Abstract
铁死亡是一种铁依赖的程序性死亡,不同于传统的细胞死亡方式(如凋亡、焦亡、坏死、自噬),其特征是活性氧诱导脂质过氧化物堆积。铁死亡在肿瘤发生发展中发挥着重要的调控作用。最新研究表明,天然药物成分可通过谷胱甘肽/谷胱甘肽过氧化物酶4途径、铁代谢、脂质代谢等调控机制诱导肿瘤细胞铁死亡。研究发现了30多种天然药物成分具有诱导肿瘤细胞铁死亡的作用,且有多通路、多靶点的特点。本文综述了天然药物成分通过干预铁死亡抑制肿瘤的作用研究进展。
Abstract
Ferroptosis is an iron-dependent programmed cell death characterized by reactive oxygen species-induced lipid peroxide accumulation, which is different from cell apoptosis, pyroptosis, necrosis or autophagy. Ferroptosis plays an important role in the regulation of tumorigenesis and tumor development. Recent studies have shown that natural medicinal ingredients can induce ferroptosis in tumor cells through glutathione (GSH)/glutathione peroxidase 4 (GPx4) pathway, iron metabolism, lipid metabolism or other mechanisms. It has been reported that more than 30 natural medicinal ingredients can induce ferroptosis in tumor cells with multiple pathways and multiple targets. This article reviews the current research progress on the antitumor effects of natural medicinal ingredients through inducing cell ferroptosis.
Keywords: Ferroptosis, Tumor, Natural medicine, Traditional Chinese medicine, Regulatory mechanism, Review
谷胱甘肽(glutathione,GSH);谷胱甘肽过氧化物酶(glutathione peroxidase,GPx);溶质载体家族7成员11(solute carrier family 7 member 11,SLC7A11);AMP活化蛋白激酶(AMP-activated protein kinase,AMPK);哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR);急性髓细胞性白血病(acute myelogenous leukemia,AML);长链脂酰辅酶A合成酶(acyl-CoA synthetase long chain family member,ACSL);
铁死亡是一种铁依赖且不同于凋亡、焦亡、自噬等传统程序性细胞死亡的细胞死亡方式,其特征是活性氧诱导脂质过氧化物堆积 [1] 。铁死亡主要表现为线粒体明显收缩、双层膜密度增加、线粒体嵴减少或消失,但细胞膜完好、细胞核大小正常、染色质未浓缩 [2] 。在细胞形态和功能方面,铁死亡无典型坏死形态特征,也无经典细胞凋亡特点,且不形成自噬的封闭双层膜结构。
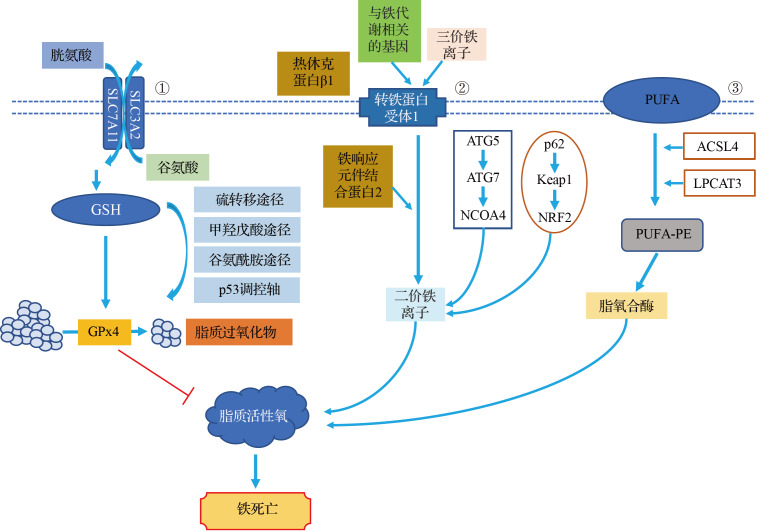
铁积累的增加、自由基的产生、脂肪酸供应和脂质过氧化是诱导铁死亡的关键。目前,铁死亡的调控机制主要可分为三类:基于GSH/GPx4途径调控机制、铁代谢调控机制、脂质代谢相关通路调控机制( 图1)。研究表明,铁死亡在肿瘤发生发展中发挥着重要的调控作用,已成为肿瘤机制探索及治疗靶点研究领域的热点和焦点 [3] 。诱导铁死亡可能成为抗肿瘤治疗的新策略,如Erastin、RSL3、索拉非尼、柳氮磺胺吡啶、铁制他汀类药物等铁死亡诱导剂,以及可诱导铁死亡和抑制肿瘤生长的电离辐射和细胞因子 [4] 。此外,越来越多的证据表明,多种天然药物成分可通过靶向铁死亡途径抑制肿瘤生长。本文基于不同调控机制综述了天然药物成分干预铁死亡发挥抗肿瘤作用的最新研究进展,以期为中药抗肿瘤治疗提供新思路。
图 1 .
铁死亡的调控机制①SLC7A11、SLC3A2可在向细胞内转运胱氨酸的同时外排等量的谷氨酸. 胱氨酸通过还原反应可生成GSH生物合成的限速底物半胱氨酸,GSH是GPx4的辅酶因子以及其降解脂质过氧化物的必需反应底物. GPx4作为调控铁死亡的关键酶,可通过催化脂质过氧化物的还原反应,抑制铁死亡的发生. 硫转移途径、甲羟戊酸途径、谷氨酰胺途径、p53调控轴等也参与铁死亡的GSH/GPx4途径. ②血液循环中的三价铁离子与转铁蛋白结合并运输,通过细胞膜上的转铁蛋白受体1进入细胞内被还原为二价铁离子后,再被转运并释放到细胞质铁池中,导致铁过量,产生活性氧,引发铁死亡. 铁蛋白代谢相关的ATG5-ATG7-NCOA4通路和p62-Keap1-NRF2通路也可参与铁死亡过程. ③ACSL4通过在内质网相关的氧合作用中心产生氧化的PE来促进铁死亡,而ACSL4催化花生四烯酸或肾上腺素的附着以产生酰基辅酶A衍生物,随后通过LPCAT3将其酯化为PE,进而被脂氧合酶氧化以产生脂质氢过氧化物,最终导致铁死亡. SLC7A11:溶质载体家族7成员11;SLC3A2:溶质载体家族3成员2;GSH:谷胱甘肽;GPx:谷胱甘肽过氧化物酶;ATG:自噬相关基因;NCOA:核受体辅激活因子;Keap:Kelch样环氧氯丙烷相关蛋白;NRF:核因子E2相关因子;ACSL:长链脂酰辅酶A合成酶;PE:磷脂酰乙醇胺;LPCAT:溶血卵磷脂酰基转移酶;PUFA:多不饱和脂肪酸.
1天然药物成分靶向GSH/GPx4途径诱导铁死亡
半胱氨酸是合成GSH的限速原料,主要由胱氨酸还原而来,而胱氨酸进入细胞则依赖胱氨酸/谷氨酸逆转运蛋白SLC7A11 [5] ;此外,GSH是GPx4降解脂质过氧化物的必需反应底物。因此,抑制GSH/GPx4通路可导致脂质过氧化物积累和铁死亡发生。
目前研究发现,新藤黄酸、猕猴桃根、青蒿琥酯、双氢青蒿素、葫芦素B、银杏素、二氢异丹参酮Ⅰ、茄碱等多种天然药物成分通过干预GSH/GPx4途径诱导铁死亡,进而抑制肿瘤生长。例如,藤黄中新藤黄酸可通过p53/SLC7A11/GPx4信号通路诱导转化生长因子β1刺激的黑色素瘤细胞发生铁死亡 [6] 。猕猴桃根可通过抑制GPx4和SLC7A11蛋白增加活性氧的积累,促进胃癌细胞铁死亡 [7] 。三萜类化合物葫芦素B可引起人鼻咽癌细胞内铁离子积累和GSH耗竭,导致脂质过氧化和GPx4表达下调,最终引发铁死亡 [8] 。二氢异丹参酮Ⅰ、茄碱也是通过抑制GPx4分别诱导乳腺癌细胞、肝癌细胞的铁死亡 [9] 。银杏叶中银杏素介导非小细胞肺癌铁死亡,与增加铁浓度和脂质过氧化、降低SLC7A11和GPx4表达及GSH/氧化型GSH比值等机制相关 [10] 。此外,双氢青蒿素和青蒿琥酯也可抑制GPx4,诱导肿瘤细胞铁死亡 [ 11- 12] 。在结肠癌中,天然产物β-榄香烯联合西妥昔单抗可下调铁死亡的负调控蛋白(如GPx4、SLC7A11等),促进结肠癌细胞铁死亡 [13] 。
研究还发现,多种天然药物成分的衍生物也能靶向GSH/GPx4途径,如苯并吡喃衍生物IMCA、小白菊内酯的衍生物DMOCPTL、姜黄素类似物EF24和ALZ003、喜树碱类似物SN38等。其中,IMCA下调SLC7A11的表达,降低半胱氨酸和GSH的含量,导致结直肠癌活性氧积累和铁死亡 [14] ;DMOCPTL通过与GPx4蛋白直接结合,诱导GPx4泛素化,导致三阴性乳腺癌细胞铁死亡和细胞凋亡 [15] ;EF24显著提高丙二醛、活性氧和细胞内铁离子水平,抑制GPx4表达,诱导骨肉瘤细胞铁死亡 [16] ;ALZ003不仅诱导胶质母细胞瘤fbxl2介导的雄激素受体泛素化,导致其降解,还可促使细胞中活性氧的积累、脂质过氧化和GPx4的抑制,进而诱导铁死亡 [17] ;SN38与电穿孔联合治疗可使结肠癌细胞内超氧化物和氢过氧化物大量产生、GSH耗竭,诱发铁死亡 [18] 。
2天然药物成分靶向铁代谢途径诱导铁死亡
铁是人体中的一种重要微量元素,二价铁离子可转移电子给胞内氧,使胞内氧与脂质发生反应形成脂质过氧化物。研究发现,双氢青蒿素能够通过与细胞游离铁结合,刺激铁调节蛋白与含有铁响应元件序列的信使RNA分子结合。因此,双氢青蒿素可破坏铁调节蛋白/铁响应元件控制的铁稳态,从而进一步增加细胞游离铁 [19] 。Du等 [20] 发现双氢青蒿素通过调节AMPK/mTOR/p70S6k信号通路的活性诱导自噬,加速铁蛋白降解,增加不稳定铁池,促进细胞活性氧积累,最终导致AML细胞铁死亡。过表达铁硫簇组装酶可调节铁代谢,降低双氢青蒿素诱导的铁死亡。另有研究发现,蒲花粉提取物香蒲新苷通过激活AMPK信号通路导致铁蛋白降解,显著增加AML细胞内和线粒体的活性氧,进而诱导AML细胞铁死亡 [21] 。大黄素甲醚8-O-β-吡喃葡萄糖苷通过调控miR-103a-3p/GSL2轴,上调活性氧、丙二醛水平以及细胞内二价铁离子,诱导胃癌细胞铁死亡 [22] 。6-姜辣素降低泛素特异性肽酶14的表达,不仅增加自噬体数量和活性氧水平,而且增加铁浓度,抑制肺癌细胞生长 [23] 。盐霉素衍生物伊诺莫霉素可将铁隔离在溶酶体中,触发铁蛋白降解,导致铁在细胞器中进一步负荷,进而杀死乳腺癌干细胞 [24] 。
综上所述,双氢青蒿素、香蒲新苷、大黄素甲醚8-O-β-吡喃葡萄糖苷、6-姜辣素等多种天然药物成分可靶向铁代谢途径诱导肿瘤细胞铁死亡。
3天然药物成分靶向脂质代谢相关通路诱导铁死亡
脂质代谢与铁死亡密切相关。例如,游离多不饱和脂肪酸是铁死亡合成脂质信号转导介质的底物,含花生四烯酸或其衍生物肾上腺素的磷脂酰乙醇胺是诱导细胞发生铁死亡的关键磷脂 [25] 。ACSL4是调节脂质组成的关键酶,可催化花生四烯酸或肾上腺素的附着以产生酰基辅酶A衍生物,溶血卵磷脂酰基转移酶3将其酯化为磷脂酰乙醇胺,进而被脂氧合酶氧化产生脂质氢过氧化物,最终导致铁死亡 [26] 。
研究发现,齐墩果酸、罗波斯塔双黄酮A等天然药物成分可靶向脂质代谢通路诱导铁死亡。齐墩果酸是一种天然存在于植物叶片、果实和根茎中的物质,具有抗癌活性。齐墩果酸可增加氧化应激水平和二价铁离子含量,以及铁死亡相关蛋白及ACSL4表达。敲低宫颈癌细胞中 ACSL4的表达后,齐墩果酸抗癌作用被抵消,活性氧和GPx4水平下降,提示齐墩果酸通过促进ACSL4的表达来激活宫颈癌细胞的铁死亡 [27] 。另一项研究从毛卷柏中分离得到6种C-3′-C-6双黄酮,其中罗波斯塔双黄酮A抗乳腺癌的活性最强,其机制为通过增强电压依赖性阴离子通道2表达,降低Nedd4 E3泛素连接酶的表达,导致脂质过氧化和活性氧的产生,从而诱发乳腺癌细胞铁死亡 [28] 。此外,桦木科 Betula etnensis Raf的树皮甲醇提取物可增加结肠癌细胞脂质过氧化和血红素加氧酶1、活性氧表达,促进细胞铁死亡 [29] 。
4天然药物成分通过其他途径诱导铁死亡
天然药物成分不仅可通过GSH/GPx4、铁代谢、脂质代谢调控机制诱导肿瘤细胞铁死亡,还可能通过其他途径调控铁死亡。例如,Du等 [30] 研究发现舒肝宁注射液通过血红素加氧酶1诱导三阴性乳腺癌细胞铁死亡。青蒿琥酯激活ATF4-CHOP-CHAC1通路导致伯基特淋巴瘤细胞铁死亡 [31] 。最近有研究发现,天然药物成分可抑制多药耐药肿瘤细胞生长也与铁死亡相关。如藤黄属植物苯甲酮类化合物Epunctanone通过改变基质金属蛋白酶和增加活性氧的产生诱导多药耐药肿瘤细胞的铁死亡 [32] ;天然齐墩烷型三萜皂苷Ardisiacrispin B能够通过铁死亡和细胞凋亡抑制多药耐药肿瘤细胞的生长 [33] ,但具体机制有待进一步探索。
此外,还有一些天然药物可促进肿瘤细胞铁死亡,但机制也不明确。例如,槐耳水提液诱导非小细胞肺癌细胞铁死亡 [34] ;穿心莲的抗结直肠癌和化疗增敏作用部分依赖铁死亡的激活 [35] ;百里香和牛蒡提取物可通过诱导细胞铁死亡抑制白血病和多发性骨髓瘤的细胞增殖 [36] ;药用球果紫堇的氯仿提取物可诱导多发性骨髓瘤细胞铁死亡 [37] ;苦瓜种子中分离的生物活性蛋白MAP30在体内通过改变代谢和诱导铁死亡,与顺铂协同发挥抑制卵巢癌的作用 [38] 。
5结语
目前已发现多种天然药物成分可诱导肿瘤细胞铁死亡,且具有多通路、多靶点的特点。然而,当前的研究多数停留在细胞水平,缺乏动物及临床水平的验证,因此天然药物成分干预铁死亡的作用有待进一步探索。此外,随着研究的不断推进,相信天然药物成分诱导肿瘤细胞铁死亡的作用机制将越来越丰富。
COMPETING INTERESTS
所有作者均声明不存在利益冲突
Funding Statement
浙江省中医药科技计划(2018ZZ006,2021ZA006)
References
- 1.DIXON S J, LEMBERG K M, LAMPRECHT M R, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death[J] Cell. . 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.STOCKWELL B R, FRIEDMANN ANGELI J P, BAYIR H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease[J] Cell. . 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MOU Y, WANG J, WU J, et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer[J] J Hematol Oncol. . 2019;12(1):34. doi: 10.1186/s13045-019-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.XIA X, FAN X, ZHAO M, et al. The relationship between ferroptosis and tumors: a novel landscape for therapeutic approach[J] Curr Gene Ther. . 2019;19(2):117–124. doi: 10.2174/1566523219666190628152137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.URSINI F, MAIORINO M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4[J] Free Radic Biol Med. . 2020;152:175–185. doi: 10.1016/j.freeradbiomed.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 6.WANG M, LI S, WANG Y, et al. Gambogenic acid induces ferroptosis in melanoma cells undergoing epithelial-to-mesenchymal transition[J] Toxicol Appl Pharmacol. . 2020;401:115110. doi: 10.1016/j.taap.2020.115110. [DOI] [PubMed] [Google Scholar]
- 7.GAO Z, DENG G, LI Y, et al. Actinidia chinensis planch prevents proliferation and migration of gastric cancer associated with apoptosis, ferroptosis activation and mesenchymal phenotype suppression[J] Biomed Pharmacother. . 2020;126:110092. doi: 10.1016/j.biopha.2020.110092. [DOI] [PubMed] [Google Scholar]
- 8.HUANG S, CAO B, ZHANG J, et al. Induction of ferroptosis in human nasopharyngeal cancer cells by cucurbitacin B: molecular mechanism and therapeutic potential[J] Cell Death Dis. . 2021;12(3):237. doi: 10.1038/s41419-021-03516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LIN Y S, SHEN Y C, WU C Y, et al. Danshen improves survival of patients with breast cancer and dihydroisotanshinone Ⅰ induces ferroptosis and apoptosis of breast cancer cells[J] Front Pharmacol. . 2019;10:1226. doi: 10.3389/fphar.2019.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LOU J S, ZHAO L P, HUANG Z H, et al. Ginkgetin derived from Ginkgo biloba leaves enhances the therapeutic effect of cisplatin via ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR wild-type non-small-cell lung cancer[J] Phytomedicine. . 2021;80:153370. doi: 10.1016/j.phymed.2020.153370. [DOI] [PubMed] [Google Scholar]
- 11.ROH J L, KIM E H, JANG H, et al. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis[J] Redox Biol. . 2017;11:254–262. doi: 10.1016/j.redox.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CHEN G Q, BENTHANI F A, WU J, et al. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis[J] Cell Death Differ. . 2020;27(1):242–254. doi: 10.1038/s41418-019-0352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CHEN P, LI X, ZHANG R, et al. Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation[J] Theranostics. . 2020;10(11):5107–5119. doi: 10.7150/thno.44705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ZHANG L, LIU W, LIU F, et al. IMCA induces ferroptosis mediated by SLC7A11 through the AMPK/mTOR pathway in colorectal cancer[J] Oxid Med Cell Longev. . 2020;2020:6901472. doi: 10.1155/2020/1675613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DING Y, CHEN X, LIU C, et al. Identification of a small molecule as inducer of ferroptosis and apoptosis through ubiquitination of GPX4 in triple negative breast cancer cells[J] J Hematol Oncol. . 2021;14(1):19. doi: 10.1186/s13045-020-01016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LIN H, CHEN X, ZHANG C, et al. EF24 induces ferroptosis in osteosarcoma cells through HMOX1[J] Biomed Pharmacother. . 2021;136:111202. doi: 10.1016/j.biopha.2020.111202. [DOI] [PubMed] [Google Scholar]
- 17.CHEN T C, CHUANG J Y, KO C Y, et al. AR ubiquitination induced by the curcumin analog suppresses growth of temozolomide-resistant glioblastoma through disrupting GPX4-mediated redox homeostasis[J] Redox Biol. . 2020;30:101413. doi: 10.1016/j.redox.2019.101413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NIKOLOVA B, SEMKOVA S, TSONEVA I, et al. Redox-related molecular mechanism of sensitizing colon cancer cells to camptothecin analog SN38[J] Anticancer Res. . 2020;40(9):5159–5170. doi: 10.21873/anticanres.14519. [DOI] [PubMed] [Google Scholar]
- 19.OOKO E, SAEED M E M, KADIOGLU O, et al. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells[J] Phytomedicine. . 2015;22(11):1045–1054. doi: 10.1016/j.phymed.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 20.DU J, WANG T, LI Y, et al. DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin[J] Free Radical Biol Med. . 2019;131:356–369. doi: 10.1016/j.freeradbiomed.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 21.ZHU H Y, HUANG Z X, CHEN G Q, et al. Typhaneoside prevents acute myeloid leukemia (AML) through suppressing proliferation and inducing ferroptosis associated with autophagy[J] Biochem Biophysl Res Commun. . 2019;516(4):1265–1271. doi: 10.1016/j.bbrc.2019.06.070. [DOI] [PubMed] [Google Scholar]
- 22.NIU Y, ZHANG J, TONG Y, et al. Physcion 8-O-β-glucopyranoside induced ferroptosis via regulating miR-103a-3p/GLS2 axis in gastric cancer[J] Life Sci. . 2019;237:116893. doi: 10.1016/j.lfs.2019.116893. [DOI] [PubMed] [Google Scholar]
- 23.TSAI Y, XIA C, SUN Z. The inhibitory effect of 6-gingerol on ubiquitin-specific peptidase 14 enhances autophagy-dependent ferroptosis and anti-tumor in vivo and in vitro[J] . Front Pharmacol. . 2020;11:598555. doi: 10.3389/fphar.2020.598555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MAI T T, HAMAÏ A, HIENZSCH A, et al. Salinomycin kills cancer stem cells by sequestering iron in lysosomes[J] Nat Chem. . 2017;9(10):1025–1033. doi: 10.1038/nchem.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.KAGAN V E, MAO G, QU F, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis[J] Nat Chem Biol. . 2017;13(1):81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DIXON S J, WINTER G E, MUSAVI L S, et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death[J] ACS Chem Biol. . 2015;10(7):1604–1609. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.XIAOFEI J, MINGQING S, MIAO S, et al. Oleanolic acid inhibits cervical cancer HeLa cell proliferation through modulation of the ACSL4 ferroptosis signaling pathway[J] Biochem Biophysl Res Commun. . 2021;545:81–88. doi: 10.1016/j.bbrc.2021.01.028. [DOI] [PubMed] [Google Scholar]
- 28.XIE Y, ZHOU X, LI J, et al. Identification of a new natural biflavonoids against breast cancer cells induced ferroptosis via the mitochondrial pathway[J] Bioorg Chem. . 2021;109:104744. doi: 10.1016/j.bioorg.2021.104744. [DOI] [PubMed] [Google Scholar]
- 29.MALFA G A, TOMASELLO B, ACQUAVIVA R, et al. Betula etnensis Raf. (betulaceae) extract induced HO-1 expression and ferroptosis cell death in human colon cancer cells[J] . Int J Mol Sci. . 2019;20(11):2723. doi: 10.3390/ijms20112723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DU J, WANG L, HUANG X, et al. Shuganning injection, a traditional Chinese patent medicine, induces ferroptosis and suppresses tumor growth in triple-negative breast cancer cells[J] Phytomedicine. . 2021;85:153551. doi: 10.1016/j.phymed.2021.153551. [DOI] [PubMed] [Google Scholar]
- 31.WANG N, ZENG G Z, YIN J L, et al. Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects ferroptosis in burkitt’s lymphoma[J] Biochem Biophysl Res Commun. . 2019;519(3):533–539. doi: 10.1016/j.bbrc.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 32.MBAVENG A T, FOTSO G W, NGNINTEDO D, et al. Cytotoxicity of epunctanone and four other phytochemicals isolated from the medicinal plants garcinia epunctata and ptycholobium contortum towards multi-factorial drug resistant cancer cells[J] Phytomedicine. . 2018;48:112–119. doi: 10.1016/j.phymed.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 33.MBAVENG A T, NDONTSA B L, KUETE V, et al. A naturally occuring triterpene saponin ardisiacrispin B displayed cytotoxic effects in multi-factorial drug resistant cancer cells via ferroptotic and apoptotic cell death[J] Phytomedicine. . 2018;43:78–85. doi: 10.1016/j.phymed.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 34.田颖颖,杨爱琳,陈孝男,等. 槐耳清膏抑制人非小细胞肺癌NCI-H1299细胞生长和转移及其作用机制研究[J]. 中国中药杂志, 2020, 45(15): 3700-3706 . [DOI] [PubMed]; TIAN Yingying, YANG Ailin, CHEN Xiaonan, et al. Effect of Huaier aqueous extract on growth and metastasis of human non-small cell lung cancer NCI-H1299 cells and its underlying mechanisms[J]. China Journal of Chinese Materia Medica, 2020, 45(15): 3700-3706. (in Chinese) . [DOI] [PubMed]
- 35.SHARMA P, SHIMURA T, BANWAIT J K, et al. Andrographis-mediated chemosensitization through activation of ferroptosis and suppression of β-catenin/Wnt-signaling pathways in colorectal cancer[J] Carcinogenesis. . 2020;41(10):1385–1394. doi: 10.1093/carcin/bgaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ADHAM A N, HEGAZY M E F, NAQISHBANDI A M, et al. Induction of apoptosis, autophagy and ferroptosis by thymus vulgaris and arctium lappa extract in leukemia and multiple myeloma cell lines[J] Molecules. . 2020;25(21):5016. doi: 10.3390/molecules25215016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ADHAM A N, NAQISHBANDI A M, EFFERTH T. Cytotoxicity and apoptosis induction by fumaria officinalis extracts in leukemia and multiple myeloma cell lines[J] J Ethnopharmacol. . 2021;266:113458. doi: 10.1016/j.jep.2020.113458. [DOI] [PubMed] [Google Scholar]
- 38.CHAN D W, YUNG M M, CHAN Y S, et al. MAP30 protein from Momordica charantia is therapeutic and has synergic activity with cisplatin against ovarian cancer in vivo by altering metabolism and inducing ferroptosis[J] . Pharmacol Res. . 2020;161:105157. doi: 10.1016/j.phrs.2020.105157. [DOI] [PubMed] [Google Scholar]