Abstract
Background
To investigate the role of adjuvant radiotherapy in patients with pancreatic cancer.
Methods and patients
The patients with pancreatic cancer from 18 registered institutions in the Surveillance Epidemiology and End Results (SEER) database were retrospectively analyzed. The characteristics of patients who would benefit from adjuvant radiotherapy were screened, as well as whether neoadjuvant or adjuvant radiotherapy conferred to a better clinical outcome. Propensity score matching was used to control for confounding features.
Results
Thirty thousand two hundred and forty-nine patients were included in this study (21,295 vs 8954 in surgery and adjuvant radiotherapy group); 1150 patients were matched in two groups. The median survivals in the surgery (S) group and adjuvant radiotherapy (S + R) group were 24 and 21 months, respectively. The 1-, 3-, and 5-year overall survival (OS) rates in the S group and S + R group were 68%, 40%, 31%, and 75%, 30%, 20%, respectively (p < 0.001), and the median OS was 22 and 25 months in S and S + R group after PSM, the former 1-, 2-, 3-, and 5-year OS were 73%, 45%, 30%, and 19%, and the later were 81%, 52%, 37%, and 24% (p = 0.0015), respectively; stratified analysis showed patients whose carcinoma located at pancreatic head with II stage infiltrating duct carcinoma (22 vs 25, p = 0.0276), T4 adenocarcinoma (28 vs 33, p = 0.0022), N1 stage adenocarcinoma (20 vs 23, p = 0.0203), and patients with infiltrating duct carcinoma received regional resection (23 vs 25, p = 0.028) and number of resected lymph node were ≥ 4 (22 vs 25, p = 0.009) had better OS after additional radiotherapy than surgery alone. Patients with pancreatic body/tail carcinoma III stage adenocarcinoma (13 vs, p = 0.0503) and T4 adenocarcinoma (14 vs, p = 0.0869) had survival advantage within 24 months for additional radiotherapy. However, patients with T2 stage adenocarcinoma located in pancreatic body/tail had better OS in surgery group than that in R + S group.
Conclusions
Additional radiotherapy may contribute to improved prognosis for patients with pancreatic head II stage infiltrating duct carcinoma, III stage adenocarcinoma, T4 stage carcinoma, N1 stage adenocarcinoma, regional resection, or number of lymphadenectomy ≥ 4 in infiltrating duct carcinoma. A specific subgroup of patients with specific stage and histological type pancreatic cancer should be considered for additional radiotherapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12094-021-02671-0.
Keywords: Pancreatic cancer, Prognosis, Surgery, Radiotherapy
Introduction
Pancreatic cancer is the seventh leading cause of cancer-related mortality, with an estimated 458,918 of new cases and 432,242 of deaths worldwide in 2018 [1]. It is predicted that pancreatic cancer will become the third cause of cancer-related death in the future [2]. Unfortunately, a multimodal therapy has not been able to improve pancreatic cancer patients’ prognosis. Surgery remains the most important treatment, but only 10–15% of patients are candidates for potentially curative resection, given that most patients would have atypical clinical symptoms and therefore present at the late advanced stage of the disease and not amenable for curative surgery. At present, the 5-year survival rate for pancreatic cancers is poor at less than 20%. Adjuvant radiotherapy and chemotherapy have been shown to improve the survival outcome of patients with pancreatic cancers, while neoadjuvant radiotherapy as well as intraoperative radiotherapy have also contributed to the improved prognosis. However, patient selection of suitability for additional radiotherapy or the optimal timing between the application of radiotherapy and surgery remains unclear.
To investigate the impact of adjuvant radiotherapy in patients with pancreatic cancer after surgical resection, we retrospectively analyzed patients from 18 registered institutions in the Surveillance Epidemiology and End Results (SEER) linked database. We aimed to determine the characteristics of patients that would benefit from adjuvant radiotherapy, and whether neoadjuvant or adjuvant radiotherapy conferred to a better clinical outcome.
Patients and methods
Data sources
The SEER*Stat software (version8.3.5) was used to retrieve data from the SEER database containing the SEER 18 registries (November 2018 submission). These registries covered approximately 90% of the US population (based on 201 censuses) from 1973 to 2016.
Patients
SEER patients were diagnosed pancreatic malignancies with site code C25.0-c25.9, and with the International Classification of Disease for Oncology, Third Edition (ICD-O-3), histological classification codes of 8000 and 9260 were included in our study. Data on diagnosis confirmation, American Joint Committee on Cancer (AJCC) 7th Editor stage, T, N, M stage, histological grade, primary site surgery, regional lymph-node surgery, the sequence of surgery and radiotherapy, chemotherapy, overall survival in months, and survival status were collected. Meanwhile, patient’s basic demographics including age, pancreatic cancer diagnosis, and race were also gathered. Patients were excluded if data on survival or any survival status were not available.
Patients who underwent surgery were compared with patients who received surgery and radiotherapy. To identify the characteristics of patients that would benefit from adjuvant radiotherapy, the association of additional radiotherapy on survival with varying tumor histology, AJCC stage, age, race, primary site, grade, surgery primary site, regional lymph-node surgery, and chemotherapy were analyzed. Covariates of interest included patient-related factors (age, gender, and race), disease-level factors (primary site, stage, grade, and histologic classification), and treatment-related factors (surgery methods, radiotherapy, the sequence between surgery and radiotherapy, chemotherapy, and diagnosis confirmation).
Statistics
The Fisher Exact Probability and ANOVA test were used to compare differences between categorical variables and continuous variables, respectively. The Cox proportional hazard regression model and Kaplan–Meier method were used to determine the effects of variables on OS and to visualize the survival curves, which were adjusted for other significant prognostic factors Propensity scores matched (PSM) were performed based on the 1:1 nearest-neighbor algorithm and logistic regression, with group (S, S + R) as the classified variable and other variables (age, gender, grade, primary site, ICD-0-3, AJCC stage, AJCC T/N/M stage, surgery T/N, and chemotherapy) as the matched variates. The cases with missing value of the paired variables were removed for good performance. All calculations were performed with SPSS version 23.0 for Windows software (IBM Corporation, Chicago, IL) and EmpowerStat software 2.0, GraphPad prism 7.0. Statistical significance was set at 0.05.
Results
A total of 243,417 patients with pancreatic malignancy were identified from the SEER data for the years of 1975–2016. Of these, 7864 patients did not have survival data, and 43,722 patients whose survival time was 0 month thus were excluded from our analysis. A total of 115,371 patients did not undergo surgery, and in 46,211 patients, surgical data were not available, and thus, these were also eliminated. Finally, 30,249 patients were included in our study. The selection of patients was shown in Figure S1. Four thousand four hundred and eighty-four patients without missing information during 2007–2016 underwent PRM and filtered 1150 pairs of patients for next analysis after PRM.
Baseline characteristics of patients
Thirty thousand two hundred and forty-nine patients were included in this study, 21,295 were in the surgery group (S group), and 8954 were in the combined surgery and radiotherapy group (S + R group). The demographics of the two groups were shown in Table S1. Patients in the S group were significantly older and more likely to be female than the S + R group. There were more tumors located at pancreatic body and tail in the S group, whereas a higher number of pancreatic head tumors were found in the S + R group. Most histological types of adenocarcinomas, carcinoma, and infiltrating duct carcinoma were identified in the S + R group, while there were more neuroendocrine neoplasms recorded in the S group. Patients with local-stage (AJCC 7th stage I, T0-2, N0, M0) disease underwent local excision (including lymph-node biopsy) in the S group and patients in the S + R group were mostly with regional-stage (AJCC 7th stage II, T 3–4, N1) and received regional surgery as well as chemotherapy. There were no significant differences in follow time and histologic grade between the two groups. More patients were still alive in the S group at the last follow-up. Age, AJCC 7th stage, and T/N stage were significant difference after PSM, and other features were identical, as shown in Table S1.
Analysis of survival and risk factor
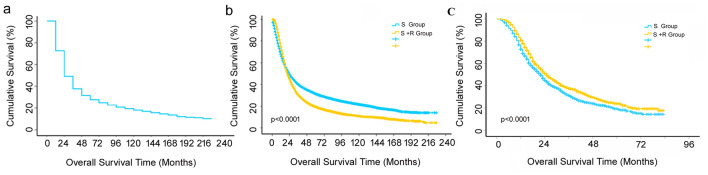
The median OS of all patients was 23 months, with the 1-, 3-, 5-year survival rate of 49%, 32%, and 25%, respectively. The median OSs in the S and S + R group were 24 and 21 months, respectively, as demonstrated in Fig. 1a. The 1-, 2-, 3-, and 5-year survival rates in the S group were 68%, 49%, 40%, and 31%, respectively, while the 1-, 2-, and 3-, 5-year survival rates in the S + R group were 75%, 45%, 30%, and 20% respectively; the differences in the two groups were statistically significance (p < 0.001), given that the 1-year OS appeared superior in the R + S group, whereas the 2-, 3-, and 5-year OSs were better in the S group, as demonstrated in Fig. 1b. However, the median OS was 22 and 25 months in S and S + R group after PSM, the former 1-, 2-, 3-, and 5-year OS were 73%, 45%, 30%, and 19%, and the later were 81%, 52%, 37%, and 24% (p = 0.0015), respectively; it was shown in Fig. 1c.
Fig. 1.
a Cumulative overall survival (OS) curves of all patients with pancreatic cancer; b OS curves of patients undergoing surgery alone (S) or surgery combined with radiotherapy (S + R). The log-rank test showed that the OS was significantly different between the two groups (p < 0.0001). S: surgery, S + R: surgery plus radiotherapy; c OS curves of patients undergoing surgery alone (S) or surgery combined with radiotherapy (S + R) after propensity score matching (PSM). The log-rank test showed that the OS was significantly different between the two groups (p < 0.0001). S: surgery, S + R: surgery plus radiotherapy
The univariate and multivariate analysis of the entire cohort showed that factors including age, gender, race, diagnosis year, primary site, ICD-0-3 classification, AJCC 7th stage, T, N, surgery primary tumor site (surgery T), surgery regional lymph node (surgery N), additional radiotherapy and chemotherapy were related to OS, as shown in Table S2. Factors such as patients in the R + S group (HR 0.88, 95% CI 0.85–0.91), additional chemotherapy (HR 0.82, 95% CI 0.79–0.85), diagnosis year between 1999 and 2006 (HR 0.88, 95% CI 0.80–0.98) and 2007–2016 (HR 0.78, 95% CI 0.69–0.87), pancreatic duct tumor (HR 0.99, 95% CI 0.96–1.03), neuroendocrine neoplasms (HR 0.17, 95% CI 0.16–0.19), mesenchymal tumor (HR 0.46, 95% CI 0.35–0.60), mucinous adenocarcinoma (HR 0.68, 95% CI 0.54–0.62), carcinoma (HR 0.48, 95% CI 0.43–0.53), and other histologic type (HR 0.56, 95% CI 0.52–0.60), and number of dissected lymph node with 1–3 (HR 0.91, 95% CI 0.84–0.98), and more than 4 (HR 0.81, 95% CI 0.76–0.87) were associated with lower risk of mortality. On the other hand, factors as independent predictors of higher mortality were age at 20–44 (HR 4.54, 95% CI 2.35–8.79), 45–64 (HR 6.31, 95% CI 3.27–12.17), 65–84 (HR 7.86, 95% CI 4.08–15.13), tumor overlapping lesion of pancreas (HR 1.1, 95% CI 1.02–1.17), adenosquamous carcinoma and squamous (HR 1.21, 95% CI 1.07–1.36), AJCC 7th stage II (HR 1.31, 1.15–1.5), III (1.73, 1.31–2.28), IV (2.53, 2.17–2.95) T2 (HR 1.49, 95% CI 1.31–1.71), T3 (HR 1.70, 95% CI 1.47–1.96), T4 (HR 1.67, 95% CI 1.28–2.18), N1 (HR 1.48, 95% CI 1.40–1.56), and resection expect for local removal. However, AJCC 7th T2 (HR 2.09, 95% CI 1.18–3.69), T3 (HR 2.58, 95% CI 1.45–4.56), T4 (HR 2.74, 95% CI 1.04–7.23), and N1 (HR 1.55, 95% CI 1.35–1.77) were correlated with adverse outcome, number of lymph-node dissection ≥ 4 (HR 0.70, 95% CI 0.52–0.94), and additional radiotherapy (HR 0.89, 95% CI 0.80–0.99) and chemotherapy (HR 0.54, 95% CI 0.40–0.72) were independent favorable prognostic factors experiencing PSM, as seen in Table S2. Histological grade was not associated with survive regardless of PSM.
Stratification analysis by subgroup population
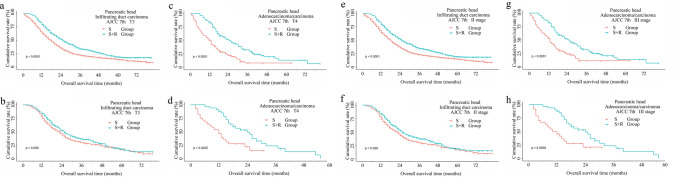
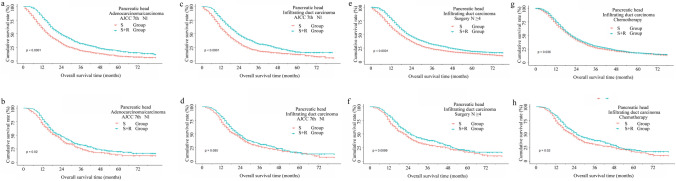
To further investigate the optimum sub-population benefited from the additional radiotherapy, we carried out analysis in the data stratified by combination among primary site, histologic type, and other clinicopathologic features. In this sub-stratified evaluation, other tumor sites and histologic behavior were excluded due to small sample size, as well as definitive survival benefits from surgery alone and reverse risk for survival, as shown in Table S3 and Table S4. For patients with pancreatic head tumors, we found patients with stage T1 disease, T2 infiltrating duct carcinomas, lymphadenectomy < 4 with infiltrating duct carcinomas, without chemotherapy of adenosquamous/squamous showed no OS benefit from radiotherapy. Other patients with pancreatic head cancer had an improved survival following additional radiotherapy, as demonstrated in Table S3 and Figs. 2, 3. However, adjuvant radiotherapy improved the OS in subgroup included patients with AJCC 7th II stage infiltrating duct carcinoma (22 vs 25, p = 0.0276), T4 (12 vs 24, p = 0.0022), and N1(20 vs 23, p = 0.0203) adenocarcinoma/carcinoma (12 vs 24, p = 0.0022), infiltrating duct carcinoma received regional resection (23 vs 25, p = 0.028) (Fig. 4a, b), more than four lymph-node dissections (22 vs 25, p = 0.009), and chemotherapy (23 vs 25, p = 0.0202) after PSM (Fig. 3). Interestingly, we noticed three sub-population (AJCC 7th III stage adenocarcinoma, AJCC 7th T3 or N1 infiltrating duct carcinoma) had survival improvement when OS cutoff was 24 months (Fig. 3).
Fig. 2.
Cumulative overall survival (OS) curves of patients with pancreatic head cancer with sub-stratification analysis in surgery alone group and surgery plus radiotherapy group with a log-rank test, which demonstrated a sub-population of patients that benefited from additional radiotherapy. Top row (a, c, e, g), before matching. Bottom row (b, d, f, h), after matching
Fig. 3.
Cumulative overall survival (OS) curves of patients with pancreatic head cancer with sub-stratification analysis in surgery alone group and surgery plus radiotherapy group with a log-rank test, which demonstrated a sub-population of patients that benefited from additional radiotherapy. Top row (a, c, e, g), before matching. Bottom row (b, d, f, h), after matching
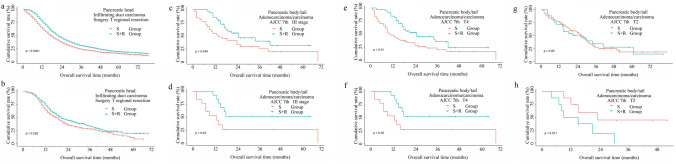
Fig. 4.
Cumulative overall survival (OS) curves of patients with pancreatic body/tail adenocarcinoma/carcinoma or infiltrating duct carcinoma, the sub-stratification analysis in surgery alone group, and surgery plus radiotherapy group with a log-rank test, which demonstrated a sub-population of patients that benefited from additional radiotherapy. Top row (a, c, e, g), before matching. Bottom row (b, d, f, h), after matching
Meanwhile, stratified analysis was performed in patients with pancreatic body/tail before PSM. The beneficiaries form additional radiotherapy were less than those lesions located at pancreatic head, as displayed in Table S4. First, radiotherapy was helpful in OS for patients who had AJCC 7th I, II, III, T3, T4, N1 stage adenocarcinoma/carcinoma and infiltrating duct carcinoma. Second, surgical resection of primary tumor might improve OS, it demonstrated local resection followed radiotherapy made patients with adenocarcinoma/carcinoma and infiltrating duct carcinoma have better OS, and total pancreatectomy combined radiotherapy improved OS of cases with infiltrating duct carcinoma. On the other hand, the patient with adenocarcinoma/carcinoma and infiltrating duct carcinoma whose number of lymph nodes dissected ≥ 4 and patients with infiltrating duct carcinoma were removed 1–3 lymph nodes received radiotherapy had better prognosis. Finally, a survival advantage remained with chemotherapy for adenocarcinoma/carcinoma, as shown in Table S4. After PSM, patients with AJCC 7th III, T4 adenocarcinoma/carcinoma had survival advantage within 24 months from additional radiotherapy, as shown in Table S4 and Fig. 4. However, patients with T2 adenocarcinoma located in pancreatic body/tail had better OS in surgery alone than that in R + S group, as displayed in Table S4 and Fig. 4.
The sequence of surgery and radiotherapy and survival, radio source
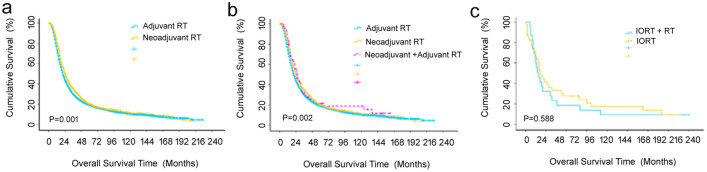
Following the identification of a specific patient population that benefited from the additional radiotherapy in addition to surgery, the next concern involving the sequence between surgery and radiotherapy was addressed, as demonstrated in Table S5. A total of 7664 and 1099 patients received adjuvant and neoadjuvant radiotherapy, respectively. The median survival was 21 months (95% CI 20–22) vs 25 months (95% CI 23–27), while the 1-, 3-, and 5-year OS were 74%, 29%, and 20% vs 81%, 35%, and 20% in the adjuvant and neoadjuvant populations, respectively, for which the differences were statistically significant (p = 0.001), as shown in Fig. 5a. There were 76 patients that received radiotherapy both before and after surgery, with the median survival of 26 months (95% CI 20–32) and the 1-, 3-, and 5-year OS of 85%, 32%, and 19%, respectively, which were significantly better than those having either radiotherapy before or after surgery only (p = 0.002), as shown in Fig. 5b. Finally, a small proportion of patients had intraoperative radiotherapy with or without further adjuvant radiotherapy, for which the survival difference between these two subgroups was not statistically significant (p = 0.588), as shown in Fig. 5c.
Fig. 5.
Cumulative overall survival (OS) curves of patients with pancreatic cancer, and the sub-stratification analysis of sequence between radiotherapy and surgery with a log-rank test. a Adjuvant and neoadjuvant radiotherapy (p = 0.001); b adjuvant, neoadjuvant, and both; c intraoperative radiotherapy plus radiotherapy and intraoperative therapy alone (p = 0.588)
Discussion
Adjuvant chemotherapy or radiotherapy in combination with surgery has been the standard of treatment for pancreatic cancer for decades [3]. External beam radiotherapy is one of the most important treatment as it contributes to better survival [3–16]. In this study, we determined the potential patient population who might be benefited from having additional radiotherapy. Our primary survival analysis indicated that the benefit from additional radiotherapy was critically time-dependent, and if extending the time window of more than 24 months, the treatment benefit from extension could be reverse. However, the multivariate analysis showed that additional radiotherapy was an independent prognostic factor for OS (HR 0.89, 95% CI 0.86–0.92, p < 0.0001). Moreover, earlier studies have demonstrated that patients treated with radiotherapy had significantly better survival than those without [7, 8, 11–21]. The conflicting results we observed might be attributed to the differences in the baseline characteristics of patients as a result of retrospective selection bias. Patients who had received additional radiotherapy were of a poor prognostic group with several high-risk factors, including advanced stage, anatomic site, and unfavorable histologic classification [22–29]. Despite these, our study showed that patients with the above unfavorable features could still benefit from additional radiotherapy.
Pancreatic head has been shown to be the most common anatomical site for cancer followed by the pancreatic body and tail, with the latter displaying poorer survival than pancreatic head lesions [29]. Adenosquamous carcinoma of the pancreas has a higher prevalence in the pancreatic tail and associated with poor survival [22]. In our study, the sample size of adenosquamous/squamous carcinoma was small and the patients with pancreatic body/tail cancer did not appear to benefit from additional radiotherapy. Patients with pancreatic neuroendocrine cancer have a better life expectancy [24, 25], and resection of the primary tumor is associated with longer survival in stages I–III as well as stage IV tumors [26]. Therefore, representing as the third most common tumor type, patients with pancreatic neuroendocrine cancer in our study had extremely good survival prognosis after surgical resection. A similarly favorable survival outcome was observed in patients with mesenchymal tumors or mucinous adenocarcinoma.
To date, pancreatic adenocarcinoma/carcinoma and infiltrating duct carcinoma are still the most difficult to treat. In addition, small pancreatic cancers have been associated with poorer survival prognosis and an extremely high rate of nodal involvement, for which they should not be regarded as early-stage cancers [30]. Similar findings were observed in our study, in which additional radiotherapy significantly contributed to improving survival in patients with stage II infiltrating duct carcinoma located in pancreatic body/tail. However, based on the AJCC 7th classification, stage I/II disease indicates a T1/T2 tumor that is localized within the pancreas. Our data showed that additional radiotherapy for patients with T2 adenosarcoma in the pancreatic body/tail had had adverse survival benefit within 2 years. In the current National Comprehensive Cancer Network (NCCN) guideline (https://www.nccn.org), an early-stage pancreatic carcinoma refers to a resectable tumor. Neoadjuvant therapy can be considered in a clinical trial or with chemotherapy alone, or induction chemotherapy followed by chemotherapy, especially in the presence of high-risk factors. In addition, chemoradiation should not be given to patients after surgery except in clinical trials [31]. Importantly, most clinical trials have been focusing on comparing chemotherapy with or without chemoradiotherapy, and a few trials have examined the effectiveness of radiotherapy alone. Our study demonstrated that patients with stage II or T2 disease had an improved survival from additional radiotherapy, suggesting that those sub-population patients especially with high-risk features and did not receive neoadjuvant treatment could be considered for adjuvant radiotherapy. The AJCC 8th stage system was prior to current stage for involved in clear T stage, [32] and future prospective clinical study should be designed to take into account patients with diseases of early stages.
Our study demonstrated that locally advanced (T3/4, N+) pancreatic adenocarcinoma/carcinoma or infiltrating duct carcinoma had improved survival prognosis following additional radiotherapy, with particular tumor location. Several studies have found adjuvant treatment after surgery contributed to improved survival in advanced pancreatic cancer [3–7, 11, 13, 33, 34]. Other clinical trials have confirmed neoadjuvant treatment was prior to adjuvant treatment [3, 5, 8, 14, 21, 35]; in fact, most patients preferentially received resection; more than 80% of patients were in the station in current study (Table S5). Surprisingly, our study showed that patients who benefited from radiotherapy were those who received chemotherapy, and this was in keeping with the results of previous studies which demonstrated that chemoradiotherapy or chemotherapy sequencing with radiotherapy was beneficial for survival improvement. An increased number of lymph-node dissection have been shown to be a poor prognostic factor for OS [36], with higher lymph-node retrieval associated with a higher proportion of positive invasion. Our study showed that patients with more than four lymph-node dissections in patients with infiltrating duct carcinoma had a favorable survival when treated with additional radiotherapy. Therefore, radiotherapy can improve cancer survival compared with cancer-directed surgery without radiation in patients with infiltrating duct adenocarcinoma of the pancreas. Patients with advanced disease regardless of tumor location should be considered for radiotherapy and chemotherapy.
Conclusions
Patients with pancreatic cancer of specific histological types (adenocarcinoma/carcinoma, infiltrating duct carcinoma, adenosquamous carcinoma, and squamous), anatomical location, and advanced stage have poor survival, and therefore, additional radiotherapy may contribute to an improved survival prognosis. A specific subgroup of patients with AJCC 7th stage II or T3 infiltrating duct carcinoma and N1 adenocarcinoma located pancreatic head had survival benefit from additional radiotherapy. Patients with adenocarcinoma/carcinoma stages AJCC 7th III, T4 whose lesions located pancreatic body/tail should be considered for additional radiotherapy. Our findings highlight the positive role of radiotherapy in highly selected patients with pancreatic malignancy, suggesting the need for future prospective randomized control study to confirm these findings before recommending such treatment to patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank NIH for its dataset during the preparation of this manuscript.
Abbreviations
- HCC
Hepatocellular carcinoma
- OS
Overall survival
- SEER
Surveillance epidemiology and end results
- MS
Median survival
- S
Surgery
- R
Radiotherapy
- ICD-3
International classification of disease for oncology, third edition
- AJCC
American Joint Committee on Cancer
- HR
Hazard rate
- CI
Confidence interval
Funding
Not applicable.
Data availability
Original data are available at Surveillance Epidemiology and End Results (SEER) database. (https://seer.cancer.gov/data-software/documentation/seerstat/).
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval and consent to participate
The study had been ethics approved for original institution.
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Footnotes
The original online version of this article was revised to correct the article title to ‘The role of radiotherapy for pancreatic malignancies: a population‑based analysis of the SEER database’ and to remove the term 'Medicare' from the text.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/24/2021
A Correction to this paper has been published: 10.1007/s12094-021-02691-w
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Partensky C, Bray F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. 2016;55:1158–1160. doi: 10.1080/0284186X.2016.1197419. [DOI] [PubMed] [Google Scholar]
- 3.Neuzillet C, Gaujoux S, Williet N, Bachet J-B, Bauguion L, Colson Durand L, et al. Pancreatic cancer: French clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, AFC) Dig Liver Dis. 2018;50:1257–1271. doi: 10.1016/j.dld.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Van Laethem J-L, Hammel P, Mornex F, Azria D, Van Tienhoven G, Vergauwe P, et al. Adjuvant gemcitabine alone versus gemcitabine-based chemoradiotherapy after curative resection for pancreatic cancer: a randomized EORTC-40013-22012/FFCD-9203/GERCOR phase II study. J Clin Oncol. 2010;28:4450–4456. doi: 10.1200/JCO.2010.30.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moureau-Zabotto L, Phélip J-M, Afchain P, Mineur L, André T, Vendrely V, et al. Concomitant administration of weekly oxaliplatin, fluorouracil continuous infusion, and radiotherapy after 2 months of gemcitabine and oxaliplatin induction in patients with locally advanced pancreatic cancer: a groupe coordinateur multidisciplinaire en oncologie phase II study. J Clin Oncol. 2008;26:1080–1085. doi: 10.1200/JCO.2007.12.8223. [DOI] [PubMed] [Google Scholar]
- 6.Sugawara A, Kunieda E. Effect of adjuvant radiotherapy on survival in resected pancreatic cancer: a propensity score surveillance, epidemiology, and end results database analysis. J Surg Oncol. 2014;110:960–966. doi: 10.1002/jso.23752. [DOI] [PubMed] [Google Scholar]
- 7.Hammel P, Huguet F, van Laethem J-L, Goldstein D, Glimelius B, Artru P, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315:1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 8.Dobiasch S, Goerig NL, Fietkau R, Combs SE. Essential role of radiation therapy for the treatment of pancreatic cancer: novel study concepts and established treatment recommendations. Strahlenther Onkol. 2018;194:185–195. doi: 10.1007/s00066-017-1227-5. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan S, Chadha AS, Suh Y, Chen H-C, Rao A, Das P, et al. Focal radiation therapy dose escalation improves overall survival in locally advanced pancreatic cancer patients receiving induction chemotherapy and consolidative chemoradiation. Int J Radiat Oncol Biol Phys. 2016;94:755–765. doi: 10.1016/j.ijrobp.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louvet C, André T, Lledo G, Hammel P, Bleiberg H, Bouleuc C, et al. Gemcitabine combined with oxaliplatin in advanced pancreatic adenocarcinoma: final results of a GERCOR multicenter phase II study. J Clin Oncol. 2002;20:1512–1518. doi: 10.1200/JCO.2002.20.6.1512. [DOI] [PubMed] [Google Scholar]
- 11.Takano N, Yamada S, Hirakawa A, Yokoyama Y, Kawashima H, Maeda O, et al. Phase II study of chemoradiotherapy combined with gemcitabine plus nab-paclitaxel for unresectable locally advanced pancreatic ductal adenocarcinoma (NUPAT 05 Trial): study protocol for a single arm phase II study. Nagoya J Med Sci. 2019;81:233–239. doi: 10.18999/nagjms.81.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas PR. Radiotherapy for carcinoma of the pancreas. Semin Oncol. 1996;23:213–219. [PubMed] [Google Scholar]
- 13.Lim YJ, Kim K, Chie EK, Kim B, Ha SW. Role of adjuvant radiotherapy in left-sided pancreatic cancer-population-based analysis with propensity score matching. J Gastrointest Surg. 2015;19:2183–2191. doi: 10.1007/s11605-015-2941-x. [DOI] [PubMed] [Google Scholar]
- 14.Landau E, Kalnicki S. The evolving role of radiation in pancreatic cancer. Surg Clin North Am. 2018;98:113–125. doi: 10.1016/j.suc.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Sajjad M, Batra S, Hoffe S, Kim R, Springett G, Mahipal A. Use of radiation therapy in locally advanced pancreatic cancer improves survival: a SEER database analysis. Am J Clin Oncol. 2018;41:236–241. doi: 10.1097/COC.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 16.Johung K, Saif MW, Chang BW. Treatment of locally advanced pancreatic cancer: the role of radiation therapy. Int J Radiat Oncol Biol Phys. 2012;82:508–518. doi: 10.1016/j.ijrobp.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Badiyan SN, Molitoris JK, Chuong MD, Regine WF, Kaiser A. The role of radiation therapy for pancreatic cancer in the adjuvant and neoadjuvant settings. Surg Oncol Clin N Am. 2017;26:431–453. doi: 10.1016/j.soc.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Huguet F, Goodman KA, Azria D, Racadot S, Abrams RA. Radiotherapy technical considerations in the management of locally advanced pancreatic cancer: American-French consensus recommendations. Int J Radiat Oncol Biol Phys. 2012;83:1355–1364. doi: 10.1016/j.ijrobp.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 19.Hazard L, Tward JD, Szabo A, Shrieve DC. Radiation therapy is associated with improved survival in patients with pancreatic adenocarcinoma: results of a study from the surveillance, epidemiology, and end results (SEER) registry data. Cancer. 2007;110:2191–2201. doi: 10.1002/cncr.23047. [DOI] [PubMed] [Google Scholar]
- 20.Hall WA, Goodman KA. Radiation therapy for pancreatic adenocarcinoma, a treatment option that must be considered in the management of a devastating malignancy. Radiat Oncol. 2019;14:114. doi: 10.1186/s13014-019-1277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stessin AM, Meyer JE, Sherr DL. Neoadjuvant radiation is associated with improved survival in patients with resectable pancreatic cancer: an analysis of data from the surveillance, epidemiology, and end results (SEER) registry. Int J Radiat Oncol Biol Phys. 2008;72:1128–1133. doi: 10.1016/j.ijrobp.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 22.Boyd CA, Benarroch-Gampel J, Sheffield KM, Cooksley CD, Riall TS. 415 patients with adenosquamous carcinoma of the pancreas: a population-based analysis of prognosis and survival. J Surg Res. 2012;174:12–19. doi: 10.1016/j.jss.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song Y, Chen Z, Chen L, He C, Huang X, Duan F, et al. A refined staging model for resectable pancreatic ductal adenocarcinoma incorporating examined lymph nodes, location of tumor and positive lymph nodes ratio. J Cancer. 2018;9:3507–3514. doi: 10.7150/jca.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fesinmeyer MD, Austin MA, Li CI, De Roos AJ, Bowen DJ. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1766–1773. doi: 10.1158/1055-9965.EPI-05-0120. [DOI] [PubMed] [Google Scholar]
- 25.Brooks JC, Shavelle RM, Vavra-Musser KN. Life expectancy in pancreatic neuroendocrine cancer. Clin Res Hepatol Gastroenterol. 2019;43:88–97. doi: 10.1016/j.clinre.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Keutgen XM, Nilubol N, Kebebew E. Malignant-functioning neuroendocrine tumors of the pancreas: a survival analysis. Surgery. 2016;159:1382–1389. doi: 10.1016/j.surg.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakashima A, et al. Number of metastatic lymph nodes, but not lymph node ratio, is an independent prognostic factor after resection of pancreatic carcinoma. J Am Coll Surg. 2010;211:196–204. doi: 10.1016/j.jamcollsurg.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 28.Makarova-Rusher OV, Ulahannan S, Greten TF, Duffy A. Pancreatic squamous cell carcinoma: a population-based study of epidemiology, clinicopathologic characteristics and outcomes. Pancreas. 2016;45:1432–1437. doi: 10.1097/MPA.0000000000000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Artinyan A, Soriano PA, Prendergast C, Low T, Ellenhorn JDI, Kim J. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB (Oxford) 2008;10:371–376. doi: 10.1080/13651820802291233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franko J, Hugec V, Lopes TL, Goldman CD. Survival among pancreaticoduodenectomy patients treated for pancreatic head cancer <1 or 2 cm. Ann Surg Oncol. 2013;20:357–361. doi: 10.1245/s10434-012-2621-y. [DOI] [PubMed] [Google Scholar]
- 31.Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:56–68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 32.Kamarajah SK, Burns WR, Frankel TL, Cho CS, Nathan H. Validation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with pancreatic adenocarcinoma: a surveillance, epidemiology and end results (SEER) analysis. Ann Surg Oncol. 2017;24:2023–2030. doi: 10.1245/s10434-017-5810-x. [DOI] [PubMed] [Google Scholar]
- 33.Gastrointestinal Tumor Study Group Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006–2010. doi: 10.1002/1097-0142(19870615)59:12<2006::AID-CNCR2820591206>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 34.Huguet F, André T, Hammel P, Artru P, Balosso J, Selle F, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 35.Vera R, Dotor E, Feliu J, González E, Laquente B, Macarulla T, et al. SEOM clinical guideline for the treatment of pancreatic cancer (2016) Clin Transl Oncol. 2016;18:1172–1178. doi: 10.1007/s12094-016-1586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He C, Zhang Y, Cai Z, Lin X, Li S. Overall survival and cancer-specific survival in patients with surgically resected pancreatic head adenocarcinoma: a competing risk nomogram analysis. J Cancer. 2018;9:3156–3167. doi: 10.7150/jca.25494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original data are available at Surveillance Epidemiology and End Results (SEER) database. (https://seer.cancer.gov/data-software/documentation/seerstat/).