Summary
Background
Children with high-risk medulloblastoma are treated with chemotherapeutic protocols which may affect heart function. We aimed to assesscardiovascular events (CVE) in children with medulloblastoma/primitive neuroectodermal tumors (PNET).
Methods
We retrospectively collected data from a case series of 22 children with high-risk medulloblastoma/PNET admitted to the Santobono-Pausilipon Hospital, Naples, Italy from 2008 to 2016. All patients received the Milan HART protocol for high-risk brain malignancies as first line treatment (induction phase), followed by a consolidation phase with Thiotepa and hematopoietic stem cells transplantation, except for 1 patient who received the Milan HART as second line therapy. Four patients also received second line treatment, while 4 patients also received maintenance therapy. Patients underwent cardiac examination, including ECG, echocardiography and serum biomarkers, before antineoplastic treatment initiation and then when clinically needed. Six patients developed CVE (CVE group); 16 patients had no CVE (NO-CVE group).
Findings
In the CVE group, 3 patients presented acute CVE during chemotherapy (2 patients with left ventricular (LV) dysfunction, 1 patient with arterial hypertension), while 3 patients presented chronic CVE after chemotherapy completion (2 patients with LV dysfunction, 1 patient with ectopic atrial tachycardia). After a 51 months median follow-up, 9 patients died: 4 from the CVE group (in 2 cases heart failure-related deaths) and 5 from the NO-CVE group (progression of disease).
Interpretation
A relevant percentage of children treated for medulloblastoma/PNET develops CVE. Heart failure potentially due to chemotherapy may represent a cause of death. Hence, in these patients, strict cardiac surveillance is essential.
Funding
No funding was associated with this study.
Keywords: Cardiovascular events, Children medulloblastoma/PNET, Milan HART
Research in context.
Evidence before this study
Little is known on the overall incidence of chemotherapy-induced-cardiotoxicity, especially in younger patients, considering that most of the studies are conducted on adults or long-term cancer survivors. Medulloblastoma is one of the most common malignancies in pediatric patients and is characterized by metastatic spread in up to 35% of patients, associated with a poor prognosis. Nowadays, the first line treatment for high-risk metastatic medulloblastoma is the Milan HART protocol. Some of the drugs included in the Milan HART protocol may have cardiac side effects, but the real incidence of cardiovascular events potentially associated with this specific protocol is yet unknown and has usually been studied in adult populations.
Added value of this study
Here we show that in children treatment for medulloblastoma/PNET is associated to a considerable risk of developing cardiovascular events during or after the completion of the antineoplastic protocol (27% of the study population), with a 9% mortality due to chemotherapy-induced cardiotoxicity.
Implications of all the available evidence
To the best of our knowledge, our current study is the first to describe a case series of different forms of cardiovascular events associated to the administration of multidrug chemotherapeutic protocols in pediatric patients with high risk medulloblastoma/PNET. We believe that a strict cardiologic monitoring is important in children undergoing the Milan HART protocol for the treatment of medulloblastoma/PNET.
Alt-text: Unlabelled box
Introduction
Advances in antineoplastic protocols have increased survival in children with cancer,1 but unfortunately subclinical, progressive, irreversible, and sometimes fatal treatment-related cardiovascular events may manifest both during and after the end of chemotherapeutic treatment.2,3
Among antineoplastic drugs, anthracyclines, widely used in many anticancer treatments, are well known for their cardiotoxicity and have been extensively studied over the past years. Furthermore, screening protocols have been suggested for early detection of anthracyclines-induced cardiotoxicity, both in adult and pediatric populations.2, 3, 4
High risk medulloblastoma in children is a severe oncologic disease that needs specific protocols with high doses of chemotherapeutic drugs that do not include anthracyclines, but that may have cardiovascular side effects as well.
The Milan hyperfractionated accelerated radiotherapy (Milan HART) protocol, approved as first line treatment for medulloblastoma, is characterized by an induction phase (IP) with vincristine, high dose (HD)-methotrexate, HD-etoposide (VP16), HD-cyclophosphamide and carboplatin, followed by radiation therapy directed to the brain. During the administration of VP16, hematopoietic cells are harvested from the patients to be re-infused later during the treatment. Patients who do not achieve complete remission before HART are then treated with HD chemotherapy with thiotepa followed by hematopoietic stem cell transplantation (HSCT).5,6 Among these drugs, HD-cyclophosphamide, etoposide, thiotepa and platinum-derived compounds have been associated with increased risk of cardiovascular toxicity (less frequently than anthracyclines, and mostly in adult populations), such as pericardial effusion, left ventricular (LV) dysfunction and heart failure (HF), arrhythmias, thrombotic events, and myocardial infarction.4,7
Few reports in pediatric populations describe non-anthracycline-induced cardiotoxicity. Furthermore, to date, the actual prevalence of chemotherapy-induced cardiovascular toxicity in pediatric patients treated with non-anthracycline based chemotherapy for high risk medulloblastoma has not been defined.
Hence, here we aim at characterizing cardiovascular events in a case-series of pediatric patients diagnosed with high risk medulloblastoma and treated according to the Milan HART protocol.
Methods
Study population
In this study, we retrospectively enrolled consecutive patients affected by high risk Medulloblastoma/primitive neuroectodermal tumors (PNETs) with indication to a 2-month schedule intensive chemotherapy, performed after brain surgery, according to the Milan HART protocol for high-risk brain malignancies admitted to the Department of Pediatric Oncology of the Santobono-Pausillipon Hospital, Naples, Italy from December 2008 to December 2016. The protocol was approved by the local ethic committee, the study was conducted following the Helsinki Declaration principles and parents or legal tutors for each patient signed a written informed consent to participate to the study.
Patients were treated with the Milan HART protocol, consisting of HD chemotherapy, with Vincristine 1.4 mg/m2 + HD-Methotrexate 8 g/m2 at week 0 and 1, HD-Etoposide (VP16) 2.4 g/m2 at week 4, then Vincristine 1.4 mg/m2 + HD-Cyclophosphamide 4 g/m2 at week 7, and Carboplatin 800 mg/m2 on the 7th week. Three or four weeks after IP completion, patients underwent HART, consisting of craniospinal radiotherapy.
Patients who did not achieve complete remission before HART were then administered with Thiotepa 300 mg/m2 for 3 consecutive days and HSCT. This procedure was repeated after a 4–6 weeks interval, (consolidation phase, CP).5,8
Patients were eligible for the study if they had received at least three doses of the HD induction chemotherapy regimen for high-risk brain malignancies, or at least two doses of the induction therapy followed by at least one course of consolidation therapy, and had undergone at least two cardiologic assessments while undergoing treatment.
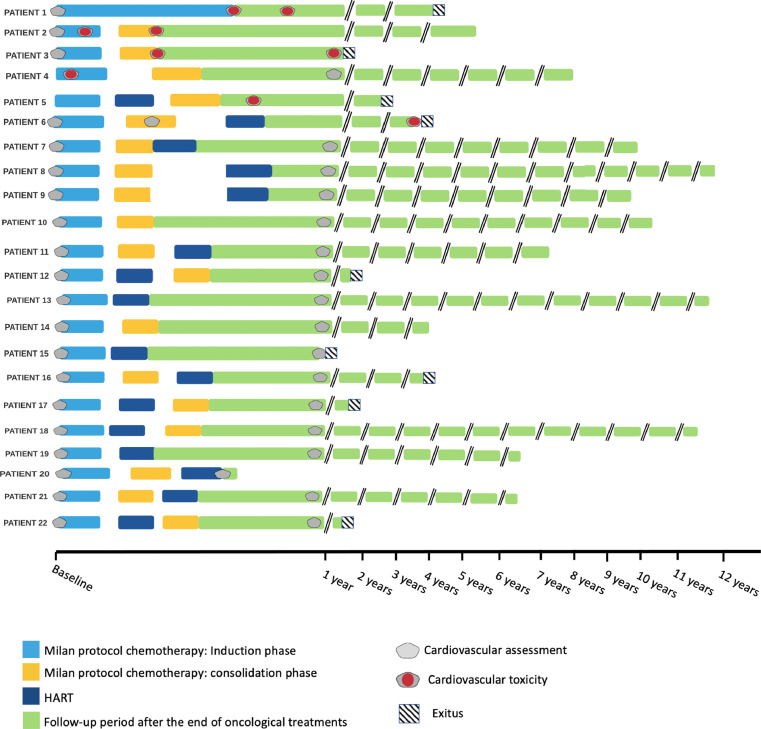
We identified 2 groups of patients, as shown in Figure 1: the CardioVascular Events (CVE) group, including all patients who experienced CVE (such as an absolute LV ejection fraction (LVEF) reduction of at least 10% from baseline to a value below 50%, rhythm disturbances, development of signs and symptoms attributable to cardiac dysfunction, arterial hypertension, or pathological increase of BNP serum values;4,10,11) the NO-CVE group, including patients who did not experience CVE during follow-up. Sixteen patients were included in the NO-CVE group, while 6 patients who experienced CVE were included in the CVE group, Specifically, 3 patients presented acute CVE, occurring during chemotherapy (2 patients with heart failure, 1 patient with arterial hypertension), while 3 patients presented chronic CVE, which manifested after chemotherapy completion (2 patients with LV dysfunction, 1 patient with ectopic atrial tachycardia). Clinical characteristics of the two subgroups are shown in Table 1.
Figure 1.
Study design
List of abbreviations: HART, hyperfractionated accelerated radiotherapy; PNET, Primitive Neuro-Ectodermal Tumor.
Table 1.
Clinical characteristics of the study group.
| Variables | Total population(n = 22) | CVE group(n = 6) | NO-CVE group(n = 16) |
|---|---|---|---|
| Females, n (%) | 11 (50) | 3 (50) | 8 (50) |
| Age, months | 61 (83) [12; 153] |
26 (32) [14; 83] |
76 (99) [12; 153] |
| BSA, mq | 0.8 (0.5) [0.4; 1.8] |
0.5 (0.3) [0.4; 0.9] |
0.9 (0.6) [0.4; 1.8] |
| Medulloblastoma | 19 (86) | 6 (100) | 13 (81) |
| PNET | 3 (14) | 0 (0) | 3 (20) |
| Metastatic | 15 (68) | 4 (67) | 11 (69) |
| Infant (<3 y.o.) | 9 (40) | 4 (67) | 5 (31) |
| Stem cell transplantation, n (%) | 18 (82) | 5 (83) | 13 (81) |
| HART | 16 (73) | 2 (33) | 14 (88) |
| Radiation dose, Gy | 60 (89) [0; 130] |
0 (45) [0; 88] |
77 (45) [0; 130] |
| Follow-up, months | 51 (89) [7; 140] |
38 (45) [12; 86] |
75 (102) [7; 140] |
| Remission/Off therapy, n (%) | 13 (59) | 2 (33) | 11 (69) |
| Non-CVE-related deaths, n (%) | 7 (32) | 2 (33) | 5 (31) |
| CVE-related deaths, n (%) | 2 (9) | 2 (33) | 0 (0) |
Data are expressed as median (interquartile range) [minimum; maximum values] or absolute number (percentage) as appropriate.
List of abbreviations: BSA, body surface area; CVE, cardiovascular events; HART, hyperfractionated accelerated radiotherapy; PNET, primitive neuroectodermal tumors; y.o., years old.
Cardiologic evaluation
Children underwent cardiologic evaluation before starting the therapies, before consolidation therapy with myeloablative courses and stem cell transplantation, at the end of the chemotherapeutic protocol, and then as clinically needed during follow-up.
Each cardiologic assessment included physical examination, ECG, two-dimensional (2D) transthoracic echocardiography, blood sampling for the evaluation of serum cardiac biomarkers including brain natriuretic peptide (BNP), troponin, creatine kinase (CK or CPK) and myoglobin.
All cardiologic examinations, including echocardiographic assessments, were performed by a board-certified cardiologist/an expert cardiologist.
Statistical analysis
Data are expressed as median (interquartile range) [minimum; maximum] for continuous variables and number (percentage) for discrete variables. Statistical analysis was performed with SPSS statistic Version 24 (IBM, Armonk, NY).
This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for observational studies.9
Figures preparation
We acknowledge the use of elements from Servier Medical Art (https://smart.servier.com/; accessed on August 6, 2021) for the preparation of Figure 1.
We acknowledge the use of elements by Freepik from Flaticons (http://www.flaticon.com/free-icon/sushi_187463#term=sushi&page=1&position=68; accessed on August 10, 2021) for the preparation of Figure 1.
We acknowledge the use of Licid.App (https://lucid.app/documents#/dashboard; accessed on October 25, 2021) for the preparation of Figure 2.
Figure 2.
Timelines schematizing each patient follow-up.
Role of funding sources
No role for any funding sources in the writing of the manuscript or the decision to submit it for publication.
Results
Patients’ general characteristics
A total of 29 patients were screened for the study. Three patients did not meet the inclusion criteria and four patients were lost at an extremely early stage of follow-up (did not complete the oncological protocol at our Institution and/or presented just a baseline cardiac evaluation). A total of 22 patients was then included in the analysis.
General characteristics of the study population are described in Table 1. The median age of the population was 61 months, ranging from 12 to 153 months, and it was composed by 11 females (50% of the whole population). 19 patients presented with medulloblastoma (9 patients had infant (<3 years old) medulloblastoma), 3 patients were affected by PNET. Out of these 22 patients, 15 already had metastatic cancer at enrollement.
Median follow-up for the entire population was 51 [7; 140] months, for CVE group was 38 [12; 86] months and for the NO-CVE group was 75 [7; 140] months.
CVE group: chemotherapeutic treatments and cardiotoxicities
All patients received the Milan HART protocol for high-risk brain malignancies as a first line therapy. Data are shown in Tables 2 and 3. Figure 2 summarizes the timeline for each patient in the CVE group.
Table 2.
Oncologic characteristics of the study group.
| Patient | Gender | Age at Diagnosis (months) | Group | Tumor Type | Radiotherapy on spinal axis | Second line | Stem Cell Transplantation | Status |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | Female | 14 | CVE | Infant Metastatic Medulloblastoma | No | None | No | Dead for cardiac complications |
| Patient 2 | Male | 32 | CVE | Infant Metastatic Medulloblastoma | No | None | Yes + 1xThiotepa | Remission |
| Patient 3 | Female | 16 | CVE | Infant Medulloblastoma | No | None | Yes + 2xThiotepa | Dead for heart failure |
| Patient 4 | Male | 20 | CVE | Infant Medulloblastoma | No | None | Yes + 2xThiotepa | Remission |
| Patient 5 | Female | 83 | CVE | Metastatic Medulloblastoma | Yes | None | Yes + 2xThiotepa | Dead for PD |
| Patient 6 | Male | 36 | CVE | Metastatic Medulloblastoma | Yes | Temozolomide, VP16 | Yes + 2xThiotepa | Dead for PD |
| Patient 7 | Female | 63 | NO-CVE | Metastatic Medulloblastoma | Yes | None | Yes + 2xThiotepa | Remission |
| Patient 8 | Female | 12 | NO-CVE | Infant Medulloblastoma | Yes | None | Yes + 1xThiotepa | Remission |
| Patient 9 | Male | 26 | NO-CVE | PNET | Yes | None | Yes + 2xThiotepa | Remission |
| Patient 10 | Female | 34 | NO-CVE | Infant Medulloblastoma | No | None | Yes + 2xThiotepa | Remission |
| Patient 11 | Male | 76 | NO-CVE | Metastatic Medulloblastoma | Yes | None | Yes + 2xThiotepa | Remission |
| Patient 12 | Male | 76 | NO-CVE | PNET | Yes | Temozolomide Irinotecan | Yes + 2xThiotepa | Dead for PD |
| Patient 13 | Male | 59 | NO-CVE | Metastatic Medulloblastoma | Yes | Maintenance: CCNU, VCR, Cisplatin | No | Remission |
| Patient 14 | Female | 15 | NO-CVE | Infant Metastatic Medulloblastoma | No | None | Yes + 2xThiotepa | Remission |
| Patient 15 | Female | 107 | NO-CVE | Metastatic Medulloblastoma | Yes | Maintenance: CCNU, VCR, Cisplatin | No | Dead for AML |
| Patient 16 | Female | 29 | NO-CVE | Infant Metastatic Medulloblastoma | Yes | Maintenance CCNU, VCR Cisplatin, Temozolomide, VP16, Gemcitabine, Oxaliplatin |
Yes + 2xThiotepa | Dead for PD |
| Patient 17 | Female | 153 | NO-CVE | Metastatic Medulloblastoma | Yes | First line: CCNU, VCR, Cisplatin |
Yes + 1xThiotepa and VP16 | Dead for PD |
| Patient 18 | Male | 136 | NO-CVE | Metastatic Medulloblastoma | Yes | None | Yes + 2xThiotepa | Remission |
| Patient 19 | Male | 78 | NO-CVE | Metastatic Medulloblastoma | Yes | Maintenance CCNU, VCR, Cisplatin | No | Remission |
| Patient 20 | Male | 150 | NO-CVE | PNET | Yes | None | Yes + 2xThiotepa | Remission |
| Patient 21 | Female | 107 | NO-CVE | Metastatic Medulloblastoma | Yes | Temozolomide Irinotecan | Yes + 2xThiotepa | Remission |
| Patient 22 | Male | 148 | NO-CVE | Metastatic Medulloblastoma | Yes | None | Yes + 1xThiotepa | Dead for PD |
List of abbreviations: CVE, cardiovascular event; CCNU, lomustine; HART, hyperfractionated accelerated radiotherapy; HR, high risk; PD, progression disease; PNET, primitive neuroectodermal tumors; VCR, vincristine; VP16, etoposide.
Table 3.
Details on the cardiovascular events and cardiologic treatments of the CVE group.
| Patient | First LVEF (%) | Last LVEF (%) | Lowest LVEF (%) | Chemotherapy administered before lower LVEF or CVE onset | CVE manifestation | Details on cardiotoxicity | Onset of CVE | Cardiac treatment |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 73 | 50 | 48 | MTX+VCR, VP16, HD-Cyclophosphamide, Carboplatin | Asymptomatic LV dysfunction | BNP elevation, abnormalities of ventricular repolarization at EKG hypokinesia of the interventricular septum, | Acute | Diuretics, β-blockers, inotropes |
| Patient 2 | 70 | 62 | 48 | MTX+VCR, VP16 | Symptomatic LV disfunction | Rest dyspnea, tachycardia, BNP elevation, prolonged QTc interval at EKG, hypokinesia of the interventricular septum, mild pericardial effusion, mild mitral and tricuspid valve regurgitation | Acute | Diuretics, β-blockers, ACE inhibitors |
| Patient 3 | 73 | 62 | 45 | MTX+VCR, VP16, HD-Cyclophosphamide, Carboplatin, Thiotepa | Asymptomatic LV dysfunction | BNP elevation, reduced QRS voltages in all EKG leads, increased left ventricular volume, mild mitral and tricuspid valve regurgitation | Chronic | Diuretics, β-blockers, inotropes |
| Patient 4 | 67 | 68 | 66 | MTX+VCR, VP16 | Arterial hypertension | None | Acute | ACE inhibitors |
| Patient 5 | Not Available | 62 | 62 | MTX+VCR, VP16, HD-Cyclophosphamide, Carboplatin, Thiotepa | Ectopic atrial tachycardia | Prolonged QTc interval at EKG | Chronic | β-blockers |
| Patient 6 | 70 | 75 | 58 | MTX+VCR, VP16, Cyclophosphamide, Carboplatin, Thiotepa, Temozolomide | Hypokinesia of the interventricular septum with dyspnea and BNP elevation | Arterial hypertension, acute prerenal renal failure, mild pericardial effusion, mild aortic, mitral and tricuspid valves regurgitation | Chronic | Diuretics, ACE inhibitors |
List of abbreviations: ACE, angiotensin converting enzyme; BNP, brain natriuretic peptide; CVE, cardiovascular events; EKG, electrocardiogram; HD, high dose; LV, left ventricular; LVEF, left ventricular ejection fraction; MTX, methotrexate; VCR, vincristine; VP16, etoposide.
Patient 1, affected by infant metastatic medulloblastoma, did not undergo radiotherapy after the induction chemotherapy and, additionally, did not undergo stem cell transplantation nor was administered with thiotepa. The patient showed acute chemotherapy-induced cardiovascular toxicity (CIC) during the IP, 32 weeks from the beginning of the chemotherapy, with BNP elevation, ventricular repolarization abnormalities at ECG, and interventricular septum hypokinesia at 2D-standard echocardiography with significant, yet asymptomatic, LVEF reduction. The patient was treated with diuretics, beta-blockers and inotropes, but died due to cardiac complications.
Patient 2, diagnosed with infant metastatic medulloblastoma, presented acute cardiovascular toxicity during chemotherapy (1 month after the beginning of the IP), symptoms of HF, such as rest dyspnea and tachycardia. BNP was increased, the ECG showed prolonged QTc interval, while hypokinesia of the interventricular septum, reduced LVEF, mild pericardial effusion and mild mitral and tricuspid valve regurgitation were present at echocardiography. The patient was treated with conventional HF therapies. He then underwent only 1 cycle of thiotepa with stem cell transplantation. At the end of follow-up, the patient was in remission.
Patient 3, affected by infant medulloblastoma, completed the IP and was administered with 2 cycles of thiotepa as consolidation treatment with stem cell transplantation. She showed asymptomatic BNP increase, increased LV dimensions and reduced LVEF, mitral and tricuspid valve regurgitation at 2D-standard echocardiography. Signs of cardiovascular toxicity developed 8 months after the beginning of chemotherapy, and 4 months after its completion. The patient was administered with diuretics, beta-blockers and inotropes, but did not survive these complications.
Patient 4, diagnosed with infant medulloblastoma, completed the IP and was then administered with 2 courses of thiotepa with stem cell transplantation. After the first 2 cycles of IP, the patient developed severe arterial hypertension and was treated with ACE-is. At the last clinical contact, the patient was in remission state.
Patient 5, affected by metastatic medulloblastoma, completed the IP, underwent radiation therapy on the spinal axis with a total of 31.2Gy and was administered with 2 courses of thiotepa and stem cell transplantation. Nine months after the beginning of chemotherapy, 1 month after its completion, the patient manifested new-onset ectopic atrial tachycardia and was treated with beta-blockers. Eventually, the patient died for progression of the oncologic disease.
Patient 6, affected by metastatic medulloblastoma, completed the IP, underwent radiation treatment with 34.2Gy, was administered with 2 cycles of thiotepa and 2 hematopoietic stem cell transplantations (HSCT) and with temozolomide and etoposide as second line therapy, after the completion of the standard first line antineoplastic treatment. 27 months after the beginning of chemotherapy (5 months after the completion of maintenance treatment with temozolomide and etoposide) the patient manifested dyspnea and polypnea. BNP levels were markedly increased, the ECG showed signs of LV overload, while 2D-standard echocardiography showed basal septum hypokinesia, mild pericardial effusion, mild aortic, mitral and tricuspid valve regurgitation. The patient also developed arterial hypertension. He was treated with diuretics and ACEis, but died due to progression of the oncologic disease.
Table 4 summarizes the different manifestations of cardiovascular events in our patients.
Table 4.
Summary of the different manifestations of cardiovascular events.
| IP (HD-Cyclo + MTX + VCR + VP16 + CBDCA) | Thiotepa | Temozolomide | |
|---|---|---|---|
| Arterial hypertension | Yes | Yes | Yes |
| Ectopic atrial tachycardia | Yes | Yes | No |
| BNP elevation | Yes | Yes | Yes |
| Interventricular septum hypokinesia | Yes | Yes | Yes |
| Symptomatic LV dysfunction | Yes | No | No |
| Asymptomatic LV dysfunction | Yes | Yes | No |
| Asymptomatic LV dilation | Yes | Yes | No |
List of abbreviations: BNP, brain natriuretic peptide; CBDCA, carboplatin; HD-Cyclo, high dose cyclophosphamide; IP, induction phase; LV, left ventricular; MTX, methotrexate; VCR, vincristine; VP16, etoposide.
NO-CVE group: chemotherapeutic treatments
Clinical information of the NO-CVE-group are shown in Table 2. Five patients had infant medulloblastoma (3 were metastatic); 3 had PNET; 10 had metastatic medulloblastoma.
All patients in this group received Milano HART protocol for high risk brain malignancies as a first line therapy, except for 1 patient who was treated with 8 six-week cycles of Vincristine (VCR) 1.5 mg/m2 weekly for 3 courses Lomustine CCNU 75 mg/m2 and Cisplatin 70 mg/m2, and was then administered with the Milan HART protocol as second line treatment, and also received one dose of thiotepa associated with one dose of etoposide (500 mg/m2) before HSCT.12
Ten patients also received consolidation therapy with 2 cycles of thiotepa + HSCT. Among these 10 patients, 2 patients also received a second line treatment with TEMIRI protocol (Temozolomide 125 mg/mq/die (days 1–5), Irinotecan 50 mg/mq/die (days 1–5) every 21 days), while 1 patient received in addition Temozolomide (150 mg/m2) and etoposide (50 mg/m2) for 9 cycles, Gemcitabine (1000 mg/m2) and Oxaliplatin (100 mg/m2) for 1 cycle.
Two patients underwent only one cycle of thiotepa and HSCT.
Three patients did not undergo consolidation treatment with thiotepa and HSCT, but were administered with maintenance chemotherapy based on 8 six-weekly cycles of Vincristine 1.5 mg/m2 weekly × 3, Lomustine CCNU 75 mg/m2 and Cisplatin 70 mg/m2.13,14
Two patients did not receive radiation therapy.
Mortality
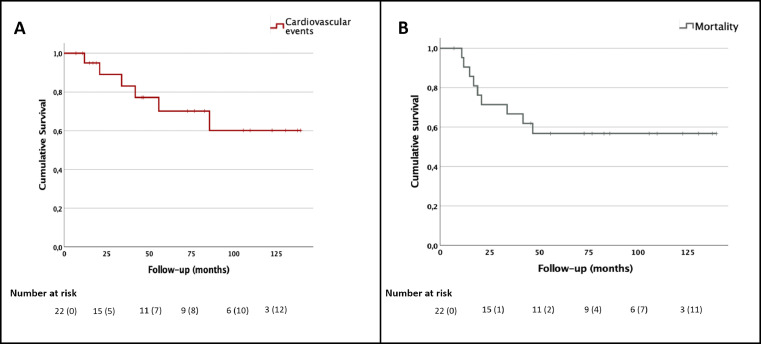
During a median follow up of 51 [7; 140] months, 9 out of 22 patients died, 4 from the CVE group (median follow-up 38 [12; 86] months) and 5 from the NO-CVE group (median follow-up of 75 [7; 140] months). Among the CVE group, patients 5 and 6 died from disease progression, while patients 1 and 3 died because of HF induced by the chemotherapeutic agents. Among the NO-CVE group, 4 patients died for disease progression and 1 patient died for acute myeloid leukemia (Table 2 and Figure 3).
Figure 3.
Kaplan-Meier curves for cardiovascular events (panel A) and for mortality (panel B).
Censored numbers are shown in parenthesis.
Discussion
Recent advances in oncological treatments have improved the prognosis of children with cancer. Unfortunately, new onset of cardiovascular events during or after chemotherapy administration remain one of the biggest burdens to such therapies, especially in younger patients. Current literature on cardiovascular side effects of antineoplastic treatments in pediatric patients has been focused mostly on the long-term effects of these treatments,15 while little is known about incidence, severity and underlying mechanisms of acute and subacute cardiac toxicity of HD-chemotherapy in pediatric patients.2,16
Medulloblastoma is one of the most common malignancies in pediatric patients (and is the most common cancer involving the central nervous system) and is characterized by metastatic spread in up to 35% of patients, associated to poor prognosis.17 Nowadays, the first line treatment for high-risk metastatic medulloblastoma is the Milan HART protocol.5,8
Many chemotherapeutic drugs produce adverse cardiovascular effects such as arterial hypertension, HF, thromboembolic events, and arrhythmias.4,18 Among antineoplastic drugs, anthracyclines have been studied extensively because of their overt cardiovascular effects and high incidence of heart failure. However, CIC might also be caused by other classes of chemotherapeutic agents, such as alkylating agents (cyclophosphamide, ifosfamide), platinum agents, antimetabolites, antibiotics, and antimicrotubule agents. Moreover, there is no published, or agreed-upon, definition of CIC for pediatric patients. Rather, the definition of CIC has come from adult data, which has been variable. This variability has led to ambiguity in the actual incidence of CIC.19,20 Although it is known that mediastinum irradiation is associated with increased risk of developing CVE,4 PNET/medulloblastoma patients usually receive craniospinal irradiation, with higher doses on the neoplastic areas (being the mediastinum spared).5 Moreover, younger patients usually are not irradiated to avoid further consequences. In our population, only 3 patients in the CVE group were treated with radiation therapy (33% of the CVE group), while 88% of the NO-CVE group was irradiated.
Interestingly, the Childhood Cancer survival study highlights that patients previously treated for cancer present an 8.2-fold increased risk of cardiac death compared to age- and sex-matched controls.21 To the best of our knowledge, our current study is the first to describe a case series of different forms of cardiovascular events due to multidrug chemotherapeutic protocols in pediatric patients with high risk medulloblastoma/PNET.
Some of the drugs included in the Milan HART protocol may have cardiac side effects, but the real incidence of cardiovascular events is yet unknown and, as said, has been usually studied in adult populations.4 In particular, etoposide seems to be associated with myocardial infarction and vasospastic angina, and, when combined with cisplatin, it seems to have synergic side effects on cardiac electrical activity.22,23
Cyclophosphamide-induced cardiovascular events has been reported in children, even if its incidence is lower than in adults (5% of pediatric patients present with HD-cyclophosphamide induced myocarditis).23 HD-cyclophosphamide in adults has been also associated to the development of chronic HF and severe hemorrhagic cardiac necrosis.4
In adults, platinum-derived compounds (PDCs) seem to be involved in arterial thrombosis, with subsequent risk of myocardial infarction and cerebrovascular ischemia.4,24 HSCT itself has been associated with increased risk of developing heart diseases, such as congestive heart failure and cardiomyopathy.25, 26, 27
Other risk factors for CIC include younger age (<4 years), Down syndrome, female sex, African American ancestry, chest irradiation, and features of metabolic syndrome (hyperlipidemia, hypertension, and obesity).19
Little is known on the overall incidence of CIC, especially in younger patients, considering that most of the studies are conducted on adults or long-term cancer survivals. Here we show that treatment for medulloblastoma/PNET in children is associated to a considerable risk of cardiovascular events (27% of the study population), with a 9% mortality due to CIC.
However, this is a single center, retrospective study conducted in a case series including a small study population. Considering that there are no international guidelines concerning the best follow-up schedule or the tests to be performed in pediatric cancer populations, some information is missing, such as cardiovascular family history, complete baseline blood analysis. In particular, as stated above, ECG, echocardiography and blood tests were performed mostly when clinically needed, without a defined schedule. It would be interesting to see whether in long term follow-up studies patients with elevated BNP or troponin have a higher risk for adverse events.
Another limitation is the rather long observation period, that might have influenced the diagnosis of cardiovascular events associated to chemotherapy, primarily in patients enrolled later during the study (i.e. ultra-sensitive troponins might anticipate the diagnosis of cardiac impairment). Further studies are needed to confirm our findings, which appear to point out that a strict cardiologic monitoring is important in children undergoing the Milan HART protocol for the treatment of medulloblastoma/PNET.
Contributors
AC, VM and MP share first authorship. AC: conceptualization, data curation, formal analysis, investigation, validation, visualization, writing – original draft, and writing – review & editing.
VM: conceptualization, data curation, formal analysis, funding acquisition, investigation, visualization, writing – original draft.
MP: conceptualization, data curation, formal analysis, investigation, methodology, resources, software, validation, visualization.
MC: data curation, formal analysis, investigation, methodology
SR: data curation, formal analysis, investigation, methodology.
AA: data curation, formal analysis, investigation, methodology.
CGT: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing – original draft, and writing – review & editing.
AP: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization.
MC, SR and AA were responsible for the raw data associated with the study; AC, VM, MP, CGT and AP accessed the raw data associated with the study and took the decision to submit the manuscript for publication.
Data sharing statement
The authors were not precluded from accessing data in the study, and they accept responsibility to submit for publication.
Declaration of interests
Dr. Cuomo, Dr. Mercurio, Dr. Pugliese, Dr. Capasso, Dr. Ruotolo, Dr. Antignano, Dr. Passariello have nothing to disclose. Dr. Tocchetti reports other from Amgen, personal fees from Vivalyfe, outside the submitted work; In addition, Dr. Tocchetti has a patent Canadian Patent No. 2,613,477, issued on Dec 3, 2013. Inventors: Nazareno Paolocci, David A Kass, Carlo G Tocchetti. Owner: Johns Hopkins University, entitled: THIOL-SENSITIVE POSITIVE INOTROPES. JHU Ref.: C04755-P04755–05 with royalties paid, and a patent P75NTR ANTAGONISTS AND TREATMENT OF ACUTE AND CHRONIC CARDIAC DISEASE. Inventors: Paolocci; Nazareno; (Baltimore, MD); Feng; Ning; (Baltimore, MD); Tocchetti; Carlo G.; (Baltimore, MD); Takahashi; Cyrus; (Baltimore, MD); Carter; Bruce; (Nashville, TN). 2020. Patent office: United States Patent and Trademark Office Granted Publication. Patent number US10786543, Publication date: Sep 29, 2020 issued.
Funding
No funding was associated with this study
References
- 1.Lam C.G., Howard S.C., Bouffet E., Pritchard-Jones K. Science and health for all children with cancer. Science. 2019;363:1182–1186. doi: 10.1126/science.aaw4892. [DOI] [PubMed] [Google Scholar]
- 2.Armenian S.H., Lacchetti C., Barac A., et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: american society of clinical oncology clinical practice guideline. J Clin Oncol. 2017 doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 3.Lipshultz S.E., Franco V.I., Miller T.L., Colan S.D., Sallan S.E. Cardiovascular disease in adult survivors of childhood cancer. Annu Rev Med. 2015;66:161–176. doi: 10.1146/annurev-med-070213-054849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zamorano J.L., Lancellotti P., Rodriguez Muñoz D., et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 5.Gandola L., Massimino M., Cefalo G., et al. Hyperfractionated accelerated radiotherapy in the Milan strategy for metastatic medulloblastoma. J Clin Oncol. 2009;27:566–571. doi: 10.1200/JCO.2008.18.4176. [DOI] [PubMed] [Google Scholar]
- 6.Vivekanandan S., Breene R., Ramanujachar R., et al. The UK experience of a treatment strategy for pediatric metastatic medulloblastoma comprising intensive induction chemotherapy, hyperfractionated accelerated radiotherapy and response directed high dose myeloablative chemotherapy or maintenance chemothera. Pediatr Blood Cancer. 2015;62:2132–2139. doi: 10.1002/pbc.25663. [DOI] [PubMed] [Google Scholar]
- 7.Bansal N., Amdani S., Lipshultz E.R., Lipshultz S.E. Chemotherapy-induced cardiotoxicity in children. Expert Opin Drug Metab Toxicol. 2017;13:817–832. doi: 10.1080/17425255.2017.1351547. [DOI] [PubMed] [Google Scholar]
- 8.Massimino M., Gandola L., Biassoni V., et al. Evolving of therapeutic strategies for CNS-PNET. Pediatr Blood Cancer. 2013;60:2031–2035. doi: 10.1002/pbc.24540. [DOI] [PubMed] [Google Scholar]
- 9.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbrouckef J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. 2007;85:867–872. doi: 10.2471/BLT.07.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Čelutkienė J., Pudil R., López-Fernández T., et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the heart failure association (HFA), the European association of cardiovascular imaging (EACVI) and the cardio-oncology council of the European society of cardiology (ESC) Eur J Heart Fail. 2020;22:1504–1524. doi: 10.1002/ejhf.1957. [DOI] [PubMed] [Google Scholar]
- 11.Pudil R., Mueller C., Čelutkienė J., et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the cardio-oncology study group of the heart failure association and the cardio-oncology council of the European society of cardiology. Eur J Heart Fail. 2020;22:1966–1983. doi: 10.1002/ejhf.2017. [DOI] [PubMed] [Google Scholar]
- 12.Lannering B., Rutkowski S., Doz F., et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol. 2012;30:3187–3193. doi: 10.1200/JCO.2011.39.8719. [DOI] [PubMed] [Google Scholar]
- 13.Taylor R.E., Howman A.J., Wheatley K., et al. Hyperfractionated accelerated radiotherapy (HART) with maintenance chemotherapy for metastatic (M1–3) medulloblastoma – a safety/feasibility study. Radiother Oncol. 2014;111:41–46. doi: 10.1016/j.radonc.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Packer R.J., Gajjar A., Vezina G., et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 15.Khanna A., Pequeno P., Gupta S., et al. Increased risk of all cardiovascular disease subtypes among childhood cancer survivors. Circulation. 2019;140:1041–1043. doi: 10.1161/CIRCULATIONAHA.119.041403. [DOI] [PubMed] [Google Scholar]
- 16.Lipshultz S.E., Sambatakos P., Maguire M., et al. Cardiotoxicity and cardioprotection in childhood cancer. Acta Haematol. 2014;132:391–399. doi: 10.1159/000360238. [DOI] [PubMed] [Google Scholar]
- 17.Millard N.E., De Braganca K.C. Medulloblastoma. J Child Neurol. 2016;31:1341–1353. doi: 10.1177/0883073815600866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lancellotti P., Suter T.M., López-Fernández T., et al. Cardio-oncology services: rationale, organization, and implementation. Eur Heart J. 2019;40:1756–1763. doi: 10.1093/eurheartj/ehy453. [DOI] [PubMed] [Google Scholar]
- 19.Loar R.W., Noel C.V., Tunuguntla H., Colquitt J.L., Pignatelli R.H. State of the art review: chemotherapy-induced cardiotoxicity in children. Congenit Heart Dis. 2018;13:5–15. doi: 10.1111/chd.12564. [DOI] [PubMed] [Google Scholar]
- 20.Hutchins K.K., Siddeek H., Franco V.I., Lipshultz S.E. Prevention of cardiotoxicity among survivors of childhood cancer. Br J Clin Pharmacol. 2017;83:455–465. doi: 10.1111/bcp.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong G.T., Kawashima T., Leisenring W., et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32:1218–1227. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemade H., Chaudhari U., Acharya A., et al. Cell death mechanisms of the anti-cancer drug etoposide on human cardiomyocytes isolated from pluripotent stem cells. Arch Toxicol. 2018;92:1507–1524. doi: 10.1007/s00204-018-2170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simbre V.C., Duffy S.A., Dadlani G.H., Miller T.L., Lipshultz S.E. Cardiotoxicity of cancer chemotherapy: implications for children. Pediatr Drugs. 2005;7:187–202. doi: 10.2165/00148581-200507030-00005. [DOI] [PubMed] [Google Scholar]
- 24.Khan S., Chen C.L., Brady M.S., et al. Unstable angina associated with cisplatin and carboplatin in a patient with advanced melanoma. J Clin Oncol. 2012;30:e163–e164. doi: 10.1200/JCO.2011.38.7852. [DOI] [PubMed] [Google Scholar]
- 25.Armenian S.H., Sun C.L., Shannon T., et al. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood. 2011;118:6023–6029. doi: 10.1182/blood-2011-06-358226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leger K.J., Cushing-Haugen K., Hansen J.A., et al. Clinical and Genetic determinants of cardiomyopathy risk among hematopoietic cell transplantation survivors. Biol Blood Marrow Transplant. 2016;22:1094–1101. doi: 10.1016/j.bbmt.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rotz S.J., Ryan T.D., Hlavaty J., George S.A., El-Bietar J., Dandoy C.E. Cardiotoxicity and cardiomyopathy in children and young adult survivors of hematopoietic stem cell transplant. Pediatr Blood Cancer. 2017;64:e26600. doi: 10.1002/pbc.26600. [DOI] [PubMed] [Google Scholar]