Abstract
Objectives—
To determine the relationship between blood flow in the fetal descending aorta and discordant umbilical arteries (UAs).
Methods—
Pulsed wave Doppler of both UAs and the descending aorta was performed at 4-weekly intervals between 14 and 40 weeks of gestation in 209 pregnant women. In datasets with discordant UAs, a linear mixed effects model was used to determine the categorical relationship between the UA pulsatility index (PI) (high, low and average) and the descending aorta PI.
Results—
Of the 209 cases, 81 had a discordance of greater than 25% in UA PI during one of their visits. There were no differences in birth outcomes between the groups with concordant and discordant UA PIs. In the cases with discordant UA PIs, the descending aorta PI was most strongly associated with both the average UA PI (P = .008), and with the UA with the lower PI (P = .008).
Conclusions—
The relationship between blood flow in the descending aorta and UAs is consistent with the law for combining resistances in parallel. Measurements of the descending aorta PI, particularly in a scenario with discordant UAs, may inform the stability of the feto-placental circulation where discordant UA PIs are found.
Keywords: descending aorta, discordant umbilical arteries, Doppler ultrasound, placenta, pulsatility index
In fetal life, nutrient depleted blood in the descending aorta reaches the placenta via the pair of umbilical arteries (UAs) and is then distributed to the villous vasculature for gas and nutrient exchange. The normal umbilical cord contains two similar sized arteries and one vein. The two UAs are usually connected at the distal end by Hyrtl’s anastomosis, typically located within 3 cm of the placental cord insertion. This anastomosis is thought to allow equalization of blood flow and pressure between the two areas of the placenta supplied by each UA.1 Significant discordance in the size of the two arteries occurs in 0.7 to 1.4% of pregnancies and has been attributed to developmental or functional impairment of Hyrtl’s anastomosis.2–5 The smaller UA typically has higher pulsatility as measured by Doppler ultrasound.6,7 The UA pulsatility index (PI) and qualitative observation of absent or reversed end-diastolic flow correlate with the severity and the clinical impact of placental dysfunction.8–10 Despite this general association, the presence of discordant UAs has not been found to correlate with pregnancy complications3,11 and is considered a benign condition.12 This suggests that even with impairment of the Hyrtl anastomosis mechanism, the functionality of the UAs is preserved. Accordingly, interrogating the descending aorta as the main upstream blood vessel may provide a more accurate measure of the impact of differential UA blood flow.
Since approximately 40 to 60% of blood from the descending aorta (DAo) is directed to the placenta,13 the DAo PI provides a secondary measurement of combined placental vascular impedance and is positively correlated with the UA PI in patients with concordant waveform patterns.14,15 An elevated DAo PI is associated with increased risk for growth restriction.14,16–18 In growth-restricted fetuses, the predictive clinical values of the UA and DAo PIs are similar19 with notable difference that UA Doppler abnormalities tend to be more pronounced and variable.20 To better understand the relationship between blood flow resistance in the DAo and the UAs, we determined the relationship between the DAo PI and the UA PI in a prospective cohort of pregnant women with concordant as well as discordant UAs. We correlated DAo and UA PIs in order to determine the predominant trend of blood flow resistance in the fetal circulation. This approach was chosen to determine if incorporating measurement of the DAo PI has the potential to be helpful to identify false-positive predictions of adverse birth outcomes among cases with discordant UAs.
Material and Methods
Women were recruited for a prospective longitudinal study from general obstetrics clinics and high-risk pregnancy clinics at Mount Sinai Hospital (Toronto, ON, Canada) and Johns Hopkins University (Baltimore, MD, USA). All patients provided written informed consent to participate and the study was approved by the Institutional Review Boards of The Hospital for Sick Children (Toronto, ON, Canada) (REB Number 1000051548), Mount Sinai Hospital (REB Number 15–0279-A), and Johns Hopkins University (IRB Number 00082717). Inclusion criteria were maternal age between 18 and 45 years, body mass index <45 kg/m2, singleton pregnancy, and no significant pre-existing maternal comorbidities such as type 1 diabetes or chronic hypertension. Datasets were excluded when a major fetal abnormality was detected or the patient withdrew at any point during the study.
Ultrasound examinations were performed on a 4-weekly interval between 14 and 40 weeks of gestation by certified sonographers using either a Philips iU22 (Philips Healthcare, Andover, MA, USA) or GE Voluson e10 (GE Healthcare, Chicago, IL, USA) ultrasound scanner. Between the two study sites, a total of seven different sonographers performed the examinations, with 84% (n = 767) of the visits conducted at Mount Sinai Hospital by one certified research sonographer. Pulsed Doppler spectra were collected for the two UAs in a free loop of umbilical cord away from the cord insertion site21 and for the DAo at a position between the diaphragm and origin of the renal arteries.17 The insonation angle was as close to 0°as possible to achieve Doppler tracing with a high signal-to-noise ratio. In addition, semiquantitative waveform analysis using the PI was utilized to account for differences in insonation angle.22 The PI for the UAs and DAo were computed as the difference between the peak systolic and end-diastolic velocities, divided by the mean velocity over the cardiac cycle, based on the average of the traced Doppler waveforms. For the purpose of analysis, we categorized the datasets based on whether the difference between the UA PI was greater than the 90th percentile of the distribution (discordant) or below (concordant).
Obstetrical data were obtained from the women’s medical records. Birth weights were categorized into percentile groups according to neonatal sex and gestational age (in completed weeks) and small for gestational age was classified as less than the 10th centile according to standard growth charts.23 After delivery, placentas were fixed in 10% formaldehyde for 48 hours and then examined for placental lesions using the Amsterdam working group definitions.24 Gross findings included placental weight and dimensions, number of cord vessels, cord insertion, and cord coiling. The umbilical cord, fetal membrane roll, and placental disc were cut into a series of 2-cm thick slices to inspect for gross lesions. The sections were then paraffin-embedded and cut into 4 μm thick sections and stained with hematoxylin and eosin to assess microscopic abnormalities.
All statistical tests were performed using the R statistical software package (www.r-project.org). To analyze the clinical characteristics, a one-way ANOVA was used for continuous variables to evaluate the effect of group (concordant, discordant) and a Fisher’s exact test was used for categorical variables. To determine the relationship between the high, low, and average UA PIs and the DAo PI, a linear mixed effects model was used with the UA PI as the fixed effect, gestational age (in completed weeks) as a covariate, and subject ID as a random factor to account for variation between cases. To account for human factors during acquisition and to assess if the relationship was different between study sites or between sonographers, we included study site and sonographer as covariates. A value of P < .05 was taken to be significant.
Results
Of the 221 women who consented to participate in the study, 209 underwent ultrasound examinations throughout gestation and were included in the study population (one had a major fetal abnormality and 11 withdrew). The 209 cases provided a total of 911 visits with measurements of both UAs and the DAo (average of 4.4 visits per case). For the entire dataset, the DAo PI was strongly correlated to the average UA PI (P < .0001).
Of the 911 measurements, the percent difference in UA PI between the two UAs was 11.4 ± 10.1%. The 90th percentile of the distribution was a discordance of 25%. The UA PIs for the 81 cases who had discordance greater than 25% during one of their visits are presented in Figure 1. The clinical characteristics of the cases (concordant versus discordant UA PIs) are summarized in Table 1. The two groups were similar in terms of clinical characteristics and birth outcomes. In the subset of cases with significant discordance in the UAs, the DAo PI was most strongly associated with the average UA PI (P = .008) and the low UA PI (P = .008). The association between the DAo PI and the high UA PI was attenuated but remained statistically significant (P = .01). Including the study site and sonographer as covariates did not alter the statistical significance.
Figure 1.
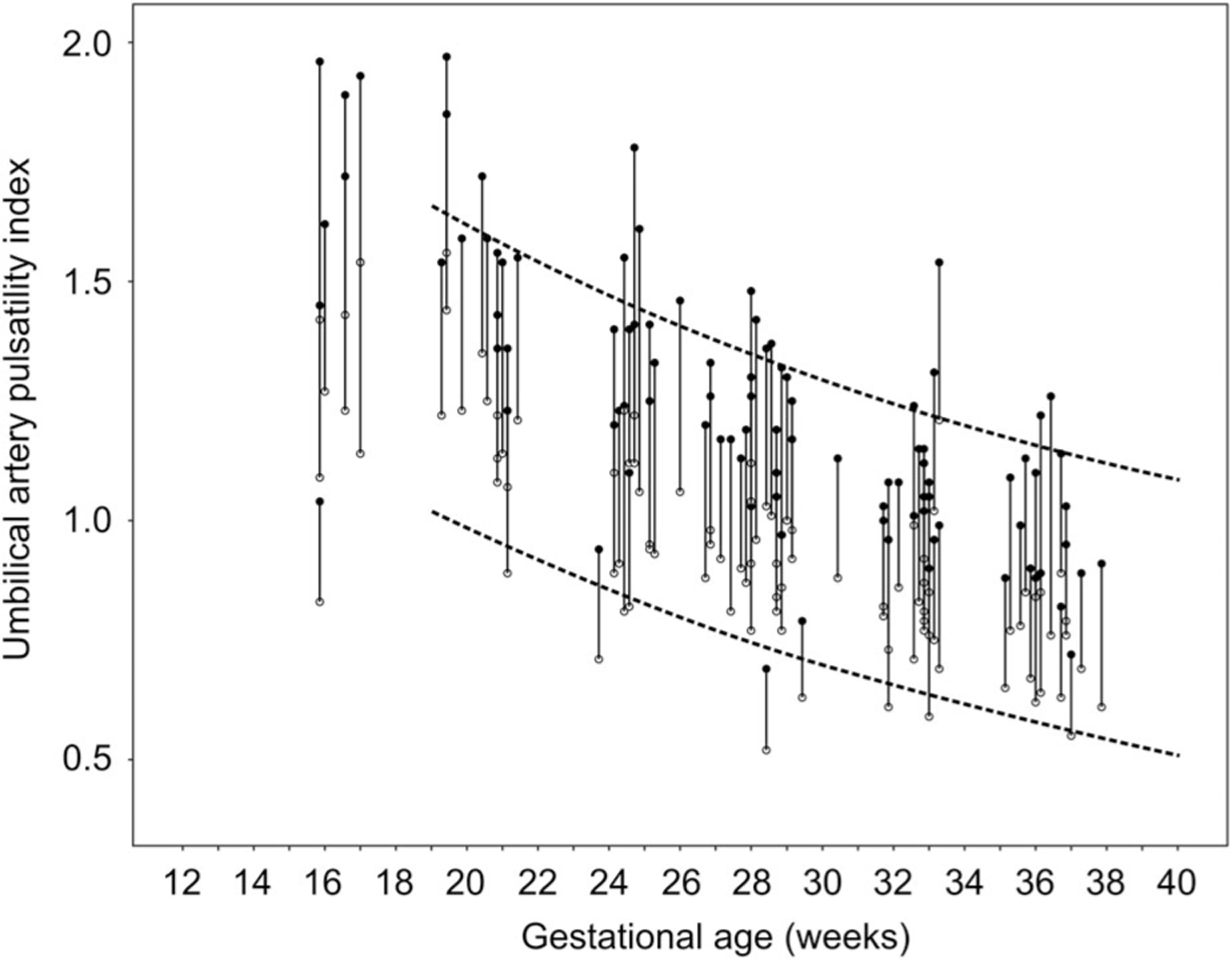
Umbilical artery pulsatility index over gestation for the cases with discordance 25% and greater (n = 81). The subjects are linked by a solid line (high pulsatility index (black circle) and low pulsatility index (open circle)). The dotted lines represent the 5th and 95th percentiles for UA PIs in low-risk pregnancies from Reference 21.
Table 1.
Characteristics of the Study Subjects Meeting the Inclusion Criteria
| Characteristic | Cases With Concordant UA PIs (n = 128) | Cases with Discordant UA PIs (n = 81) | P Value |
|---|---|---|---|
|
| |||
| Maternal age at delivery (years) | 34 [23–43] | 34 [18–43] | .17 |
| Maternal prepregnancy body mass index (kg/m2) | 25.5 [17.5–43.6] | 25.1 [16.5–40.0] | .60 |
| Race (%) | .88 | ||
| Asian | 22 (29/128) | 27 (22/81) | |
| Black | 12 (15/128) | 12 (10/81) | |
| White | 62 (79/128) | 57 (46/81) | |
| Other | 4 (5/128) | 4 (3/81) | |
| Gestational age at delivery (weeks) | 38 [30–41] | 38 [32–41] | .20 |
| Preeclampsia (%) | 4 (5/128) | 9 (7/81) | .22 |
| Cesarean delivery (%) | 39 (50/128) | 40 (32/81) | 1.00 |
| Birth weight (g) | 3136 [1390–4630] | 3226 [1615–4490] | .29 |
| Infant female sex (%) | 53 (68/128) | 43 (35/81) | .20 |
| Small for gestational age (%) | 9 (12/128) | 11 (9/81) | .75 |
| 1-min Apgar score < 7 | 9 (11/128) | 10 (8/81) | .81 |
| 5-min Apgar score < 7 | 1 (1/128) | 1 (1/81) | 1.00 |
| NICU admission | 10 (13/128) | 5 (4/81) | .21 |
| Cord abnormalitya,b | 26 (28/107) | 26 (15/58) | .98 |
| Placental pathologyb,c | 11 (12/107) | 10 (6/58) | .91 |
Data are mean [range] or % (n/N).
Velamentous or marginal insertion (<3 cm from the placental disc margin).
Forty four placentas did not have an umbilical cord insertion or placental pathology assessed.
Defined as maternal vascular malperfusion or fetal vascular malperfusion.
A UA PI above the 95th percentile is one of the defining criteria of placental dysfunction.25,26 Fifteen of the 81 cases (19%) with discordant UA PI measurements at one visit had a high UA PI value above the 95th percentile for gestational age. Figure 2 shows a representative example of the Doppler waveforms, and Table 2 shows the umbilical cord characteristics, DAo PI, and birth outcomes for these cases. Despite an abnormal UA PI in one of the UAs, all 15 of the cases had a normal birth outcome. Moreover, the DAo PI was normal (10–90th percentile) for 14 of the 15 cases.
Figure 2.
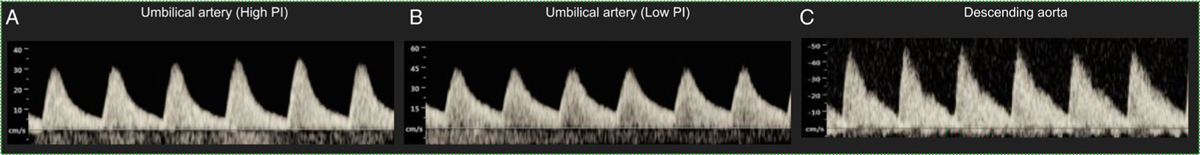
Representative Doppler waveforms for a case with discordant umbilical artery pulsatility indices (Subject 5 in Table 2). A, The umbilical artery pulsatility index of one of the umbilical arteries is higher than the 95th centile for gestational age. B and C, The pulsatility indices of the second umbilical artery and the descending aorta are normal (10–90th centile for gestational age).
Table 2.
Summary of Ultrasound and Clinical Findings in Cases With a UA PI >95th Percentile for Gestational Age in the Subset of Cases With Discordant UA PIs
| Subject ID | Gestational Age (wk+day) | Highest UA PI | Lowest UA PI | Average UA PI | DAo PI | DAo PI Centile | Birth Outcome |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 19+3 | 1.97 | 1.56 | 1.77 | 1.80 | 50–90 | Normal |
| 2 | 19+3 | 1.85 | 1.44 | 1.65 | 2.40 | >90 | Normal |
| 3 | 20+3 | 1.72 | 1.35 | 1.54 | 2.04 | 50–90 | Normal |
| 4 | 24+3 | 1.55 | 1.23 | 1.39 | 1.89 | 50–90 | Normal |
| 5 | 24+5 | 1.78 | 1.22 | 1.50 | 1.59 | 10–50 | Normal |
| 6 | 24+6 | 1.61 | 1.06 | 1.34 | 1.72 | 10–50 | Normal |
| 7 | 26+0 | 1.46 | 1.06 | 1.26 | 1.82 | 50–90 | Normal |
| 28+4 | 1.37 | 1.01 | 1.19 | 1.81 | 10–50 | ||
| 8 | 28+0 | 1.48 | 1.12 | 1.30 | 1.93 | 50–90 | Normal |
| 9 | 28+1 | 1.42 | 0.96 | 1.19 | 2.01 | 50–90 | Normal |
| 10 | 28+3 | 1.36 | 1.03 | 1.20 | 1.83 | 10–50 | Normal |
| 37+3 | 1.26 | 0.76 | 1.01 | 2.12 | 50–90 | ||
| 11 | 28+6 | 1.32 | 0.86 | 1.09 | 1.77 | 10–50 | Normal |
| 12 | 32+4 | 1.24 | 0.99 | 1.12 | 2.07 | 50–90 | Normal |
| 13 | 33+1 | 1.31 | 1.02 | 1.17 | 2.16 | 50–90 | Normal |
| 14 | 33+2 | 1.54 | 1.21 | 1.38 | 2.03 | 50–90 | Normal |
| 15 | 37+1 | 1.22 | 0.85 | 1.04 | 1.90 | 50–90 | Normal |
Discussion
The pair of UAs originate from the fetal internal iliac arteries and perfuse their respective placental territories with Hyrtl’s anastomosis as an equalizing mechanism to ensure balanced placental perfusion and size of the arteries.1,5,27 Although abnormal UA Doppler is associated with placental dysfunction, fetal growth restriction, and hypoxia, there does not appear to be an impact on outcome when this is only observed in one of the vessels.3,11 The overall normal outcome suggests measuring PI at a different vessel may more accurately represent placental vascular resistance in cases of discordant UAs.28 The DAo PI is often used as a surrogate for feto-placental hemodynamics and is associated with increased risk for fetal growth restriction and hypoxia.14,16–18,29 Since the DAo perfuses both UAs, we evaluated the relationship between the blood flow impedance in these vessels under different clinical scenarios. Consistent with previous studies, we observed that discordance in the UA PI was not associated with adverse birth outcomes. In addition, we found that when there is significant discordance in the PI between the two UAs, the blood flow resistance in the DAo is more closely correlated with the more normal PI in the UAs. This finding is consistent with the law for combining resistances in parallel, where the resistance in the feto-placental vascular network is represented as an analogue of an electrical circuit.
Adequate nutrient supply to essential fetal organs and waste disposal is achieved by vascular partitioning across four sequential shunts: the ductus venosus, the foramen ovale, the aortic isthmus, and the UAs as they originate from the iliac vessels.28,30 Of these shunts, the mechanisms that dictate the recirculation of descending aortic blood flow to the placenta are the least well studied. The “hind-limb” reflex is a recognized vascular mechanism where the elevation of distal arterial blood flow resistance favors recirculation of iliac blood flow via the UAs to the placenta.31 Whether this safety mechanism is passive or active is unknown.
Of the cases in our study with discordant UA Doppler, 15 had one UA PI that was above the 95th percentile by gestational age. Fourteen of these had normal DAo PIs, 12 of the 15 had a normal average UA PI, and all 15 cases had a normal birth outcome (delivery at term (>37 weeks’ gestation) with neonatal birth weight appropriate for gestational age). These findings are consistent with those of Harrington et al, indicating that in fetuses with placental dysfunction, variations and degree of abnormality tend to be less in the DAo than in the remainder of the fetal arterial system.20 While current surveillance guidelines recommend collecting multiple UA Doppler blood velocity waveforms,32 they do not specify measurements to be obtained from both UAs, and therefore the information about fetal well-being obtained from one UA could be misleading. Confirming prior results, our study supports collecting UA PI measurements at both arteries to avoid false positives. It is also critical to avoid false negatives as illustrated by a recent study of high-risk pregnancies, which looked at absent or reverse end-diastolic flow in UA Doppler waveforms, the clinical gold standard for detecting compromised fetuses.33 Park et al. reported that 28.6% of cases had a discrepancy between UAs in the end-diastolic flow (present vs. absent) in Doppler waveforms.34 When the UA PI is high, measurement of the DAo PI is likely to be more helpful because it more accurately reflects the sum of the placental afterload in the fetal heart than the UA. The present study illustrates that this is particularly important for cases with discordant UA PIs.
One limitation of the study is that we did not evaluate the Hyrtl’s anastomosis in the study population. An absent anastomosis may explain the discordant UA PIs observed in this study.5 Another limitation is that the measurements for this dataset were collected at two study sites and by different operators, which may have introduced interobserver variability. However, including the study site and sonographer as covariates in the analysis did not change the findings. Finally, we did not have a large population of women with preeclampsia or more severe growth restriction to evaluate the numerical relationships between the DAo and UA PIs. Accordingly, we have no cases where there was discordance for end-diastolic velocities, a circumstance that has potentially great clinical relevance. Here, we only evaluated the DAo PI, while previous studies have reported that classifying DAo waveforms into “blood flow classes” based on both the PI and the presence or absence of end-diastolic velocity was more accurate for detection of growth restriction and fetal distress.18
The principal finding of this work is that in cases with discordance in the UA PI, the DAo PI trends toward the more normal artery. Measuring the DAo PI, particularly when the UA PIs are discordant and one is abnormally high, may have merit for fetal surveillance to avoid false-positive predictions of adverse birth outcome.
Acknowledgments
The authors thank the women who participated in the study. Funding for this work was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of Health Grant 1U01HD087177–01 and the Banting Research Foundation.
Abbreviations
- DAo
descending aorta
- PI
pulsatility index
- UAs
umbilical arteries
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Lindsay S. Cahill, Department of Chemistry, Memorial University of Newfoundland, St John’s, Newfoundland and Labrador, Canada.
Grace V. Mercer, Department of Chemistry, Memorial University of Newfoundland, St John’s, Newfoundland and Labrador, Canada
Dakshita Jagota, Department of Chemistry, Memorial University of Newfoundland, St John’s, Newfoundland and Labrador, Canada.
Anjana Ravi Chandran, Mount Sinai Hospital, Toronto, Ontario, Canada.
Natasha Milligan, Division of Cardiology, Department of Paediatrics, The Hospital for Sick Children, Toronto, Ontario, Canada.
Shiri Shinar, Mount Sinai Hospital, Toronto, Ontario, Canada.
Clare L. Whitehead, Pregnancy Research Centre, Department of Obstetrics and Gynaecology, Royal Women’s Hospital, Parkville, Australia.
Sebastian R. Hobson, Mount Sinai Hospital, Toronto, Ontario, Canada.
Lena Serghides, Toronto General Hospital Research Institute, University Health Network, Toronto, Ontario, Canada; Department of Immunology and Institute of Medical Sciences, University of Toronto, Toronto, Ontario, Canada; Women’s College Research Institute, Women’s College Hospital, Toronto, Ontario, Canada.
W. Tony Parks, Department of Pathology, Mount Sinai Hospital, Toronto, Ontario, Canada; Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada.
Christopher K. Macgowan, Translational Medicine, The Hospital for Sick Children, Toronto, Ontario, Canada; Department of Medical Biophysics, University of Toronto, Toronto, Ontario, Canada.
John C. Kingdom, Mount Sinai Hospital, Toronto, Ontario, Canada; Department of Obstetrics and Gynecology, University of Toronto, Toronto, Ontario, Canada.
John G. Sled, Translational Medicine, The Hospital for Sick Children, Toronto, Ontario, Canada; Department of Medical Biophysics, University of Toronto, Toronto, Ontario, Canada; Department of Obstetrics and Gynecology, University of Toronto, Toronto, Ontario, Canada; Mouse Imaging Centre, The Hospital for Sick Children, Toronto, Ontario, Canada.
Ahmet A. Baschat, Centre for Fetal Therapy, Johns Hopkins Medicine, Baltimore, Maryland, USA.
References
- 1.Raio L, Ghezzi F, Di Naro E, et al. In-utero characterization of the blood flow in the Hyrtl anastomosis. Placenta 2001; 22:597–601. [DOI] [PubMed] [Google Scholar]
- 2.Aoki S, Hata T, Ariyuki Y, Makihara K, Hata K, Kitao M. Antenatal diagnosis of aberrant umbilical vessels. Gynecol Obstet Invest 1997; 43:232–235. [DOI] [PubMed] [Google Scholar]
- 3.Raio L, Ghezza F, Di Naro E, Gomez R, Saile G, Bruhwiler H. The clinical significance of antenatal detection of discordant umbilical arteries. Obstet Gynecol 1998; 91:86–91. [DOI] [PubMed] [Google Scholar]
- 4.Harper MA, Murnaghan A. Discordant umbilical artery flow velocity waveforms and pregnancy outcome. Br J Obstet Gynecol 1989; 96:1449–1452. [DOI] [PubMed] [Google Scholar]
- 5.Hitschold T, Braun S, Weiss E, Berle P, Beck T, Muntfering H. A case of discordant flow velocity waveforms in non-anastomosing umbilical arteries: a morphometric analysis. J Matern Fetal Investig 1992; 2:215–219. [Google Scholar]
- 6.Dolkart LA, Reimers FT, Kuonen CA. Discordant umbilical arteries: ultrasonographic and Doppler analysis. Obstet Gynecol 1992; 79:59–63. [PubMed] [Google Scholar]
- 7.Predanic M, Kolli J, Yousefzadeh P, Pennisi J. Disparate blood flow patterns in parallel umbilical arteries. Obstet Gynecol 1998; 91:757–760. [DOI] [PubMed] [Google Scholar]
- 8.Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, Kingdom JC. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am J Obstet Gynecol 1996; 175:1534–1542. [DOI] [PubMed] [Google Scholar]
- 9.Baschat AA, Hecher K. Fetal growth restriction due to placental disease. Semin Perinatol 2004; 28:67–80. [DOI] [PubMed] [Google Scholar]
- 10.Viero S, Chaddha V, Alkazaleh F, et al. Prognostic value of placental ultrasound in pregnancies complicated by absent end-diastolic flow velocity in the umbilical arteries. Placenta 2004; 25:735–741. [DOI] [PubMed] [Google Scholar]
- 11.Predanic M, Perni SC. Antenatal assessment of discordant umbilical arteries in singleton pregnancies. Croat Med J 2006; 47:701–708. [PMC free article] [PubMed] [Google Scholar]
- 12.Di Naro E, Ghezzi F, Raio L, Franchi M, D’Addario V. Umbilical cord morphology and pregnancy outcome. Eur J Obstet Gynecol Reprod Biol 2001; 96:150–157. [DOI] [PubMed] [Google Scholar]
- 13.Rudolph AM, Heymann MA. Circulatory changes during growth in the fetal lamb. Circ Res 1970; 26:289–299. [DOI] [PubMed] [Google Scholar]
- 14.Madazli R, Uludag S, Ocak V. Doppler assessment of umbilical artery, thoracic aorta and middle cerebral artery in the management of pregnancies with growth restriction. Acta Obstet Gynecol Scand 2001; 80:702–707. [DOI] [PubMed] [Google Scholar]
- 15.Baschat AA. Fetal responses to placental insufficiency: an update. Br J Obstet Gynaecol 2004; 111:1031–1041. [DOI] [PubMed] [Google Scholar]
- 16.Soothill PW, Nicolaides KH, Bilardo CM, Campbell S. Relation of fetal hypoxia in growth retardation to mean blood velocity in the fetal aorta. Lancet 1986; 15:1118–1120. [DOI] [PubMed] [Google Scholar]
- 17.Tonge HM, Wladimiroff JM, Noordam MJ, van Kooten C. Blood flow velocity waveforms in the descending fetal aorta: comparison between normal and growth-retarded pregnancies. Obstet Gynecol 1986; 67:851–855. [DOI] [PubMed] [Google Scholar]
- 18.Laurin J, Lingman G, Marsal K, Persson PH. Fetal blood flow in pregnancies complicated by intrauterine growth retardation. Obstet Gynecol 1987; 69:895–902. [PubMed] [Google Scholar]
- 19.Gudmundsson S, Marsal K. Blood velocity waveforms in the fetal aorta and umbilical artery as predictors of fetal outcome: a comparison. Am J Perinatol 1991; 8:1–6. [DOI] [PubMed] [Google Scholar]
- 20.Harrington K, Thompson MO, Carpenter RG, Nguyen M, Campbell S. Doppler fetal circulation in pregnancies complicated by pre-eclampsia or delivery of a small for gestational age baby: 2. Longitudinal analysis. Br J Obstet Gynaecol 1999; 106:453–466. [DOI] [PubMed] [Google Scholar]
- 21.Acharya G, Wilsgaard T, Berntsen GK, Malta JM, Kiserud T. Reference ranges for serial measurements of umbilical artery Doppler indices in the second half of pregnancy. Am J Obstet Gynecol 2005; 192:937–944. [DOI] [PubMed] [Google Scholar]
- 22.Gosling RG, King DH. Ultrasound angiology. In: Marcus AW, Adamson L (eds). Arteries and Veins. Edinburgh: Churchill Livingstone; 1975:61–98. [Google Scholar]
- 23.Kramer MS, Platt RW, Wen SW, et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics 2001; 108:E35. [DOI] [PubMed] [Google Scholar]
- 24.Khong TY, Mooney EE, Ariel I, et al. Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch Pathol Lab Med 2016; 140:698–713. [DOI] [PubMed] [Google Scholar]
- 25.Lees C, Marlow N, Arabin B, et al. Perinatal morbidity and mortality in early-onset fetal growth restriction: cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE). Ultrasound Obstet Gynecol 2013; 42: 400–408. [DOI] [PubMed] [Google Scholar]
- 26.Unterscheider J, Daly S, Geary MP, et al. Optimizing the definition of intrauterine growth restriction: the multicenter prospective PORTO study. Am J Obstet Gynecol 2013; 208:290.e1–290.e6. [DOI] [PubMed] [Google Scholar]
- 27.Priman J A note on the anastomosis of the umbilical arteries. Anat Rec 1959; 134:1–5. [DOI] [PubMed] [Google Scholar]
- 28.Baschat AA. The fetal circulation and essential organs—a new twist to an old tale. Ultrasound Obstet Gynecol 2006; 27:349–354. [DOI] [PubMed] [Google Scholar]
- 29.Jouppila P, Kirkinen P. Increased vascular resistance in the descending aorta of the human fetus in hypoxia. Br J Obstet Gynaecol 1984; 91:853–856. [DOI] [PubMed] [Google Scholar]
- 30.Sheldon RE, Peeters LL, Jones MD Jr, Makowski EL, Meschia G. Redistribution of cardiac output and oxygen delivery in the hypoxemic fetal lamb. Am J Obstet Gynecol 1979; 135: 1071–1078. [DOI] [PubMed] [Google Scholar]
- 31.Akalin-Sel T, Campbell S. Understanding the pathophysiology of intrauterine growth retardation: the role of the ‘lower limb reflex’ in redistribution of blood flow. Eur J Obstet Gynecol Reprod Biol 1992; 46:79–86. [DOI] [PubMed] [Google Scholar]
- 32.Antepartum Fetal Surveillance. ACOG practice bulletin no. 145. American College of Obstetricians and Gynecologists. Obstet Gynecol 2014; 124:182–192. [DOI] [PubMed] [Google Scholar]
- 33.Spinillo A, Gardella B, Bariselli S, Alfei A, Silini E, Dal BB. Placental histopathological correlates of umbilical artery Doppler velocimetry in pregnancies complicated by fetal growth restriction. Prenat Diagn 2012; 32:1263–1272. [DOI] [PubMed] [Google Scholar]
- 34.Parks JW, Lee SM, Kang H-S, Shim S-S, Jun JK. Discrepancy in Doppler waveforms between two umbilical arteries in fetuses with absent or reversed end-diastolic flow: a prospective observational study. J Clin Ultrasound 2019; 47:526–530. [DOI] [PubMed] [Google Scholar]
- 35.Bahlmann F, Wellek S, Reinhardt I, Krummenauer F, Merz E, Welter C. Reference values of fetal aortic flow velocity waveforms and associated intra-observer reliability in normal pregnancies. Ultrasound Obstet Gynecol 2001; 17:42–49. [DOI] [PubMed] [Google Scholar]


