To the editor:
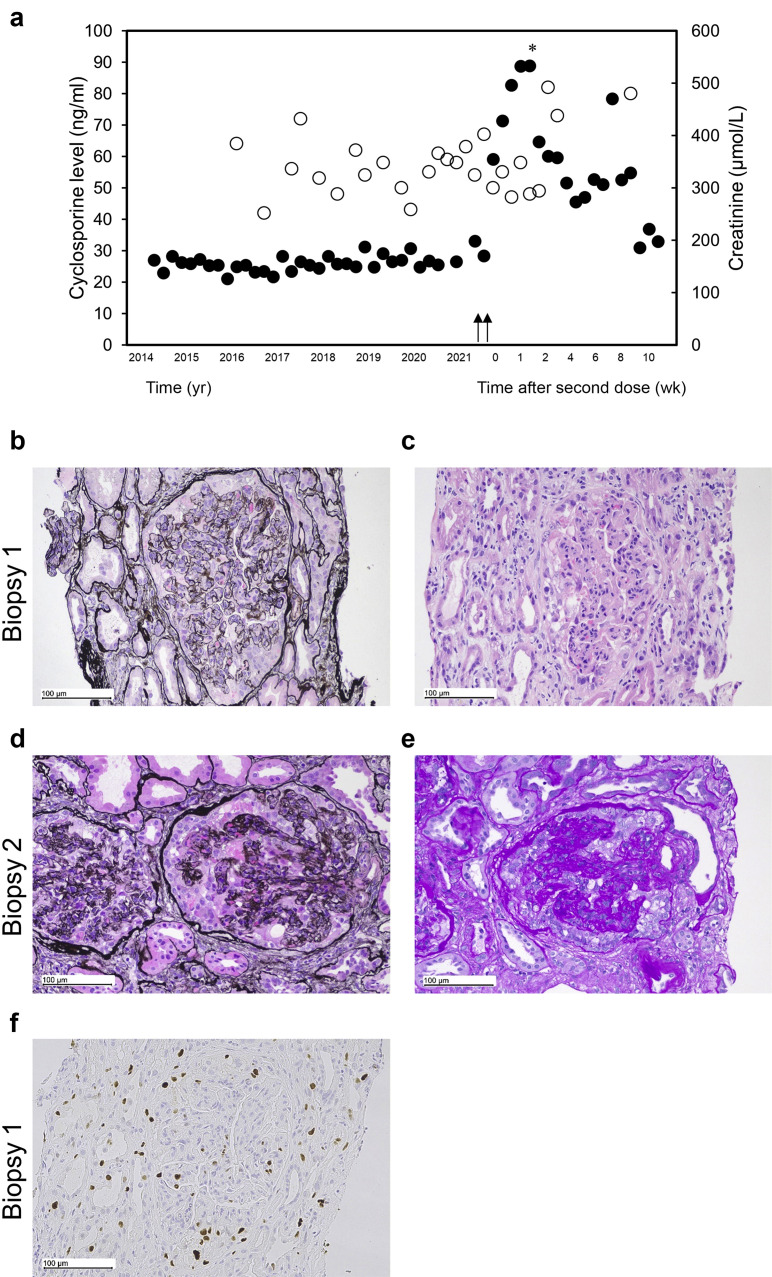
A stable kidney transplant recipient (KTR) of 14 years received the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) ChAdOx1 (AZD1222) vaccine and, within a fortnight, presented with severe acute kidney injury (creatinine, 533 μmol/L; baseline, 125–187 μmol/L) and nephrotic range proteinuria (urine protein creatinine ratio, 2000 g/mol, with no hematuria). The allograft, her first, was from a deceased white donor, following kidney failure from IgA vasculitis, and was stable for many years (Figure 1 a). Initial kidney biopsy showed glomerulomegaly, glomerulitis, podocyte enlargement with protein droplets, and collapse of the glomerular tufts along with glomerular and interstitial immune infiltrates (Banff score, i3 t0 v0 g3 ptc0 ci1 ct1 cv0 cg1b mm0 ah3 ti3 i-IFTA3) (Figure 1b and c); electron microscopy was unavailable. Immunofluorescence for Igs and C4d immunohistochemistry and antibodies against donor-specific human leukocyte antigen and angiotensin 2R1 were absent. Treatment response for suspected antibody-mediated rejection with methylprednisolone, i.v. Ig, and plasma exchange was poor. Repeated kidney biopsy showed the absence of immune cell infiltration and features consistent with collapsing glomerulopathy (CG) with acute tubular injury and mild glomerulitis (Banff score, i0 t1 v0 g1 ptc0 ci1 ct1 cv0 cg3 mm0 ah3 ti0 i-IFTA3; Figure 1d and e). Electron microscopy revealed the detachment of podocytes with extensive foot process effacement, microvillous hyperplasia, protein droplets in some podocytes, and normal glomerular basement membranes, with no evidence of immune complex deposition. Secondary causes of CG, including cytomegalovirus, BK polyomavirus, HIV, parvovirus, and SARS-CoV-2 infection, were excluded. The patient required hemodialysis at 3 months with persisting proteinuria (15 g/d). Further immunohistochemistry confirmed the following: (i) expression of Ki-67, a marker of glomerular expansion/proliferation in CG, detected in biopsy 1, with epithelial cell reactivity in Bowman’s space (Figure 1f); (ii) reduced glomerular expression of synaptopodin and podocalyxin, evidence of podocyte dedifferentiation previously associated with CG, on biopsy 1 (Supplementary Figure S1); and (iii) significantly increased levels of inflammatory subpopulations on the initial biopsy to be significantly attenuated following intervention (Supplementary Figure S2).1 , 2 SARS-CoV-2 vaccination has been associated with many glomerular diseases, minimal change and membranous nephropathy, whereas SARS-CoV-2 infection has been associated with CG, including in a KTR.3, 4, 5 We report the first case of a KTR developing CG after SARS-CoV-2 ChAdOx1 vaccination, with no identifiable cause besides temporal association with vaccination.
Figure 1.
Timeline of kidney function and sequential biopsy results in a kidney transplant recipient. (a) Timeline of renal function/creatinine (black circles) and cyclosporine (white circles) levels, with arrows indicating timing of first and second AstraZeneca vaccinations. The asterisk indicates initiation of 1 g methylprednisolone, second daily plasma exchange, and i.v. Ig for 2 weeks, continued 60 mg prednisolone/D. (b) Kidney biopsy 1 before treatment shows enlarged glomeruli with features of glomerulitis with endocapillary lymphocytes, some neutrophils, and capillary loop narrowing (periodic Schiff–methenamine [PASM], original magnification ×200). Bar = 100 μm. (c) In biopsy 1, enlarged podocytes with protein droplets in their cytoplasm were noted in 2 glomeruli (hematoxylin and eosin, original magnification ×200). Bar = 100 μm. (d,e) The second biopsy (biopsy 2) 2 weeks later revealed 2 glomeruli containing large podocytes with prominent protein globules and collapse of underlying capillary loops ([d] PASM, original magnification ×200; [e] periodic acid–Schiff, original magnification ×200). Bars = 100 μm. (f) Ki-67 immunohistochemistry shows reactivity in multiple cells in Bowman’s space, consistent with either visceral or parietal epithelial cells. Some of the cells contain cytoplasmic protein droplets. Bar = 100 μm. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors would like to thank the tissue donors for provision of renal biospecimens. The authors also gratefully acknowledge the staff of QIMR Berghofer Core Histology and Australian Cancer Research Foundation Centre for Comprehensive Biomedical Imaging (QIMR Berghofer) for expert technical assistance with staining and image analysis and Dr Kim Oliver, nephropathologist at the Princess Alexandra Hospital, Brisbane, for reviewing the biopsy slides. The work was funded by Pathology Queensland and a National Health and Medical Research Council (NHMRC) project grant (GNT1161319).
Footnotes
Supplementary Methods.
Supplementary Results.
Supplementary Reference.
Figure S1. Reduced expression of glomerular markers in kidney transplant recipient (KTR) biopsy tissue with collapsing glomerulopathy associated with SARS-CoV-2 vaccination.
Figure S2. Immune cell infiltration in kidney transplant recipient (KTR) biopsy tissue with collapsing glomerulopathy associated with SARS-CoV-2 vaccination.
Supplementary Material
References
- 1.Barisoni L., Kriz W., Mundel P., et al. The dysregulated podocyte phenotype. J Am Soc Nephrol. 1999;10:51. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- 2.Fogo A.B., Lusco M.A., Najafian B., et al. AJKD atlas of renal pathology: collapsing glomerulopathy. Am J Kidney Dis. 2015;66:e3–e4. doi: 10.1053/j.ajkd.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Izzedine H., Bonilla M., Jhaveri K.D. Nephrotic syndrome and vasculitis following SARS-CoV-2 vaccine: true association or circumstantial? Nephrol Dial Transplant. 2021;36:1565–1569. doi: 10.1093/ndt/gfab215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meliambro K., Li X., Salem F., et al. Molecular analysis of the kidney from a patient with COVID-19-associated collapsing glomerulopathy. Kidney Med. 2021;3:653–658. doi: 10.1016/j.xkme.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazareth H., Péré H., Binois Y., et al. COVID-19-related collapsing glomerulopathy in a kidney transplant recipient. Am J Kidney Dis. 2020;76:590–594. doi: 10.1053/j.ajkd.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.