Highlights
-
•
Saccharides assessed as combined cryoprotectant, preservative and prebiotic.
-
•
Application is freeze dried topical probiotic of Lactobacillus plantarum.
-
•
Inulin was best as cryoprotectant, but did not protect cells over storage.
-
•
Best combined performance using sucrose with storage at 4 °C.
-
•
Room temperature storage only feasible with skimmed milk (positive control).
Keywords: Probiotic, Prebiotic, Cryoprotectant, Freeze drying, Lactobacillus
Abstract
Probiotic formulations must contain the right strain(s) in sufficient numbers when administered to confer the desired health benefit. However, significant cell death can occur during freeze-drying and over storage. This study assesses various saccharides for their ability to protect Lactobacillus plantarum cells over freeze-drying and storage, as well as their potential to act as prebiotics. The cryoprotective potential of 10% (m/v) of skimmed milk, inulin, maltodextrin, and sucrose were investigated during freeze-drying. Storage was assessed over 12 weeks at 4 °C and room temperature. Improved cell survival over freeze drying was observed with all the saccharides. However, only maltodextrin and sucrose retained cell viability over storage at 4 °C. Overall, skimmed milk demonstrated the highest survival up to 91%. Despite good cryoprotectant performance, inulin provided the least protection over storage, with <1% cell survival. Prebiotic potential was determined through growth experiments with 2% (m/v) of the saccharides in glucose-free MRS. All saccharides supported cell growth, with sucrose performing best and inulin worst.
1. Introduction
The use of probiotics for treating diseases has in recent year's sparked increasing interest amongst researchers and pharma industries. Numerous studies have shown that the establishment of commensal and sometimes mutualistic microbes may hinder the growth of disease-causing microbes found in the same host microbial environment and additionally benefit the population of beneficial bacteria. Probiotics are defined as live microorganisms that when administered in the adequate amount confers health benefits on the host [20]. These beneficial microbes eliminate disease-causing bacteria through antagonistic mechanisms such as competitive adhesion advantage to host cells, production of lactic acid, production of hydrogen peroxide, and production of bacteriocins. Lowering of the pH by probiotic microbes can further make the environment unsuitable for pathogens to survive [15, 25, 27].
For probiotic treatments to be effective, it is important that strains in a probiotic formulation have been clinically proven to confer the desired benefits and they must be present in the sufficient number upon administration, as the definition implies. However, the functionality and efficiency of probiotics can be compromised due to loss of viability over storage [4]. Cell dehydration is a frequently used final step in probiotic production to keep the probiotic microbes in an inactivated form during storage, thereby ensuring that there are enough viable cells still present when the probiotic is taken. However, the drying process itself is detrimental to the probiotic cells and can result in reduced viability and stability of cells over storage.
Freeze drying is a preferred method due to its low operating temperature and pressure which help to retain the native structure, biochemical properties, and activities of bacterial cells [10, 24]. To minimise cell damage during freeze drying and to achieve high cell viability yields, protectants known as cryoprotectants can be added to the biomaterial before drying. A good cryoprotectant can be easily vitrified and can protect the embedded bacterial cells throughout the whole freeze drying process and during subsequent storage. The identification of the right protective agents to enhance cellular survival during storage is the key challenge [30]. The choice of cryoprotectants by manufacturers has been on a trial-and-error case based on factors such as protection performance, availability, cost and resulting physical characteristics of the final product [11]. Commonly used probiotic cryoprotectants include disaccharides (saccharose, lactose, trehalose), polyols (mannitol, sorbitol), and polysaccharides (maltodextrin, dextran, inulin) [1]. Disaccharide sugars and oligomeric sugars are preferred as additives for freeze drying not only because they can be easily vitrified but also because they are small molecular structures that could easily replace the water molecules removed during drying, and thus maintain the cell integrity [1]. The protective ability of sugars in freeze drying media can be linked to various mechanisms, one of which is the ability of sugar molecules to permeate through the cell membranes and replace the removed water molecules that were initially present between the bi-phospholipid layers within the cells. The water molecules act as spacers between the phospholipid heads that prevent them from rubbing against each other and subsequently causing an acyl chain reaction. This chain reaction leads to Van der Waals forces that shift the fluid state within cells to a more viscous gel-like phase. Upon rehydration, the transition back to the liquid crystal-like phase is non-homogenous due to the inhomogeneous nature of the phospholipid layers. This result in packing defects within cells and the cells lose their membrane integrity which consequently leads to death [1]. Therefore, its replacement during dehydration by the sugar molecules helps to retain the native robust structure of cell membranes after the freeze drying process. However, this protective mechanism favours systems with low sugar concentrations [3]. For higher concentration of sugar molecules (>0.2 M), there exists a preferential hydration between the solute and water molecules. This leads to a network of arrangement between the sugar and excluded water molecules on the cell membrane surfaces. As a result, the native state of the cell membrane is retained [1, 3]. Another mechanism that can assist the improved survival of cells with the inclusion of cryoprotectants in the drying media is the vitrification hypothesis, that is the ability of the cryoprotectants to form a protective glassy matrix during the freezing process, in which the cells are embedded [1]. In a protective glassy matrix, all diffusion-controlled processes that could be detrimental are slowed down, thus enhancing bacterial viability during long-term storage [4]. Furthermore, Tymczyszyn et al. [32] suggests that survival during dehydration can be improved when cells are pre-cultivated in the growth media supplemented with sugar used for cryoprotection. Tymczyszyn et al. [32] attributes this improved survival to a lowered water activity in the growth media which subsequently enhances microbial response to osmotic stress during dehydration.
The efficacy of probiotics upon administration can be improved through the use of prebiotics, which are substrates that promote probiotic habitation. In the past, prebiotics (by definition) were limited to substances that could remain available in the gut flora after the journey through the gastrointestinal tract. These included non-digestible fibres such as inulin and other oligosaccharides. In recent times, the field of probiotics has extended the utilization of microbes to confer health benefits beyond the gastrointestinal tract. An example of this is evidence of improved vaginal and skin health with the application of Lactobacillus plantarum [8, 21, 35]. With this advancement, a new definition of the term was established by a panel of experts convened by the International Scientific Association for Probiotics and Prebiotics (ISAPP) [14]: “A substrate that is selectively utilized by host microorganisms conferring a health benefit”. This opens up far more options for prebiotics, especially for topical administration. For example, Succi et al. [34] reported that strains belonging to Lactobacillus rhamnosus taxa have a genetic makeup that allows them to have a wide range of adaptability and selectivity to various substrates. These strains can grow in environments rich in lactose, inulin, sucrose, trehalose, starch, fructans, maltose, cellobiose and raffinose.
The combined use of prebiotics in probiotic formulations is known as “synbiotics”. An example of this is when prebiotics are contained in probiotic foods such as yogurt that act to promote growth in the bowel when ingested [30]. Furthermore, some prebiotics are being considered for their possible protective role on probiotic bacteria [2, 7, 34]. This approach is of great interest since prebiotics could be used to play a double role both as sugars “for their historical role by stimulating proliferation and activity of probiotic bacteria in the colon and as protective agents against various environmental stresses” [34].
In this study, different typical cryoprotectant saccharides were assessed for their ability to fulfill multiple roles in a probiotic product: (1) protect probiotic cells during freeze drying, (2) preserve the viability of the cell concentrates over storage at different typical temperatures, and (3) act as a prebiotic substrate for the probiotic cells. This is a step towards the development of an effective synbiotic product for the topical treatment of conditions such as bacterial vaginosis and acne vulgaris. Lactobacillus plantarum was used as a model strain, where L. plantarum is known to utilize carbohydrates such as glucose in a homofermentative metabolism to produce lactic acid as the main fermentation product [18, 33]. The cryoprotectants considered were the saccharides inulin, maltodextrin, glucose and sucrose. These were considered for their availability, commercial feasibility, non-animal origin and for being Generally Recognized as Safe (GRAS). Skimmed milk was included as a freeze drying “positive control” due to the commonness of its industrial use.Material and methods
2. Materials
The following reagents were used: de Man, Rogosa & Sharpe (MRS) broth (product number 69,966, 99% purity), skimmed milk for bacteriological purposes (product number 70,166, ≥50% reducing sugars), d-(+)-glucose (product number G8270, ≥99.5% purity) and l-Cysteine (product number 168,149, 97% purity), all purchased from Sigma Aldrich, Germany; inulin, purchased from NOW foods, USA; food-grade sucrose, purchased from Pick n Pay stores, South Africa; maltodextrin, purchased from Supplement Factory, South Africa.
3. Collection and isolation of Lactobacillus strain
Lactobacillus plantarum was isolated in the work by Happel et al. [17], in which microbial samples were collected and isolated from bacterial vaginosis negative South African women (ages 18–25). Primary L. plantarum isolate stocks were frozen in 60% glycerol stocks (20% v/v) and stored at −80 °C. Frozen glycerol stocks were transferred to the Centre for Bioprocessing Engineering Research (CeBER) at the University of Cape Town (UCT), re-grown twice in MRS media and stored at −80 °C in 50% glycerol stocks (25% v/v). The working glycerol stocks of L. plantarum were regrown again twice in 50% glycerol stocks (25% v/v) frozen and stored at −80 °C.
4. Freeze drying and storage with cryoprotectants
4.1. Fermentation of L. plantarum
Fermentation media were prepared by dissolving MRS broth in deionized water to make up a solution of 0.5 g/L concentration. The prepared media was autoclaved after preparation using a Hirayama HG50 autoclave at 121 °C for 20 min and then cooled to room temperature for use.
Fermentations were performed in 100 ml serum bottles sealed with a rubber septum in aluminium caps. Fresh bacterial sub-culture was prepared from thawed working glycerol stocks, as discussed in 2.2. From the bacterial glycerol stocks, 2 ml was inoculated into a 50 ml working volume of sterile MRS media. The fermentation was run overnight at 37 °C and 140 rpm using the Stuart SSL1 shaker. At the end of the fermentation, the overnight culture was inoculated into 100 ml fresh sterile MRS fermentation media to make up an initial optical density (OD) reading of 0.1 (see section 2.5.1). The fermentation was run under the same conditions as that of the inoculum fermentation until stationary phase was obtained. Samples were taken using a syringe at 1 h time intervals to measure the cell density and pH (Lasec pH 50+ DHS metre) for determining the growth kinetic parameters. Experiments were performed in triplicate.
4.2. Cell harvesting
Once stationary phase was attained, the cells were concentrated and separated from the supernatant by centrifugation at 8000 g for 15 min at 4 °C (Beckman JA-25 / J-A centrifuge). The supernatant was discarded, and the cell pellet was twice washed by resuspending in sterile deionized water and homogenizing at a medium speed (Benchmark Scientific BV1000 vortex) before reconcentrating by centrifugation.
4.3. Preparation of freeze drying suspensions
The various cryoprotectants were suspended in demineralized water to achieve a 10% m/v solution and autoclaved for 20 min at 121 °C. Freshly harvested cells were suspended in 30 ml of the prepared solution and vortexed to achieve a homogenous 1% m/v biomass feed suspension.
2 ml samples were collected from the feed suspensions and stored at 4 °C to quantify the number and vitality of viable cells before freeze drying. 100 µl was collected from this for serial dilution to enumerate the cells in suspension. The remainder of the bacterial suspension from each fermentation experiment was separated into 5 ml ampoules for freeze drying.
4.4. Freeze drying
The bacterial suspensions were frozen in a freezer at −80 °C for 24 h, following which they were transferred to a freeze drier (Instruvac 5KL V) operated at 300 mTorr and −35 °C for 24 h.
4.5. Storage of freeze dried samples
The freeze dried samples were sealed in 5 ml airtight vials sealed with rubber stoppers embedded in aluminium caps. Storage was performed under two different conditions: at room temperature (20 ± 2 °C) and 4 °C (standard refrigeration temperature). At equal time intervals of 4 weeks for a total of 3 months, a vial representing each experimental system was taken, rehydrated to the original volume, and analysed to determine the impact of the various cryoprotectants on the cells over the storage period.
5. Prebiotic potential of cryoprotectant saccharides
L. plantarum was fermented in sterilized 100 ml glucose-free MRS media in serum bottles, supplemented with 2% (m/v) of inulin, maltodextrin, or sucrose. Fermentations were performed as described in section 2.3.1, at 37 °C and for 24 h.
6. Analytical methods
6.1. Cell density
The density of cells in solution was tracked using OD measurements, read at 660 nm using the Thermo Scientific Genesys 10S UV–VIS spectrophotometer. Fresh MRS media was used as the blank.
A linear trendline standard curve was used to relate the dry biomass in grams to the OD measurement, given by Eq. (1). For readings above 0.6, the culture was diluted 10 times using fresh MRS media. The curve was developed using fresh bacterial suspensions of specific OD. The suspensions were concentrated by centrifugation at 13,000 rpm (Eppendorf 5418R microfuge) for 15 min in pre-weighed and pre-dried (80 °C, 48 h) Eppendorf microfuge tubes, washed in sterile deionized water and centrifuged again. The pellets were dried at 80 °C (Labotec Eco Therm 277 oven) for 48 h and placed in a desiccator for 2 h to cool. The biomass dry weight was determined by weighing with a Radwag AS220.R2 balance.
| (1) |
6.2. Cell growth kinetic parameters
Cell growth was quantified through enumeration of the maximum specific growth rate , lag time and maximum cell density (ODmax). The was calculated using the natural integral of Eq. (2), where is the amount of cells (mass/volume) at time t (Maier, 2000).
| (2) |
6.3. Cell viability
Cell survival was measured in terms of viability, calculated using the values of enumerated colony forming units (CFU). The bacterial suspension from each experiment was diluted serially in sterile deionized water and spread on MRS agar plates (50 g/L MRS supplemented with 12 g/L bacteriological agar) in duplicate. The agar plates were incubated at 37 °C for 48 h after which the CFU were counted.
Counts were performed before freeze drying, after freeze drying, and after storage. In the case of freeze dried cells, the cells were rehydrated to the initial sample volumes using sterile deionized water in an ice bath.
Eq. (3) shows the formula applied in calculating the percentage viability. N0 [CFU/ml] represents the number of cell counts before freeze drying and Nt [CFU/ml] represents the number of cell counts at time t (after freeze drying or after storage).
| (3) |
6.4. Moisture content
The moisture content of the freeze dried material was determined by the gravitational method [10]. Weighing boats were made from cut aluminium foil sheets in equal sizes, labelled, and oven dried at 80 °C for 24 h (Labotec Eco Therm 277 oven). The weights of the boats were measured using the Radwag AS220.R2 balance. After freeze drying, samples were transferred on to the boats and weighed immediately (Wfs, [g]) using the weighing balance. The samples were then oven dried at 80 °C for 24 h and placed in a desiccator for 2 h, after which they were reweighed (Wos, [g]). The moisture content (Mw) was calculated as a percentage of the loss in weight of the samples using Eq. (4).
| (4) |
6.5. Transmission electron microscopic (TEM) imaging
TEM imaging was performed at the Aaron Klug Centre for Imaging and Analysis, UCT.
7. Results and discussion impact of cryoprotectants on L. plantarum over freeze drying
The moisture content of freeze dried probiotic bacteria is important in ensuring stability throughout its shelf life and the viability over storage [31]. Table 1 reports the moisture content of the L. plantarum after freeze drying in the presence of the various drying media each containing 10% (m/v) of the cryoprotectants skimmed milk (positive control), inulin, maltodextrin, and sucrose, and after freeze drying without cryoprotectants (negative control). Tests with glucose were also performed, but this system produced a rubbery matrix instead of the desired glassy matrix (data qualitatively assessed and hence not shown) and was therefore excluded from the analysis.
Table 1.
Moisture content of freeze dried L. plantarum in the presence of various cryoprotectant drying media.
| Moisture content (wt%) | |||||
|---|---|---|---|---|---|
| Drying media | Water (negative control) | Skimmed milk (positive control) | Inulin | Maltodextrin | Sucrose |
| Run 1 | 2.62 | 1.02 | 4.00 | 0.87 | 6.06 |
| Run 2 | 3.77 | 1.76 | 5.68 | 2.29 | 8.25 |
| Run 3 | 2.36 | 1.38 | 4.73 | 6.12 | No data |
| Average | 2.92 | 1.39 | 4.80 | 3.09 | 7.15 |
| SD | 0.43 | 0.21 | 0.49 | 1.57 | 0.89 |
The average final moisture content achieved under these conditions was in a relatively wide range of 1.39±0.21% (with skimmed milk) to 7.15 ± 0.89% (with sucrose). The moisture content values correspond with the molecular weights of the cryoprotectants, with the lowest molecular weight sucrose resulting in the highest moisture content and the moisture content in the samples then reducing as the molecular weight of the carbohydrates increased. It is generally accepted that probiotic moisture content should be kept below 5% for the stability of Lactobacillus [6]. This was not achieved for the sucrose freeze drying experiments, and not all of the inulin runs.
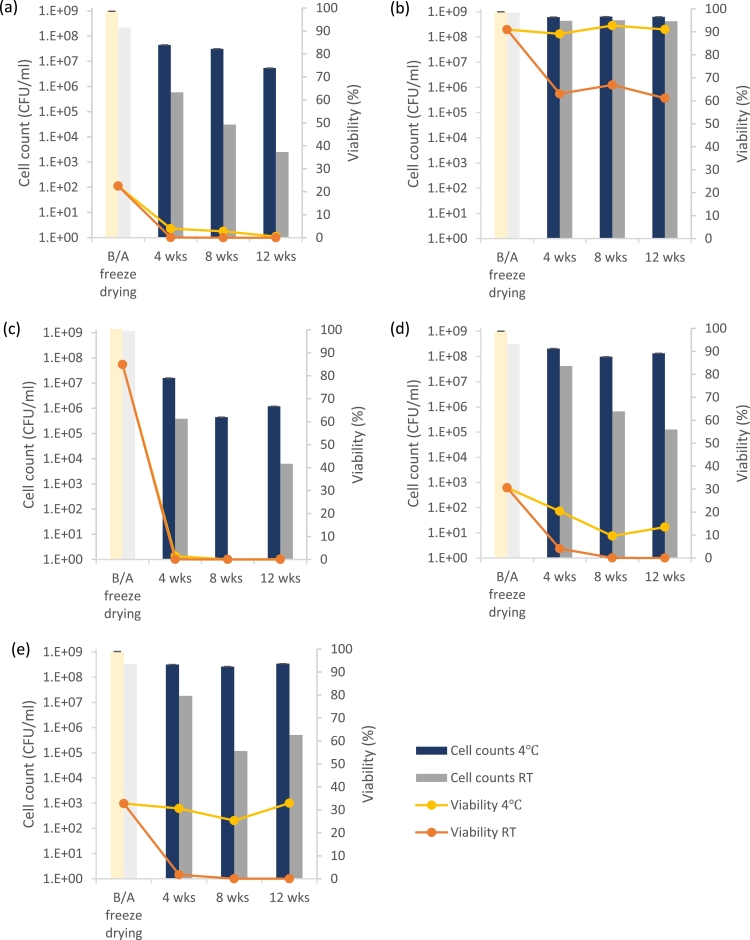
The survival of L. plantarum when the cells were freeze dried in the various drying media and over storage are presented in Fig. 1. An initial survival rate of up to 91% was achieved for cells freeze dried with skimmed milk, compared to the cells freeze dried in water which demonstrated a survival rate of only 22%. Amongst the saccharide cryoprotectant candidates, inulin followed skimmed milk by demonstrating the second-highest survival rate of 85%, followed by sucrose and maltodextrin with survival rates of 33% and 31% respectively.
Fig. 1.
Survival of L. plantarum freeze dried in (a) water (negative control) and 10% (m/v) (b) skimmed milk (positive control), (c) inulin, (d) maltodextrin, and (e) sucrose. Results are reported for immediately before and after (B/A) freeze drying and over 12 weeks of storage at 4 °C and at room temperature (RT).
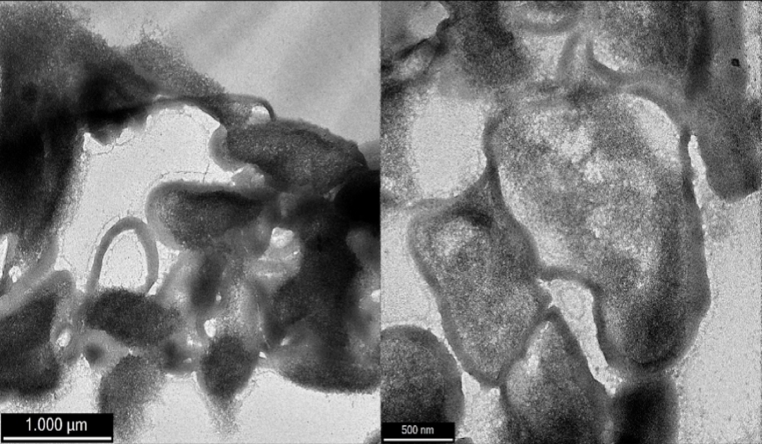
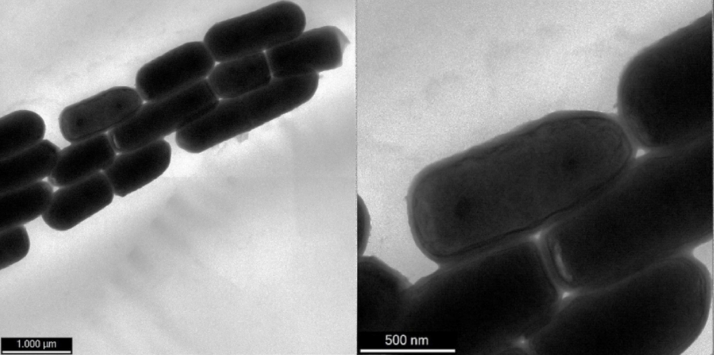
These results are consistent with observations of the state of the cell membranes post-freeze drying, visualised with TEM. The loss in the native cell membrane is observable in Fig. 2, when the L. plantarum cells were freeze dried in water without cryoprotectants. By contrast, protection of the native cell membrane form of the L. plantarum cells when freeze dried in the presence of inulin is seen in Fig. 3.
Fig. 2.
TEM images of freeze dried L. plantarum cells without cryoprotectants (negative control).
Fig. 3.
TEM images of freeze dried L. plantarum cells embedded in inulin Prebiotic potential of cryoprotectants on L. plantarum.
This result is similar to Reddy et al. [26], where L. plantarum demonstrated high survival rates of 100% when freeze dried in skimmed milk. However, the survival rates obtained in that study when L. plantarum was freeze dried in maltodextrin and sucrose ( 90%) and in water without cryoprotectants ( 40%), were higher than the rates obtained in this work. One possible reason for this is that Reddy et al. [26] pre-exposed their cells to cold shock prior to freeze drying, such that the cells developed cold shock resistance. Derzelle et al. [9] explain that this developed resistance by L. plantarum to cold shock is linked to the release of three cold shock proteins CspC, CspP and CspL during exposure to cold shock in the early log phase, confirmed to have been expressed in Reddy et al. [26]. Pre-stressing cells prior to the freeze drying process is also known to improve the integrity of cells membranes [36]. Another probable explanation for the higher rates of survival obtained by Reddy et al. [26] is the higher concentration of cryoprotectants in the drying media −20% (m/v) compared to 10% used in this study.
8. Preservative effect of cryoprotectants on L. plantarum over storage
Conditions known to affect the survival of probiotics during storage are the temperature and duration of shelf life [23]. This is due to the biochemical activity of cells being enhanced when cells are stored at a higher temperature compared, and these reactions release metabolites which are detrimental to cells and eventually cause cell death. Furthermore, an absence of protective material around stored cells will contribute to a decrease in cell viability.
When freeze dried L. plantarum samples without excipients were rehydrated after being stored under dry airtight conditions at 4 °C or room temperature, there was a further decline in the viability beyond that found at the end of the dehydration process. After only 4 weeks of storage, the viable cell numbers had reduced to only 2.2 × 108 CFU/ml (4% viability) at 4 °C and 5.8 × 108 CFU/ml (0.05% viability) at room temperature. By the 12th week, less than 1% of the cells remained viable in both cases.
There was a significant improvement in the survival rates with skimmed milk as the cryoprotectant compared to that of the negative control at both 4 °C and room temperature. After 4 weeks of storage, 89% of the cells (6.2 × 108 CFU/ml) remained viable at 4 °C and 63% (4.4 × 108 CFU/ml) remained viable at room temperature. This degree of viability remained approximately constant at the 8- and 12-week measurement marks. Therefore, a high degree of product stability was observed under refrigerated conditions, with negligible degradation of the cell viability. The product also appeared to be relatively stable at room temperature following the initial decrease in viability during the first month (to just over 60%). The high survival rates observed for dehydration with skimmed milk when compared to the saccharides can be attributed to the stabilizing effect on cell membrane contents, with the presence of protein in skimmed milk known to provide additional protective coating to cells [5].
Despite the initially high survival rate of the cells over freeze drying in the presence of inulin, the CFU counts after 4 weeks of storage showed negligible cell survival. A reduction in the number of cells was observed from 1.1 × 109 CFU/ml before freeze drying to 1.6 × 107 CFU/ml (representing a viability of 1%) and 3.8 × 105 CFU/ml (representing a viability of less than 1%) after 4 weeks storage at 4 °C and room temperature respectively. This low protective capacity of inulin over storage is proposed to be due to its high hygroscopic nature and low dry state Tg [12, 22, 28].
L. plantarum stored under ambient conditions in the presence of maltodextrin demonstrated a lesser decline in survival under refrigeration, from 3.1 × 108 CFU/ml immediately after freeze drying, to 1.3 × 108 CFU/ml (13% viability) by the end of the 12 week storage period. By comparison, only 4% of the cells were viable at 4 weeks and less than 1% at 8 weeks when the cells were stored at room temperature. Overall, the protective performance of maltodextrin over storage was therefore significantly better than that of inulin but still not as good as skimmed milk.
Incorporation of sucrose as a cryprotectant led to a maintained cell viability of between 25% and 33% at 4 °C, with no further reduction in the number of viable cells following the initial decrease over the freeze drying process to 3.4 × 108 CFU/ml. However, at room temperature the viability had decreased to only 2% after 4 weeks of storage and less than 1% at 12 weeks. In contrast to this result, a much higher protective performance by sucrose was obtained by Gul et al. [16] when L. curvatus was freeze dried in the presence of sucrose (10% (m/v)). This suggest that certain strains possess higher resistance to stress when dehydrated in combination with cryoprotectants.
Having investigated the cryoprotective and preservative potential of the selected saccharides, their prebiotic potential and hence ability to play a third role in the probiotic formulation efficacy was investigated. To determine this, the microorganism was cultured in glucose-free MRS media in which the glucose carbon source was substituted with 2% (m/v) of inulin, maltodextrin, and sucrose. These experiments were performed to compare the ability of L. plantarum to selectively utilize these carbohydrates for cell growth and decrease pH, both of which are essential probiotic pharmabiotic outcomes for restoring and maintaining vaginal and skin health. Across all experiments, the control was standard MRS media (already containing 2% (m/v) of glucose) without addition of the cryoprotectants.
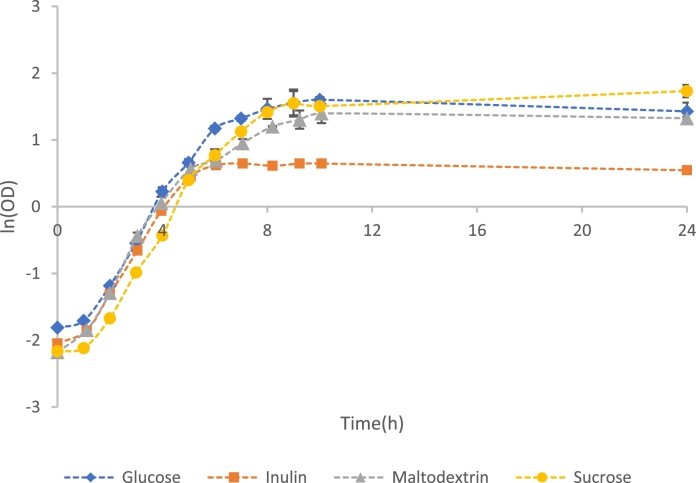
The growth curves presented in Fig. 4 show that all cryoprotectant candidates supported the growth of L. plantarum. Table 2 presents the obtained from the experimental data. The values remained the same across all runs at approximately 1 h. No significant differences are observed for the values of the different systems, all of which fall between 0.59 h−1 and 0.63 h−1. There were, however, differences between the final cell densities achieved. The highest ODmax value of 5.65 ± 0.09 was obtained for sucrose-containing MRS media, followed by 4.97 ± 0.03 for the glucose-containing control and 3.76 ± 0.02 for maltodextrin, with these three systems achieving stationary phase at circa10 h. The use of inulin resulted in the lowest ODmax value of 3.46, achieved at hour 5 when the system entered stationary phase.
Fig. 4.
Fermentation profiles of L. plantarum in the control (glucose containing MRS media) and glucose-free-MRS media supplemented with 2% (m/v) inulin, maltodextrin, and sucrose. The error bars are representative of the standard deviation between triplicate repeat runs.
Table 2.
Prebiotic test ,, and ODmax values, and the corresponding average (Ave) and standard deviation (SD) values calculated from experimental data for L. plantarum cultured in 2% (m/v) of inulin, maltodextrin and sucrose supplemented glucose-free-MRS media and the 2% (m/v) glucose-containing-MRS media positive control.
| Systems | (h − 1) | Ave | SD | (h) | Ave | SD | ODmax | Ave | SD | |
|---|---|---|---|---|---|---|---|---|---|---|
| MRS media (already containing 2% (m/v) glucose | Run 1 | 0.59 | 0.59 | 0.00 | 1 | 1 | 0 | 5.00 | 4.97 | 0.03 |
| Run 2 | 0.60 | 1 | 4.95 | |||||||
| Run 3 | 0.59 | 1 | 4.95 | |||||||
| Inulin | Run 1 | 0.59 | 0.59 | 0.02 | 1 | 1 | 0 | 1.85 | 1.85 | 0.05 |
| Run 2 | 0.58 | 1 | 1.89 | |||||||
| Run 3 | 0.61 | 1 | 1.80 | |||||||
| Maltodextrin | Run 1 | 0.64 | 0.62 | 0.02 | 1 | 1 | 0 | 3.77 | 3.76 | 0.02 |
| Run 2 | 0.63 | 1 | 3.74 | |||||||
| Run 3 | 0.60 | 1 | 3.78 | |||||||
| Sucrose | Run 1 | 0.63 | 0.63 | 0.00 | 1 | 1 | 0 | 5.70 | 5.65 | 0.09 |
| Run 2 | 0.62 | 1 | 5.70 | |||||||
| Run 3 | 0.63 | 1 | 5.54 | |||||||
The results signify that L. plantarum has high affinity for the digestion of sucrose, like that of glucose. This could be linked to the dimer nature of sucrose which is made up of shorter glucose units compared to the longer polymer units of maltodextrin and inulin [19]. The higher OD achieved in the sucrose system may be correlated with the higher carbon content of sucrose compared to glucose (42 wt% vs 40 wt%), though this argument does not hold for the lower maltodextrin and inulin ODmax as they comprise of 42 wt% – 44 wt% carbon. Gänzle and Follador [13] reported that Lactobacillus possesses the capacity to hydrolyse starch such as maltodextrin by the release of MalL, MalN and DexB which are α-Glucosidases intracellular enzymes. Therefore, it is possible that the maltodextrin nutrients were catabolised for other cell functions such as the conversion of carbon to energy for transportation of substances, to maintain osmotic balance or the production of cell metabolites rather than channelling this substrate nutrient for cell division. A similar mechanism could explain for the much lower ODmax values obtained for inulin. This means that in the culture, inulin acted as a limiting substrate to a greater degree compared to that glucose substrate and other cryoprotectants.
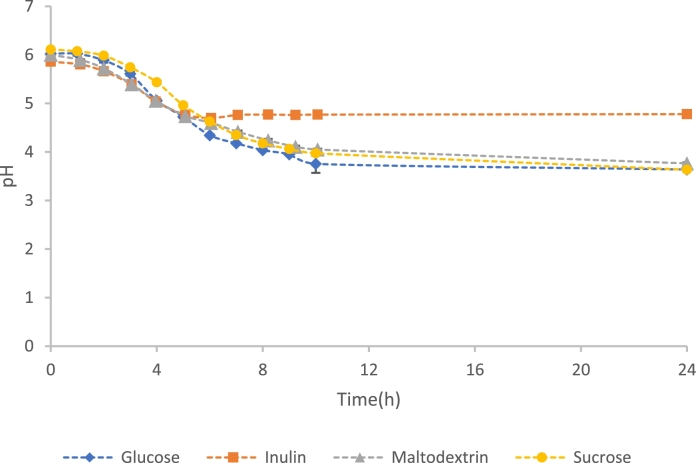
The pH reduction profiles of L. plantarum cultivated in 2% (m/v) cryoprotectant supplemented glucose-free MRS media are presented in Fig. 5. The profiles show that during fermentation of L. plantarum, all cryoprotectant candidates stimulated a reduction in pH of the growth media from the initial pH of 6.0 to a more acidic level. In the case of inulin, the pH was reduced to a value of 4.78 ± 0.01 by hour 5 after which it remained constant. This corresponds to when stationary phase was reached in the growth curve plot. In all the other runs, there was no significant difference in the pH reduction profiles of the control and sucrose or maltodextrin supplemented media, with the pH only plateauing after hour 10 to a final value of between 3.64 - 3.78.
Fig. 5.
pH reduction profiles of L. plantarum in the control (glucose containing MRS media) and glucose-free-MRS media supplemented with 2% (m/v) inulin, maltodextrin, and sucrose. The error bars are representative of the standard deviation between triplicate repeat runs.
9. Conclusions
The incorporation of 10% (m/v) skimmed milk, inulin, maltodextrin, and sucrose demonstrated a significant role in protecting Lactobacillus plantarum during freeze drying. The type and extent of protection was dependant on the cryoprotectant used. Inulin was the most effective saccharide over freeze drying, achieving more than twice the cell survival than maltodextrin and sucrose, but it did not perform as well as skimmed milk. It also proved unable to preserve the cells over storage. By contrast, both sucrose and maltodextrin were able to retain cell viability when the freeze dried cells were stored at 4 °C. Interestingly, this is despite the sucrose dried product having a moisture content above the literature recommended value of 5%. Only skimmed milk was found to be effective at preserving the cell viability at room temperature storage conditions.
The in vitro growth tests revealed that L. plantarum was able to use the various saccharides (inulin, maltodextrin and sucrose) for cell growth and production of metabolites which caused a decrease in pH. Similar maximum specific growth rates were found for all carbon sources. Therefore inulin, maltodextrin and sucrose all possess potential as prebiotics to promote the topical delivery of desired outcomes by pharmabiotic containing L. plantarum when administered. However, maltodextrin and inulin supported a significantly lower final cell density than the sucrose system (67% and 33% respectively), despite the same carbon availability. This indicates that the cells are less efficient at converting these substrates to biomass, even if the same pH reduction profiles were seen.
Therefore, sucrose comes out as the best choice when combining the findings to identify the best candidate that can fulfil the multiple roles of a prebiotic, cryoprotectant and preservative.
To further improve the survival over freeze drying and due to the poor preservation results at room temperature, it is recommended that further strategies be explored to strengthen the protective efficiency of sucrose. These include cold shock exposure prior to freeze drying to improve survival during freeze drying, and microencapsulation to improve the survival over storage.
It should also be noted that behaviour and interaction between the microorganism and the substrates during in vitro tests might translate differently when in vivo tests are performed. Prebiotics able to stimulate the propagation in media may not promote growth in the same way on the human body where other carbon sources are present and other microorganisms may compete for the prebiotic.
10. Availability of data and material
The data presented in this paper is stored on the ZivaHub platform, available at: https://doi.org/10.25375/uct.15089883
11. Funding
This work is based on research supported in part by the National Research Foundation of South Africa (UID 86,054 and UID 116,290) including funding under the South African Research Chairs Initiative (SARChI Chair in Bioprocess Engineering (UID 64,778). Any opinion, finding, conclusion or recommendation expressed in this material is that of the authors and the SARChI and NRF do not accept any liability in this regard.
12. CRediT author statement
Sumbo Oluwatoyin Oluwatosin: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - Original Draft, Visualization Siew Leng Tai: Conceptualization, Methodology, Resources, Writing - Review & Editing, Supervision, Project administration Marijke Antonia Fagan-Endres: Conceptualization, Methodology, Resources, Writing - Review & Editing, Supervision, Project administration, Funding acquisition
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Special thanks to following persons for their contributions to this work: Mohammed Jaffar from the Aaron Klug Centre for Imaging and Analysis, UCT for assistance with the TEM and Brian Kullin, from the Mucosal Infections Group, Faculty of Health Sciences, UCT for assistance with acquisition and identification of the microbial culture.
References
- 1.Aschenbrenner M., Foerst P., Kulozik U. Freeze-drying of probiotics. Adv. Probiotic Technol. 2015:213–241. doi: 10.1201/b18807-15. (August) [DOI] [Google Scholar]
- 2.Avila-Reyes S.V., Garcia-Suarez F.J., Jiménez M., San Martín-Gonzalez M.F., Bello-Perez L.A. Protection of L. rhamnosus by spray-drying using two prebiotics colloids to enhance the viability. Carbohydr. Polym. 2014;102(1):423–430. doi: 10.1016/j.carbpol.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 3.Broeckx G., Vandenheuvel D., Claes I.J.J., Lebeer S., Kiekens F. Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. Int. J. Pharm. 2016;505(1–2):303–318. doi: 10.1016/j.ijpharm.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Broeckx G., Vandenheuvel D., Henkens T., Kiekens S., van den Broek M.F.L., Lebeer S., Kiekens F. Enhancing the viability of Lactobacillus rhamnosus GG after spray drying and during storage. Int. J. Pharm. 2017;534(1–2):35–41. doi: 10.1016/j.ijpharm.2017.09.075. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho A., Silva J., Ho P., Teixeira P., Malcata F.X., Gibbs P. Relevant factors for the preparation of freeze-dried lactic acid bacteria. Int. Dairy J. 2004;14(10):835–847. doi: 10.1016/j.idairyj.2004.02.001. [DOI] [Google Scholar]
- 6.Chávez B.E., Ledeboer A.M. Drying of probiotics: optimization of formulation and process to enhance storage survival. Drying Technol. 2007;25(7–8):1193–1201. doi: 10.1080/07373930701438576. [DOI] [Google Scholar]
- 7.Cheow W.S., Kiew T.Y., Hadinoto K. Effects of adding resistant and waxy starches on cell density and survival of encapsulated biofilm of Lactobacillus rhamnosus GG probiotics. LWT - Food Sci. Technol. 2016;69:497–505. doi: 10.1016/j.lwt.2016.02.010. [DOI] [Google Scholar]
- 8.Cianci A., Cicinelli E., de Leo V., Fruzzetti F., Massaro M.G., Bulfoni A., Parazzini F., Perino A. Observational prospective study on Lactobacillus plantarum P 17630 in the prevention of vaginal infections, during and after systemic antibiotic therapy or in women with recurrent vaginal or genitourinary infections. J. Obstet. Gynaecol. (Lahore) 2018;38(5):693–696. doi: 10.1080/01443615.2017.1399992. [DOI] [PubMed] [Google Scholar]
- 9.Derzelle S., Hallet B., Ferain T., Delcour J., Hols P. Improved adaptation to cold-shock, stationary-phase, and freezing stresses in Lactobacillus plantarum overproducing cold-shock proteins. Appl. Environ. Microbiol. 2003;69(7):4285–4290. doi: 10.1128/AEM.69.7.4285-4290.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fatemeh S., Mustafa S., Ariff A., Manap Y.A. Optimization of a cryoprotective medium and survival of freeze-dried Bifidobacterium infantis 20088 throughout storage, rehydration and gastrointestinal tract transit for infant formula probiotic applications. Afr. J. Microbiol. Res. 2011;5(21):3373–3384. doi: 10.5897/ajmr11.319. [DOI] [Google Scholar]
- 11.Flores-Ramírez A.J., García-Coronado P., Grajales-Lagunes A., García R.G., Archila M.A., Ruiz Cabrera M.A. Freeze-concentrated phase and state transition temperatures of mixtures of low and high molecular weight cryoprotectants. Adv. Polym. Technol. 2019 doi: 10.1155/2019/5341242. 2019. [DOI] [Google Scholar]
- 12.Franck A. Technological functionality of inulin and oligofructose. Br. J. Nutr. 2002;87(S2):S287–S291. doi: 10.1079/bjn/2002550. [DOI] [PubMed] [Google Scholar]
- 13.Gänzle M.G., Follador R. Metabolism of oligosaccharides and starch in Lactobacilli: a review. Frontiers in microbiology. Front. Res. Foundation. 2012 doi: 10.3389/fmicb.2012.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., Verbeke K., Reid G. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Na. Rev. Gastroenterol. Hepatol. 2017:491–502. doi: 10.1038/nrgastro.2017.75. Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- 15.Goldin B.R. Health benefits of probiotics. Br. J. Nutr. 2019;80(S2):S203–S207. doi: 10.1017/s0007114500006036. [DOI] [PubMed] [Google Scholar]
- 16.Gul L.B., Con A.H., Gul O. Storage stability and sourdough acidification kinetic of freeze-dried Lactobacillus curvatus N19 under optimized cryoprotectant formulation. Cryobiology. 2020;96:122–129. doi: 10.1016/j.cryobiol.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Happel, A.U. 2018. Evaluation of probiotic and vaginal Lactobacillus species for the treatment of bacterial vaginosis and promotion of vaginal health in South African women. Ph.D. Thesis, University of Cape Town.
- 18.Melgar-Lalanne G., Rivera-Espinoza Y., Hernández-Sánchez H. Lactobacillus plantarum: an overview with emphasis in biochemical and healthy properties. Lactobacillus: Classification, Uses and Health Implications. 2012 [Google Scholar]
- 19.Hernandez-Hernandez O., Muthaiyan A., Moreno F.J., Montilla A., Sanz M.L., Ricke S.C. Effect of prebiotic carbohydrates on the growth and tolerance of Lactobacillus. Food Microbiol. 2012;30(2):355–361. doi: 10.1016/j.fm.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., Calder P.C., Sanders M.E. Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 21.Kim M.J., Kim K.P., Choi E., Yim J.H., Choi C., Yun H.S., Ahn H.Y., Oh J.Y., Cho Y. Effects of Lactobacillus plantarum CJLP55 on clinical improvement, skin condition and urine bacterial extracellular vesicles in patients with acne vulgaris: a randomized, double-blind, placebo-controlled study. Nutrients. 2021;13(4) doi: 10.3390/nu13041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moayyedi M., Eskandari M.H., Rad A.H.E., Ziaee E., Khodaparast M.H.H., Golmakani M.T. Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469. J. Funct. Foods. 2018;40:391–399. doi: 10.1016/j.jff.2017.11.016. [DOI] [Google Scholar]
- 23.Nader-Macías M.E.F., Juárez Tomás M.S. Profiles and technological requirements of urogenital probiotics. Adv. Drug Deliv. Rev. 2015;92:84–104. doi: 10.1016/J.ADDR.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Perdana J., Bereschenko L., Fox M.B., Kuperus J.H., Kleerebezem M., Boom R.M., Schutyser M.A.I. Dehydration and thermal inactivation of Lactobacillus plantarum WCFS1: comparing single droplet drying to spray and freeze drying. Food Res. Int. 2013;54(2):1351–1359. doi: 10.1016/j.foodres.2013.09.043. [DOI] [Google Scholar]
- 25.Plaza-Diaz J., Ruiz-Ojeda F.J., Gil-Campos M., Gil A. Mechanisms of action of probiotics. Adv. Nutr. 2019;10:S49–S66. doi: 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy K.B.P.K., Awasthi S.P., Madhu A.N., Prapulla S.G. Role of cryoprotectants on the viability and functional properties of probiotic lactic acid bacteria during freeze drying. Food Biotechnol. 2009;23(3):243–265. doi: 10.1080/08905430903106811. [DOI] [Google Scholar]
- 27.Reid G., Bruce A.W. Urogenital infections in women: can probiotics help? Postgrad. Med. J. 2003;79(934):428–432. doi: 10.1136/pmj.79.934.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saavedra-Leos M.Z., Leyva-Porras C., Martínez-Guerra E., Pérez-García S.A., Aguilar-Martínez J.A., Álvarez-Salas C. Physical properties of inulin and inulin-orange juice: physical characterization and technological application. Carbohydr. Polym. 2014;105(1):10–19. doi: 10.1016/j.carbpol.2013.12.079. [DOI] [PubMed] [Google Scholar]
- 30.Savini M., Cecchini C., Verdenelli M.C., Silvi S., Orpianesi C., Cresci A. Pilot-scale production and viability analysis of freeze-dried probiotic bacteria using different protective agents. Nutrients. 2010;2(3):330–339. doi: 10.3390/nu2030330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trujillo-de Santiago G., Rojas-de Gante C. In: In Water Stress in Biological, Chemical, Pharmaceutical and Food Systems. Gutiérrez-López G.F., Alamilla-Beltrán L., del Pilar Buera M., Welti-Chanes J., Parada-Arias E., Barbosa-Cánovas G.V., editors. Springer; New York: 2015. Influence of moisture content and temperature on the stability of a dehydrated probiotic dairy product containing Bifidobacterium infantis or Lactobacillus acidophilus. (Eds.) [DOI] [Google Scholar]
- 32.Tymczyszyn E.E., Gómez-Zavaglia A., Disalvo E.A. Effect of sugars and growth media on the dehydration of Lactobacillus delbrueckii ssp. bulgaricus. J. Appl. Microbiol. 2007;102(3):845–851. doi: 10.1111/j.1365-2672.2006.03108.x. [DOI] [PubMed] [Google Scholar]
- 33.Sharma R., Garg P., Kumar P., Bhatia S.K., Kulshrestha S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation. 2020 doi: 10.3390/fermentation6040106. MDPI AG. [DOI] [Google Scholar]
- 34.Succi M., Tremonte P., Pannella G., Tipaldi L., Cozzolino A., Romaniello R., Sorrentino E., Coppola R. Pre-cultivation with selected prebiotics enhances the survival and the stress response of Lactobacillus rhamnosus strains in simulated gastrointestinal transit. Front Microbiol. 2017;8(JUN):1–11. doi: 10.3389/fmicb.2017.01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vicariotto F., Mogna L., Piano M.D. Effectiveness of the two microorganisms Lactobacillus fermentum LF15 and Lactobacillus plantarum LP01, formulated in slow-release vaginal tablets, in women affected by bacterial vaginosis: a pilot study. J. Clin. Gastroenterol. 2014;48:S106–S112. doi: 10.1097/MCG.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 36.Wang G.-.Q., Pu J., Yu X.-.Q., Xia Y.-.J., Ai L.-.Z. Influence of freezing temperature before freeze-drying on the viability of various Lactobacillus plantarum strains. J. Dairy Sci. 2020;103(4):3066–3075. doi: 10.3168/jds.2019-17685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this paper is stored on the ZivaHub platform, available at: https://doi.org/10.25375/uct.15089883