Abstract
Antibodies are antigen recognizing immunoglobulins with an amazingly diverse repertoire in the antigen specific domain. The diversity of the antibody response is further increased by modifications such as somatic recombination and hypermutation. Furthermore, variation in the isotype and post-translational modifications such as Fc glycosylation further increase diversity of the effector functions. In particular variations in the glycan structures contribute significantly to the functional capacities of the antibodies. This is of particular interest given the dynamic nature of these modifications that is strongly influenced by the inflammatory environment.
Intriguingly, the glycan profile of antibodies has been unravelled in great detail in inflammatory (auto)immune diseases but received only limited attention in the area of infectious diseases and vaccination. Here, we reviewed the current knowledge on immunoglobulin glycosylation and specifically focussed on studies in the field of infectious diseases and vaccination against infectious diseases, an area with a lot of interesting opportunities.
Keywords: Antibody, Glycosylation, Infectious diseases, Vaccination, Glycan, Function
1. Antibody structure and function
Since the origin of uni- and multi-cellular organisms, the release and secretion of anti-microbial peptides has played a key role in cell and organismal survival by driving cell:cell communication and pathogen evasion. Among the pathogen-targeting secreted proteins, antibodies represent an evolutionary marvel, enabling both pathogen-adaptable recognition, and providing a mechanism for exquisitely specific instructions to the innate immune system to direct immune cell function and regulate inflammation. These functions are tightly regulated through the co-evolution of 2 separate protein-domains, the Fragment:antibody binding (Fab) and the Fragment:crystallizable (Fc) domain of an antibody, each responsible for directing the bi-functional activity of these molecules.
Antibodies arose approximately 500 million years ago in the jawed fish [1], enabling the generation of highly diverse repertoires of antigen-recognizing immunoglobulins that serve as both antigen targeting and effector molecules in a single molecule. The emergence of these molecules enabled an adaptive opportunity to not only recognize pathogens directly and provide a cellular basis for immunological memory upon re-encounter, but also to provide a means to enable the adaptive immune response to direct the biological activity of the innate immune system, through complement or Fc-receptors, which are expressed on all innate immune cells. Further evolutionary events led to development of machinery that enables remarkable structural diversification of the antigen-binding domain of antibodies, via somatic recombination and hypermutation [2], but also through the capacity to link these unique antigen-binding domains to one of many different possible Fc-domains [3], each endowed with their own functionality.
Antibodies are heterodimeric glycoproteins, and were first reported by von Behring and Kitasato [4] in 1890 as serum molecules able to neutralize diphtheria toxin. Antibodies are composed of two identical heavy chains (H) and two identical light chains (L), held together by inter-chain disulfide bonds [5] (Fig. 1). Each heavy and light chain possesses a variable (V) N-terminal region (VHor VL), consisting of 3 hypervariable regions, also known as complementary determining regions (CDR) [6,7]. The variable domains are in turn recombined with one of 9 different Fc constant domains [8], each able to interact with the immune system in a distinct and different manner [9,10], thereby directing the effector functions of the antibody molecule. The positioning of the disulfide bones between heavy and light chains, creates a Y-like shape, that is further stabilized by a flexible hinge region, that vary among the Fc-variants [10]. This gives rise to structural diversity in Fab arm flexibility, allowing the identical Fab arms to move freely, while binding to identical epitopes, providing bi-valent binding, and avid interactions [5]. In contrast, the Fc domain of the antibody can be classified into one of 5 isotypes: in humans these are IgD, IgM, IgG, IgA, and IgE [9], each mediating unique effector functions within distinct anatomical compartments, thus directing distinct effector functions. Moreover, among the isotypes, further diversification exists in humans, with 4 subclasses of IgG (1, 2, 3, 4) and 2 for IgA (1 and 2) each with additional structural differences and related innate immune receptor binding, complement activation, or transport across mucosal surfaces.
Fig. 1.
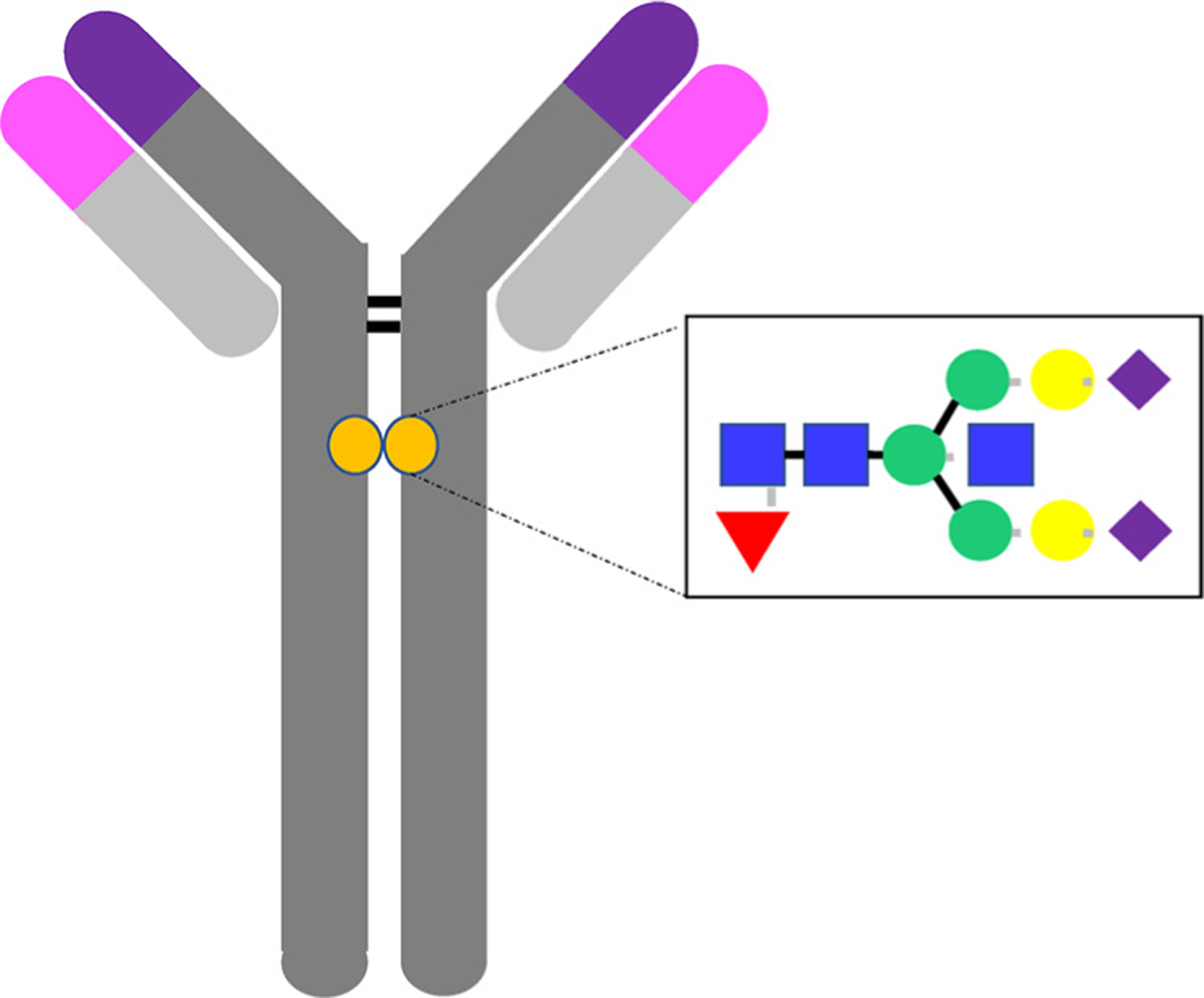
The Antibody and its glycan. IgG1 are glycosylated at asparagine-297 in the Fc-domain, to which one of 30 documented structures can be attached, known to have an essential role on antibody structure and effector function The antibody glycan is composed of a biantennary core heptasaccharide composed of a chain of 2 n-acetylglucosamine (GlcNAc) residues (blue squares), followed by a mannose (green circle), followed by a 1,3 and a 1,6 mannose branching and an additional GlcNAc residue on each mannose. Variable addition of an additional fucose (red triangle), an additional bisecting GlcNAc, up to 2 galactoses (yellow circle) or up to 2 sialic acids (purple triangle) then give rise to antibody microheterogeneity.
However, beyond this protein diversification, immunoglobulins as glyco-proteins also vary in numbers of glycosylation sites [5,11] (Fig. 1). For most immunoglobulins, alterations in glycosylation regulates structural stability and half-life [12,13]. Moreover, like most cell surface and secreted proteins, glycosylation varies widely, depending on the inflammatory state [14]. However, unlike other proteins, these changes contribute directly to altered effector function [12,13], offering an additional dimension of antibody functional diversity. Remarkably, changes in IgG glycosylation have been observed across health and disease [15,16], with dramatic changes noted in antibody glycosylation with age, during pregnancy, across geographic areas [17,18], and even among the sexes [17,19–22]. Antibody glycosylation varies furthermore in a specific IgG subclass-dependent manner, aimed at selectively regulating specific antibody functions [23,24]. Additionally, profound changes in IgG glycosylation have been observed in autoimmune [25], infectious [26], and malignant diseases [22,27–29]. Because these changes in glycosylation have been causally linked to altered Fc-effector function for IgGs [12], the monoclonal therapeutics field has begun to extensively exploit these IgG modifications to improve effector function for therapeutic purposes.
2. Antibody glycosylation
Glycosylation of proteins is a remarkably dynamic posttranslational modification that governs the biology of the majority of cell surface and secreted proteins [14,30]. Specifically, glycosylation can impact secretion, stability, solubility, packing, binding, conformation, biological activity, and antigenicity [30]. Because antibody glycosylation is not templated, but influenced by immune state, it is a remarkably adaptive process, creating an unprecedented level of microheterogeneity in proteins, which endow proteins with a broader range of potential functional roles, some silent, some subtle and some more profound [11,14]. Critically, variation in protein glycosylation is conserved and not random under normal physiological conditions, and variation is reproducible and controlled [26,31], following specific patterns when perturbed [32–34], suggesting exquisite control of this biological process. Glycans are added to proteins through one of 2 molecular linkages, linkages on asparagine residues (N-glycans) or on serine/threonine residues (O-glycans) [30]. However, unlike other proteins, to which dozens of different N- or O-linked glycans may be added, a stricter repertoire of N-linked glycans can be coupled to IgG [26,30]. Importantly, all IgG species are glycosylated at a single asparagine-297 residue in the Fc-domain of the IgG antibody, to which one out of 30 documented structures can be attached, which are known to have an essential role on antibody structure and effector function [12,13]. Additionally, approximately 20% of Fab domains can evolve to include a N-linked glycan site [35]. However, this site is glycosylated more uniformly, with a more restricted glycan profile than that found in the Fc-domain of the antibody.
The antibody glycan is composed of a biantennary core heptasaccharide composed of a chain of 2 n-acetylglucosamine (GlcNAc) residues, followed by a mannose, followed by a 1,3 and a 1,6 mannose branching and an additional GlcNAc residue on each mannose [30] (Fig. 1). Then variable addition of a fucose, a bisecting GlcNAc, two galactoses, and two sialic acids residues to the core sugar forms the basis of antibody glycan diversity [11] of which 30 structures have been observed in healthy human plasma. Bisection, the addition of an extra GlcNAc to the core mannose, hampers fucosylation by steric hindering of the antibody, thereby enhancing functionality of the antibody. Glycan diversity can be classified based on their inflammatory and functional capacity [25]. Specifically, heterogeneous glycans can be classified based on their level of galactose incorporation, as glycans that have no galactose residues (G0), one galactose residue (G1), or two galactose residues (G2). Striking enrichment of agalactosylated (G0) glycans is observed in inflammatory diseases [25] (Fig. 2). Conversely, elevated levels of galactosylation are associated with reduced inflammatory activity in antibody preparations [16,36].
Fig. 2.
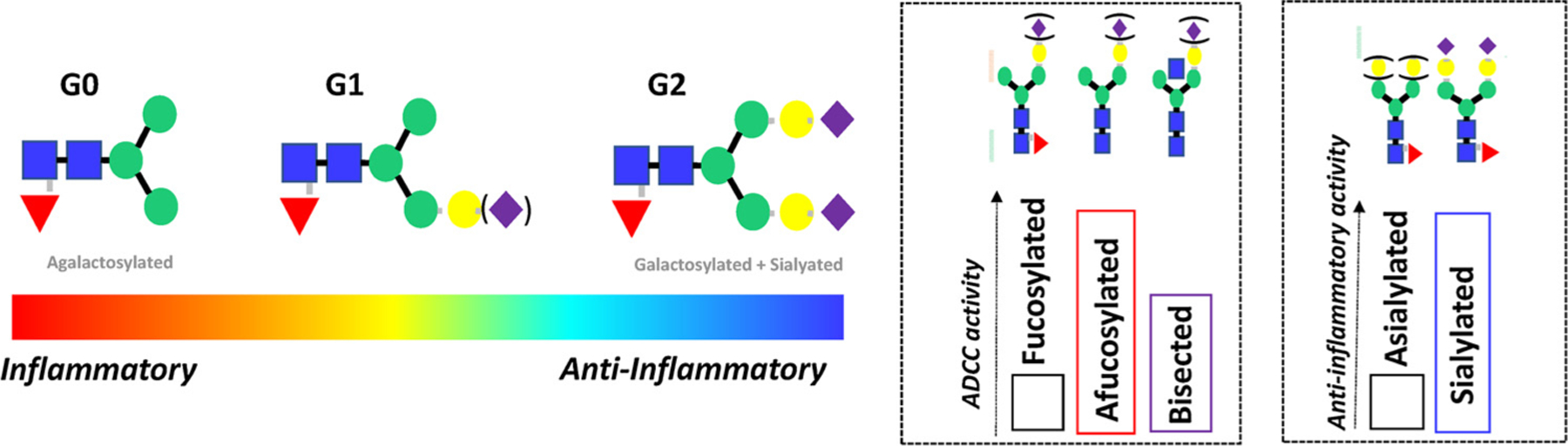
The functional consequences in antibody glycan changes. Changes in galactosylation have been observed across a multitude of human diseases, linked to alterations in antibody inflammatory activity. Specifically, an accumulation of agalactosylated antibodies observed across inflammatory diseases (auto-immunity, infections, malignancy), whereas increased galactosylation is associated with less-inflammatory conditions (pregnancy). Moreover, specific glycan changes have been exploited in the monoclonal therapeutics field demonstrating the critical role of glycans in shaping antibody effector function. For example, removal of fucose reproducibly enhances ADCC activity, whereas changes in bisecting GlcNAc alone have a more modest effect. Additionally, sialylation reproducibly enhance the anti-inflammatory activity of IVIG in vivo.
Galactosylation shows unique age-distributions with G0 antibodies enriched in the very young [20] and the elderly [21,22,24] (Fig. 3). Moreover, levels of galactosylation increase during pregnancy, with a significant increase in galactosylation and sialylation and decreased bisection that normalizes post-partum [19,37,38]. Furthermore, Fc-glycosylation also varies significantly across geographic regions of the world [18], and again is dramatically altered in autoimmune [25], infectious [26], and oncological diseases [22,27,29] (Fig. 3).
Fig. 3.
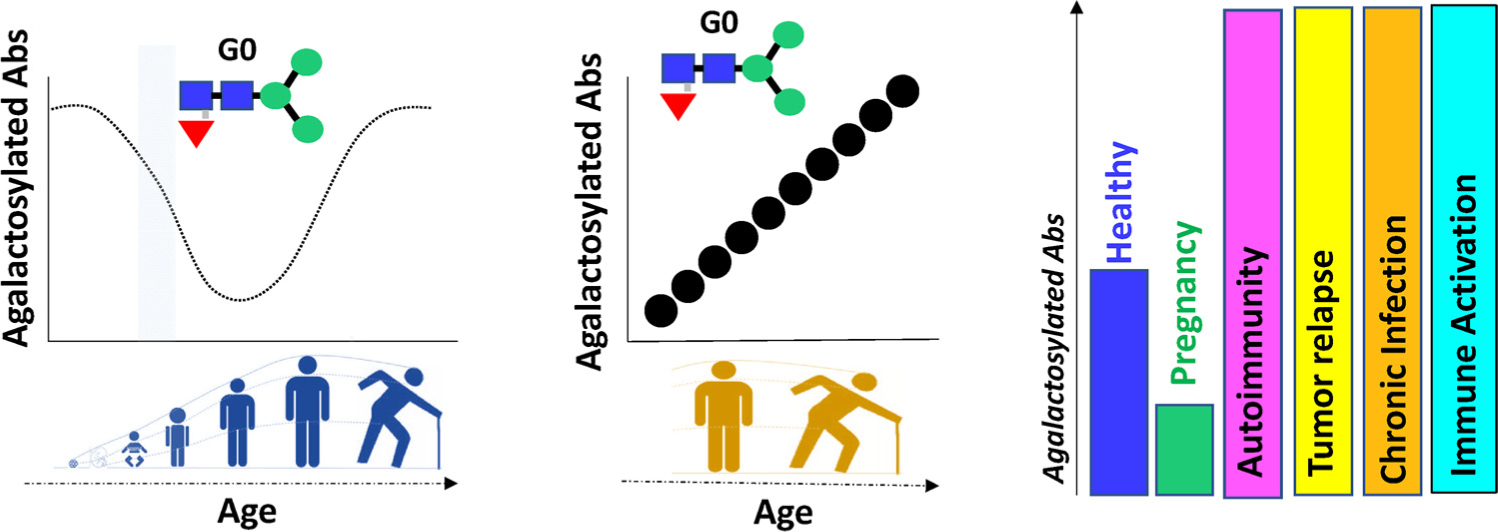
Galactosylation in health and disease. Changes in galactosylation have been widely documented across health and disease. Groundbreaking work pointed to an interesting distribution of glycans across the ages, with increased levels of inflammatory – agalactosylated- antibodies in early life, which re-emerged with age. Moreover, compelling evidence points to an accumulation of agalactosylated antibodies with inflammaging- which may be unlinked from numerical age. Finally, agalactosylated antibodies accumulate across inflammatory diseases of dramatically different etiologies, but decrease in anti-inflammatory states, such as pregnancy.
Beyond alterations in galactosylation, the removal of fucose [39] has been clearly linked to improved antibody-dependent cellular cytotoxicity [40,41] (Fig. 2). Likewise, improvement of ADCC has also been linked to the addition of a bisecting GlcNAc, though this improvement is related to the indirect loss of fucose upon addition of a bisecting GlcNAc [42] (Fig. 2). Moreover, because each Fc domain is glycosylated, and each arm may possess a different glycoform, heterogeneity is further increased across IgG antibodies in a polyclonal pool. Variable addition of glycans give rise to a diverse set of glycoforms that affect stability, pharmacokinetics, distribution by altering the shape of the Fc-backbone, all ultimately impacting antibody function by altering Fc-interactions with a wide array of innate immune receptors or complement that regulate innate immunity [9].
3. Antibody glycosylation and function
IgG antibodies are able to deploy effector functions following Fc-binding to both type 1 and 2 Fc-receptors found on all innate immune cells, or classical or non-classical initiators of the complement cascade [43] (Fig. 4). In humans, 3 Fc-receptor groups exist that interact with IgG, including 1 high affinity FcγR1, and 2 low affinity Fc-receptors including FcγR2 and FcγR3 [9,44]. Moreover, three variants of FcγR2 exist, including 2 activating variants: FcγR2a expressed in all humans and FcγR2c only expressed in a fraction of the population due to a splice polymorphism; and an inhibitory variant, FcγR2b. Similarly, two variants of FcγR3 exist, including a transmembrane activating FcγR3a and an activating GPI anchored variant of this Fc-receptor, FcR3b. Additionally, high and low affinity polymorphic variants have been identified for both FcγR2a and FcγR3a [45]. In addition, IgG is also able to interact with the classical initiator of the complement cascade, C1q to drive both direct cytotoxicity as well as direct immunoregulatory functions [46]. Furthermore, complexed antibodies have also been implicated in binding to non-classical Fc-receptors, lectin like receptors, including DC-SIGN [47], CD22 [48], CD23 [49,50], etc. (Fig. 4) Thus, because IgG antibodies are able to interact with a remarkably broad array of receptors/proteins, expressed broadly across immune and non-immune cells, antibodies have the capacity to direct and regulate the immune system broadly. However, with our growing appreciation of the role of both immune-protective and -pathological antibodies in disease, it is plausible that antibodies, and specifically complexes of antibodies, may interact with an even broader array of receptors than currently known.
Fig. 4.
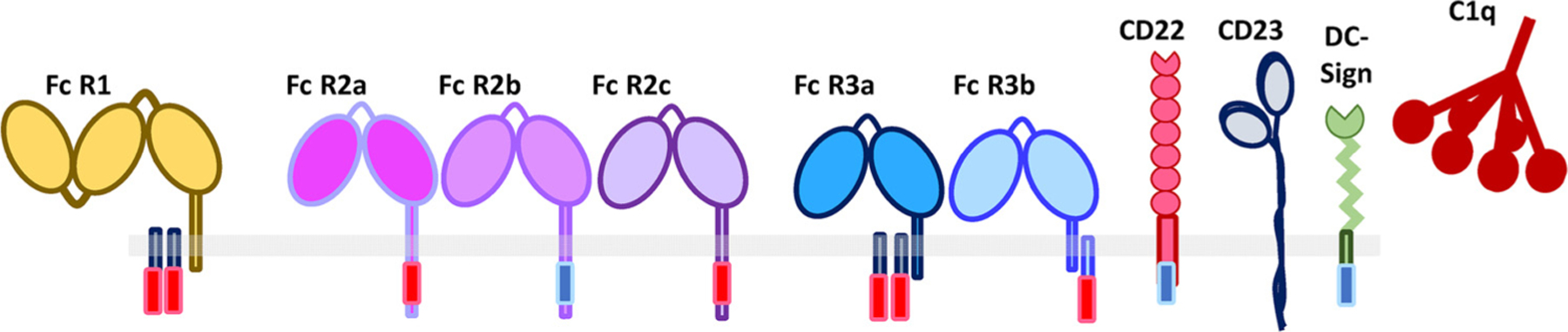
The landscape of antibody-Fc binding receptors. Beyond canonical Fc-receptors, mounting evidence supports a broader role of additional lectin-like proteins in interacting with immune complexes and deploying a broader array of antibody functions including both activating and inhibitory activities.
Importantly, most antibody interactions with canonical and non-canonical receptors/proteins are low-affinity, requiring complexes of antibodies [44], rather than single antibodies, to bind simultaneously in order to drive highly avid interactions and subsequent cellular activation. Cellular activation can then drive phagocytosis, antibody dependent cellular cytotoxicity (ADCC), complement dependent cytotoxicity (CDC), neutrophil activation, etc., all of which are abrogated by IgG deglycosylation [12]. However, as mentioned above, IgG crystal structures demonstrate that the IgG glycan is tucked snuggly between the 2 CH2 domains of the antibody [51,52], and early crystal structures suggested that the Fc-glycan made minimal contacts with Fc-receptors, despite the fact that removal of the glycan resulted in the abrogation of IgG binding to Fc-receptors. Conversely, removal of fucose has a profound impact on modulating FcγR3a binding [39], through a glycan:glycan interaction with a second fucose attached to the Fc-receptor [53]. However, beyond fucose interactions, the glycan contributes significantly to the Fc-conformation, where larger more processed glycans show the widest conformation, that then enables differential binding to both canonical and non-canonical Fc-receptors/proteins [52]. Additionally, highly galactosylated IgG trigger enhanced complement activity through C1q binding [41,54]. However lack of galactosylation may also make GlcNAc residues more accessible for mannose-binding protein (MBP) binding and thus activation of the alternative complement cascade [55]. Thus, complex roles exist for distinct sugar modifications, that finely tune the shape of IgG Fc, enabling unique interactions with Fc-receptor or Fc-receptor like proteins. Yet the functional consequences of these different binding profiles are most clearly illustrated in the context of disease.
4. Antibody glycosylation and anti-inflammatory activity
IgG glycosylation changes have been widely documented across autoimmune diseases [15,25]. Specifically, altered glycosylation has been observed in rheumatoid arthritis [19,37,38,56–61], lupus [62], multiple sclerosis [63,64], diabetes [65], Sjogren’s disease [66], inflammatory bowel disease [29,67,68], alzheimer’s [69], myasthenia gravis [70], and Guillain-Barre [71]. Specifically, autoimmune diseases are largely associated with a dramatic, common, and significant loss of galactosylation, likely related to the critical linkage between changes in IgG galactosylation and generalized immune activation [72]. While glycosyltransferases and glycosidases are elevated in autoimmune, inflammatory diseases, changes in antibody glycosylation do not correlate with changes in circulating enzymes [73]. Instead, transcriptional changes in enzymes [50,74] and alterations in sugar precursors have been observed in the setting of disease, potentially regulating antibody glycan levels at an intracellular level [75]. However, since the inflammatory changes are conserved across distinct autoimmune diseases of highly diverse etiologies, it is likely that generalized inflammation regulates galactosylation in a common and universal manner. Given the intimate role of galactosylation in tuning antibody effector function, including complement activity, it is plausible that alteration in galactose levels is a non-specific mechanism exploited across diseases to rapidly drive immune complex clearance. However, because these complexes persist, chronic immune activation likely also contributes to the pathology of many autoimmune disease.
However, significant differences in antibody glycosylation are observable on antigen-specific antibodies and within disease-specific compartments. Specifically, changes in anti-citrullinated protein-specific antibody (ACPA) Fc-glycosylation, but not Fab-glycosylation [76], both preceded and predicted the recurrence of autoimmune flares [59] and the amelioration of symptoms and disease activity [19,37,57]. Moreover, beyond antigen-specific differences, Fc-glycosylation changes are more profoundly altered at the site of disease, where more dramatic differences are observed in IgG Fc-glycosylation in the synovium during rheumatoid arthritic disease [60] and within the cerebrospinal fluid in multiple sclerosis [64].
Beyond their roles as biomarkers, the biological role of IgG glycosylation was most clearly demonstrated in a number of autoimmune mouse models [16,25]. Interestingly, injection of endoglycosidase S (EndoS), that removes the majority of the Fc-glycan excluding the final core GlcNAc, resulted in significantly attenuated immunopathology and tissue injury in a number of autoimmune mouse models [77]. Similarly, in the lupus-prone BXSB mice, efficient removal of the serum IgG Fc-glycan with EndoS did not alter the production of disease associated antibodies, including anti-dsDNA or ANA-specific antibodies, but did prevent glomerulonephritis and improved survival [78,79]. Additionally, IgG galactosylation was shown to contribute to FcγR3-mediated autoimmune hemolytic anemia [36] whereas highly galactosylated IgG1, but not other subclasses, reduced inflammation in a complement dependent manner, via the inhibitory FcγR2b and lectin-like Dectin-1 receptors [80]. Furthermore, sialic acid residues have been extensively associated with reduced inflammation and pathology. For example, terminal sialylation was associated with reduced FcγR3 binding and reduced platelet clearance in a mouse model of thrombocytopenia [81] and to significantly reduce ADCC activity [82,83] and complement activity [84] in vitro via reduced binding to Fc-receptors [85].
Most revelatory, the broad impact of intravenous gammaglobulin (IVIG) has clearly illustrated the critical nature of antibody glycosylation on inflammation. Specifically, IVIG is used widely to treat a large number of autoimmune, infectious, and inflammatory diseases [16]. The anti-inflammatory properties of this mix of polyclonal antibodies from thousands of blood donors is driven by a small subset of antibodies that harbor α2,6-sialylation [81,85]. Specifically, removal of the Fc-glycan with PNGaseF resulted in reduced immunoprotective activity, which was additionally recapitulated with the removal of sialic acid residues alone, using neuraminidase. Thisclearly implicates sialylation of IVIG as the key to anti-inflammatory activity of the IgG Fc-glycan. Remarkably, dissection of the biological mechanism of this anti-inflammatory activity pointed to a complex network of interactions where sialylated IgG required the c-type lectin receptor SIGN-R1 (specific intracellular adhesion molecule-grabbing non-integrin R1) or the human orthologue DC-SIGN (dendritic-cell-specific ICAM-3 grabbing non-integrin), that was linked to the induction of a novel Th2 response, marked by interleukin-33 secretion (IL-33) [85,86]. A similar interaction between SIGN-R1 and IVIG has been observed in a mouse model of immune thrombocytopenia (ITP) [85]. Moreover IVIG has also been linked to reduced B cell activation via direct interactions with CD22 during B cell signalling [85]. However, precisely how polyclonal IVIG competes with disease-specific antibodies, that are likely found in complexes, is unclear, but both in vivo and in vitro data clearly highlight the critical role of IVIG glycan profiles in driving the disease modifying activity of this surprisingly effective treatment.
5. Antibody glycosylation and infection
Like autoimmune diseases, chronic infections also induce chronic inflammation, and are marked by a loss of galactosylated antibodies [74,87,88], likely also linked to infection associated immune activation. Specifically, an expansion of agalactosylated IgG antibodies was observed in HIV-infected individuals compared to healthy controls. Strikingly higher levels of agalactosylated antibodies were observed in HIV compared to autoimmunity [87]. Moreover, further studies highlighted that this expansion of agalactosylated antibodies occurred in both individuals with progressive HIV infection off therapy as well as in those that spontaneously control HIV infection, despite lower levels of immune activation in the “controllers”. Moreover, unlike in pregnancy and autoimmunity, where glycosylation changes resolve post-partum or after flare termination, respectively, agalactosylated antibody levels did not revert following anti-retroviral therapy, despite a reduction in immune activation due to the elimination of circulating virus [74]. These data argue that infection, unlike pregnancy and autoimmunity, likely perturb the B cell compartment, resulting in permanent changes in antibody glycosylation, reflecting a permanently more inflamed antibody glycome. Interestingly, altered antibody glycosylation was also observed in Mycobacterium tuberculosis (Mtb) infection, however, glycosylation shifted with disease severity, with an accumulation of agalactosylated glycans in individuals with active Mtb infection and normal levels of galactosylation in individuals with latent, controlled, Mtb infection [88]. These data suggest that unlike HIV infection which appears to irreversibly affect the humoral immune response [89], antibody glycosylation appears to not be irrepairably altered in the context of Mtb infection. Whether irreversible changes occur across other diseases, with respect to antibody glycosylation is uncertain, but could point to the mechanism(s) by which antibody glycosylation is regulated at a molecular level within B cells.
Beyond inflammatory glycan changes, additional changes were observed within the antigen-specific antibody compartment, associated with enhanced functionality, in both HIV and Mtb infections. Both agalactosylation and afucosylation were enriched in HIV-specific antibodies among spontaneous controllers of HIV, and were linked to enhanced NK cell activity [74]. Similarly, latently infected Mtb controllers also exhibited an expansion of afucosylated antibodies, which were also able to drive enhanced NK cell activity and bind more effectively to FcγR3a [88]. These data suggest that for both of these intracellular infections, where antibody-dependent cellular cytotoxicity may be key to infected cell control and clearance, antigen-specific antibody glycosylation may be selectively skewed to promote more effective destruction of the pathogen. Thus collectively, these data highlight similar changes in bulk IgG glycosylation in infectious diseases similar to those observed in autoimmune disease, however antigen-specific glycosylation appears to be directed in a deliberate manner during infection, enabling individuals who control their infections by driving enhanced antibody functionality. These exciting data strongly argue for immunological control of antibody glycosylation, and suggest that vaccination may have the capacity to harness the unexplored functional potential of the antibody Fc-domain via glyco-regulation.
6. Vaccination and antibody glycosylation
Vaccination against infectious diseases has been initiated in the late 1800 s by Edward Jenner, who discovered protection against smallpox be exposing people to the related cow pox virus. Although the mechanism of protection remained elusive for a considerable time, the use of a related virus to reduce the burden and incidence of an infection like smallpox was a giant step forward for global health. Subsequent vaccination strategies were based on isolation of the pathogens, inactivation and injection, and more recently live attenuated pathogens were replaced by antigenic fragments [90]. Vaccine induced protection against most pathogens depends on the induction of neutralizing antibodies, in particular for protection against most viruses. More complex pathogens may require additional (cellular) protective mechanisms. Several of the vaccines that have been developed many years ago are still used on a daily basis. For example, BCG (Bacillus Calmette Guerin) the vaccine against tuberculosis (TB) was developed in the 1920 s and is still administered to newborns in large parts of the world.
Vaccine induced antibodies often act through neutralization of critical antigens for cell entry, such that antibody binding inhibits infection and contributes to elimination of the pathogen (Fig. 5). Moreover, antibody binding to pathogens may contribute to opsonisation and subsequent clearance of the pathogen. Antibody neutralization appears highly efficient for small pathogens such as most viruses but seems less efficient in case of more complex pathogens such as parasites and (intracellular) bacteria.
Fig. 5.
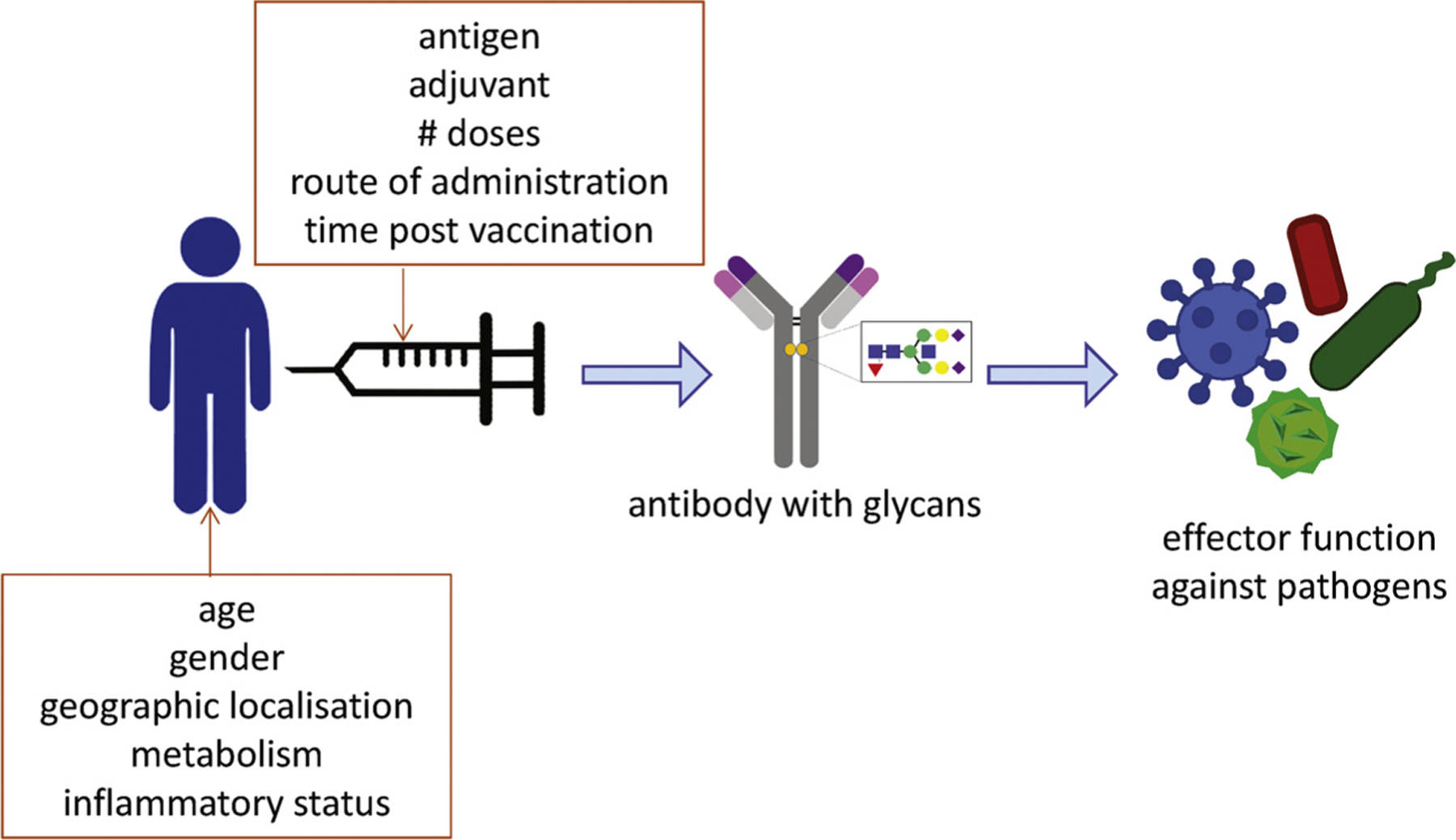
Factors that may affect vaccine efficacy. Vaccination against infectious agents aims in the induction of antibodies that subsequently contribute to elimination of the pathogen. The function of these antibodies is shaped by the glycan repertoire on the Fc portion of the antibody. Different factors may influence the glycosylation profile of antibodies, both host related factor such as age, gender and origin, inflammatory status, as well as vaccine factors determine the end result. Vaccine factors that may influence antibody induction and modification are poorly characterized but may involve antigen, adjuvant, doses, administration routes and the time window since vaccination.
Studies on vaccine induced antibodies have comprised not only analysis of antigen recognition and viral neutralization but also the characterization of the properties of the antigen specific antibodies.
However, in contrast to the highly detailed characterization of antibody properties in health and disease only a limited number of studies have assessed the glycosylation profiles of antibodies evoked by vaccination. Antibody induction by vaccination not only depends on the specific vaccine antigen, but also the adjuvant used, which will influence antibody properties, including glycosylation patterns. Moreover, depending on the type of vaccine single or multiple doses are required to induce optimal protection, but subsequent B-cell activation by booster vaccination may not only increase antibody levels but may also alter glycan structures.
Thus vaccination is an interesting “model” to assess glycosylation profiles and learn about in vivo regulation of glycosylation mechanisms and phenotypes. Firstly, the antigen and immune-activating adjuvant can be controlled and compared. Antibody responses to single recombinant antigens may be functionally completely different from antibodies formed in response to complete pathogens, either live attenuated or killed. Multiple different vaccine antigens can be administered simultaneously to compare regulation of glycosylation by the antigen rather than the host. Secondly, priming vs. booster responses can be compared. Priming vaccines represent the first exposure of the immune system to the particular antigen. In contrast to the permanent persistence of autoantigens or the long-term exposure to tumour-derived antigens, antigens in vaccines may be cleared from the body and thus only temporarily trigger the immune system. It would be highly interesting to assess the stability of glycosylation stages for antigens that are only temporarily present. In addition, re-exposure to the antigen may modify the glycosylation profile, and this process might be evoked by revaccination or subsequent encounter of the pathogen. Thirdly, in a vaccination setting the time since primary antigen encounter is known and responses can be monitored over time. Booster vaccinations may not only alter the magnitude of the antibody response but potentially also the glycosylation signature. Time between primary exposure and subsequent re-encounters may be varied to assess waning and boosting capacities. Fourthly, vaccination generally occurs in healthy individuals and thus in the absence of a persisting systemic inflammatory environment. In the absence of strong inflammatory signals B-cells may be more strongly influenced by antigen properties, rather than by the cytokine milieu. Fifthly, vaccination in healthy adults does not conflict with any immune-modulating treatment regimens, as may be the result in many individuals treated for auto-immune diseases.
Given the strong advantages and feasibility of monitoring antigen-specific antibody glycosylation in a vaccination setting in humans, it is surprising to see how limited the studies into this area have been thus far. The limited number of studies that have been performed do confirm the interesting patterns of antigen specific antibodies induced by vaccination and strongly support further investigation of the glycan repertoire of antigen specific IgG following vaccination. However, given the natural variation in IgG-Fc glycosylation between sexes, but also during normal physiological processes such as ageing and pregnancies as elaborated on above, vaccine studies should be carefully designed and balanced (Fig. 5). Only then vaccine-induced changes in IgG Fc glycosylation can be identified. Moreover, vaccination in infants and young children may result in different IgG glycan profiles as compared to vaccination with the same combination of antigen and adjuvant in adolescents, adults or the elderly. Therefore results from these studies should be interpreted carefully, taking into consideration natural physiological differences between the populations.
In mice, steady-state IgG glycosylation patterns already differs between the IgG subclasses [34]. Moreover, not all IgG subclasses respond with similar glycosylation changes to vaccination with the influenza nucleoprotein antigen, such that not only pro and anti-inflammatory signals but also IgG subclass determine which precise glycoforms are induced [34]. T-cell independent vaccines (4-hydroxy-3-nitrophenyl) acetyl - lipopolysaccharide (NP-LPS)) resulted in a stronger induction of sialylated antibodies in experimental vaccination studies [34]. High level sialylation may reduce binding to activating Fc receptors and thereby result in reduced levels of pro-inflammatory signals. Similarly, T-cell independent vaccination with the TNP (2,4,6-trinitrophenyl) antigen in mice, induced suppressive sialylated antibodies, whereas T-cell dependent antigen induced antibodies that lacked galactose and sialic acids [91]. T-cell help in the presence of proinflammatory stimuli (by Th1 and Th17) is also involved in the induction of proinflammatory IgG responses [91]. T-cell dependent antigens in the presence of proinflammatory stimuli resulted in IgG antibodies that lacked sialic acids [91,92]. By contrast, if co-stimulatory immune activation signals are lacking, antibodies are induced that contain sialic acids and thus can mediate immunosuppressive functions.
Mice vaccinated with BSA (bovine serum albumin) in incomplete Freund’s adjuvant initially had anti-BSA antibodies with low levels of galactosylation, but when the titres decreased the antibodies were found to contain more galactoses [93]. This indicates that time is also a critical factor, in particular in the vaccination setting where antigen is only present temporarily. It would have been interesting to boost these animals and assess IgG galactosylation following secondary antigen encounter. Others have shown that repeated vaccination with ovalbumin (OVA), in the absence of adjuvant, resulted in antigen specific fucose containing IgG antibodies, and that the degree of fucosylation increased with repeated booster vaccinations [94]. However, no differences in galactose, mannose or sialic acid were detected between the differently boosted groups, and unfortunately no group that only received the priming dose was included [94].
Non-human primates were vaccinated with HIV gp140 envelope protein (Env) adjuvanted with alum, MF59 or adjuvant nanoemulsion (ANE) in the absence or presence of TLR activators and antibody induction assessed. Antibody titers were highest in animals vaccinated with Env in alum + TLR7, MF59 or ANE + TLR4 ligand. Antibodies induced by alum + TLR7 and MF59 had the highest avidity. Antibodies induced by Env adjuvanted with MF59 maintained the highest titers during follow up [95]. Serological analysis revealed clear differences between the adjuvants that correlated with the functional capacities of the antibodies [95].
Together, these animal studies illustrate that IgG subclasses respond differently to vaccination, antigen, adjuvant, number of doses, the environment and the time since vaccination, and that all these factors an influence the resulting glycosylation signature. However, all studies report rather anecdotal analyses and this field would benefit from more systematic analysis of the glycan repertoire as a result of vaccination.
Comparison of human volunteers vaccinated with meningococcal, pneumococcal or influenza vaccines revealed different glycosylation responses for the IgG subclasses depending on the vaccine. Global Fc glycosylation profiles were not altered by vaccination [96]. In a small group of humans, 2 influenza vaccines were compared, a pandemic influenza vaccine adjuvanted with squalene and a seasonal influenza vaccine without adjuvant [96]. The vaccine adjuvanted with squalene induced fewer glycoforms and these glycoforms were less complex [96]. In the same study an adjuvanted meningococcal vaccine (Al(OH)3) did not show the same effects, indicating that adjuvants may have specific effects on glycosylation. Although the numbers of individuals were highly limited, repeated annual vaccination against influenza seemed not to influence the complexity of IgG glycosylation [96]. The presence or absence of specific glycan groups such as fucoses will determine functional properties, e.g. the presence of fucoses greatly diminishes ADCC. Although functional assessments were not performed in this study differences in fucosylation suggest differences in functional capacities [96].
Moreover, the IgG1 Fc glycosylation repertoire was assessed following vaccination with the MF59 adjuvanted influenza vaccine in adults [97]. Vaccine specific IgG1 antibodies had increased levels of galactosylation and sialylation, but decreased bisecting GlcNAc [97]. The number of sialic acids per galactose increased over time post vaccination [97]. Again, functional implications of these results were not assessed and the number of samples analysed is limited and relatively heterogeneous.
Glycosylation of Fc IgG1 was assessed upon vaccination with the trivalent inactivated influenza vaccine. Sialic acid on IgG1 peaked at day 7 post vaccination, after which it decreased again, but persisted at higher levels compared to pre-vaccination [50]. In contrast, fucose was consistently present on IgG1 molecules after vaccination. Galactose also peaked at day 7 but returned to baseline afterwards, while no regulation of bisected GlcNac was observed [50]. The presence of sialic acid on the IgG1 molecules correlated with the affinity for antigen on week 3 post vaccination [50].
Vaccination with the trivalent influenza vaccine was found not to alter the total IgG glycosylation profile [98]. However, if the glycosylation repertoire was analysed in responders compared to non-responders important differences were identified. Responders had increased levels of galactosylation of the bisected glycans and high mannose containing glycoforms [98]. The glycosylation differences observed may be used as predictive biomarkers of vaccine responsiveness as they were already detectable prior to vaccination and persisted post vaccination [98].
Natural antibodies against HIV have been analysed in great detail for their variation in Fc glycosylation and antiviral activity. Intriguingly, HIV status and the capacity to control viral replication was associated with changes in total Fc IgG glycosylation [74]. Spontaneous control of HIV was associated with a shift towards agalactosylated glycoforms for the total IgG fraction. This was much more prominent among HIV specific antibodies (gp120), that had a greater frequency of antibodies that lacked galactoses, fucoses and sialic acids [74]. The glycoforms of these gp120 specific antibodies resulted in enhanced Fc mediated viral control [74]. Different antigens may evoke either more or less immune activating glycosylation profiles: during HIV infection p24 specific antibodies expressed more of the highly inflammatory agalactosyated glycan as compared to gp120 specific antibodies [18].
In contrast, vaccination of HIV negative individuals with different formulations of the gp120 antigen elicited differently glycosylated IgGs. Gp120 presented in an adenoviral vector was compared to gp120 adjuvanted with alum [18]. Indeed, significant differences were identified in glycosylation profiles between the differently adjuvanted vaccines. Gp120 specific antibodies elicited by adenoviral vectors had higher proportions of di-galactosylated and sialylated glycans, fitting with a more anti-inflammatory profile. Moreover, the viral vectored vaccine elicited increased proportions of bisected glycan structures [18]. Bisected glycans in general have been reported to elicit greater ADCC activity [99], but this has not been shown specifically for these adenoviral vector elicited gp120 specific antibodies. Combined, the viral vector induced antibodies that activate less inflammatory antibody glycosylation patterns but very efficient viral elimination [18]. Intriguingly, the glycan profile of the vaccine induced antibodies was completely different from the antibodies involved in control following natural infection. The same antigen thus can trigger completely different Ab glycan profiles depending on the context, either infection or vaccination, modified by the type of vaccine/adjuvant, indicating regulation of glycosylation in vivo by the environment.
In summary, vaccination offers a highly interesting clinical human model to study and dissect the different aspects that contribute to the induction, diversification as well as the persistence of (human) IgG Fc glycosylation patterns, which has been underexplored. We strongly support more in depth analysis of vaccine induced glycosylation pro-files, not only to understand the protective efficacy of specific vaccines but also to explore the mechanisms, regulation and stability of IgG glycosylation (Fig. 5). The limited amount of data available to date suggest that subtle changes are functionally highly significant.
7. Summary and future perspective
Immunoglobulins and in particular IgG undergo dynamic post-translational modifications, glycosylations, that strongly influence secretion, stability, solubility, conformation and biological activity. Since the process of glycosylation is highly dynamic and determined by individual B-cells in response to the environment, IgG glycosylation responses may be functionally very different even for antibodies with the same specificity. Natural variation in IgG Fc glycosylation occurs between sexes, but also during ageing and pregnancies. Inflammatory conditions such as autoimmune diseases but also chronic persisting infections may further contribute to modification of glycosylation profiles.
Diversity in glycosylation profiles may affect both the inflammatory as well as the functional properties of the antibody (Fig. 6). Agalactosylated antibodies are increased in inflammatory diseases, whereas antibodies with sialic acids are associated with anti-inflammatory functions and the presence of fucose is associated with ADCC and subsequent phagocytosis (Fig. 6). Expression of glycosyl transferases and other enzymes involved in glycosylation is regulated by B-cells individually. However, it is unknown how the glycosylation machinery of individual B-cells is regulated, it may be influenced by many factors including host metabolism, sugar abundance, Golgi function and other factors, and may very well respond to inflammatory signals in the environment. Therefore comparison of antibody responses against the same antigen in the context of infection or vaccination may result in highly different glycan profiles. Similarly, treatment of inflammatory flares in autoimmune diseases may significantly alter the glycan repertoire irrespective of antigen specificity. Antibody glycosylation profiles and subsequent functional properties have mostly been investigated in auto-immune diseases, for antibodies specific to auto-antigens and under strong inflammatory conditions. However, antibody responses to neo-antigens such as those derived from pathogens have received only limited attention (Fig. 6). Glycosylation profiles of antibodies to pathogen derived antigens may not only be different because the antibodies are directed against neo-antigens but also the inflammatory signals are different. Infections generally result in activation of danger signals such as triggering of Toll-like receptors (TLR) or Nod-like receptors (NLR). Ligation of these pathways may strongly alter glycosylation capacities of individual B-cells. Moreover, vaccination is absolutely under-explored in this setting (Fig. 6). Although it is uncertain which parts of the biological phenomenon of glycosylation can be controlled for in future studies, understanding the rules of natural Fc-engineering and it’s relation to function could provide a path to actively leverage the full biology of the humoral immune response. Vaccination studies may be instrumental to explore the effect of antigen specificity on immunoglobulin effector functions. In a vaccination context, antigens can be administered at dedicated times points, permitting kinetic analyses, with variation in dose, route, interval and the number of boosts. Vaccine antigens can be administered in the absence or presence of a variety of adjuvants, including live viral vectors or isolated TLR ligands to provide variable inflammatory conditions.
Fig. 6.
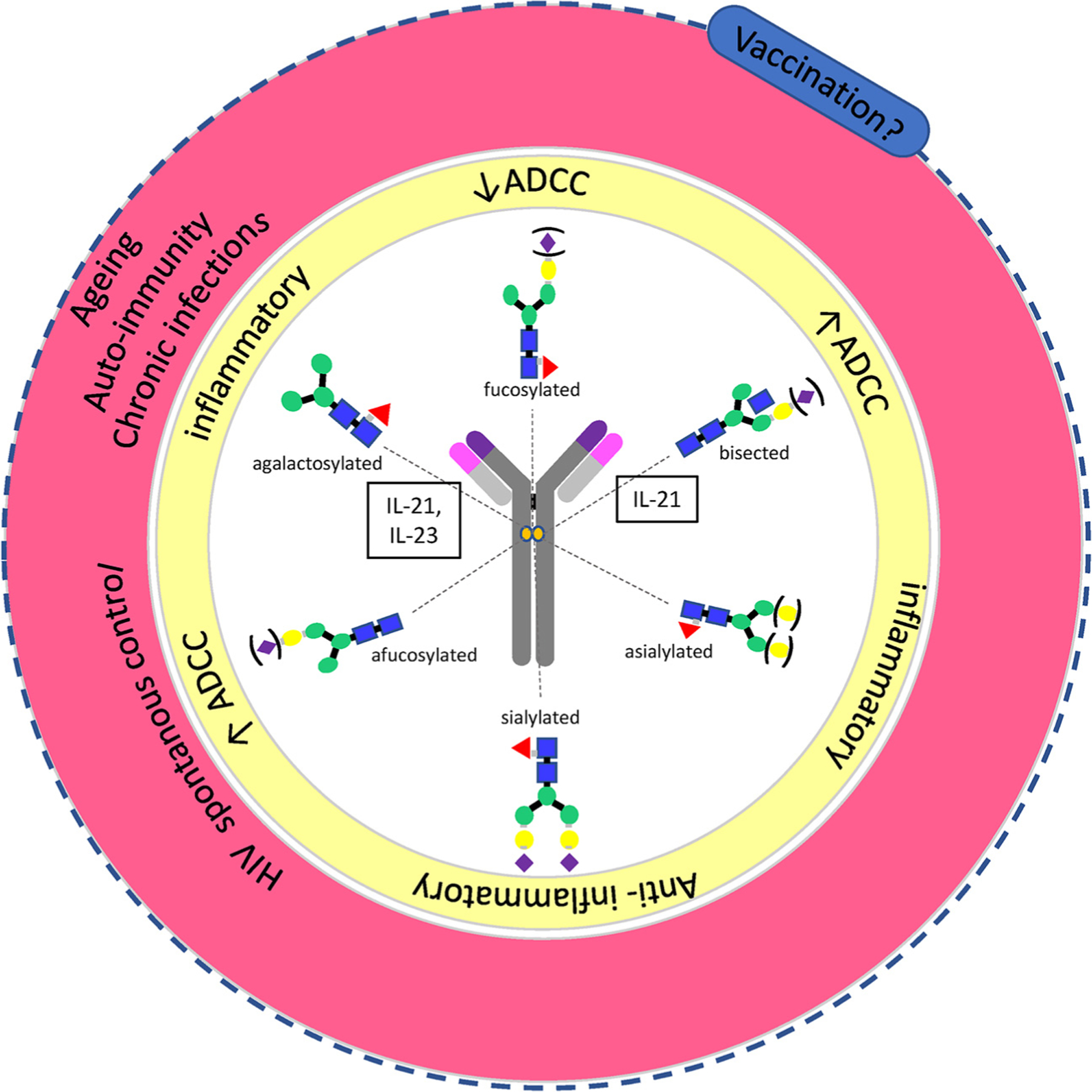
Summary. The antibody glycosylation repertoire determines the functional consequences of the antibodies. Different glycosylation structures (inner ring) have functional implications (middle ring). Several of these glycosylation profiles have been associated with autoimmunity, but limited information is available on antigen specific responses during infection or preventive vaccination (outer ring).
In conclusion, modifications in antibody glycosylation repertoires seem highly relevant for the functional capacity of the antibodies and thus should be incorporated in the assessment of antibody properties. Antibody glycosylation profiles have been quite well established in autoimmune diseases and monoclonal antibody therapies, however in the field of infectious diseases and vaccination much work as yet needs to be done. We expect that this will not only guide the design of improved vaccines, but will also yield significant biological and mechanistic insights into regulation of antibody glycosylation and functional significance of these modifications.
Acknowledgements
We gratefully acknowledge funding by EC HORIZON2020 TBVAC2020 (Grant Agreement No. 643381); EC FP7 EURIPRED (FP7-INFRA-2012 Grant Agreement No. 312661); EC-FP7 Infrastructure Project TRANSVAC2: Infrastructural project on systems analyses (Grant Agreement No. 730964); The Netherlands Organization for Scientific Research (NWO-TOP Grant Agreement No. 91214038); EC IMI2 VSV EBOPLUS (Grant Agreement No. 116068). Research reported in this publication was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R21AI127133. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or any funder. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- [1].Schroeder HW Jr., The evolution and development of the antibody repertoire, Front. Immunol 6 (2015) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Market E, Papavasiliou FN, V(D)J recombination and the evolution of the adaptive immune system, PLoS Biol. 1 (2003) E16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bournazos S, Ravetch JV, Fcgamma receptor function and the design of vaccination strategies, Immunity 47 (2017) 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Behring Kitasato, On the development of immunity to diphtheria and tetanus in animals, Dtsch. Med. Wochenschr 90 (1965) 2183. [PubMed] [Google Scholar]
- [5].Schroeder HW Jr., Cavacini L, Structure and function of immunoglobulins, J. Allergy Clin. Immunol 125 (2010) S41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rajewsky K, Forster I, Cumano A, Evolutionary and somatic selection of the antibody repertoire in the mouse, Science 238 (1987) 1088–1094. [DOI] [PubMed] [Google Scholar]
- [7].Maizels N, Somatic hypermutation: how many mechanisms diversify V region sequences? Cell 83 (1995) 9–12. [DOI] [PubMed] [Google Scholar]
- [8].Stavnezer J, Schrader CE, IgH chain class switch recombination: mechanism and regulation, J. Immunol 193 (2014) 5370–5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nimmerjahn F, Ravetch JV, Divergent immunoglobulin g subclass activity through selective Fc receptor binding, Science 310 (2005) 1510–1512. [DOI] [PubMed] [Google Scholar]
- [10].Vidarsson G, Dekkers G, Rispens T, IgG subclasses and allotypes: from structure to effector functions, Front. Immunol 5 (2014) 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rudd PM, Leatherbarrow RJ, Rademacher TW, Dwek RA, Diversification of the IgG molecule by oligosaccharides, Mol. Immunol 28 (1991) 1369–1378. [DOI] [PubMed] [Google Scholar]
- [12].Jefferis R, Glycosylation as a strategy to improve antibody-based therapeutics, Nat. Rev. Drug Discov 8 (2009) 226–234. [DOI] [PubMed] [Google Scholar]
- [13].Jefferis R, Isotype and glycoform selection for antibody therapeutics, Arch. Biochem. Biophys 526 (2012) 159–166. [DOI] [PubMed] [Google Scholar]
- [14].Dwek RA, Biological importance of glycosylation, Dev. Biol. Stand 96 (1998) 43–47. [PubMed] [Google Scholar]
- [15].Seeling M, Bruckner C, Nimmerjahn F, Differential antibody glycosylation in autoimmunity: sweet biomarker or modulator of disease activity? Nat. Rev. Rheumatol 13 (2017) 621–630. [DOI] [PubMed] [Google Scholar]
- [16].Schwab I, Nimmerjahn F, Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat. Rev. Immunol 13 (2013) 176–189. [DOI] [PubMed] [Google Scholar]
- [17].Pucic M, et al. , High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations, Mol. Cell Proteom 10 (M111) (2011) 010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mahan AE, et al. , Antigen-specific antibody glycosylation is regulated via vaccination, PLoS Pathog. 12 (2016) e1005456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bondt A, et al. , Association between galactosylation of immunoglobulin G and improvement of rheumatoid arthritis during pregnancy is independent of sialylation, J. Proteome Res 12 (2013) 4522–4531. [DOI] [PubMed] [Google Scholar]
- [20].de Haan N, Reiding KR, Driessen G, van der Burg M, Wuhrer M, Changes in healthy human IgG Fc-glycosylation after birth and during early childhood, J. Proteome Res 15 (2016) 1853–1861. [DOI] [PubMed] [Google Scholar]
- [21].Dall F, Olio, et al. , N-glycomic biomarkers of biological aging and longevity: a link with inflammaging, Ageing Res. Rev 12 (2013) 685–698. [DOI] [PubMed] [Google Scholar]
- [22].Zhang D, et al. , Disease-specific IgG Fc N-glycosylation as personalized biomarkers to differentiate gastric cancer from benign gastric diseases, Sci. Rep 6 (2016) 25957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wuhrer M, et al. , Glycosylation profiling of immunoglobulin G (IgG) subclasses from human serum, Proteomics 7 (2007) 4070–4081. [DOI] [PubMed] [Google Scholar]
- [24].Plomp R, et al. , Subclass-specific IgG glycosylation is associated with markers of inflammation and metabolic health, Sci. Rep 7 (2017) 12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Goulabchand R, Vincent T, Batteux F, Eliaou JF, Guilpain P, Impact of auto-antibody glycosylation in autoimmune diseases, Autoimmun. Rev 13 (2014) 742–750. [DOI] [PubMed] [Google Scholar]
- [26].Jennewein MF, Alter G, The immunoregulatory roles of antibody glycosylation, Trends Immunol. 38 (2017) 358–372. [DOI] [PubMed] [Google Scholar]
- [27].Tanaka T, et al. , Aberrant N-glycosylation profile of serum immunoglobulins is a diagnostic biomarker of Urothelial Carcinomas, Int. J. Mol. Sci 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Theodoratou E, et al. , Glycosylation of plasma IgG in colorectal cancer prognosis, Sci. Rep 6 (2016) 28098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Trbojevic Akmacic I, et al. , Inflammatory bowel disease associates with proinflammatory potential of the immunoglobulin G glycome, Inflamm. Bowel Dis 21 (2015) 1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].C. R. Varki A, Esko JD, Essentials of Glycobiology, (Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015–2017., 2015–2017), vol. 3rd Edition. [PubMed] [Google Scholar]
- [31].Mahan AE, et al. , Correction: antigen-specific antibody glycosylation Is regulated via vaccination, PLoS Pathog. 12 (2016) e1005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang J, et al. , Fc-glycosylation of IgG1 is modulated by B-cell stimuli, Mol. Cell Proteom 10 (2011) M110 004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Selman MH, et al. , Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination, Mol. Cell Proteom 11 (2012) M111 014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kao D, et al. , IgG subclass and vaccination stimulus determine changes in antigen specific antibody glycosylation in mice, Eur. J. Immunol 47 (2017) 2070–2079. [DOI] [PubMed] [Google Scholar]
- [35].van de Bovenkamp FS, Hafkenscheid L, Rispens T, Rombouts Y, The emerging importance of IgG fab glycosylation in immunity, J. Immunol 196 (2016) 1435–1441. [DOI] [PubMed] [Google Scholar]
- [36].Yamada K, et al. , Galactosylation of IgG1 modulates FcgammaRIIB-mediated inhibition of murine autoimmune hemolytic anemia, J. Autoimmun 47 (2013) 104–110. [DOI] [PubMed] [Google Scholar]
- [37].Bondt A, et al. , ACPA IgG galactosylation associates with disease activity in pregnant patients with rheumatoid arthritis, Ann. Rheum. Dis (April) (2018), 10.1136/annrheumdis-2018-212946 pii: annrheumdis-2018–212946. [DOI] [PubMed] [Google Scholar]
- [38].van de Geijn FE, et al. , Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study, Arthritis Res. Ther 11 (2009) R193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shinkawa T, et al. , The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity, J. Biol. Chem 278 (2003) 3466–3473. [DOI] [PubMed] [Google Scholar]
- [40].Mimura Y, et al. , Glycosylation engineering of therapeutic IgG antibodies: challenges for the safety, functionality and efficacy, Protein Cell 9 (2018) 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Raju TS, Terminal sugars of Fc glycans influence antibody effector functions of IgGs, Curr. Opin. Immunol 20 (2008) 471–478. [DOI] [PubMed] [Google Scholar]
- [42].Tobinai K, Klein C, Oya N, Fingerle-Rowson G, A review of obinutuzumab (GA101), a novel type II anti-CD20 monoclonal antibody, for the treatment of patients with B-cell malignancies, Adv. Ther 34 (2017) 324–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Anthony RM, Nimmerjahn F, The role of differential IgG glycosylation in the interaction of antibodies with FcgammaRs in vivo, Curr. Opin. Organ Transpl 16 (2011) 7–14. [DOI] [PubMed] [Google Scholar]
- [44].Nimmerjahn F, Ravetch JV, Fcgamma receptors: old friends and new family members, Immunity 24 (2006) 19–28. [DOI] [PubMed] [Google Scholar]
- [45].Bruhns P, et al. , Specificity and affinity of human fcgamma receptors and their polymorphic variants for human IgG subclasses, Blood 113 (2009) 3716–3725. [DOI] [PubMed] [Google Scholar]
- [46].Sorman A, Zhang L, Ding Z, Heyman B, How antibodies use complement to regulate antibody responses, Mol. Immunol 61 (2014) 79–88. [DOI] [PubMed] [Google Scholar]
- [47].Bruckner C, Lehmann C, Dudziak D, Nimmerjahn F, Sweet SIGNs: IgG glycosylation leads the way in IVIG-mediated resolution of inflammation, Int. Immunol 29 (2017) 499–509. [DOI] [PubMed] [Google Scholar]
- [48].Seite JF, et al. , IVIg modulates BCR signaling through CD22 and promotes apoptosis in mature human B lymphocytes, Blood 116 (2010) 1698–1704. [DOI] [PubMed] [Google Scholar]
- [49].Gustavsson S, Hjulstrom S, Liu T, Heyman B, CD23/IgE-mediated regulation of the specific antibody response in vivo, J. Immunol 152 (1994) 4793–4800. [PubMed] [Google Scholar]
- [50].Wang TT, et al. , Anti-HA glycoforms Drive B cell affinity selection and determine influenza vaccine efficacy, Cell 162 (2015) 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sondermann P, Huber R, Oosthuizen V, Jacob U, The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex, Nature 406 (2000) 267–273. [DOI] [PubMed] [Google Scholar]
- [52].Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P, Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity, J. Mol. Biol 325 (2003) 979–989. [DOI] [PubMed] [Google Scholar]
- [53].Ferrara C, et al. , Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose, Proc. Natl. Acad. Sci. U. S. A 108 (2011) 12669–12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hodoniczky J, Zheng YZ, James DC, Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro, Biotechnol. Prog 21 (2005) 1644–1652. [DOI] [PubMed] [Google Scholar]
- [55].Malhotra R, et al. , Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein, Nat. Med 1 (1995) 237–243. [DOI] [PubMed] [Google Scholar]
- [56].Gornik I, Maravic G, Dumic J, Flogel M, Lauc G, Fucosylation of IgG heavy chains is increased in rheumatoid arthritis, Clin. Biochem 32 (1999) 605–608. [DOI] [PubMed] [Google Scholar]
- [57].Gudelj I, et al. , Low galactosylation of IgG associates with higher risk for future diagnosis of rheumatoid arthritis during 10years of follow-up, Biochim. Biophys. Acta 1864 (2018) 2034–2039. [DOI] [PubMed] [Google Scholar]
- [58].Rombouts Y, et al. , Extensive glycosylation of ACPA-IgG variable domains modulates binding to citrullinated antigens in rheumatoid arthritis, Ann. Rheum. Dis 75 (2016) 578–585. [DOI] [PubMed] [Google Scholar]
- [59].Rombouts Y, et al. , Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis, Ann. Rheum. Dis 74 (2015) 234–241. [DOI] [PubMed] [Google Scholar]
- [60].Scherer HU, et al. , Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid, Arthritis Rheum. 62 (2010) 1620–1629. [DOI] [PubMed] [Google Scholar]
- [61].Scherer HU, et al. , Immunoglobulin 1 (IgG1) Fc-glycosylation profiling of anti-citrullinated peptide antibodies from human serum, Proteom. Clin. Appl 3 (2009) 106–115. [DOI] [PubMed] [Google Scholar]
- [62].Vuckovic F, et al. , Association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome, Arthritis Rheumatol 67 (2015) 2978–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Decker Y, et al. , Abnormal galactosylation of immunoglobulin G in cerebrospinal fluid of multiple sclerosis patients, Mult. Scler 22 (2016) 1794–1803. [DOI] [PubMed] [Google Scholar]
- [64].Wuhrer M, et al. , Pro-inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid, J. Neuroinflammation 12 (2015) 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bermingham ML, et al. , N-glycan profile and kidney disease in type 1 diabetes, Diabetes Care 41 (2018) 79–87. [DOI] [PubMed] [Google Scholar]
- [66].Youinou P, et al. , Galactose terminating oligosaccharides of IgG in patients with primary Sjogren’s syndrome, J. Autoimmun 5 (1992) 393–400. [DOI] [PubMed] [Google Scholar]
- [67].Miyoshi E, et al. , Role of aberrant IgG glycosylation in the pathogenesis of inflammatory bowel disease, Proteom. Clin. Appl 10 (2016) 384–390. [DOI] [PubMed] [Google Scholar]
- [68].Varadi C, et al. , Combination of IgG N-glycomics and corresponding transcriptomics data to identify anti-TNF-alpha treatment responders in inflammatory diseases, Electrophoresis 36 (2015) 1330–1335. [DOI] [PubMed] [Google Scholar]
- [69].Lundstrom SL, et al. , Blood plasma IgG Fc glycans are significantly altered in Alzheimer’s disease and progressive mild cognitive impairment, J. Alzheimers Dis 38 (2014) 567–579. [DOI] [PubMed] [Google Scholar]
- [70].Selman MH, et al. , IgG fc N-glycosylation changes in Lambert-Eaton myasthenic syndrome and myasthenia gravis, J. Proteome Res 10 (2011) 143–152. [DOI] [PubMed] [Google Scholar]
- [71].Fokkink WJ, et al. , IgG Fc N-glycosylation in Guillain-Barre syndrome treated with immunoglobulins, J. Proteome Res 13 (2014) 1722–1730. [DOI] [PubMed] [Google Scholar]
- [72].de Jong SE, et al. , IgG1 Fc N-glycan galactosylation as a biomarker for immune activation, Sci. Rep 6 (2016) 28207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Catera M, et al. , Identification of novel plasma glycosylation-associated markers of aging, Oncotarget 7 (2016) 7455–7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ackerman ME, et al. , Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity, J. Clin. Invest 123 (2013) 2183–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hills AE, Patel A, Boyd P, James DC, Metabolic control of recombinant monoclonal antibody N-glycosylation in GS-NS0 cells, Biotechnol. Bioeng 75 (2001) 239–251. [DOI] [PubMed] [Google Scholar]
- [76].Bondt A, Wuhrer M, Kuijper TM, Hazes JM, Dolhain RJ, Fab glycosylation of immunoglobulin G does not associate with improvement of rheumatoid arthritis during pregnancy, Arthritis Res. Ther 18 (2016) 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Albert H, Collin M, Dudziak D, Ravetch JV, Nimmerjahn F, In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner, Proc. Natl. Acad. Sci. U. S. A 105 (2008) 15005–15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Keser T, et al. , Increased plasma N-glycome complexity is associated with higher risk of type 2 diabetes, Diabetologia 60 (2017) 2352–2360. [DOI] [PubMed] [Google Scholar]
- [79].Lemmers RFH, et al. , IgG glycan patterns are associated with type 2 diabetes in independent European populations, Biochim. Biophys. Acta 1861 (2017) 2240–2249. [DOI] [PubMed] [Google Scholar]
- [80].Karsten CM, et al. , Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1, Nat. Med 18 (2012) 1401–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kaneko Y, Nimmerjahn F, Ravetch JV, Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation, Science 313 (2006) 670–673. [DOI] [PubMed] [Google Scholar]
- [82].Thomann M, et al. , In vitro glycoengineering of IgG1 and its effect on Fc receptor binding and ADCC activity, PLoS One 10 (2015) e0134949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Scallon BJ, Tam SH, McCarthy SG, Cai AN, Raju TS, Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality, Mol. Immunol 44 (2007) 1524–1534. [DOI] [PubMed] [Google Scholar]
- [84].Quast I, et al. , Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity, J. Clin. Invest 125 (2015) 4160–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Anthony RM, et al. , Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc, Science 320 (2008) 373–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Anthony RM, Kobayashi T, Wermeling F, Ravetch JV, Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway, Nature 475 (2011) 110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Moore JS, et al. , Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals, AIDS 19 (2005) 381–389. [DOI] [PubMed] [Google Scholar]
- [88].Lu LL, et al. , A functional role for antibodies in tuberculosis, Cell 167 (433–443) (2016) e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sciaranghella G, Tong N, Mahan AE, Suscovich TJ, Alter G, Decoupling activation and exhaustion of B cells in spontaneous controllers of HIV infection, AIDS 27 (2013) 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Bragazzi NL, et al. , Vaccines meet Big data: State-of-the-art and future prospects. From the classical 3Is (“isolate-inactivate-inject”) vaccinology 1.0 to vaccinology 3.0, vaccinomics, and beyond: a historical overview, Front. Public Health 6 (2018) 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hess C, et al. , T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies, J. Clin. Invest 123 (2013) 3788–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Oefner CM, et al. , Tolerance induction with T cell-dependent protein antigens induces regulatory sialylated IgGs, J. Allergy Clin. Immunol. Pract 129 (2012) 1647–1655 e1613. [DOI] [PubMed] [Google Scholar]
- [93].Lastra GC, Thompson SJ, Lemonidis AS, Elson CJ, Changes in the galactose content of IgG during humoral immune responses, Autoimmunity 28 (1998) 25–30. [DOI] [PubMed] [Google Scholar]
- [94].Guo N, et al. , Repeated immunization induces the increase in fucose content on antigen-specific IgG N-linked oligosaccharides, Clin. Biochem 38 (2005) 149–153. [DOI] [PubMed] [Google Scholar]
- [95].Francica JR, et al. , Innate transcriptional effects by adjuvants on the magnitude, quality, and durability of HIV envelope responses in NHPs, Blood Adv. 1 (2017) 2329–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Vestrheim AC, et al. , A pilot study showing differences in glycosylation patterns of IgG subclasses induced by pneumococcal, meningococcal, and two types of influenza vaccines, Immun., Inflamm. Dis 2 (2014) 76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Selman MH, et al. , Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination, Mol. Cell. Proteom. MCP 11 (2012) M111.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Wang JR, et al. , Glycomic signatures on serum IgGs for prediction of post-vaccination response, Sci. Rep 5 (2015) 7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Davies J, et al. , Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FC gamma RIII, Biotechnol. Bioeng 74 (2001) 288–294. [PubMed] [Google Scholar]


