34 (15.0) | − 0.01 |
| Clinical illness severity | |||
| SOFA score | 11 (8–14) | 11 (8–14) | 0 |
| Baseline PaO2/FiO2, mmHg | 67 (56–77) | 66 (55–80) | − 0.01 |
| Before ECMO | |||
| Days of mechanical ventilation before ECMO | 4 (1–8) | 3 (1–8) | − 0.03 |
| Prone positioning before ECMO | 126 (55.5) | 124 (54.6) | − 0.02 |
| Mobile ECMO transfer | 152 (67.0) | 150 (66.1) | − 0.02 |
| p-value | |||
| Outcome variables | |||
| ECMO duration, days | 9 (5–25) | 15 (10–25) | < 0.001 |
| ECMO successful weaning | 135 (59.5) | 148 (65.2) | 0.208 |
| ICU LOS, days | 24 (12–41) | 31 (19–48) | < 0.001 |
| Survivors | 27 (15–49) | 34 (22–49) | 0.024 |
| Non-survivors | 19 (7–33) | 23 (15–47) | 0.005 |
| Time to successful ECMO weaning within 60 days from ECMO start, days | 56 (8–60) | 24 (12–60) | 0.705 |
| ICU mortality | 109 (48.0) | 90 (39.6) | 0.072 |
| Hospital mortality | 113/226 (50.0) | 94/226 (41.6) | 0.073 |
Data are presented as count (percentage) or median (25th-75th percentile). SOFA, Simplified Organ Failure Assessment; LOS, length of stay
ECMO duration was significantly lower in the Supine group, whereas both ICU and hospital survival rates were 8.4% higher in the Prone group (p = 0.072 and 0.073, respectively).
Kaplan–Meier survival analysis showed a lower 60-day mortality in the Prone group (log-rank test p = 0.002, see Fig. 1).
Fig.1.
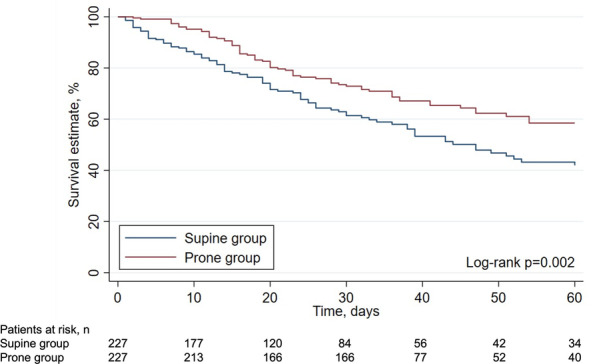
Survival estimation over 60-day follow-up in matched groups of patients. For each time interval, the survival probability was calculated as the number of alive subjects divided by the number of patients at risk
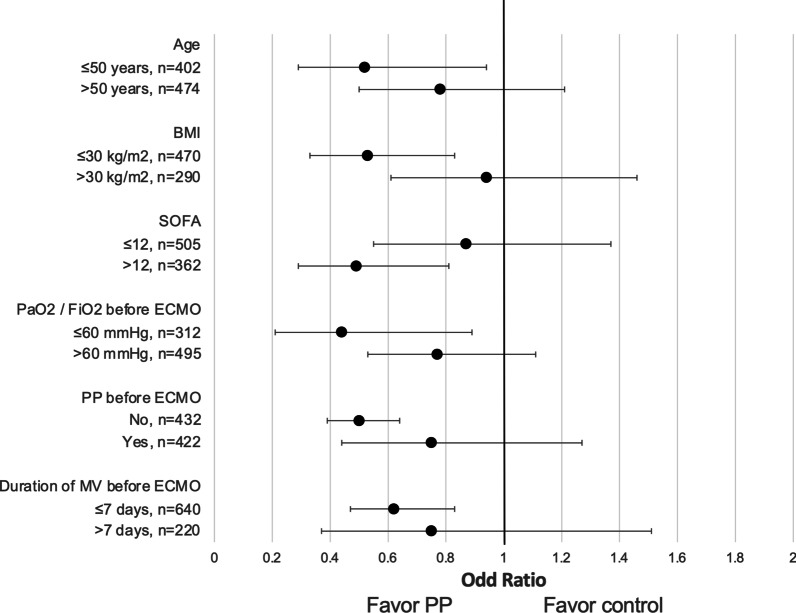
As an exploratory endpoint, we assessed the association between the use of PP during ECMO and hospital mortality in pre-defined clinically relevant subgroups (Fig. 2). The strongest association between PP during ECMO and decrease of hospital mortality was found in patients ≤ 50 years of age, BMI ≤ 30 kg/m2, SOFA > 12, PaO2 to FiO2 ratio ≤ 60 mmHg, in patients who were not proned PP before ECMO and in those with a duration of mechanical ventilation before ECMO ≤ 7 days.
Fig.2.
Post-hoc analysis on risk of hospital mortality of patients who underwent prone positioning (PP) during ECMO versus patients managed in supine position (control) in predefined clinically relevant subgroups. Risk of hospital mortality was expressed using OR (points) with 95% CI (error bars) adjusted by robust clustering taking into account the 5 original cohorts of ECMO patients (i.e. clusters). BMI, Body Mass Index; SOFA, Sequential Organ Failure Assessment; MV, mechanical ventilation
Discussion
In this pooled individual patient data analysis of European cohort studies including 889 patients, the use of prone positioning during ECMO was not independently associated with a significant reduction of ICU mortality. Although matched patients in the Prone group had an absolute decrease of ICU mortality of 8.4% (39.6% vs 48%), the difference in mortality did not reach statistical significance (p = 0.072). However, when patients were matched on baseline characteristics using a propensity score, those in the Prone group had a lower 60-day mortality.
Low volume-low pressure ventilation and repeated and prolonged prone positioning proved to decrease mortality in moderate to severe ARDS patients [1].
Although mechanical ventilation during ECMO [17] and other aspect of V-V ECMO management vary significantly among centers [18], very low tidal volume (< 4 ml/kg/predicted weight) and driving pressure are usually utilized to minimize the risk of ventilator induced lung injury.
The application of ultraprotective ventilation after V-V ECMO implantation may lead to derecruitment and decrease of respiratory system compliance [19]. PP may contribute to maintain lung recruitment while avoiding high airway pressures [20]. Previous studies [2] showed that the highest benefit of PP on survival is observed in the most severe patients. Therefore, it seems reasonable to investigate the efficacy of PP in patients supported by V-V ECMO.
To date, despite a sound physiologic rationale, the quality of evidence supporting the use of PP during ECMO is low and derives only from retrospective cohort studies. These studies gave conflicting results, since three of them [3, 4, 6] showed an independent association between the use of PP during extracorporeal support and reduced 90-day or hospital mortality, whereas the fourth one failed to show a beneficial effect of PP during ECMO on patient outcome. Another small cohort study [5] found an increased mortality of ECMO patients who underwent PP, which however was used only as rescue therapy for refractory hypoxia. Of note, this was the only study on patients with COVID-19 associated ARDS, in whom the efficacy of PP remains to be demonstrated [21]. COVID-19-related ARDS has a different pathophysiology than “traditional” ARDS, resulting in an ARDS phenotype with lower lung elastance and less recruitability[22]. Therefore, the benefit of PP on mortality might not be translated to COVID-19 ARDS.
Recently, the findings of the available studies on the use of PP during ECMO were pooled in in a systematic review and meta-analysis [23] that showed a non-significant trend towards decreased mortality in patients who underwent PP during ECMO. However, this meta-analysis by Poon et al. (which also included 4 out of 5 studies included in our analysis) was performed on aggregate data (i.e. the authors did not have access to patient data), and may be biased by an imbalance between the characteristics of patients treated with PP and controls. Our analysis, on the contrary, was performed on individual patient data, thus allowing to adjust for confounders. Individual patient data analysis may allow to homogenize and to minimize bias, while increasing the sample size and, consequently, the statistical power [12]. Due to the nature of the studies included in our analysis, our findings cannot support the routine use of PP. Nonetheless, the trend towards reduced mortality suggests that it is very unlikely that PP during V-V ECMO is associated with harm. Indeed, our study reports a low incidence of adverse events related to this procedure. However, it should be noted that PP during ECMO is a challenging procedure, and might be associated with more complications when performed in ECMO centers without a specific expertise. Moreover, PP is time-consuming and might be difficult to perform as requires four to six health care providers. This might be challenging especially in the COVID-era. For these reasons, we speculate that PP may be considered in experienced centers for the most severe ECMO patients, who present with a high degree of consolidation and lung collapse. Moreover, we might hypothesize that anticipating the commencement and increasing the number of PP procedures during ECMO (median of 2 (1–3) procedures in our study versus an average of 4 ± 4 in the PROSEVA trial [1]) might provide a greater benefit on survival.
In an exploratory analysis, we found a stronger association between PP and lower hospital mortality in patients who were younger (≤ 50 years of age), more severe (SOFA > 12, PaO2 to FiO2 ratio ≤ 60 mmHg), in patients who were not proned PP before ECMO and in those with a shorter duration of mechanical ventilation before ECMO (less than ≤ 7 days). This might indicate a greater benefit of PP in the most acute and severe patients, who have higher mortality. In addition, BMI over 30 was not associated with beneficial effects of PP. We hypothesize that ECMO patients with BMI < 30 may have sicker lungs, and thus may benefit more from PP compared to obese patients, where severe hypoxia may be partly secondary to airway or lung collapse due to high pleural pressure. Therefore, the risk/benefit ratio of proning in this subgroup should be carefully evaluated.
This study presents several limitations. First, only baseline variables were used for statistical corrections. Multiple regression and propensity score analysis do not take into account several other unmeasured variables. Data were aggregated from five studies which were not randomized trials. Therefore, we cannot exclude that clinicians decided to use PP in specific subsets of patients (i.e. selection bias). However, we may expect that clinicians tend to use PP in patients who do not improve or even transiently worsen after ECMO connection, whereas a significant improvement in oxygenation after the ECMO start might suggest keeping the patient in the supine position. Hence, this potential bias might play “against” PP in our analysis. This might explain the longer ECMO support in the Prone group, even if we cannot exclude that a longer duration of ECMO might be due to a “selection” of patients for which clinicians want to struggle more. Second, the studies included in the analysis were not homogeneous: only one study [3] was multi-centric and gathered control patients from two large “non-pronating” ECMO centers in order to minimize selection bias. Third, our search cannot be considered systematic, as we did not include all the small studies and case series who reported the use of PP in ECMO patients without comparing outcomes to (matched or unmatched) controls. Fourth, date of hospital discharge (or date of death after ICU discharge) was not available. For this reason, we could not perform a time-to-event analysis on hospital survival. Fifth, when gathering data on complications, we did not collect information on pressure sores and other skin lesions attributable to PP. Last, we did not gather physiologic data before and after prone positioning. Therefore, we did not assess if variation of oxygenation, CO2 clearance and respiratory system compliance are associated with patient outcome.
Conclusion
In our pooled individual patient data analysis, the use of prone positioning during ECMO was not associated with reduced mortality. Ongoing prospective randomized controlled trials will have to assess the impact of this procedure on mortality and other patient-centered outcomes.
Supplementary Information
Additional file 1. Supplemental methods and results.
Acknowledgements
Group Information: Investigators of the European Prone positioning During Extracorporeal Membrane Oxygenation (EuroPronECMO) study group: Giacomo Bellani, Gennaro Martucci, Antonio Arcadipane, Alberto Lucchini, Eugenio Garofalo, Mirko Belliato, Vito Fanelli, Laurent Papazian, Jean-Marie Forel, Sami Hraiech, Antoine Roch, Eloi Prud'homme, Charles Edouard Luyt, Guillaume Hekimian, Juliette Chommeloux, Marc Pineton de Chambrun, Nicolas Brechot, Dawid L Staudacher, Alexander Supady, Paul Biever, Viviane Zotzmann, Xavier Bemtgen, Asieb Sekandarzad, Kirsten Krüger, Annabelle Flügler, Erika Parmentier-Decrucq, Julien Poissy, Alexandre Gaudet, Mouhamed D Moussa, André Vincentelli
Abbreviations
- PP
Prone positioning
- ARDS
Acute respiratory distress syndrome
- ECMO
Extracorporeal membrane oxygenation
- V-V
Veno-venous
- ICU
Intensive care unit
- SOFA
Sequential organ failure assessment
- PaO2
Arterial oxygen tension
- FiO2
Inspiratory oxygen fraction
- MV
Mechanical ventilation
- BMI
Body mass index
- LOS
Length of stay
- OR
Odds ratio
- HR
Hazard ratio
- CI
Confidence interval
Authors' contributions
MG and ER conceived and coordinated the study and wrote the manuscript. ER, CG, JR, TD, TW, AP, GF, GG, AC and MS participated in the study design. ER performed the statistical analysis. MP, LT and BG contributed to data collection. All authors revised the manuscript and approved the final version.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All studies have been independently reviewed and approved by the local Institutional Review Boards.
Consent for publication
Not applicable.
Competing interests
All authors declare no conflict of interest related to the submitted work. CG reports fees from Xenios Fresenus Medical Care; GG received personal fees (payment for lectures) and travel/congress registration support from Getinge, MSD, Draeger Medical and Fisher & Paykel; MS reported personal fees from Getinge, Drager, and Xenios, all unrelated with the present work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Marco Giani and Emanuele Rezoagli contributed equally to this work.
Contributor Information
Marco Giani, Email: marco.giani@unimib.it.
Emanuele Rezoagli, Email: emanuele.rezoagli@unimib.it.
Christophe Guervilly, Email: christophe.guervilly@ap-hm.fr.
Jonathan Rilinger, Email: jonathan.rilinger@uniklinik-freiburg.de.
Thibault Duburcq, Email: thibault.duburcq@chru-lille.fr.
Matthieu Petit, Email: matthieu.petit@aphp.fr.
Laura Textoris, Email: laura.textoris@ap-hm.fr.
Bruno Garcia, Email: bruno.garcia@chru-lille.fr.
Tobias Wengenmayer, Email: tobias.wengenmayer@uniklinik-freiburg.de.
Giacomo Grasselli, Email: giacomo.grasselli@unimi.it.
Antonio Pesenti, Email: antonio.pesenti@unimi.it.
Alain Combes, Email: alain.combes@aphp.fr.
Giuseppe Foti, Email: giuseppe.foti@unimib.it.
Matthieu Schmidt, Email: matthieu.schmidt@aphp.fr.
EuroPronECMO Investigators:
Giacomo Bellani, Gennaro Martucci, Antonio Arcadipane, Alberto Lucchini, Eugenio Garofalo, Mirko Belliato, Vito Fanelli, Laurent Papazian, Jean-Marie Forel, Sami Hraiech, Antoine Roch, Eloi Prud’homme, Charles Edouard Luyt, Guillaume Hekimian, Juliette Chommeloux, Marc Pineton de Chambrun, Nicolas Brechot, Dawid L. Staudacher, Alexander Supady, Paul Biever, Viviane Zotzmann, Xavier Bemtgen, Asieb Sekandarzad, Kirsten Krüger, Annabelle Flügler, Erika Parmentier-Decrucq, Julien Poissy, Alexandre Gaudet, Mouhamed D. Moussa, and André Vincentelli
References
- 1.Guérin C, Reignier J, Richard J-C, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 2.Taccone P, Pesenti A, Latini R, Polli F, Vagginelli F, Mietto C, et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009;302:1977–1984. doi: 10.1001/jama.2009.1614. [DOI] [PubMed] [Google Scholar]
- 3.Giani M, Martucci G, Madotto F, Belliato M, Fanelli V, Garofalo E, et al. Prone Positioning during Venovenous Extracorporeal Membrane Oxygenation in Acute Respiratory Distress Syndrome. A Multicenter Cohort Study and Propensity-matched Analysis. Ann Am Thorac Soc. 2021;18:495–501. [DOI] [PubMed]
- 4.Petit M, Fetita C, Gaudemer A, Treluyer L, Lebreton G, Franchineau G, et al. Prone-Positioning for Severe Acute Respiratory Distress Syndrome Requiring Extracorporeal Membrane Oxygenation. Crit Care Med. 2021; [DOI] [PubMed]
- 5.Garcia B, Cousin N, Bourel C, Jourdain M, Poissy J, Duburcq T, et al. Prone positioning under VV-ECMO in SARS-CoV-2-induced acute respiratory distress syndrome. Crit Care. 2020;24:428. doi: 10.1186/s13054-020-03162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guervilly C, Prud’homme E, Pauly V, Bourenne J, Hraiech S, Daviet F, et al. Prone positioning and extracorporeal membrane oxygenation for severe acute respiratory distress syndrome: time for a randomized trial? Intensive Care Med. 2019; [DOI] [PubMed]
- 7.Rilinger J, Zotzmann V, Bemtgen X, Schumacher C, Biever PM, Duerschmied D, et al. Prone positioning in severe ARDS requiring extracorporeal membrane oxygenation. Crit Care. 2020;24:397. doi: 10.1186/s13054-020-03110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorusso R, Combes A, Coco VL, De Piero ME, Belohlavek J, Delnoij T, et al. ECMO for COVID-19 patients in Europe and Israel. Intensive Care Med. 2021;47:344–348. doi: 10.1007/s00134-020-06272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabie AA, Azzam MH, Al-Fares AA, Abdelbary A, Mufti HN, Hassan IF, et al. Implementation of new ECMO centers during the COVID-19 pandemic: experience and results from the Middle East and India. Intensive Care Med. 2021;47:887–895. doi: 10.1007/s00134-021-06451-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 11.Goligher EC, Tomlinson G, Hajage D, Wijeysundera DN, Fan E, Jüni P, et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome and Posterior Probability of Mortality Benefit in a Post Hoc Bayesian Analysis of a Randomized Clinical Trial. JAMA. 2018;320:2251–2259. doi: 10.1001/jama.2018.14276. [DOI] [PubMed] [Google Scholar]
- 12.Combes A, Peek GJ, Hajage D, Hardy P, Abrams D, Schmidt M, et al. ECMO for severe ARDS: systematic review and individual patient data meta-analysis. Intensive Care Med. 2020;46:2048–2057. doi: 10.1007/s00134-020-06248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raith EP, Udy AA, Bailey M, McGloughlin S, MacIsaac C, Bellomo R, et al. Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults With Suspected Infection Admitted to the Intensive Care Unit. JAMA. 2017;317:290–300. doi: 10.1001/jama.2016.20328. [DOI] [PubMed] [Google Scholar]
- 14.Broman LM, Taccone FS, Lorusso R, Malfertheiner MV, Pappalardo F, Di Nardo M, et al. The ELSO Maastricht Treaty for ECLS Nomenclature: abbreviations for cannulation configuration in extracorporeal life support - a position paper of the Extracorporeal Life Support Organization. Crit Care. 2019;23:36. doi: 10.1186/s13054-019-2334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laffey JG, Bellani G, Pham T, Fan E, Madotto F, Bajwa EK, et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med. 2016;42:1865–1876. doi: 10.1007/s00134-016-4571-5. [DOI] [PubMed] [Google Scholar]
- 16.Azoulay E, Lemiale V, Mourvillier B, Garrouste-Orgeas M, Schwebel C, Ruckly S, et al. Management and outcomes of acute respiratory distress syndrome patients with and without comorbid conditions. Intensive Care Med. 2018;44:1050–1060. doi: 10.1007/s00134-018-5209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt M, Pham T, Arcadipane A, Agerstrand C, Ohshimo S, Pellegrino V, et al. Mechanical Ventilation Management during Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. An International Multicenter Prospective Cohort. Am J Respir Crit Care Med. 2019;200:1002–12. [DOI] [PubMed]
- 18.Karagiannidis C, Brodie D, Strassmann S, Stoelben E, Philipp A, Bein T, et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med. 2016;42:889–896. doi: 10.1007/s00134-016-4273-z. [DOI] [PubMed] [Google Scholar]
- 19.Rozé H, Doassans G, Repusseau B, Ouattara A. Decrease of thoracopulmonary compliance with pressure assist controlled ventilation in ARDS patients under ECMO and transported to a referral centre. Intensive Care Med. 2017;43:148–149. doi: 10.1007/s00134-016-4616-9. [DOI] [PubMed] [Google Scholar]
- 20.Franchineau G, Bréchot N, Hekimian G, Lebreton G, Bourcier S, Demondion P, et al. Prone positioning monitored by electrical impedance tomography in patients with severe acute respiratory distress syndrome on veno-venous ECMO. Ann Intensive Care. 2020;10:12. doi: 10.1186/s13613-020-0633-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guérin C, Albert RK, Beitler J, Gattinoni L, Jaber S, Marini JJ, et al. Prone position in ARDS patients: why, when, how and for whom. Intensive Care Med. 2020;46:2385–2396. doi: 10.1007/s00134-020-06306-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poon WH, Ramanathan K, Ling RR, Yang IX, Tan CS, Schmidt M, et al. Prone positioning during venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care. 2021;25:292. doi: 10.1186/s13054-021-03723-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplemental methods and results.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.