Abstract
Purpose
To better understand the impact of cancer and treatment on outcomes and guide program development, we evaluated breast cancer survivors at risk for long-term medical and psychosocial issues who participated in survivorship care visits (SVs) at Johns Hopkins Hospital.
Methods
We conducted a prospective survey study of women with stage I-III breast cancer who participated in SVs from 2010–2016. The same 56-item questionnaire administered at SV and follow-up included an assessment of symptoms, social factors, demographics, anxiety, depression, and comorbidities. We added the Godin Exercise questionnaire to the follow-up.
Results
In 2018, 74 participants were identified as disease-free and mailed a follow-up survey; 52 (70.3%) completed the survey. At a median follow-up time of 3.1 years after diagnosis, participants were less likely to be employed (54% vs. 67%) than at the SV. About two-thirds were sedentary, and this was associated with high body mass index (p = 0.02). Sufficiently active participants (≥ 150 min per week of moderate-intensity activity) were less likely to report pain (p = 0.02) or fatigue (p = 0.001). Although 19% had moderate/severe anxiety or depression at follow-up, participants who reported employment satisfaction were less likely to be depressed (p = 0.02).
Conclusions
Awareness of issues faced by survivors is critical for enhancing care and developing models to identify patients who might benefit most from targeted long-term interventions.
Implications for cancer survivors
Interventions to address physical activity, persistent symptoms, and mental health are critical for breast cancer survivors.
Keywords: Breast cancer, Physical activity, Mental health, Obesity, Patient-reported outcomes
Introduction
There are over 3.5 million breast cancer survivors in the USA, and this growing population presents unique challenges and opportunities [1], including care for treatment-related issues and coexisting medical conditions [2]. Several longer-term comorbidities observed in cancer survivors, such as weight gain, obesity, infertility, psychological distress, depression, sexual dysfunction, second cancers, bone loss, and body image issues, can have lasting effects on quality of life [3]. A growing body of data shows that adverse effects of breast cancer treatment can negatively affect survivors’ ability to work and to remain physically activity [4, 5]. Prospective studies have shown that 21–29% of patients had not returned to work nearly two years after diagnosis, and that odds of not returning to work were significantly increased for those with treatment consisting of chemotherapy and HER2 therapy, African American race, depression or anxiety, fatigue, and higher grade toxicities. In fact, excess body weight is one of the strongest determinants of reduced life expectancy and morbidity [6-8]. Therefore, it is important to identify subsets of patients who might be at greater risk for adverse events long-term and who could potentially benefit from more targeted interventions early on [9, 10].
In 2006, the Institute of Medicine proposed standardizing practices for survivorship care by improving patient education and facilitating communication between providers with a survivorship care plan (SCP) [11]. While SCPs demonstrated high levels of patient satisfaction and self-reported understanding, obstacles to general administration include time, lack of role clarity between providers, and limited evidence on effect on cancer outcomes [12, 13]. Thus, many survivorship programs have tailored these to their population’s needs, such as a one-time visit to discuss survivorship concerns. To address this, the breast cancer survivorship program at Johns Hopkins Hospital (JHH) was established in 2008. Since 2010, it has offered a one-time survivorship visit (SV) with a nurse practitioner following completion of local and systemic therapy for patients with early stage breast cancer [14]. At the SV, participants complete questionnaires to assess lingering side effects and to screen for depression and anxiety, and receive a SCP. Our group previously reported on a cohort of JHH breast cancer patients (n = 87), most of whom received adjuvant chemotherapy and were subsequently referred by their medical oncology providers for a single SV [15].Compared to those in the 2010–2015 JHH Cancer Registry (n = 2,942), SV participants were younger, more likely to be African American, and more likely to have a higher TNM stage, hormone receptor-negative disease, and HER2-positive disease. They were also more likely to have received chemotherapy and radiation therapy. In this new analysis, we report on their long-term follow-up outcomes (employment, physical activity, symptoms, comorbidities, and mental health) based on subsequent review of medical records and a follow-up survey. We have added an employment and exercise evaluation for most recent survey evaluation.
A better understanding of long-term issues affecting cancer survivors has informed our breast cancer survivorship program, and influenced the build and design of various targeted intervention studies. For example, we have examined the impact of mindfulness meditation and survivorship education on behavioral health, including depressive symptoms, fatigue, sleep disturbance, and vasomotor symptoms for long-term survivors as far as 5 years post end of treatment. Our previous weight management studies have tested the efficacy of remote behavioral interventions with weight loss [16], and our recent studies have examined the effective of a sleep intervention prior to a behavioral weight loss strategy (NCT03542604). Our current weight management studies are determining the effects of pharmacotherapy and remote behavioral weight loss intervention, and the impact of weight loss on serum biomarkers and gut microbiome (NCT04499950). The aim of this study is to evaluate the impact of survivorship care visits (SVs) at Johns Hopkins Hospital and guide program development for breast cancer survivors at risk for long-term medical and psychosocial issues.
Methods
This study was approved by the Johns Hopkins Institutional Review Board (IRB Number: NA_00079523) with informed consent obtained from each participant or each participant’s guardian. Inclusion criteria included adults at least 18 years of age, a diagnosis of breast cancer, and receipt of at least a portion of breast cancer care or participation in educational activated coordinated by the Breast Cancer Survivorship Program at participating Johns Hopkins site. We retrospectively reviewed the charts of a cohort of patients who participated in a 60- to 90-min SV at JH medical oncology clinics at the JH Hospital or at JH Green Spring Station from January 2012 through December 2016. SVs were conducted by one of two nurse practitioners after referral by patients’ medical oncology providers and occurred about 1–3 months after completion of locoregional therapy and initial systemic therapy. To obtain data, each patient chart was accessed once between February 2018 and May 2018. Data from two time points were collected as follows: (1) at the time of the SV and (2) at the most recent follow-up. We also mailed a 56-item survey to a cohort of patients who were disease-free. The same 56-item questionnaire was previously administered at the SV and included: a locally developed patient symptom questionnaire, reassessment of behavioral factors (e.g., alcohol, smoking, employment) and demographic characteristics, the General Anxiety Disorder-7 (GAD-7) [17, 18] and Patient Health Questionnaire-9 (PHQ-9), and comorbidity assessment [19]. Our team of breast medical oncologists and advanced practice providers internally developed a symptom questionnaire that was piloted in a small group of patients in an exercise conducted by our internal experts and felt to comprehensively address a spectrum of symptoms that patients have reported [15]. It used a 4-item Likert scale ranging from none to severe to rate their concerns in the following areas: musculoskeletal pain, mobility, neuropathy, fatigue, sleep difficulty, memory decline, hot flashes, menstrual cycle pattern, sexuality, vaginal dryness, fertility, weight changes, inability/difficulty working, and difficulty with family/relationships. Lymphedema data was not collected in the survey, as other studies have shown inconsistency between self-perceived (subjective) lymphedema and objective lymphedema [20]. To evaluate employment, we assessed domains including being laid off, discrimination after diagnosis and current job satisfaction at follow-up but not at baseline. These were also internally developed and selected to address employment discrimination. We added the 3-item Godin Exercise questionnaire that was not included in the clinical SV to the mailed survey [21].
In order to ensure maximum mailed survey response, we implemented mailed survey best practices as described by Dillman et al. [22], including a survey format that is easy to read and understand, a plan for up to five contacts, inclusion of a stamped and addressed return envelope, and personalization of all correspondence. All eligible subjects were mailed a pre-notice letter informing them of the upcoming survey mailing (contact #1) to enhance participation rates [23]. One week later, all eligible subjects were mailed a packet including (1) cover letter on institutional stationary explaining the study and the purpose of the study, (2) two copies of the informed consent document, (3) survey instructions for survey completion, (4) survey packet, (5) a stamped self-addressed return envelope, and (6) an opt-out form (contact #2). Disease status was confirmed prior to contact of subjects. The opt-out form included a request for reason for disinterest and was to be returned if a subject did not wish to participate in the study or receive additional study-related mailings. One week later, a thank you letter was sent to all eligible subjects expressing appreciation for those who completed the survey; this also served as a reminder for those who had not yet returned the survey (contact #3). Those who responded to the survey also had a $10 honorarium mailed with the thank you letter. For those who had not responded after the initial three contacts, a second survey packet was sent (contact #4) three weeks later [24]. One week after that, a telephone call was to be placed to non-responders to request survey completion (contact #5).
We also performed a retrospective chart review to assess data from the most recent follow-up. These data supplemented an existing database on JHH Survivorship Visit that has previously been described [15]. Participant characteristics at diagnosis (e.g., age, race and ethnicity, insurance, marital status, employment status, menopausal status, parity, BMI, family history, comorbidities, and genetic testing), cancer characteristics (stage and tumor phenotype), and treatment (surgery, radiation, and systemic therapy) had already been collected [15]. We supplemented these with updated information on BMI, comorbidities and survival data (e.g., vital status, cancer status), time from diagnosis to survivorship visit, and time from diagnosis to most recent recorded follow-up. We calculated Charlson Comorbidity Index (CCI) [25] at time of diagnosis and at most recent follow-up. The pre-specified date of February 1, 2018, was used for determination of vital status.
Data were described using descriptive statistics. Fisher’s exact testing was utilized to make a conservative estimate of association (e.g., endocrine therapy, employment, employment satisfaction, activity, anxiety, and depression) due to limited sample size. Univariate associations were explored between survey elements (e.g., employment, activity) and abstracted data from the medical record (e.g., obesity, Charlson Comorbidity Index) using univariate logistic regression, while changes in continuous variable measurements between time points were assessed using the paired t test. Adjusting for age and race, multivariate logistic regression was used to compare symptoms at SV to those at most recent follow-up. All statistical tests were two-tailed. P values were considered significant if less than 0.05. All analyses were performed using Stata 14.1/MP for Windows (College Station, Texas).
Results
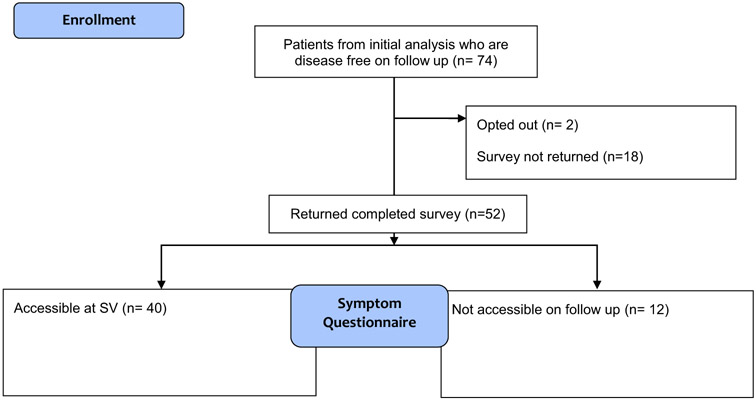
Participants who primarily received adjuvant chemotherapy and were subsequently referred by their medical oncology providers for a single SV were included in this follow-up survey study. Of 87 participants in the initial SV analysis, 74 were alive with no evidence of disease in February 2018. The surveys were mailed to these 74 individuals with a return rate of 70% (54 of 74). In total, there were 52 returned completed surveys, two opt outs, and 18 without a return (Fig. 1). Forty participants had the same questionnaires completed at SV visit and 12 did not have these accessible in the medical record.
Fig. 1.
CONSORT flow diagram
Participant demographics and cancer characteristics and treatment
This analysis includes the 52 women with early-stage breast cancer (stages I–III) who participated in a SV between January 2012 and December 2016 and returned the follow-up survey (Table 1). Median age at follow-up was 54.5 (inter-quartile range [IQR] 43, 65.5) years old. Most were Caucasian (71%), married (61%), and had private insurance (73%). Overall, the median time from diagnosis to most recent follow-up in survey responders was 3.1 years. The median time from SV to most recent follow-up was 1.91 years.
Table 1.
Participant demographics, cancer characteristics, and treatment at most recent follow-up
| Characteristic (N = 52) | Frequency | |
|---|---|---|
| Age at diagnosis (years), median (IQR) | 54.5 (43, 65.5) | |
| Current age, median (IQR) | 59 (47, 71.5) | |
| Race | Caucasian | 71% |
| African American | 27% | |
| Other | 2% | |
| Ethnicity | Hispanic | 2% |
| Non-Hispanic | 98% | |
| Education | Less than high school | 4% |
| High school/GED | 17% | |
| Vocational and Associates | 21% | |
| Undergraduate degree BA/BS | 25% | |
| Graduate degree MS/MBA/JD/PhD/MD | 33% | |
| Insurance status | Private | 73% |
| Medicare | 27% | |
| Marital status* | Single | 12% |
| Married/Partnered | 61% | |
| Separated/Divorced | 17% | |
| Widowed | 10% | |
| Menopausal at diagnosis | Yes | 62% |
| No | 38% | |
| Mutation present in those with genetic testing (n = 25) | BRCA1 (n = 4) | 16% |
| BRCA2 (n = 1) | 4% | |
| PALB2 (n = 1) | 4% | |
| Stage | 1 | 31% |
| 2 | 52% | |
| 3 | 17% | |
| Node status | Positive | 54% |
| Negative | 46% | |
| Hormone receptor status | Positive | 56% |
| Negative | 44% | |
| HER-2 receptor status | Positive | 42% |
| Negative | 58% | |
| Surgery | Lumpectomy | 44% |
| Bilateral mastectomy | 31% | |
| Bilateral nipple sparing mastectomy | 8% | |
| Bilateral skin-sparing mastectomy | 6% | |
| UL mastectomy | 11% | |
| Lymph node assessment | Sentinel lymph node (SLN) biopsy | 73% |
| Axillary lymph node dissection (ALND) | 19% | |
| Chemotherapy | Yes | 94% |
| No | 6% | |
| Endocrine therapy | Overall | 56% |
| Among HR-positive | 97% | |
| Tamoxifen | 31% | |
| Aromatase inhibitor 20 | 69% | |
| Anti-HER2 therapy | Yes | 42% |
| No | 58% | |
| Radiation | Breast | 56% |
| Chest | 44% | |
| Boost | 18% | |
| LN | 59% | |
| Time from diagnosis to SV (years), median (IQR) | 0.9 (0.7, 1.3) | |
| Time from diagnosis to most recent follow-up (years), median (IQR) | 3.1 (2.4, 4.2) | |
| Time from SV to most recent follow-up (years), median (IQR) | 1.9 (1.5, 3.1) |
At SV, 13% were single, 66% were married/partnered, 9% were separated/divorced, and 11% were widowed
Employment
Compared to the time of diagnosis, the proportion of participants who reported they were currently employed decreased from 67 to 54% (Table 2). Three participants did report that they felt discriminated against at work following their breast cancer diagnosis. While job satisfaction was not assessed with the baseline survey at the SV, specific questions were added to the follow-up survey. Among those who completed the recent survey, 46% and 29% reported they were very satisfied and somewhat satisfied with their current employment, respectively, while 25% were not too satisfied or not at all satisfied.
Table 2.
Employment, body mass, and physical activity
| Characteristic (N = 52) | Frequency at present |
|---|---|
| Employment status | |
| Employed | 54% |
| Homemaker | 36% |
| Retired | 6% |
| Unemployed | 4% |
| Current employment type | |
| Regular | 86% |
| Independent contractor | 7% |
| On call | 4% |
| Agency | 3% |
| Employment length | |
| < 6 months | 3% |
| 6–12 months | 11% |
| > 12 months | 86% |
| Employment years, median (IQR) | 9.5 (5.8, 12) |
| Employment satisfaction | |
| Very | 46% |
| Somewhat | 29% |
| Not too | 14% |
| Not at all | 11% |
| BMI, mean (IQR) | 28.9 (24.5, 32.7) |
| BMI class | |
| < 18.5 (underweight) | 0% |
| 18.5–24.9 (normal) | 31% |
| 25–29.9 (overweight) | 23% |
| > 30 (obese) | 46% |
| Exercise | |
| Sedentary or inactive | 60% |
| Insufficiently active | 19% |
| Sufficiently active | 21% |
At diagnosis [15]: 67% were employed, 29% retired, 4% homemaker, and 0% unemployed. Mean BMI (IQR) was 28.5 (24.3, 31.6). BMI classes include 2% underweight, 29% normal BMI, 31% overweight, and 38% obese
BMI body mass index
Physical activity
At both diagnosis and median follow-up of 3.1 (2.4, 4.2) years, the mean body mass index (BMI) was similar (28.5 and 28.9 kg/m2, respectively), and less than one-third had a normal BMI (29% v 31%). However, there were more participants in the obese category (46% vs 38%) and fewer in the overweight category (23% vs 31%) at follow-up compared to diagnosis. The only factor associated with normal BMI was sufficient physical activity (≥ 150 min per week of moderate-intensity physical activity per guidelines [26]) (p = 0.02), while endocrine therapy, employment, anxiety, and depression were not. Level of daily activity was sufficient in 21% and insufficient (10–149 min per week) in 19%, while 60% were sedentary or inactive (< 10 min per week) (Table 2).
Participant symptoms
Forty of 52 participants had symptom data available at both the SV and most recent follow-up. Commonly reported symptoms at most recent follow-up included the following: insomnia (33%), weight change (33%), myalgias (28%), paresthesias (28%), hot flashes (23%), weakness (21%), fatigue (21%), and pain (18%) (Table 3). Among the 40 participants with questionnaires at both SV and follow-up, after adjusting for age and race, both pain (p = 0.02, OR 0.15 [CI 0.02–0.77]) and fatigue (p = 0.001, OR 0.03 [CI 0.004–0.22]) were significantly associated with less activity at follow-up. After adjusting for age and race, paresthesias were significantly associated with unemployment (p = 0.03, OR 0.12 (CI 0.02–0.81)). At most recent follow-up, there was a significant association with more pain among those who were obese compared to those who have normal BMI (p = 0.003). There were no significant associations of other symptoms (myalgias, weakness, paresthesias, fatigue, insomnia, or hot flashes) with employment, mental health, BMI, or activity at follow-up.
Table 3.
Symptom questionnaire among those with paired data (at both SV and follow-up)
| Symptom | Frequency of moderate or severe symptom at SV (n = 40) |
Frequency of moderate or severe symptom at follow-up (n = 40) |
|---|---|---|
| Insomnia | 31% | 33% |
| Weight change | 36% | 33% |
| Myalgias | 28% | 28% |
| Paresthesias | 25% | 28% |
| Hot flashes | 18% | 23% |
| Weakness | 10% | 21% |
| Fatigue | 28% | 21% |
| Pain | 21% | 18% |
| Sexuality | 17% | 15% |
| Memory | 13% | 13% |
| Menstrual changes | 7% | 13% |
| Vaginal dryness | 21% | 13% |
| Difficulty at work | 10% | 11% |
| Difficulty with relationships | 11% | 8% |
| Fertility | 3% | 3% |
| % weight change, median (IQR) | 1.8% (−3.6%, 6.0%) | n/a |
| Significant weight gain (> 10%) | 15% | n/a |
Patients used a 4-item Likert scale ranging from none to severe to rate their concerns. For analysis, scores of none and mild, as well as moderate and severe, were grouped together
Comorbidities and mental health
Comorbidity data were similar between diagnosis and the most recent follow-up (Table 4). Median Charlson Comorbidity Index (CCI) at both diagnosis and most recent follow-up was 0 (IQR 0, 1). There was a 12% incidence of deep vein thrombosis at follow-up; none of these participants with DVT was on tamoxifen. Among the same 40 participants who completed the generalized anxiety disorder 7-item scale (GAD-7) and the depression module of patient health questionnaire (PHQ-9) at both the SV visit and follow-up, the median GAD-7 score for anxiety was 0 (“none”) with an IQR of 1 (“none”) to 7 (“mild”), and the median PHQ-9 score for depression was 1 (“minimal”) with an IQR of 1 (“minimal”) to 6 (“mild”). A higher proportion of participants reported moderate or severe anxiety (17% vs. 12%) and depression (19% vs. 7%) at most recent follow-up than at SV. While employment status was not significantly associated with depression, employment satisfaction was associated with lower likelihood of depression (p = 0.02). Moderate or severe depression at most recent follow-up was significantly associated with higher likelihood of myalgias (p = 0.006), pain (p < 0.001), weakness (p = 0.007), fatigue (p < 0.001), insomnia (p < 0.001), and hot flashes (p = 0.007). There were no significant differences in levels of depression or anxiety based on use of endocrine therapy, obesity, activity level, and paresthesias.
Table 4.
Comorbidity and mental health
| Comorbidity | Frequency at follow-up (N = 52) |
|---|---|
| HTN | 42% |
| Thyroid issues | 15% |
| DVT | 12% |
| Pulmonary issues | 10% |
| Diabetes without damage | 6% |
| MI | 6% |
| PVD | 4% |
| Diabetes with damage | 2% |
| Liver disease, mild | 2% |
| CKD | 2% |
| Stroke | 2% |
| Hemiplegia | 2% |
| CT | 2% |
| Charlson comorbidity score, median (IQR)* | 0 (0, 1) |
| Mental health** | At recent survey (N = 40) |
| Anxiety score, median (IQR) | 0 (0.1) |
| None/mild | 83% |
| Moderate/severe | 17% |
| Depression score, median (IQR) | 1 (1.2) |
| None/mild | 81% |
| Moderate/severe | 19% |
Charlson comorbidity score includes moderate/severe liver disease, metastatic cancer, AIDS, CHF, dementia, PUD, and leukemia/lymphoma/local cancer (all of which were 0% for participants)
At SV, 12% had moderate/severe anxiety and 7% had moderate/severe depression (n = 52)
Discussion
As advances in the management of early-stage breast cancer reduce the risk of cancer recurrence and death, recognition of issues faced by survivors is essential for optimizing survivorship care and future research. In this long-term follow-up study, we surveyed a cohort of patients who were initially referred by their medical oncology providers for a SV after locoregional and initial systemic therapy. We observed a large proportion of participants who were dissatisfied at work or who dropped out of the work force, along with a significant association with presence of symptoms like paresthesias. Over two-thirds of our breast cancer survivors were sedentary and had excess weight (BMI ≥ 25 kg/m2) at diagnosis and at the SV, which is higher than expected when compared to cancer survivors at large [27]. Use of endocrine therapy, employment, anxiety, and depression was not associated with BMI, while the only factor associated with BMI was activity. Activity was also associated with lower odds of pain or fatigue, though there were no significant changes in comorbidities between diagnosis and most recent follow-up. Moderate or severe depression was observed in 19% of participants, which is increased from 6% at SV and higher than the reported prevalence of depression in the general cancer survivor population [28]. Moreover, for those with moderate or severe depression at most recent follow-up, there was an association with myalgias, pain, weakness, fatigue, insomnia, and hot flashes. Moderate or severe anxiety was observed in 17% of participants, which is increased from 12% at and comparable to observed estimates in general cancer survivors.
Our data suggest that certain breast cancer survivors, such as those who have received systemic chemotherapy, may face heightened issues after completing initial treatments. Additionally, given the association between depression and employment satisfaction, detection and treatment of underlying depression and sequela of therapy, like paresthesias, could favorably impact their ability to work. Increasing physical activity could also help improve BMI and possibly symptoms such as pain and fatigue. Thus, helping patients return to work and increase physical activity may improve the quality of their survivorship. Further studies examining work discrimination and work satisfaction after diagnosis may better inform us of the long-term challenges that patients experience.
Over a quarter of participants in our study were African American, and continued inclusion of minorities is essential for understanding long-term effects in all breast cancer survivor population. Black women have lower disease-free survival compared and are 1.2 times more likely to suffer from breast cancer mortality than White women. Additionally, the prevalence of obesity in Black women is almost twice than that of White women (Arnold, 2016) and there is a modest exploration of how both obesity and race impact cancer outcomes. Future studies will need to address racial disparities in cancer and non-cancer outcomes.
Our study has limitations. First, our study population is small, has incomplete data at SV, may underestimate comorbidities, and is insufficiently powered to test many associations of interest. While all participants who received SVs completed an initial survey, these were not all scanned into the medical record at intake and not accessible upon chart review. The survey return rate was lower than ideal at 70%, as not all who completed it at SV completed it at follow-up. The SVs were not designed to assess their effectiveness, but instead to better understand the individual impact of cancer diagnosis and treatment, as well as to guide program development. Additionally, not all patients were systematically offered to participate in them, resulting in a potential referral bias with clinicians identifying patients felt to be at greater risk or challenges after treatment and late effects. Despite these limitations, the major strengths of the study include high survey participation rate and a broad assessment of domains with long-term follow-up.
Finally, increasing efforts in wellness promotion are vital for cancer survivors. Similar to other survivorship programs, the current care models at our institution lack emphasis on assessment and cultivation of interpersonal relationships, review of employment, reinforcement of lifestyle interventions, and conversations about mental health. Future studies should focus on clear selection criteria for higher risk patients for targeted interventions. Moving forward, we will be testing a recently developed behavioral battery, which assesses demographics, social determinants of health, mental health, medication adherence, lifestyle factors, and symptoms. Risk models incorporating a behavioral battery may help health systems deploy meaningful interventions for those at greatest risk for worse outcomes, address their needs as cancer survivors, more effectively use health care resources, and ultimately improve their overall health-related quality of life.
Funding
This study is funded by Susan G. Komen Leadership Grant SAC110053 and SAC 170001 (ACW), Susan G. Komen Maryland (ACW and EDT), and National Institutes of Health Grant P30CA006973.
Footnotes
Ethics approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animal performed by any of the authors.
Consent to participate Informed consent was obtained on all individual participants included in the study.
Conflict of interest SJS, NZ, CR, JR, KCS, and ACW declare no competing interests. JYS received funding from Pfizer through the institution. EDT and CS received funding from Genentech and Pfizer through the institution. KLS receives funding from Pfizer and has a family member with stock in Abbot Labs and Abbvie. VS receives funding (to the institution) from Pfizer, Novartis, Puma Biotechnology, Biocept, and AbbVie, and as a Member, Data Safety Monitoring Board, Immunomedics, Inc.
References
- 1.Shapiro CL (2018) Cancer Survivorship. N Engl J Med 379(25):2438–2450 [DOI] [PubMed] [Google Scholar]
- 2.Jacobs LA, Shulman LN (2017) Follow-up care of cancer survivors: challenges and solutions. Lancet Oncol 18(1):e19–29 [DOI] [PubMed] [Google Scholar]
- 3.Sheng JY, Visvanathan K, Thorner E, Wolff AC (2019) Breast cancer survivorship care beyond local and systemic therapy. Breast Edinb Scotl 48(Suppl 1):S103–S109 [DOI] [PubMed] [Google Scholar]
- 4.Dumas A, Vaz Luis I, Bovagnet T et al. (2019) Impact of breast cancer treatment on employment: results of a multicenter prospective cohort study (CANTO). J Clin Oncol 38(7):734–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekenga CC, Pérez M, Margenthaler JA, Jeffe DB (2018) Early-stage breast cancer and employment participation after 2 years of follow-up: A comparison with age-matched controls. Cancer [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu H-S, Davis JE, Chen L (2018) Impact of comorbidity on symptoms and quality of life among patients being treated for breast cancer. Cancer Nurs [DOI] [PubMed] [Google Scholar]
- 7.Cho WK, Choi DH, Park W, et al. (2018) Effect of body mass index on survival in breast cancer patients according to subtype, metabolic syndrome, and treatment. Clin Breast Cancer [DOI] [PubMed] [Google Scholar]
- 8.Sung H, Siegel RL, Torre LA, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin [Internet] 2018. [cited 2018 Dec 25];0(0). Available from: http://onlinelibrary.wiley.com/doi/abs/10.3322/caac.21499 [DOI] [PubMed] [Google Scholar]
- 9.Palmer SC, Stricker CT, Panzer SL et al. (2015) Outcomes and satisfaction after delivery of a breast cancer survivorship care plan: results of a multicenter trial. J Oncol Pract 11(2):e222–e229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulloch KJ, Irwin ML, Chagpar AB et al. (2015) Systematic approach to providing breast cancer survivors with survivorship care plans: a feasibility study. J Oncol Pract 11(2):e170–e176 [DOI] [PubMed] [Google Scholar]
- 11.National Research Council and Institute of Medicine (2005) From cancer patient to cancer survivor: lost in transition. [cited 2018 Sep 25]. Available from: https://www.nap.edu/catalog/11468/from-cancer-patient-to-cancer-survivor-lost-in-transition [Google Scholar]
- 12.Ganz PA (2009) Quality of care and cancer survivorship: the challenge of implementing the institute of medicine recommendations. J Oncol Pract 5(3):101–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coyle D, Grunfeld E, Coyle K, Pond G, Julian JA, Levine MN (2014) Cost effectiveness of a survivorship care plan for breast cancer survivors. J Oncol Pract 10(2):e86–92 [DOI] [PubMed] [Google Scholar]
- 14.Peairs KS, Wolff AC, Olsen SJ et al. (2011) Coordination of care in breast cancer survivors: an overview. J Support Oncol 9(6):210–215 [DOI] [PubMed] [Google Scholar]
- 15.Skuli SJ, Sheng JY, Bantug ET et al. (2019) Survivorship care visits in a high-risk population of breast cancer survivors. Breast Cancer Res Treat 173(3):701–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santa-Maria CA, Coughlin JW, Sharma D et al. (2020) The effects of a remote-based weight loss program on adipocytokines, metabolic markers, and telomere length in breast cancer survivors: the POWER-remote trial. Clin Cancer Res 26(12):3024–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams N (2014) The GAD-7 questionnaire. Occup Med 64(3):224–224 [Google Scholar]
- 18.Kroenke K, Spitzer RL, Williams JBW, Monahan PO, Löwe B (2007) Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med 146(5):317–325 [DOI] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JBW (2001) The PHQ-9. J Gen Intern Med 16(9):606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bulley C, Gaal S, Coutts F et al. (2013) Comparison of breast cancer-related lymphedema (upper limb swelling) prevalence estimated using objective and subjective criteria and relationship with quality of life. BioMed Res Int 2013:e807569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amireault S, Godin G, Lacombe J, Sabiston CM (2015) The use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in oncology research: a systematic review. BMC Med Res Methodol 15(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dillman DA (2006) Mail and internet surveys: the tailored design method -- 2007 update with new internet, visual, and mixed-mode guide. Wiley [Google Scholar]
- 23.Mindell JS, Giampaoli S, Goesswald A et al. (2015) Sample selection, recruitment and participation rates in health examination surveys in Europe – experience from seven national surveys. BMC Med Res Methodol 15(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claycomb C, Porter SS, Martín CL (2000) Riding the wave: response rates and the effects of time intervals between successive mail survey follow-up efforts [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383 [DOI] [PubMed] [Google Scholar]
- 26.U.S. Department of Health and Human Services (2018) Physical Activity Guidelines for Americans, 2nd edition. Wash DC: 118 [Google Scholar]
- 27.Underwood JM, Townsend JS, Stewart SL, et al. (2002) Surveillance of demographic characteristics and health behaviors among adult cancer survivors--Behavioral Risk Factor Surveillance System, United States, 2009. Morb Mortal Wkly Rep Surveill Summ Wash DC 61(1):1–23 [PubMed] [Google Scholar]
- 28.Mitchell AJ, Ferguson DW, Gill J, Paul J, Symonds P (2013) Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol 14(8):721–732 [DOI] [PubMed] [Google Scholar]