Abstract
Breast cancers often exhibit elevated expression of tyrosine kinase growth factor receptors; these pathways influence breast cancer cell growth in part by targeting steroid hormone receptors, including progesterone receptors (PR). To mimic activation of molecules downstream of growth factor-initiated signaling pathways, we overexpressed mitogen-activated protein kinase (MAPK; also known as extracellular signal-regulated kinase) kinase kinase 1 (MEKK1) in T47D human breast cancer cells expressing the B isoform of PR. MEKK1 is a strong activator of p42 and p44 MAPKs. MEKK1 expression increased progestin-mediated transcription 8- to 10-fold above normal PR-driven transcription levels. This was dependent on the presence of a progesterone response element and functional PR. PR protein levels were unchanged by MEKK1 alone but were extensively down-regulated by MEKK1 plus the progestin R5020. MEKK1 expression resulted in phosphorylation of PR on Ser294, a MAPK consensus site known to mediate ligand-dependent PR degradation. MEK inhibitors blocked phosphorylation of Ser294 and attenuated PR transcriptional hyperactivity in response to MEKK1 plus R5020; stabilization of PR by inhibition of the 26S proteasome produced similar results. T47D cells stably expressing mutant S294A PR, in which serine 294 is replaced by alanine, fail to undergo ligand-dependent down-regulation and are resistant to MEKK1-plus-R5020-induced transcriptional synergy but respond to progestins alone. Similarly, c-myc protein levels are synergistically increased by epidermal growth factor and R5020 in cells expressing wild-type PR, but not S294A PR. Thus, highly stable mutant PR are functional in response to progestins but are incapable of cross talk with MAPK-driven pathways. These studies demonstrate a paradoxical coupling between steroid receptor down-regulation and transcriptional hyperactivity. They also suggest a link between phosphorylation of PR by MAPKs in response to peptide growth factor signaling and steroid hormone control of breast cancer cell growth.
Many solid tumors, including breast cancers, exhibit elevated mitogen-activated protein kinase (MAPK) expression and/or activities (13, 40), presumably due to increased expression of growth factor receptors that couple to MAPK activation. Overexpression of type I tyrosine kinase growth factor receptors in the epidermal growth factor (EGF) receptor/c-ErbB family is believed to contribute to proliferative signaling in breast cancer and to be indicative of a poor prognosis. Analogous to other members of the steroid receptor superfamily, human estrogen receptors (ER) and progesterone receptors (PR) are highly phosphorylated and therefore sensitive to growth factor-initiated signaling pathways. Indeed, the same phosphorylation sites on ER and/or PR can be regulated in response to steroid hormone or growth factor treatment of cells (reviewed in references 18 and 49). Although the role of direct phosphorylation of steroid hormone receptors and the exact kinase-signaling pathways involved remain largely undefined, phosphorylation is influenced by ligand binding and may affect both ligand-dependent and -independent receptor functions and/or interactions with coregulatory molecules (reviewed in references 44 and 47).
A variety of agents, including EGF, can activate unliganded ER (5). Furthermore, ligand-dependent ER transcriptional activity is enhanced by activated Ras and/or growth factors like EGF (1), insulin-like growth factor (16), and mitogen-activated protein (also known as extracellular signal-regulated protein kinase) kinase kinase kinase 1 (MEKK1) (23) that feed into activation of MAPK pathways. In contrast, activation of human PR appears to be entirely ligand dependent, although examples of ligand-independent activity have been reported (3). Additionally, growth factors greatly influence PR signaling in the presence of progestins (4, 11, 19, 20, 32, 38). Activation of cyclic AMP-dependent protein kinase by 8-Br–cyclic AMP produces synergy with PR agonists on progesterone response element (PRE)-regulated promoters and converts the PR antagonists, RU486 and ZK112993, to transcriptional agonists (37, 38). Transcriptional synergy between progestins and EGF occurs at several promoters, including those regulating the mouse mammary tumor virus (12), p21WAF1, and c-fos genes (32). EGF and progestins up-regulate cyclin D1, cyclin E, and p21WAF1 protein levels (11) in a MAPK-dependent manner in T47D human breast cancer cells (20).
Several endogenously regulated phosphorylation sites on human PR have been well characterized (reviewed in references 44 and 47). For example, Ser400 is both basally phosphorylated and regulated by ligand in vivo; Ser400 phosphorylation is mediated by cyclin-dependent protein kinase 2 in vitro (50). Two MAPK phosphorylation sites, Ser294 and Ser345, are predominantly phosphorylated after treatment of cells with progestins (51). These residues reside within an inhibitory functional domain of the PR N terminus (14); the contribution of either of these sites to repression is unknown. However, we recently found that Ser294 plays an essential role in PR protein turnover (21). In the presence of ligand, Ser294 phosphorylation by MAPK leads to rapid PR degradation by the ubiquitin-proteasome pathway (21). Inhibition of the 26S proteasome by lactacystin, inhibition of MAPKs by MEK inhibitors, or mutation of Ser294 to alanine stabilized PR in the presence of ligand and prevented the formation of ubiquitinylated PR species. ER are also substrates for the ubiquitin-proteasome pathway, although the role of phosphorylation in this process, if any, remains undefined (29). Lonard et al. (24) recently found that stabilization of ERα by 26S proteasome inhibitors blocks ER transcriptional activity. Mutational analysis of ER demonstrated that protein interactions with ERα coactivator-binding surfaces are also important for ligand-dependent receptor down-regulation, suggesting that turnover of ER and its coactivators may be functionally coupled to ER transcriptional activity. These interactions are likely to be affected by phosphorylation (21; for a review, see reference 49).
Herein, we examine the relationship between ligand-induced PR turnover and transcriptional activity in response to activation of MAPKs by expression of constitutively active MEKK1. We show that MEKK1 both enhances PR transcriptional activation by progestins and augments ligand-dependent PR down-regulation. Stabilization of PR by ubiquitin pathway inhibitors, MEK inhibitors, or mutation of Ser294 blocks MEKK1-induced transcriptional hyperactivation. Thus, phosphorylation of Ser294 by MAPK may induce maximal transcription in the presence of progesterone by coupling this activity to efficient PR turnover.
MATERIALS AND METHODS
Cell lines and reagents.
Monoclonal T47D-YB human breast cancer cells engineered to stably express PR-B were previously described (37). Monoclonal T47D-YB-S294A cells (S294A) containing mutant PR-B with serine 294 replaced by alanine were engineered by stable transfection of mutant S294A PR-B into PR-negative T47D-Y cells as previously described (21). T47D-YB and S294A cells were routinely seeded at 106 cells/dish, cultured in 10-cm dishes, and incubated in 5% CO2 at 37°C in a humidified environment as described (37). Stably transfected cells were also maintained in 700-μg/ml neomycin analog G418 (Life Technologies, Gaithersburg, Md.). HeLa cells were seeded at 350,000 cells/dish. For experiments involving steroid hormone (R5020; 10 nM) or EGF (30 ng/ml) treatments or MAPK assays, cultures were placed in serum-free media for 18 to 24 h prior to addition of growth factor. Phospho-specific and total p42 and p44 MAPK antibodies, phospho-specifc p38 MAPK antibodies, phospho-specific Jun N-terminal kinase (JNK) antibodies, and the MEK1 inhibitor (PD98059) were purchased from New England Biolabs (Beverly, Mass.). The p38 MAPK inhibitor (SB203580) and the MEK1-MEK2 inhibitor (U0126) were purchased from Upstate Biotechnology (Lake Placid, N.Y.). Horseradish peroxidase-conjugated secondary antibodies were obtained from Collaborative Biomedical Products, Inc. (Bedford, Mass.). R5020 was obtained from NEN Life Sciences Products, Inc. (Boston, Mass.). Lactacystin and calpain inhibitor I (N-acetyl-Leu-Leu-norleucinal [LLnL]) were purchased from Calbiochem (La Jolla, Calif.). The anti-PR monoclonal antibodies, AB-52 and B-30, were produced in the K. B. Horwitz laboratory (9). c-Myc antibodies were a kind gift of S. R. Hann (42). Phospho-294-specific PR antibodies were a gift of D. P. Edwards (6).
Immunoblotting.
For detection of PR-B or MAPK family kinases in whole-cell lysates, cells growing in 10-cm dishes were washed twice in 4 ml of phosphate-buffered saline and lysed by scraping in extraction buffer (1% [vol/vol]) Triton X-100, 10 mM Tris-HCl [pH 7.4], 5 mM EDTA, 50 mM NaCl, 50 mM NaF, aprotinin [20 μg/ml], 1 mM phenylmethylsulfonyl fluoride, and 2 mM Na3VO4). Lysates were clarified by centrifugation for 10 min at maximum speed in a refrigerated microfuge. Soluble proteins in clarified lysates were quantified by the method of Bradford (Gibco BRL), and equal amounts of protein were resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (10% acrylamide for MAPKs; 7.5% acrylamide for PR) and detected by immunoblotting. For detection of c-myc proteins, cells were washed as above and lysed in 1× Laemmli sample buffer (60 mM Tris [pH 6.8], 5% [vol/vol] β-mercaptoethanol, 10% [vol/vol] glycerol, and 0.01% [wt/vol] bromophenol blue). Lysates were passed through a 27-gauge needle five times to shear DNA prior to loading equal volumes onto each gel lane. Alternatively, cells were lysed in RIPA buffer (10 mM sodium phosphate [pH 7.0], 150 mM NaCl, 2 mM EDTA, 1% [wt/vol] Nonidet P-40, 0.1% [wt/vol] SDS, 1% sodium deoxycholate, 20 μg of aprotinin/ml, 50 mM sodium flouride, 200 μM Na3VO4, 0.1% [vol/vol] β-mercaptoethanol, and 1 mM phenlymethylsulfonyl fluoride); protein was quantified as above; and equal amounts of protein were resolved by SDS-polyacrylamide gel electrophoresis, transferred onto nitrocellulose filters, and immunoblotted with specific antibodies.
Transient transfections and luciferase assays.
T47D-YB or S294A cells plated at 500,000 cells/10-cm dish or HeLa cells plated at 350,000 cells/10-cm dish were transiently transfected with 1 μg of pCMV5 control vector or MEKK1 (in pCMV5) or with 25 ng of pSG5 control vector or PR-B (in pSG5), 3 μg of PRE 2× TATA in the luciferase reporter plasmid pA3-LUC, 3 μg of the β-galactosidase expression plasmid pCH110 (Amersham Pharmacia Biotech) to check transfection efficiency, and Bluescript plasmid (Stratagene, La Jolla, Calif.) as a DNA carrier, for a total of 20 μg of DNA with calcium phosphate precipitation as described previously (38). For T47D-YB and S294A cells, medium was aspirated 3 h after transfection, and the cells were shocked with 2 ml of phosphate-buffered saline containing 20% glycerol. Cells were washed twice with serum-free medium to remove the glycerol, and 10 ml of minimal essential medium containing 5% charcoal-stripped fetal bovine serum was added for 18 h. Triplicate cultures of cells were then treated with 10 nM R5020 or ethyl alcohol (EtOH) vehicle for 24 h prior to harvesting in lysis solution (Analytical Luminescence Laboratories, Ann Arbor, Mich.), and 100 μl of lysate was analyzed for luciferase activity with the Enhanced Luciferase Assay kit and a Monolight 2010 Luminometer (Analytical Luminescence Laboratories), as described by the manufacturer. Transfected HeLa cells were not glycerol shocked but were washed 18 h after transfection and then treated with hormone as described above.
RESULTS
Hormone-dependent down-regulation of endogenous PR-B in T47D-YB cells.
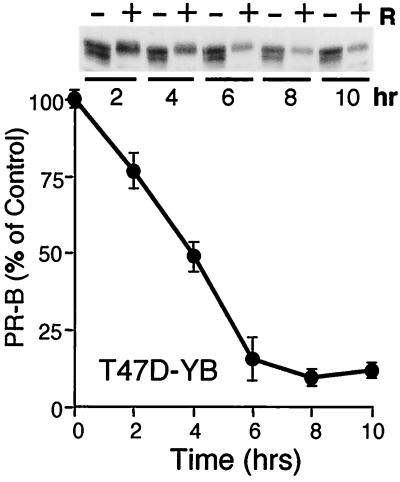
We previously demonstrated a role for p42 and p44 MAPKs in targeting PR to the ubiquitin-proteasome pathway via phosphorylation of Ser294 (21). T47D human breast cancer cells containing mutant PR in which Ser294 is replaced by alanine (S294A) fail to undergo ubiquitin-dependent down-regulation in the presence of ligand (21). In contrast, wild-type PR-B, stably expressed in T47D breast cancer cells (T47D-YB) (37), were extensively down-regulated within 6 to 8 h of exposure to progestins and degraded with an apparent half-life of approximately 4 h (Fig. 1). This is comparable to the half-life of 6 h for endogenous PR in progestin-treated cells labeled with 2H, 13C, and 15N dense amino acids (28) and indicates that the predominant mechanism for ligand-dependent down-regulation involves loss of receptor protein, rather than its diminished transcription.
FIG. 1.
Ligand-dependent PR down-regulation. T47D-YB cells were treated without or with R5020 (10 nM) for 2 to 10 h; protein levels were measured with PR-specific antibodies as described in Materials and Methods. A total of 100 μg of protein was loaded per lane for Western immunoblotting (inset); band density was plotted as a percentage of untreated control for each time point; graphed data represent the mean and standard error of the mean (error bars) from three independent experiments.
MAPK hyperactivates PR in the presence of progestins.
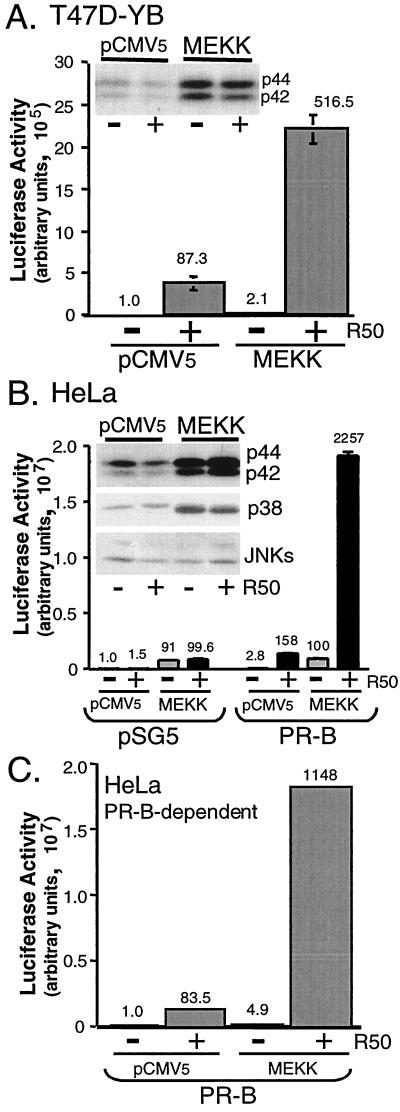
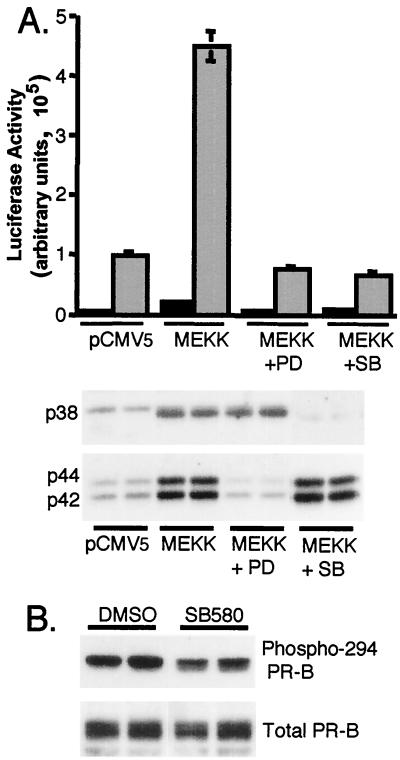
Advanced breast cancer cells often overexpress persistently activated MAPKs (13, 40), and ligand-induced PR down-regulation by the 26S proteasome is MAPK dependent (21). To study PR signaling under conditions of persistent MAPK activation, the constitutively active C-terminal kinase domain of MEKK1 (22) was overexpressed in T47D-YB cells together with a PRE-driven luciferase reporter construct. MEKK1 is a strong activator of p42 and p44 MAPKs in these cells (Fig. 2A, inset). Transient expression of MEKK1 resulted in increased PR transcriptional activity in the presence of R5020 (Fig. 2A). Typically, MEKK1 expression resulted in a five- to eightfold increase in ligand-dependent PR transcriptional activity compared to that in vector controls; MEKK1 also induced a slight increase in basal transcriptional activity in T47D-YB cells. Progestin treatment did not further increase the ability of MEKK1 to activate MAPK (Fig. 2A, inset). MEKK1 also increased the transcriptional activity of PR-A in the presence of progestins, but to a much lesser extent than PR-B (not shown).
FIG. 2.
MEKK1 increases PR transcriptional activity in the presence of progestins. (A) Triplicate cultures of T47D-YB cells were transiently transfected with a PRE-luciferase reporter construct and either pCMV5 control vector or MEKK1. Cells were placed in serum-free medium for 18 h and then treated without or with R5020 (R50; 10 nM) for 24 h, and luciferase activity in cell lysates was determined as described (see Materials and Methods). Numbers above bars indicate relative fold inductions above untreated vector controls. In a separate experiment, fold inductions for control vector, vector plus R5020, MEKK1, and MEKK1 plus R5020 were 1.0, 100, 1.4, and 380, respectively. (Inset) p42 and p44 MAPK activity in cell lysates was measured from the same experimental set using phospho-specific MAPK antibodies that recognize only activated p42 and p44 MAPKs; total MAPK protein levels remained constant (not shown). (B) MEKK1 increases PR transcriptional activity in the presence of progestins in HeLa cells. Triplicate cultures of HeLa cells were transiently transfected with a PRE-luciferase reporter construct and either pSG5 control vector or human PR-B and/or pCMV5 control vector or MEKK1. Cells were placed in serum-free medium for 18 h and then treated without (gray bars) or with (black bars) R5020 (R50; 10 nM) for 24 h, and luciferase activity in cell lysates was determined as described (see Materials and Methods). Numbers above the bars indicate relative fold inductions above untreated vector controls. (Inset) MAPK activities were measured as described in the legend to panel A with phospho-specific MAPK antibodies that recognize only activated p42 and p44 MAPKs, p38 MAPK, and JNKs. (C) PR-B-dependent transcriptional activity in HeLa cells. Luciferase activity (B) was corrected for background by subtraction of the activity in vector control (pSG5) containing cell lysates from that in PR-B-containing cell lysates for each condition to yield PR-B-dependent transcriptional activities. Numbers above the bars indicate relative fold inductions above untreated vector control.
MEKK1-induced hyperactivation of PR-dependent transcription in HeLa cells was also tested. Like T47D-YB cells, MEKK1 expression in HeLa cells resulted in robust activation of p42 and p44 MAPKs (Fig. 2B, inset). Basal levels of p38 MAPK activity were also further increased by MEKK1 expression, while JNKs were weakly activated in the same whole-cell lysates (Fig. 2B, inset). Following their transient transfection with the appropriate control vectors or PR-B and without or with MEKK1, along with the PRE-luciferase reporter, MEKK1 again resulted in greatly increased transcription induced by R5020. The MEKK-induced increase in transcription was both PR-B and ligand dependent and required the presence of a PRE. Reporter constructs lacking a PRE or containing an estrogen reponse element were not induced above basal control levels (not shown). Thus, the remarkable increase in PR-dependent transcription observed when MAPK signaling is activated is independent of cell type. In contrast to activity in T47D-YB cells (Fig. 2A), MEKK1 induced a significant increase in basal transcriptional activity in HeLa cells; this was independent of the presence of PR-B or ligand and may reflect increased sensitivity of the basal transcriptional machinery in these cells (Fig. 2B). Activated MAPKs likely exert important effects on the activity of components within the basal transcription machinery independently of specific transcription factors, making it necessary to separate the effects of MEKK1 into basal versus PR-specific events. PR-B-dependent changes in transcriptional activity in HeLa cells were therefore determined by subtracting the MEKK-induced changes in background activity in cells lacking PR-B (Fig. 2B, left) from the total activity (Fig. 2B, right). The resultant PR-B-dependent transcription is shown in Fig. 2C. This correction demonstrates that MEKK1-induced effects on PR-B-specific transcriptional activity are very similar in HeLa (Fig. 2C) and T47D cells (Fig. 2A).
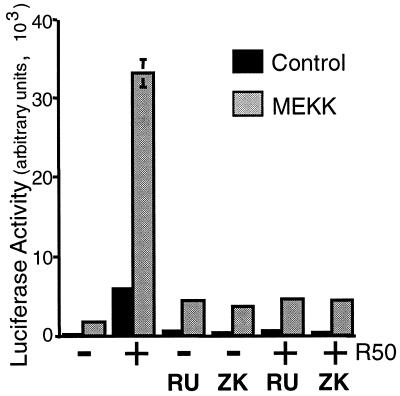
The PR antagonists, RU486 and ZK98299, blocked the R5020-induced transcriptional activity of PR-B in HeLa cells, in both the presence and absence of MEKK1, returning it to basal levels (Fig. 3). That PR antagonists had no effect on MEKK1-induced basal transcription demonstrates a separation of the effects of MEKK1 into PR-dependent and -independent components. Thus, MEKK1 appears to affect both basal transcriptional activity and PR-specific activity (Fig. 2 and 3). Herein, we have focused on alterations in ligand-dependent PR function in cells containing elevated MAPK activities.
FIG. 3.
PR antagonists block MEKK1- and R5020-induced transcription. Triplicate cultures of HeLa cells were transiently transfected with a PRE-luciferase reporter construct and human PR-B and either pCMV5 control vector (black bars) or MEKK1 (gray bars). Cells were placed in serum-free medium for 18 h and then treated without or with R5020 (10 nM), RU486 (100 nM), ZK98299 (100 nM), or R5020 plus each antagonist for 24 h. Luciferase activity in cell lysates was determined as described (see Materials and Methods).
Because MEKK1 is a strong activator of p42 and p44 MAPKs (Fig. 2A and 2B, insets) and ligand-dependent PR down-regulation is MAPK dependent (21), we asked whether MEKK1 overexpression influences this process (Fig. 4A). HeLa cells were cotransfected with PR-B and either control vector or MEKK1 and then treated with vehicle or R5020 for 24 h. PR protein levels were measured by Western immunoblotting of whole-cell lysates from duplicate cultures (Fig. 4A). R5020 induced a characteristic upshift in PR-B mobility in both control and MEKK1-expressing cells, associated with phosphorylation of PR at multiple serine residues (44, 45, 47) However, in contrast to T47D-YB cells (Fig. 1), PR-B in HeLa cells were not significantly down-regulated by R5020. This confirms our previous observation that transiently overexpressed PR-B are generally more resistant to ligand-dependent down-regulation than are endogenous PR-B (21). We speculated that the high PR levels, typical of transient systems, exceed the capacity of the 26S proteasome to degrade them (21). Surprisingly, however, MEKK1 promoted nearly complete PR-B down-regulation under the same conditions (Fig. 4A). This suggests that kinase activation or availability, rather than proteasome saturation, is likely to be the limiting factor when receptor levels are excessive and confirms the critical role of MAPK in this process.
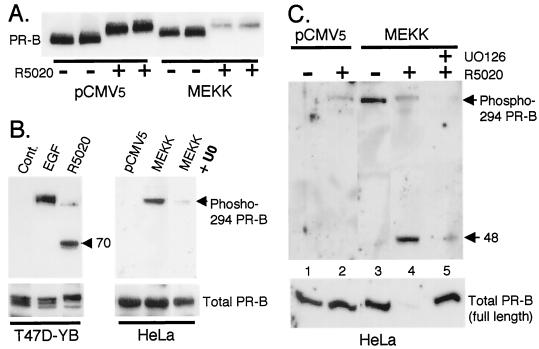
FIG. 4.
MEKK1 expression increases ligand-induced PR turnover and Ser294 phosphorylation. (A) MEKK1 augments PR protein turnover in the presence of progestins. Duplicate cultures of HeLa cells were transiently transfected with PR-B and either pCMV5 control vector or MEKK1 as described in Materials and Methods. Cells were treated without or with R5020 (10 nM) for 24 h, and PR protein levels in whole-cell lysates were determined with PR monoclonal antibodies. (B) Regulation of Ser294 phosphorylation in T47D-YB and HeLa cells. T47D-YB cells were treated with EGF (30 ng/ml) for 5 min or with R5020 (10 nM) for 1 h. HeLa cells were transfected with pCMV5 control vector or MEKK1 and treated without or with the MEK inhibitor (U0126) (20 μM) for 12 h. Phospho-Ser294 PR levels in cell lysates were measured with specific monoclonal antibodies to PR phospho-Ser294; a lower-molecular-weight phospho-Ser294-containing PR fragment of approximately 70 kDa in molecular mass was present in lysates from R5020-treated cells only (arrow). Total full-length PR levels remained constant (bottom); PR-B monoclonal antibodies failed to recognize PR fragments (not shown). (C) Regulation of Ser294 by MEKK1 plus R5020. HeLa cells were transfected with pCMV5 control vector or MEKK1 and pretreated without or with the MEK inhibitor, U0126 (20 μM), for 30 min prior to R5020 (10 μM) treatment for 12 h. Phosphorylated PR levels in cell lysates were measured with phospho-Ser294-specific monoclonal antibodies; a lower-molecular-weight phospho-Ser294-containing PR fragment of approximately 48 kDa was present in lysates from R5020-treated HeLa cells in the presence of MEKK1 (arrow). Total full-length PR levels were measured in the same lysates (lower panel); PR-B monoclonal antibodies failed to recognize PR fragments (not shown).
Ser294 of human PR is rapidly hyperphosphorylated in response to ligand (51), and this event triggers ligand-dependent PR down-regulation (21). Our data (Fig. 4A) suggest that perhaps chronic activation of MAPKs in response to growth factors (analogous to expression of constitutively active MEKK1) augments ligand-dependent PR down-regulation via phosphorylation of Ser294 (Fig. 4A). To directly monitor the phosphorylation status of Ser294, we utilized recently described monoclonal antibodies that recognize only the phosphorylated Ser294 residue but fail to recognize PR that are unphosphorylated at this site (6). Treatment of T47D-YB cells with EGF, a well-characterized activator of p42 and p44 MAPKs, resulted in robust phosphorylation of Ser294 in 5 min (Fig. 4B) and 1 h (not shown). R5020 treatment for 1 h, as a positive control, resulted in slight but detectable phosphorylation of full-length PR at Ser294. Interestingly, a lower-molecular-weight form of PR-B containing the phospho-Ser294 residue was the predominant peptide. We were also unable to detect phospho-Ser294 PR of any size at earlier time points (not shown). These data suggest that the phospho-S294 species is a very transient intermediate, which is rapidly down-regulated in the presence of ligand (discussed below). Fragments of phosphorylated PR-B were undetectable with PR monoclonal antibodies directed against N-terminal receptor epitopes (9), suggesting that proteolysis of the PR N terminus is an early event following ligand binding. In HeLa cells, activation of MAPKs by MEKK1 also resulted in the phosphorylation of PR-B at Ser294 (Fig. 4B). This was blocked by the MEK1-MEK2 inhibitor, U0126, indicating a requirement for p42 and p44 MAPKs. Total PR-B remained constant in the presence of control vector, MEKK1, and MEKK1 plus U0126.
To determine whether phospho-294 intermediates could be trapped in cells simultaneously expressing MEKK1 and being treated with R5020, HeLa cells were transfected with PR-B and either control vector or MEKK1 and treated with or without R5020 (Fig. 4C). Whole-cell lysates were blotted with phospho-294-specific or total PR monoclonal antibodies. Under control conditions (Fig. 4C, lane 1), no phosphorylated PR were detectable, and only weak Ser294 phosphorylation was observed following R5020 treatment alone (lane 2, bottom), consistent with little or no PR down-regulation. In contrast to T47D-YB cells, lower-molecular-weight forms of phospho-294 containing PR were not apparent in lysates from HeLa cells treated with R5020 alone, presumably due to retarded PR down-regulation in these cells (reference 21 and Fig. 4A). However, MEKK1 alone led to robust phosphorylation of Ser294 (Fig. 4C, lane 3). Addition of R5020 under these conditions led to loss of full-length phospho-Ser294 PR-B, while a low-molecular-weight phosphorylated species appeared and total PR (full-length) were completely down-regulated (lane 4). These data suggest that phosphorylation of S294 is necessary but not sufficient for receptor down-regulation and that ligand occupancy of the PR C terminus is also absolutely required. Both PR-B phosphorylation at Ser294 and PR down-regulation by R5020 plus MEKK1 were blocked by inhibition of p42 and p44 MAPKs using the MEK inhibitor, U0126, demonstrating a requirement for MAPK in this process (lane 5). Thus, MAPK activation augments ligand-dependent PR down-regulation via phosphorylation of Ser294 (Fig. 4).
Stabilization of PR blocks PR hyperactivation.
We noted that transcription of the luciferase reporter is paradoxically highest (Fig. 2B) when PR protein levels are down-regulated (Fig. 4A and C). This prompted us to more closely examine the role of PR turnover in PR-dependent transcriptional activity. We showed previously that ligand-dependent PR down-regulation is blocked by inhibition of p42 and p44 MAPKs with MEK inhibitors and/or by selective inhibition of the 26S proteasome (21). We therefore tested PR transcriptional activity under conditions in which PR are stabilized using the same pharmacological approaches (Fig. 5 and 6). HeLa cells were transfected with PR-B and either control vector or MEKK1 and treated with or without R5020 in the presence or absence of the MEK1 inhibitor, PD98059 (PD). The transcriptional hyperactivation induced by MEKK1 plus R5020 was completely blocked by inhibition of p42 and p44 MAPKs (Fig. 5A). A selective inhibitor of p38 MAPK, SB203580, also blocked progestin-stimulated PR transactivation in MEKK1 expressing cells, indicating that MEKK1 effects are mediated in part by activation of p38 MAPK. MAPK assays indicated that the inhibitors were selective for their respective kinase activities and that no cross-reactivity was observed (Fig. 5A). Interestingly, MEKK1-induced basal PR transcriptional activity was not significantly inhibited by either agent. In contrast to U0126 (Fig. 4B and C), the SB203580 compound did not appreciably block phosphorylation of Ser294 by MEKK1 (Fig. 5B), indicating that p38 MAPK must function to augment PR transcription largely independently of Ser294 phosphorylation. SB203580 does not block ligand-dependent PR down-regulation, as do PD98059 (21) and U0126 (Fig. 4C).
FIG. 5.
Inhibition of MEKK1-induced transcription by MEK inhibitors. (A) Triplicate cultures of HeLa cells were transiently transfected with a PRE-luciferase reporter construct and PR-B and either pCMV5 control vector or MEKK1. Cells were pretreated for 30 min without or with MEK inhibitors specific for p42 and p44 MAPKs (PD98059; 50 μM) or p38 MAPK (SB203580; 20 μM) and then treated without (black bars) or with (gray bars) R5020 (10 nM) for 12 h. Luciferase activity in cell lysates was determined as described above (see Materials and Methods). MAPK activities were measured in duplicate samples from the same experimental set with phospho-specific MAPK antibodies that recognize only activated p42 and p44 MAPKs or activated p38 MAPK. (B) Phosphorylation of PR Ser294 in the presence of MEKK1 and the p38 MAPK inhibitor (SB580). Duplicate cultures of HeLa cells were transfected with MEKK1 and treated with either dimethyl sulfoxide (vehicle) or the p38 MAPK inhibitor (SB203580; 20 μM) for 12 h. Phospho-Ser294 PR levels in cell lysates were measured with monoclonal antibodies specific to PR phospho-Ser294. Total PR levels in the same lysates were measured (bottom).
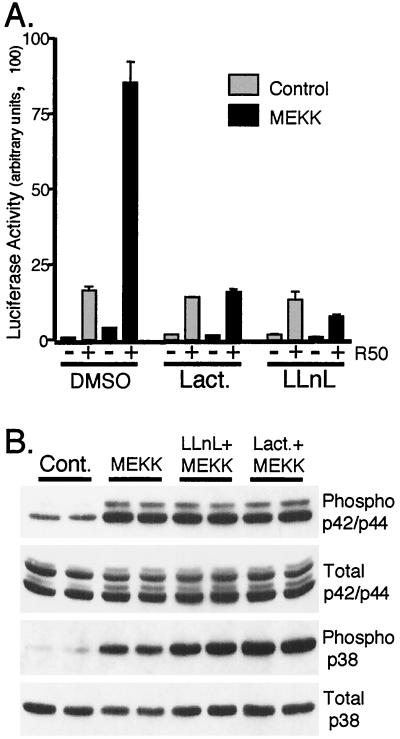
FIG. 6.
Inhibition of the 26S proteasome blocks MEKK1-induced transcription without effecting MAPK activities. (A) Triplicate cultures of HeLa cells were transiently transfected with a PRE-luciferase reporter construct and PR-B and either pCMV5 control vector (gray bars) or MEKK1 (black bars) as described in Materials and Methods. Cells were pretreated for 30 min without or with specific inhibitors of the 26S proteasome (10 μM lactacystin; 25 μM LLnL) and then treated without or with R5020 (R50; 10 nM) for 16 h. Luciferase activity in cell lysates was determined as described above (see Materials and Methods). Proteasome inhibitors block ligand-dependent PR degradation under these conditions in several cell lines (21). (B) Duplicate cultures of HeLa cells were transiently transfected with either pCMV5 control vector (Cont.) or MEKK1 and treated as described in the legend to panel A, and activated (phospho) and total p42-p44 and p38 MAPKs were measured in cell lysates with specific antibodies. Proteasome inhibitors did not alter the ability of MEKK1 to activate MAPKs.
Inhibitors of the 26S proteasome also block ligand-dependent down-regulation of PR (21). We therefore also tested the effects of lactacystin and LLnL on R5020-plus-MEKK1-dependent PR transcriptional hyperactivation (Fig. 6A). Both agents blocked the MEKK1-dependent component of PR transcriptional activity. Importantly, however, the PR transcriptional activity induced by R5020 alone was unaffected by either inhibitor. This suggests that the transcriptional hyperactivation observed in the presence of MEKK1 plus R5020 requires coordinated PR down-regulation involving the 26S proteasome. Importantly, inhibition of the 26S proteasome by either lactacystin or LLnL did not effect the ability of MEKK1 to activate either p42-p44 or p38 MAPKs (Fig. 6B).
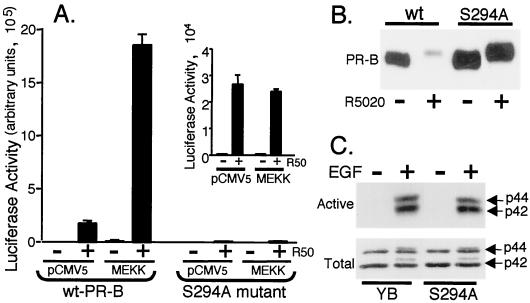
To study the role of Ser294 phosphorylation in this process, we compared the ability of MEKK1 plus R5020 to hyperactivate transcription of stably expressed wild-type and S294A mutant PR-B in T47D cells (Fig. 7). The PRE-driven luciferase construct was coexpressed with control vector or MEKK1, and cells were treated with or without R5020 for 24 h. Cells containing wild-type PR-B are highly responsive to R5020-induced transcriptional activation of the PRE-luciferase reporter gene (Fig. 7A and 2A), and expression of MEKK1 induced PR hyperactivation. In contrast, cells expressing mutant S294A PR-B were less responsive to R5020 alone and unresponsive to MEKK1-induced hyperactivation (Fig. 7A). Thus, mutant S294A PR-B are functional in the presence of R5020 alone but do not synergize with active MEKK1. To insure that variations in wild-type versus mutant PR-B protein expression levels were not responsible for these differences, Western immunoblot analysis was performed with whole-cell lysates (Fig. 7B). In the absence of ligand, PR protein levels were similar in both cell lines; S294A cells expressed slightly more PR-B than wild-type cells. Progestin treatment for 24 h resulted in nearly complete ligand-dependent down-regulation of wild-type PR-B, while mutant S294A PR-B underwent the characteristic ligand-induced gel mobility upshift due to multisite phosphorylation but remained entirely stable in the presence of R5020.
FIG. 7.
Mutation of PR Ser294 to alanine blocks MEKK1-induced transcription. (A) Triplicate cultures of T47D-YB cells stably expressing wild-type PR or PR-negative T47D cells stably expressing S294A mutant PR (S294A) were transiently transfected with a PRE-luciferase reporter construct and either pCMV5 control vector or MEKK1. Cells were treated without or with R5020 (R50; 10 nM) for 24 h, and luciferase activity in cell lysates was determined as described in Materials and Methods. (Inset) Expanded scale showing R5020-induced transcription in S294A mutant PR-containing cells. Both cell lines exhibited similar transfection efficiencies (not shown). (B) PR protein turnover in T47D cells stably expressing either wild-type or S294A mutant PR-B. Cells were treated without or with R5020 (10 nM) for 24 h, and PR-B protein levels in whole-cell lysates were measured using PR monoclonal antibodies. (C) MAPK activity in T47D cells stably expressing either wild-type or S294A mutant PR-B. Cells were placed in serum-free medium 24 prior to treatment without or with EGF (30 ng/ml) for 5 min, and activated p42 and p44 MAPKs (top) or total MAPKs (bottom) in the same whole-cell lysates were measured with specific antibodies for phosphorylated or total MAPK, respectively.
PR serine 294 is a MAPK consensus site. Mutation of this site to alanine or treatment of cells with the MEK inhibitor, PD98059, both stabilizes PR (21) and blocks MEKK1-plus-R5020-induced transcriptional synergy (Fig. 5). To insure that the MAPK pathway is intact in T47D-YB cells expressing mutant S294A PR-B, cells were treated with EGF for 5 min in order to fully activate p42 and p44 MAPKs. Western blot analysis indicated that MAPKs in S294A cells are equally well activated by EGF and contain total MAPK levels similar to those in wild-type cells (Fig. 7C).
c-myc expression in T47D-YB cells expressing wild-type and mutant PR-B.
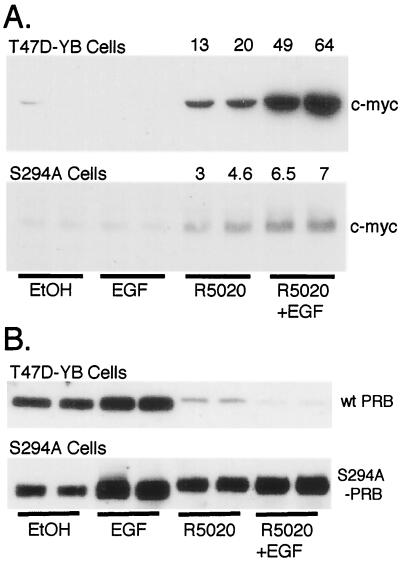
Since MAPK activation by MEKK1 greatly increases R5020- and PR-dependent transcription of a synthetic promoter-reporter (Fig. 2), we asked whether an endogenous protein can be similarly regulated. c-myc mRNA and protein levels are known to be up-regulated by progestins in human breast cancer cells (27) and the c-myc gene promoter contains a putative consensus PRE sequence (26). We therefore examined the effects of EGF and R5020 on c-myc and PR protein expression in T47D cells stably expressing either wild-type PR-B or the S294A mutant (Fig. 8A). c-myc protein Western immunoblot analysis was performed with whole-cell lysates from cells treated with vehicle control, EGF, R5020, or both EGF and R5020 for 12 h to allow protein accumulation (Fig. 8A). EGF alone had no effect on c-myc protein levels in either cell line, while R5020 up-regulated c-myc protein in both cell lines (27). Simultaneous treatment of T47D-YB cells containing wild-type PR-B with both R5020 and EGF synergistically increased c-myc protein levels. However, this effect was markedly attenuated in cells expressing mutant S294A PR-B (Fig. 8A).
FIG. 8.
Regulation of c-myc and PR protein expression in T47D cells stably expressing wild-type or mutant S294A PR-B. (A) Duplicate cultures of T47D-YB cells stably expressing wild-type PR or PR-negative T47D cells stably expressing S294A mutant PR were placed in serum-free medium for 24 h prior to treatment without or with EtOH vehicle control, EGF (30 ng/ml), R5020 (10 nM), or EGF plus R5020 for 12 h; levels of c-myc protein in whole-cell lysates were determined with specific antibodies. Numbers above the lanes indicate fold increases over EtOH-treated controls as measured by densitometric analysis of immunoblots. (B) Duplicate cultures of T47D cells stably expressing either wild-type or mutant S294A PR-B were treated as described in the legend to panel A, except that treatment continued for 6 h and PR-B protein levels in whole-cell lysates were measured using PR monoclonal antibodies.
Changes in PR protein levels were monitored under the same conditions in each cell line following 6 h of treatment (Fig. 8B), just prior to the nadir of PR-B down-regulation in the presence of ligand (Fig. 1). Interestingly, EGF treatment increased PR levels in both cell lines; this was most apparent in cells expressing stabilized mutant S294A PR (Fig. 8B). As expected, R5020 treatment down-regulated wild-type PR-B to trace levels but had no effect on mutant PR-B. EGF treatment augmented down-regulation of wild-type PR in the presence of progestin but had no effect on mutant PR. Wild-type PR-B was undetectable in cells treated with R5020 plus EGF, while mutant PR-B levels remained essentially unchanged. Similar results were observed following 12-h treatments (not shown).
Taken together, our data suggest that, paradoxically, PR that are capable of ligand-dependent down-regulation function as more efficient transcriptional activators. We propose that, in the case of PR, steroid hormone receptor turnover is functionally coupled to transcriptional hyperactivation during cross talk with growth factor-initiated signaling pathways.
DISCUSSION
At least 10 serine residues on human PR are known to be phosphorylated in vivo, either basally or as a result of hormonal stimuli and/or following protein kinase activation (18, 31, 50; for a review, see references 44 and 47). The significance of phosphorylation of these sites with regard to PR function remains largely undefined. Phosphorylation of human steroid hormone receptors is generally believed to positively or negatively modify their transcriptional activity rather than act as an on-off switch. Phosphorylation-dephosphorylation events may instead predominantly serve to fine-tune aspects of receptor regulation, perhaps by regulating the integration of signals from other pathways or subcellular localization, or trafficking of receptor complexes or by degradation of receptor proteins (reviewed in references 44 and 47). However, the traditional view that phosphorylation of human steroid hormone receptors, including PR, is largely a means of accomplishing relatively subtle alterations in receptor regulation is changing, as novel phosphorylation sites continue to be identified and characterized (18).
Similar to human PR, ER are also heavily phosphorylated. However, in contrast to PR, phosphorylation of ER-α is a well-known mechanism for the modulation of its transcriptional activity. Phosphorylation of Ser118, located in AF1 (activation of function 1), is mediated in vitro by MAPK and in vivo in cells treated with growth factors and enhances the transcriptional activity of ER elicited by either estrogen or tamoxifen (16). Lee et al. (23) recently reported that MEKK1 increased the agonist activity of ER-α induced by either estradiol or 4-hydroxytamoxifen in endometrial and ovarian cancer cells. Interestingly, this effect was mediated through activation of JNK and p38 MAPK, but not p42 and/or p44 MAPKs. Although independent of known phosphorylation sites on ER-α, p38 MAPK efficiently phosphorylated the receptor in immunocomplex kinase assays in vitro (23). Thus, in the presence of estrogen, ER appear to undergo a state of hyperactivation, following growth-factor stimulation of MAPK pathways. Our results suggest that this regulatory paradigm also holds true for PR signaling. In addition to p42 and/or p44 MAPKs, p38 MAPK appears to play a role in PR hyperactivation (Fig. 5A). However, p38 MAPK mediates this effect independently of Ser294 phosphorylation (Fig. 5B) and ligand-dependent PR degradation (21); p38 MAPK may contribute to PR transcriptional activation by phosphorylation of other as-yet-undefined regulatory sites on human PR or its coregulators. This is an important topic for further studies.
We have uncovered a novel link between transcriptional hyperactivation following stimulation of MAPKs and ligand-dependent PR down-regulation; both events are mediated by phosphorylation of Ser294. We propose that Ser294 serves to integrate signals from growth factor-initiated pathways with progesterone in order to amplify PR transcriptional activity. The surprising finding that stabilized PR are incapable of such cross talk suggests that hyperactivation is functionally coupled to ligand-dependent PR down-regulation. How might this happen?
Studies of very short-lived transcription factor oncoproteins provide some mechanistic clues. For example, c-myc protein is a substrate for the ubiquitin-proteasome pathway, and transforming mutations stabilize this protein (35). Interestingly, sequences required for c-myc protein destruction by the 26S proteasome map to its transcriptional activation domain (35). Further studies by Salghetti et al. (36) noted a similar overlap between the activation domains and destruction elements or “degrons” of several unstable transcription factors, including E2F-1, fos, jun, and p53. A close correlation exists between the ability of an acidic activation domain to both activate transcription and signal proteolysis. Destruction elements derived from the yeast cyclins Cln2 and Cln3 activated transcription when fused to a DNA-binding domain (36). Phosphorylation is a prerequisite for degron function of Cln2 (41) and likely for Cln3 (48). Salghetti et al. (36) speculate that the negative charge provided by phosphorylation of these degrons mimics the environment provided by an acidic activation function. Thus, short-lived transcription factors may be destroyed because of their ability to activate transcription well, perhaps through a common cellular machinery (36).
Although unproven, the functional linkage of transcriptional activation and ubiquitin-mediated proteolysis by common regulatory elements is emerging as a potentially important cellular control mechanism. This linkage most likely occurs at the transcriptional level. McNally et al. (25) reported a continuous exchange of liganded glucocorticoid receptors with genomic targets and the nucleoplasmic compartment. Thus, the interaction of transcription factors with target sites in chromatin is a highly dynamic process. This exchange may provide liganded receptors the opportunity to interact with the cellular machinery required for their degradation. Indeed, several points of interaction have been documented. Proteasome subunits Sug1 and Sug2 interact with transcriptional activation domains (33, 34, 43), and Sug1 interacts with a subunit of the basal transcription factor, TFIIH (46). The ubiquitin-protein ligases, hRPF1 (15) and E6-AP (30), have been shown to function as coactivators for liganded steroid hormone receptors, including PR. Finally, histones are substrates for the ubiquitin-proteasome pathway and their ubiquitinylation correlates with increased transcriptional activity (7, 8). Thus, although their significance remains to be defined, it appears that complex interactions between regulatory molecules governing both transcription and ubiquitinylation-mediated degradation exist.
Lonard et al. (24) recently demonstrated that the 26S proteasome was required for ER-α turnover and efficient ER-α transactivation. Indeed, these activities also appear to be functionally coupled. The steroid receptor coactivator E6-AP associates with ER-α and was previously described as a ubiquitin protein ligase (39). Similar to what occurs with PR, stabilization of ER by 26S proteasome inhibitors blocks ER transcriptional activity (24). Thus, the transcriptional activities and/or hormone responsiveness of both ER and PR appears to be tightly linked to receptor stability and/or turnover. These results favor a model whereby, in the presence of ligand, a coactivator(s) is recruited to steroid receptor complexes; this same factor either functions directly in the ubiquitin pathway or associates with enzymes required for receptor ubiquitinylation (24). Although some likely candidates exist (15, 30), the binding of such a coupling factor(s) has not been demonstrated for human PR. However, the MAPK consensus site containing Ser294 is nested within a larger nine-amino-acid motif known as a destruction box. This motif is found in both cyclin A- and B-type molecules and is required for their degradation by the ubiquitin-proteosome pathway during cell cycle traverse (10, 17). The destruction box motif in cyclins may serve as a binding site for a specific ubiquitin ligase enzyme or an associated protein(s) (10, 17). Thus, in human PR, it is possible that phosphorylation of Ser294 may induce the association of an analogous factor that is both a ubiquitin ligase (or associated protein) as well as a transcriptional coactivator. This may explain why R5020-plus-MEKK1-induced transcriptional activity is highly sensitive to proteasome inhibition, while that induced by R5020 alone is not (Fig. 6A); such a regulatory molecule(s) may be stably recruited only during robust MAPK activation. It is equally possible that in addition to mediating the rapid destruction of PR, phosphorylation of Ser294 induces conformational changes in PR which render it more transcriptionally active, perhaps via more efficient exposure of its AFs. These exciting possibilities remain to be explored.
What is the purpose of rapid destruction of liganded receptor-transcription factors? Why should these activities be coupled? Such functional coupling obviously provides an efficient means of attenuating potent transcriptional responses. PR that have been hyperactivated in response to MAPK signaling would thus provide a robust yet transient transcriptional response. Indeed, phospho-Ser294 PR appear to be highly transient species in the presence of ligand (Fig. 4). Additionally, receptor degradation could serve to reset the transcriptional apparatus following a specific stimulus, so that activated receptors may be continuously replaced. Thus, genomic targets would be readily available to receive subsequent and/or alternate stimuli. Finally, coupling of rapid receptor degradation with transcriptional activity provides a means to tightly regulate inputs from multiple signaling pathways. Perhaps breast cancers that are unresponsive to endocrine therapy, but express apparently functional receptors, have somehow uncoupled receptor turnover from transcription at certain genes. Thus, gene regulation may be altered due to abnormally stabilized receptors. Indeed, breast cancer cells harboring stable S294A PR that cannot down-regulate are much less responsive to hormonal regulation than cells expressing PR that are capable of efficient turnover (Fig. 7 and 8).
Independent protein kinase cascades, hormones, and antihormones are predicted to induce differential phosphorylation of steroid hormone receptors (2); these heterogeneously phosphorylated receptors may regulate gene activity differently. Our results suggest that changes in cellular phosphorylation state are likely to be important in determining the relative stability and thus biological activity of PR at specific promoters and may provide important insight into possible mechanisms of steroid hormone resistance in advanced breast cancer.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants DK53825 (to C.A.L.) and DK48238 and CA26869 and by the National Foundation of Cancer Research (to K.B.H.).
We are grateful to S. R. Hann (Vanderbilt University School of Medicine, Nashville, Tennessee) and D. P. Edwards (University of Colorado Health Sciences Center, Denver, Colorado) for the gift of c-myc (42) and phospho-Ser294 PR antibodies (6), respectively.
REFERENCES
- 1.Aronica S M, Katzenellenbogen B S. Stimulation of estrogen receptor-mediated transcription and alteration in the phosphorylation state of the rat uterine estrogen receptor by estrogen, cyclic adenosine monophosphate, and insulin-like growth factor-I. Mol Endocrinol. 1993;7:743–752. doi: 10.1210/mend.7.6.7689695. [DOI] [PubMed] [Google Scholar]
- 2.Bagchi M K, Tsai S Y, Tsai M J, O'Malley B W. Ligand and DNA-dependent phosphorylation of human progesterone receptor in vitro. Proc Natl Acad Sci USA. 1992;89:2664–2668. doi: 10.1073/pnas.89.7.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bamberger A M, Bamberger C M, Gellersen B, Schulte H M. Modulation of AP-1 activity by the human progesterone receptor in endometrial adenocarcinoma cells. Proc Natl Acad Sci USA. 1996;93:6169–6174. doi: 10.1073/pnas.93.12.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck C A, Weigel N L, Edwards D P. Effects of hormone and cellular modulators of protein phosphorylation on transcriptional activity, DNA binding, and phosphorylation of human progesterone receptors. Mol Endocrinol. 1992;6:607–620. doi: 10.1210/mend.6.4.1316549. [DOI] [PubMed] [Google Scholar]
- 5.Bunone G, Briand P A, Miksicek R J, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 6.Clemm D L, Sherman L, Boonyaratanakornkit V, Schrader W T, Weigel N L, Edwards D P. Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site-specific monoclonal antibody. Mol Endocrinol. 2000;14:52–65. doi: 10.1210/mend.14.1.0413. [DOI] [PubMed] [Google Scholar]
- 7.Davie J R, Murphy L C. Inhibition of transcription selectively reduces the level of ubiquitinated histone H2B in chromatin. Biochem Biophys Res Commun. 1994;203:344–350. doi: 10.1006/bbrc.1994.2188. [DOI] [PubMed] [Google Scholar]
- 8.Davie J R, Murphy L C. Level of ubiquitinated histone H2B in chromatin is coupled to ongoing transcription. Biochemistry. 1990;29:4752–4757. doi: 10.1021/bi00472a002. [DOI] [PubMed] [Google Scholar]
- 9.Estes P A, Suba E J, Lawler H J, Elashry S D, Wei L L, Toft D O, Sullivan W P, Horwitz K B, Edwards D P. Immunologic analysis of human breast cancer progesterone receptors. 1. Immunoaffinity purification of transformed receptors and production of monoclonal antibodies. Biochemistry. 1987;26:6250–6262. doi: 10.1021/bi00393a045. [DOI] [PubMed] [Google Scholar]
- 10.Glotzer M, Murray A W, Kirschner M W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- 11.Groshong S D, Owen G I, Grimison B, Schauer I E, Todd M C, Langan T A, Sclafani R A, Lange C A, Horwitz K B. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1) Mol Endocrinol. 1997;11:1593–1607. doi: 10.1210/mend.11.11.0006. [DOI] [PubMed] [Google Scholar]
- 12.Haraguchi S, Good R A, Engelman R W, Greene S, Day N K. Prolactin, epidermal growth factor or transforming growth factor-alpha activate a mammary cell-specific enhancer in mouse mammary tumor virus- long terminal repeat. Mol Cell Endocrinol. 1997;129:145–155. doi: 10.1016/s0303-7207(97)04053-7. [DOI] [PubMed] [Google Scholar]
- 13.Hoshino R, Chatani Y, Yamori T, Tsuruo T, Oka H, Yoshida O, Shimada Y, Ari-i S, Wada H, Fujimoto J, Kohno M. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene. 1999;18:813–822. doi: 10.1038/sj.onc.1202367. [DOI] [PubMed] [Google Scholar]
- 14.Hovland A R, Powell R L, Takimoto G S, Tung L, Horwitz K B. An N-terminal inhibitory function, IF, suppresses transcription by the A-isoform but not the B-isoform of human progesterone receptors. J Biol Chem. 1998;273:5455–5460. doi: 10.1074/jbc.273.10.5455. [DOI] [PubMed] [Google Scholar]
- 15.Imhof M O, McDonnell D P. Yeast RSP5 and its human homolog hRPF1 potentiate hormone-dependent activation of transcription by human progesterone and glucocorticoid receptors. Mol Cell Biol. 1996;16:2594–2605. doi: 10.1128/mcb.16.6.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 17.King R W, Glotzer M, Kirschner M W. Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol Biol Cell. 1996;7:1343–1357. doi: 10.1091/mbc.7.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knotts T A, Orkiszewski R S, Cook R G, Edwards D P, Weigel N L. Identification of a phosphorylation site in the hinge region of the human progesterone receptor and additional amino-terminal phosphorylation sites. J Biol Chem. 2001;276:8475–8483. doi: 10.1074/jbc.M009805200. [DOI] [PubMed] [Google Scholar]
- 19.Lange C A, Richer J K, Horwitz K B. Hypothesis: progesterone primes breast cancer cells for cross-talk with proliferative or antiproliferative signals. Mol Endocrinol. 1999;13:829–836. doi: 10.1210/mend.13.6.0290. [DOI] [PubMed] [Google Scholar]
- 20.Lange C A, Richer J K, Shen T, Horwitz K B. Convergence of progesterone and epidermal growth factor signaling in breast cancer. Potentiation of mitogen-activated protein kinase pathways. J Biol Chem. 1998;273:31308–31316. doi: 10.1074/jbc.273.47.31308. [DOI] [PubMed] [Google Scholar]
- 21.Lange C A, Shen T, Horwitz K B. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci USA. 2000;97:1032–1037. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lange-Carter C A, Pleiman C M, Gardner A M, Blumer K J, Johnson G L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993;260:315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- 23.Lee H, Jiang F, Wang Q, Nicosia S V, Yang J, Su B, Bai W. MEKK1 activation of human estrogen receptor alpha and stimulation of the agonistic activity of 4-hydroxytamoxifen in endometrial and ovarian cancer cells. Mol Endocrinol. 2000;14:1882–1896. doi: 10.1210/mend.14.11.0554. [DOI] [PubMed] [Google Scholar]
- 24.Lonard D M, Nawaz Z, Smith C L, O'Malley B W. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol Cell. 2000;5:939–948. doi: 10.1016/s1097-2765(00)80259-2. [DOI] [PubMed] [Google Scholar]
- 25.McNally J G, Muller W G, Walker D, Wolford R, Hager G L. The glucocorticoid receptor: rapid exchange with regulatory sites in living cells. Science. 2000;287:1262–1265. doi: 10.1126/science.287.5456.1262. [DOI] [PubMed] [Google Scholar]
- 26.Moore M R, Zhou J L, Blankenship K A, Strobl J S, Edwards D P, Gentry R N. A sequence in the 5′ flanking region confers progestin responsiveness on the human c-myc gene. J Steroid Biochem Mol Biol. 1997;62:243–252. doi: 10.1016/s0960-0760(97)00036-8. [DOI] [PubMed] [Google Scholar]
- 27.Musgrove E A, Lee C S, Sutherland R L. Progestins both stimulate and inhibit breast cancer cell cycle progression while increasing expression of transforming growth factor alpha, epidermal growth factor receptor, c-fos, and c-myc genes. Mol Cell Biol. 1991;11:5032–5043. doi: 10.1128/mcb.11.10.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nardulli A M, Katzenellenbogen B S. Progesterone receptor regulation in T47D human breast cancer cells: analysis by density labeling of progesterone receptor synthesis and degradation and their modulation by progestin. Endocrinology. 1988;122:1532–1540. doi: 10.1210/endo-122-4-1532. [DOI] [PubMed] [Google Scholar]
- 29.Nawaz Z, Lonard D M, Dennis A P, Smith C L, O'Malley B W. Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci USA. 1999;96:1858–1862. doi: 10.1073/pnas.96.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nawaz Z, Lonard D M, Smith C L, Lev-Lehman E, Tsai S Y, Tsai M J, O'Malley B W. The Angelman syndrome-associated protein, E6-AP, is a coactivator for the nuclear hormone receptor superfamily. Mol Cell Biol. 1999;19:1182–1189. doi: 10.1128/mcb.19.2.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao K V, Peralta W D, Greene G L, Fox C F. Cellular progesterone receptor phosphorylation in response to ligands activating protein kinases. Biochem Biophys Res Commun. 1987;146:1357–1365. doi: 10.1016/0006-291x(87)90799-6. [DOI] [PubMed] [Google Scholar]
- 32.Richer J K, Lange C A, Manning N G, Owen G, Powell R, Horwitz K B. Convergence of progesterone with growth factor and cytokine signaling in breast cancer. Progesterone receptors regulate signal transducers and activators of transcription expression and activity. J Biol Chem. 1998;273:31317–31326. doi: 10.1074/jbc.273.47.31317. [DOI] [PubMed] [Google Scholar]
- 33.Rubin D M, Coux O, Wefes I, Hengartner C, Young R A, Goldberg A L, Finley D. Identification of the gal4 suppressor Sug1 as a subunit of the yeast 26S proteasome. Nature. 1996;379:655–657. doi: 10.1038/379655a0. [DOI] [PubMed] [Google Scholar]
- 34.Russell S J, Sathyanarayana U G, Johnston S A. Isolation and characterization of SUG2. A novel ATPase family component of the yeast 26 S proteasome. J Biol Chem. 1996;271:32810–32817. doi: 10.1074/jbc.271.51.32810. [DOI] [PubMed] [Google Scholar]
- 35.Salghetti S E, Kim S Y, Tansey W P. Destruction of Myc by ubiquitin-mediated proteolysis: cancer-associated and transforming mutations stabilize Myc. EMBO J. 1999;18:717–726. doi: 10.1093/emboj/18.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salghetti S E, Muratani M, Wijnen H, Futcher B, Tansey W P. Functional overlap of sequences that activate transcription and signal ubiquitin-mediated proteolysis. Proc Natl Acad Sci USA. 2000;97:3118–3123. doi: 10.1073/pnas.050007597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sartorius C A, Groshong S D, Miller L A, Powell R L, Tung L, Takimoto G S, Horwitz K B. New T47D breast cancer cell lines for the independent study of progesterone B- and A-receptors: only antiprogestin-occupied B-receptors are switched to transcriptional agonists by cAMP. Cancer Res. 1994;54:3868–3877. [PubMed] [Google Scholar]
- 38.Sartorius C A, Tung L, Takimoto G S, Horwitz K B. Antagonist-occupied human progesterone receptors bound to DNA are functionally switched to transcriptional agonists by cAMP. J Biol Chem. 1993;268:9262–9266. [PubMed] [Google Scholar]
- 39.Scheffner M, Huibregtse J M, Vierstra R D, Howley P M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 40.Sivaraman V S, Wang H, Nuovo G J, Malbon C C. Hyperexpression of mitogen-activated protein kinase in human breast cancer. J Clin Investig. 1997;99:1478–1483. doi: 10.1172/JCI119309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skowyra D, Craig K L, Tyers M, Elledge S J, Harper J W. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 1997;91:209–219. doi: 10.1016/s0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- 42.Spotts G D, Hann S R. Enhanced translation and increased turnover of c-myc proteins occur during differentiation of murine erythroleukemia cells. Mol Cell Biol. 1990;10:3952–3964. doi: 10.1128/mcb.10.8.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swaffield J C, Melcher K, Johnston S A. A highly conserved ATPase protein as a mediator between acidic activation domains and the TATA-binding protein. Nature. 1995;374:88–91. doi: 10.1038/374088a0. [DOI] [PubMed] [Google Scholar]
- 44.Takimoto G, Horwitz K. Progesterone receptor phosphorylation—complexities in defining a functional role. Trends Endocrinol Metab. 1993;4:1–7. doi: 10.1016/1043-2760(93)90056-k. [DOI] [PubMed] [Google Scholar]
- 45.Takimoto G S, Tasset D M, Eppert A C, Horwitz K B. Hormone-induced progesterone receptor phosphorylation consists of sequential DNA-independent and DNA-dependent stages: analysis with zinc finger mutants and the progesterone antagonist ZK98299. Proc Natl Acad Sci USA. 1992;89:3050–3054. doi: 10.1073/pnas.89.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weeda G, Rossignol M, Fraser R A, Winkler G S, Vermeulen W, van't Veer L J, Ma L, Hoeijmakers J H, Egly J M. The XPB subunit of repair/transcription factor TFIIH directly interacts with SUG1, a subunit of the 26S proteasome and putative transcription factor. Nucleic Acids Res. 1997;25:2274–2283. doi: 10.1093/nar/25.12.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weigel N L. Steroid hormone receptors and their regulation by phosphorylation. Biochem J. 1996;319:657–667. doi: 10.1042/bj3190657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yaglom J, Linskens M H, Sadis S, Rubin D M, Futcher B, Finley D. p34Cdc28-mediated control of Cln3 cyclin degradation. Mol Cell Biol. 1995;15:731–741. doi: 10.1128/mcb.15.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yee, D., and C. Lange. Crosstalk between estrogen receptors and growth factor signaling. In A. Manni (ed.) Contemporary endocrinology; selective estrogen receptor modulators: research and clinical applications, in press. Humana Press, Totowa, N.J.
- 50.Zhang Y, Beck C A, Poletti A, Clement J P, Prendergast P, Yip T T, Hutchens T W, Edwards D P, Weigel N L. Phosphorylation of human progesterone receptor by cyclin-dependent kinase 2 on three sites that are authentic basal phosphorylation sites in vivo. Mol Endocrinol. 1997;11:823–832. doi: 10.1210/mend.11.6.0006. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Beck C A, Poletti A, Edwards D P, Weigel N L. Identification of a group of Ser-Pro motif hormone-inducible phosphorylation sites in the human progesterone receptor. Mol Endocrinol. 1995;9:1029–1040. doi: 10.1210/mend.9.8.7476977. [DOI] [PubMed] [Google Scholar]