Abstract
Background
We assessed the effect of supervised, combined aerobic and resistance exercise on diabetic parameters in Japanese patients with type 2 diabetes mellitus (T2DM).
Methods
This 12-week, multicenter (17 medical institutions), open-label, parallel-group study (clinicaltrials.jp; JapicCTI-184002), randomized (1:1) Japanese patients aged 20–75 years with T2DM and hemoglobin A1c (HbA1c) of 7.0–10.0% to supervised exercise (n = 113) or standard therapy (n = 115). The supervised exercise group undertook supervised aerobic (30 min) and resistance exercise 3 times/week (20 designated gyms). Primary endpoint was change in HbA1c from baseline at week 13. Secondary endpoints were change in fasting blood glucose (FBG), glycoalbumin, fasting insulin, homeostatic model assessment of insulin resistance (HOMA-IR), and HOMA-β at week 13.
Results
Of 228 randomized patients, 97 (85.8%) in the supervised exercise group and 108 (93.9%) in the standard therapy group completed the study. Supervised exercise significantly lowered HbA1c at week 13 versus standard therapy (estimated difference in change from baseline [95% confidence interval]: − 0.44% [− 0.61, − 0.28], p < 0.001). Supervised exercise also significantly decreased FBG (estimated difference: − 13.0 [− 19.2, − 6.7] mg/dL) and glycoalbumin (estimated difference: − 1.52% [− 2.10, − 0.93]) compared with standard therapy. Fasting insulin (− 0.5 µIU/mL) and HOMA-IR (− 0.3) decreased with supervised exercise, but group differences were not significant. Treatment-emergent adverse events were more frequent in the supervised exercise group (42.5%) than in the standard therapy group (29.6%); however, no major safety concerns were identified.
Conclusions
A structured, supervised, aerobic and resistance exercise program improved HbA1c and was well accepted among patients with T2DM.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13340-021-00506-5.
Keywords: Diabetes mellitus, Type 2, Exercise, HbA1c, Japan, Randomized controlled trial
Introduction
Regular exercise is well known to provide substantial benefits to patients with type 2 diabetes mellitus (T2DM) [1–4]. Specifically, regular aerobic and/or resistance exercise is associated with lower hemoglobin A1c (HbA1c), triglycerides, blood pressure, and insulin resistance, as well as reduced fat mass, increased strength, and increased lean body mass [1–4]. Although the benefits of exercise are well known, the best combination of aerobic and resistance exercise, as well as the duration and frequency for maximum impact, remains unclear [1, 2, 5–7]. Japanese T2DM treatment guidelines recommend that moderate-intensity exercise be implemented for 20–60 min per day or 3–5 times a week for a total of 150 min or longer per week [8, 9]. In addition, these guidelines specify that resistance exercise should be implemented 2–3 times a week [8, 9]; however, the exact form of exercise and the best combination of aerobic and resistance exercise are not specified in Japanese guidelines or in guidelines from other countries [8–10].
Despite healthcare professionals suggesting patients implement regular exercise regimens for management of T2DM, in Japan, 30% of patients do not implement the prescribed exercise program and less than 50% of patients implement the exercise regimen on a regular basis [11]. Factors such as guidance on frequency of exercise therapy and detailed exercise prescription have been shown to have a positive effect on patients who were performing exercise therapy [12]. Supervised exercise has also been shown to have a greater effect on HbA1c levels compared with unsupervised exercise [6]. Despite a large number of studies assessing the impact of exercise as a form of therapy for T2DM, trials in Japan [13, 14] have not analyzed a large number of patients, and the best exercise prescription remains to be elucidated. This is the first randomized controlled trial to assess the effect of a specific exercise program conducted under supervision in a relatively large cohort of Japanese patients.
Materials and methods
Study design
This 12-week, multicenter, randomized, open-label, parallel-group study in Japanese patients with T2DM was conducted at 17 medical institutions from July 2018 to November 2019. Patients were randomized (1:1) to a supervised exercise group or a standard therapy group. Randomization was conducted using a dynamic allocation method, with medical institution, screening HbA1c level (< 8.0%, ≥ 8.0%), body mass index (BMI) (< 25 kg/m2, ≥ 25 kg/m2), sex, and age (≤ 64 years, ≥ 65 years) as allocation factors. Patients randomized to the supervised exercise group conducted a specific exercise program at any of 20 designated sports gyms under one-on-one guidance from a trainer who recorded the session and noted whether exercises were carried out correctly and whether patients met intensity targets (Supplementary Table S1). Patients were fully compensated for all costs associated with visiting the gym, including usage fees and transportation costs. All patients in both groups continued with their current diet therapy and normal daily activity; patients randomized to the supervised exercise group performed the supervised exercise in addition to their normal activity.
The exercise program was divided into 3 periods (initial period: weeks 1–2; titration period: weeks 3–4; and maintenance period: weeks 5–12) (Supplementary Table S1), and both aerobic and resistance exercises were conducted during each session, 3 times a week. Aerobic exercise was conducted for 30 min at each session, and the intensity increased gradually throughout the study, with a target to achieve light intensity (based on heart rate reserve) in the first 2 weeks, moderate intensity in weeks 3–4, and vigorous intensity from week 5 to the end of the study, as recommended [15–18]. Heart rate reserve was measured using a Polar M430 running watch (Polar Electro Oy, Kempele, Finland). For resistance exercise, patients aimed to achieve moderate-to-vigorous intensity (as recommended [15–18]), based on the repetition maximum (maximum amount of weight that a person can possibly lift for 1 repetition) assessed on the first day of weeks 1, 5, and 9.
This study was performed in compliance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects and the Declaration of Helsinki. The study protocol was approved by the local ethics committee at each medical institution, the Medical Corporation Toukeikai Kitamachi Clinic Ethical Review Board (approval number AST05921, approved June 28, 2018), or the Joint Ethical Review Board (approval number 14000050.20180817-4654, approved August 17, 2018). All patients provided written informed consent before participating. The study was registered at clinicaltrials.jp (JapicCTI-184002).
Study population
Eligible patients were male or female, aged 20–75 years at the time of consent, were diagnosed with T2DM at least 12 weeks before providing consent, and had a BMI of 20.0–35.0 kg/m2 at screening. All patients had to have HbA1c levels between 7.0% and 10.0% at screening and fluctuations of ≤ ± 1.0% in HbA1c levels within 3 months from screening to analysis. Patients agreed not to participate in any other intervention study. All patients who consented also agreed to conduct the exercise program 3 times a week. In addition, female patients were included if they were menopausal at the time of consent and had not menstruated for ≥ 1 year, had undergone surgical sterilization, or if of reproductive age, had a negative pregnancy test at screening and agreed to use contraception during the study. The need for documenting the use of contraception was based on the terms set by the sports gyms providing the exercise equipment, with no documentation of the type of contraceptive medication used, and patients were permitted to discontinue the study if they wished to become pregnant.
Patients were not eligible if they had received any insulin preparations, sulfonylureas, or rapid-acting insulin secretagogues at the time of consent or during the study or had a change in administration of any other oral diabetes drug following consent. Patients who planned to start or change their dietary intervention during the study, had been exercising regularly for ≥ 20 min for 3 or more times a week, or conducted any other regular resistance exercise in the 6 months before providing consent were excluded. Patients were also excluded if they had: a diagnosis of type 1 diabetes, diabetic retinopathy, diabetic neuropathy, diabetic nephropathy, or severe hypertension; a history or diagnosis of severe heart disease, cerebrovascular accident, or peripheral occlusive arterial disease; a combination of musculoskeletal disorders or severe respiratory illness that would make it difficult to carry out the prescribed exercise program, as determined by the attending physician; acute or severe infection; an immune disease requiring steroids or immunosuppressants; malignant tumor (unless treatment was not required for ≥ 5 years before the study); systolic blood pressure (SBP) ≥ 160 mmHg, diastolic blood pressure (DBP) ≥ 100 mmHg, pulse rate ≥ 130 beats/min, or electrocardiogram (ECG) abnormality grade > 2; brain natriuretic peptide (BNP) ≥ 100 pg/mL; fasting blood glucose (FBG) ≥ 250 mg/dL; or moderate or high urinary ketone bodies.
Outcome measures
The primary endpoint was change in HbA1c from baseline at week 13. The secondary endpoints were change in FBG, glycoalbumin, fasting insulin, homeostatic model assessment of insulin resistance (HOMA-IR, calculated as FBG × fasting insulin ÷ 405), and HOMA-β (calculated as [fasting insulin × 360] ÷ [FBG − 63]) at week 13. Clinic visits were conducted at screening and at weeks 0, 5, 9, 13, and 15 (follow-up), and the following clinical measurements were conducted: fasting insulin, triglycerides, low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, glycoalbumin, BNP, FBG, HbA1c (calculated using the National Glycohemoglobin Standardization Program method), urinary ketone bodies, infectious disease test results, height, body weight, waist circumference, blood pressure, pulse rate, and ECG. For patients in the supervised exercise group, exercise data, muscle mass, and body fat percentage were recorded by the trainer at weeks 1 (before the exercise program was started), 5, 9, and 12.
Safety measures comprising treatment-emergent adverse events (TEAEs) that occurred from the time of enrollment to week 15 were identified by self-report or from clinical findings at each visit. The incidence of each TEAE was calculated by referring to the Japanese translation of the Medical Dictionary for Regulatory Activities and summarized by system organ class and preferred term. The rates of exercise-related TEAEs, serious TEAEs, and TEAEs leading to discontinuation were also assessed.
Statistical analysis
The statistical analysis plan was finalized before the availability of the first patient’s primary endpoint, and only minor changes were made subsequently. The planned sample size was 109 patients in each group. This sample size was based on an assumed discontinuation rate of 20% and calculated to give 80% power for statistical significance with a 2-sided significance level of 0.05.
Efficacy analyses were performed on the full analysis set (FAS), which included patients with data for at least 1 efficacy variable measured after randomization. For patients in the supervised exercise group, at least 1 efficacy variable had to be evaluated after the first exercise.
For the primary endpoint, change from baseline in HbA1c at week 13 was calculated as least squares (LS) means using mixed-model repeated measures (MMRM). Treatment group and week were used as factors, with baseline levels used as a covariate, as well as an interaction of treatment by week and an interaction of baseline measurement by week. The differences between groups for HbA1c, FBG, glycoalbumin, fasting insulin, HOMA-IR, and HOMA-β were estimated using MMRM with 2-sided 95% confidence intervals (CIs).
Safety outcomes were assessed on the safety analysis set, which included patients who were randomized to each group, and were reported using descriptive statistics. All analyses were performed using SAS® software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patient disposition
A total of 285 patients were enrolled and provided informed consent (Fig. 1). Of these, 228 patients were considered eligible at screening and randomized into 2 groups (supervised exercise = 113; standard therapy = 115) comprising the safety analysis set population. One patient who had previously received rapid-acting insulin secretagogue treatment was enrolled and randomized to the standard therapy group; this patient was included in both the safety analysis set and the FAS. Of the 228 patients randomized, 3 patients from the supervised exercise group were excluded from the FAS population as they did not conduct any of the exercises. One patient in the standard therapy group did not have any efficacy variables measured after baseline and was excluded from the FAS. Of the randomized patients, 97 (85.8%) in the supervised exercise group and 108 (93.9%) in the standard therapy group completed the study. The primary reason for discontinuation was adverse events in the supervised exercise group (n = 5) and protocol deviation in the standard therapy group (n = 2). In the supervised exercise group, other reasons for discontinuation were shutdown of the medical institution (n = 3), difficulty attending the gym (n = 2), too busy (n = 2), inconvenient to go to the gym, patient decision, patient desire to exercise on their own and not at a gym, and unable to attend the exercise session in the first week following registration (n = 1 each).
Fig. 1.
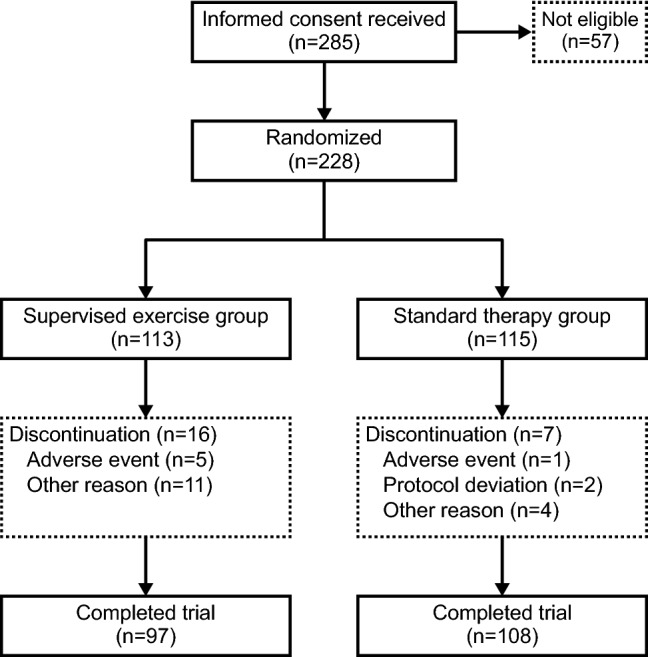
Patient disposition and discontinuations
Demographics and baseline clinical characteristics
Demographics and baseline characteristics were generally balanced between groups (Table 1). Approximately 60% of patients were male, with a mean age of approximately 55 years. Patients in both groups were overweight with an average BMI of approximately 26–27 kg/m2 and had average HbA1c levels of 7.7%. Baseline SBP and DBP were well controlled, and more than 75% of patients in both groups were on antidiabetic drug therapy, most commonly biguanides or dipeptidyl peptidase-4 inhibitors (Supplementary Table S2).
Table 1.
Demographics and baseline characteristics for FAS
| Variable | Supervised exercise (N = 110) | Standard therapy (N = 114) |
|---|---|---|
| Age, mean (SD) | 54.6 (9.2) | 55.7 (10.2) |
| Female, n (%) | 42 (38.2) | 45 (39.5) |
| Anthropometrics, mean (SD) | ||
| Weight, kg | 73.15 (12.64) | 71.88 (12.37) |
| BMI, kg/m2 | 26.66 (3.77) | 26.25 (3.59) |
| Waist circumference, cm | 92.25 (9.47) | 92.35 (9.70) |
| Diabetes factors, mean (SD) | ||
| HbA1c, % | 7.68 (0.60) | 7.71 (0.60) |
| FBG, mg/dL | 147.5 (27.8) | 151.4 (30.5) |
| Glycoalbumin, % | 19.32 (2.94) | 19.75 (2.99) |
| Fasting insulin, µIU/mL | 9.22 (5.84) | 7.36 (4.55) |
| Urinary ketone bodiesa, n (%) | ||
| Negative ( − ) | 97 (88.2) | 106 (93.0) |
| Trace (±) | 1 (0.9) | 2 (1.8) |
| 1 + | 10 (9.1) | 4 (3.5) |
| 2 + | 2 (1.8) | 2 (1.8) |
| Diabetes durationb, months | 89.7 (69.5)c | 87.9 (72.7) |
| Antidiabetic drug therapyd, n (%) | 85 (77.3) | 87 (76.3) |
| Medical diet therapy, n (%) | 50 (45.5) | 51 (44.7) |
| Exercise instruction, n (%) | 22 (20.0) | 24 (21.1) |
| Cardiovascular factors, mean (SD) | ||
| Systolic BP, mmHg | 126.2 (11.8) | 126.0 (14.2) |
| Diastolic BP, mmHg | 78.1 (10.5) | 78.1 (10.4) |
| Pulse rate, beats/min | 79.1 (10.5) | 76.8 (11.7) |
| LDL-cholesterol, mg/dL | 124.9 (31.1) | 128.1 (34.8) |
| HDL-cholesterol, mg/dL | 55.6 (16.0) | 58.5 (13.8) |
| Triglycerides, mg/dL | 165.8 (134.9) | 154.6 (118.5) |
| BNPa, pg/mL | 8.70 (7.04) | 10.43 (7.72) |
BMI body mass index, BNP brain natriuretic peptide, BP blood pressure, FAS full analysis set, FBG fasting blood glucose, HbA1c hemoglobin A1c, HDL high-density lipoprotein, LDL low-density lipoprotein, SD standard deviation
aAt screening
bAt consent
cN = 109
dSee Supplementary Table S2 for antidiabetic drug classes
Primary efficacy endpoint
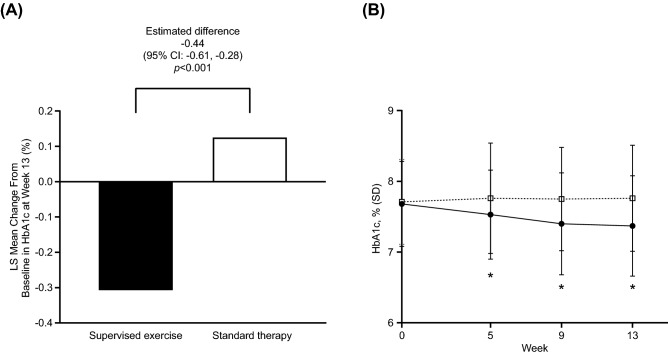
Patients in the standard therapy group had an increase in HbA1c levels at week 13 (LS mean change from baseline = 0.13%). In contrast, exercise intervention had a significant effect on HbA1c levels, with an LS mean change from baseline at week 13 of −0.31% in the supervised exercise group and an estimated difference between the 2 groups of − 0.44% (95% CI −0.61, −0.28, p < 0.001) (Fig. 2a). A significant decrease in HbA1c levels was observed in patients after just 5 weeks of supervised exercise, which continued to decrease for the duration of the study (Fig. 2b), whereas HbA1c levels remained constant for patients in the standard therapy group. The mean (standard deviation) HbA1c level decreased from 7.68% (0.60%) at baseline to 7.37% (0.71%) at week 13 in the supervised exercise group and increased from 7.71% (0.60%) at baseline to 7.76% (0.75%) at week 13 in the standard therapy group.
Fig. 2.
a LS mean change from baseline in HbA1c at week 13 and b change over time in HbA1c (FAS). Closed circles = supervised exercise group; open squares = standard therapy group. CI confidence interval; FAS full analysis set; HbA1c hemoglobin A1c; LS least squares; MMRM mixed-model repeated measures; SD standard deviation. *p < 0.001 between groups for the LS mean change from baseline, MMRM with an unstructured covariance matrix within patients. The model considered randomization group (supervised exercise, standard therapy), week, baseline HbA1c, and week-by-randomization group interaction and week-by-baseline interaction as explanatory variables
Key secondary endpoints
Overall, exercise had a significant effect on a number of laboratory measures (Table 2). Significantly greater decreases in FBG and glycoalbumin levels were observed in patients in the supervised exercise group compared with the standard therapy group (estimated difference: FBG = −13.0 mg/dL [95% CI −19.2, −6.7], p < 0.001; glycoalbumin = −1.52% [95% CI −2.10, −0.93], p < 0.001), and both parameters increased slightly from baseline to week 13 in the standard therapy group (Table 2; Supplementary Fig. S1). Improvement in insulin resistance was observed in patients in the supervised exercise group as shown by decreases in fasting insulin (− 0.482 µIU/mL) and HOMA-IR (− 0.2778); however, the differences between the 2 groups were not statistically significant for either measure at week 13 (Table 2; Supplementary Fig. S1 and S2). Homeostatic β-cell dysfunction assessed by HOMA-β was slightly improved in the supervised exercise group at week 13 (LS mean change = 0.7340) compared with patients in the standard therapy group (LS mean change = −1.6589); however, the difference between the 2 groups was not significant (Table 2; Supplementary Fig. S2). No significant changes were observed in SBP, DBP, body weight, or waist circumference (Table 2; Supplementary Fig. S3). Exercise intervention significantly decreased triglyceride levels at weeks 5 and 9, but not at week 13 (Supplementary Fig. S4). No significant difference was observed in LDL-cholesterol; however, a significant increase was observed at week 13 in HDL-cholesterol in the supervised exercise group compared with the standard therapy group (2.6 mg/dL [95% CI 0.8, 4.4], p = 0.004) (Table 2; Supplementary Fig. S4).
Table 2.
Results of key secondary endpoints for the FAS
| Variable | Treatment group | Mean at baseline (SD) [n] | Mean at week 13 (SD) [n] | LS mean change from baseline at week 13a | Estimated differencea,b (95% CI) | p valuea,b |
|---|---|---|---|---|---|---|
| FBG, mg/dL | Supervised exercise | 147.5 (27.8) [109] | 143.0 (25.7) [97] | − 6.1 | − 13.0 (− 19.2, − 6.7) | < 0.001 |
| Standard therapy | 151.4 (30.5) [114] | 155.3 (28.7) [107] | 6.9 | |||
| Glycoalbumin, % | Supervised exercise | 19.32 (2.94) [110] | 18.57 (3.15) [97] | − 0.75 | − 1.52 (− 2.10, − 0.93) | < 0.001 |
| Standard therapy | 19.75 (2.99) [114] | 20.18 (3.19) [108] | 0.77 | |||
| Fasting insulin, µIU/mL | Supervised exercise | 9.22 (5.84) [109] | 8.77 (5.98) [97] | − 0.48 | − 0.47 (− 1.46, 0.52) | 0.348 |
| Standard therapy | 7.36 (4.55) [114] | 7.51 (5.48) [107] | − 0.01 | |||
| HOMA-IR | Supervised exercise | 3.41 (2.33) [109] | 3.11 (2.19) [97] | − 0.28 | − 0.31 (− 0.73, 0.10) | 0.138 |
| Standard therapy | 2.74 (1.71) [114] | 2.84 (1.98) [107] | 0.04 | |||
| HOMA-β | Supervised exercise | 42.04 (27.02) [109] | 42.52 (30.84) [97] | 0.73 | 2.39 (− 2.90, 7.68) | 0.373 |
| Standard therapy | 33.41 (24.88) [114] | 32.55 (30.75) [107] | − 1.66 | |||
| Triglycerides, mg/dL | Supervised exercise | 165.8 (134.9) [110] | 154.8 (117.4) [97] | − 12.4 | − 6.9 (− 28.2, 14.4) | 0.523 |
| Standard therapy | 154.6 (118.5) [114] | 148.5 (107.0) [108] | − 5.5 | |||
| LDL-cholesterol, mg/dL | Supervised exercise | 124.9 (31.1) [110] | 127.8 (32.5) [97] | 2.0 | 0.9 (− 4.1, 5.9) | 0.715 |
| Standard therapy | 128.1 (34.8) [114] | 127.4 (30.8) [108] | 1.0 | |||
| HDL-cholesterol, mg/dL | Supervised exercise | 55.6 (16.0) [110] | 57.5 (15.8) [97] | 1.7 | 2.6 (0.8, 4.4) | 0.004 |
| Standard therapy | 58.5 (13.8) [114] | 57.6 (12.9) [108] | –0.9 | |||
| Systolic BP, mmHg | Supervised exercise | 126.2 (11.8) [110] | 124.9 (13.9) [97] | − 0.8 | 1.2 (− 1.7, 4.1) | 0.407 |
| Standard therapy | 126.0 (14.2) [114] | 123.6 (11.6) [108] | − 2.0 | |||
| Diastolic BP, mmHg | Supervised exercise | 78.1 (10.5) [110] | 76.1 (10.6) [97] | − 2.0 | − 0.5 (− 2.5, 1.4) | 0.587 |
| Standard therapy | 78.1 (10.4) [114] | 76.3 (10.9) [108] | − 1.5 | |||
| Body weight, kg | Supervised exercise | 73.15 (12.64) [110] | 73.44 (12.84) [97] | − 0.42 | − 0.31 (− 0.77, 0.15) | 0.188 |
| Standard therapy | 71.88 (12.37) [114] | 71.58 (12.46) [108] | − 0.11 | |||
| Waist circumference, cm | Supervised exercise | 92.25 (9.47) [110] | 91.69 (9.01) [97] | − 1.01 | − 0.91 (− 1.87, 0.06) | 0.066 |
| Standard therapy | 92.35 (9.70) [114] | 92.01 (9.90) [108] | − 0.10 |
BP blood pressure, CI confidence interval, FAS full analysis set, FBG fasting blood glucose, HDL high-density lipoprotein, HOMA-IR homeostatic model assessment for insulin resistance, HOMA-β homeostatic model assessment for beta-cell function, LDL low-density lipoprotein, LS least squares, MMRM mixed-model repeated measures, SD standard deviation
aMMRM with an unstructured covariance matrix within patients was used. The differences between groups were estimated using MMRM with 2-sided 95% CIs. Significance level: 0.05 (2-sided)
bEstimated difference between supervised exercise and standard therapy groups
Overview of TEAEs
Overall, the incidence of TEAEs was higher in the supervised exercise group (42.5%) compared with the standard therapy group (29.6%) (Table 3); however, no major safety concerns were identified. The most common TEAE was nasopharyngitis (supervised exercise = 19.5%; standard therapy = 12.2%). Musculoskeletal and connective tissue disorders were more common in the supervised exercise group (10.6%) compared with the standard therapy group (5.2%). More patients in the supervised exercise group (2.7%) experienced upper respiratory tract inflammation compared with patients in the standard therapy group (0.9%). Exercise-related TEAEs were reported in 11 (9.7%) patients in the supervised exercise group, which were muscle pain (n = 4), back pain (n = 2), muscle strain, blood pressure decreased, osteoarthritis, headache, and dizziness (n = 1 each). Of the patients who reported an exercise-related TEAE, 3 (2.7%) patients discontinued the study due to muscle strain, back pain, and osteoarthritis (n = 1 each). Only 1 patient (0.9%) in the supervised exercise group and 2 patients (1.7%) in the standard therapy group reported a serious TEAE. Head distortion was reported as a serious TEAE by 1 patient in the supervised exercise group, who recovered after 16 days of follow-up. In the standard therapy group, 1 patient reported stress cardiomyopathy and recovered after 49 days of follow-up. The other patient reported macular degeneration, but did not recover during the follow-up period; however, the incident was judged to be no longer clinically significant by the investigator. No exercise-related serious TEAEs or hypoglycemic events were reported for either group.
Table 3.
Summary of TEAEsa by SOC and preferred term occurring in ≥ 2 patients in either group (SAF)b
| SOC Preferred term, n (%) |
Supervised exercise (N = 113) |
Standard therapy (N = 115) |
|---|---|---|
| Overall | 48 (42.5) | 34 (29.6) |
| Gastrointestinal disorders | 2 (1.8) | 4 (3.5) |
| Dental caries | 2 (1.8) | 1 (0.9) |
| Infections and infestations | 28 (24.8) | 20 (17.4) |
| Bronchitis | 2 (1.8) | 0 |
| Gastroenteritis | 1 (0.9) | 2 (1.7) |
| Herpes zoster | 0 | 2 (1.7) |
| Influenza | 3 (2.7) | 2 (1.7) |
| Nasopharyngitis | 22 (19.5) | 14 (12.2) |
| Injury, poisoning, and procedural complications | 5 (4.4) | 2 (1.7) |
| Wound | 2 (1.8) | 1 (0.9) |
| Musculoskeletal and connective tissue disorders | 12 (10.6) | 6 (5.2) |
| Back pain | 3 (2.7) | 3 (2.6) |
| Myalgia | 4 (3.5) | 0 |
| Respiratory, thoracic, and mediastinal disorders | 5 (4.4) | 3 (2.6) |
| Upper respiratory tract inflammation | 3 (2.7) | 1 (0.9) |
| Hypoglycemic eventsc | 0 | 0 |
MedDRA/J Japanese translation of Medical Dictionary for Regulatory Activities, SAF safety analysis set, SOC system organ class, TEAE treatment-emergent adverse event
aTEAEs are as per MedDRA/J v21.1
bThe SAF population consists of patients who were randomized to each group
cHypoglycemic events were TEAEs that were judged by physicians as being associated with hypoglycemia
Discussion
This is the first randomized study to assess the impact of a supervised exercise program combining aerobic and resistance exercise, detailing the type of activity, duration, and frequency in a relatively large cohort of Japanese patients with T2DM over a 12-week period. The study demonstrated that supervised combined aerobic and resistance exercise significantly lowered HbA1c levels and significantly decreased other laboratory measures such as FBG and glycoalbumin. Improvements were also observed in insulin resistance parameters (fasting insulin and HOMA-IR) and β-cell dysfunction; however, these were not statistically significant. Overall, exercise was well accepted by patients and no serious TEAEs were associated with exercise intervention.
Regular exercise intervention significantly lowered HbA1c levels after 12 weeks in the supervised exercise group compared with the standard therapy group, which had a slight increase in HbA1c. These results are consistent with previous small (< 40 participants) and/or non-Japanese randomized studies, indicating that a combination of aerobic and resistance exercise is associated with decreases in HbA1c levels and improvements in insulin resistance [13, 19–21]. Japanese clinical practice guidelines specify that patients should aim to achieve an HbA1c target of < 7.0% to prevent complications and < 6.0% when aiming for normal glycemia [16]. Although a significant change in mean HbA1c was observed with exercise intervention in this study, not all patients in the supervised exercise group achieved HbA1c < 7.0% and none achieved HbA1c < 6.0%. This could be due to the short duration of the study as similar results were observed in 2 small (< 40 patients) Japanese exercise intervention studies conducted over 12–16 weeks [13, 14], whereas HbA1c levels of approximately 6.5% were achieved in Canadian patients who partook in a randomized controlled trial of combined exercise intervention for 6 months [20]. Importantly, in the current study, a significant decrease in HbA1c levels was observed as early as week 5, suggesting that exercise intervention had a rapid effect. Such early reductions in HbA1c may help encourage patients to continue with the exercise program, resulting in sustained glycemic control.
Structured exercise has been reported to be as effective as antidiabetic medication in improving glycemic control [22]. Metformin has been shown to lower HbA1c levels by approximately 0.2% after 4 weeks and by approximately 0.4% after 13 weeks of treatment, which is similar to the changes observed in this study [23]. In that same study, dulaglutide 1.5 mg lowered HbA1c levels by approximately 0.5% after 4 weeks of treatment and by approximately 0.7% by 13 weeks [23]. A similar effect was reported for alogliptin 12.5 mg (− 0.60%) after 12 weeks of treatment in combination with sulfonylurea [24]. Interestingly, a similar decrease was observed in a randomized, placebo-controlled trial of alogliptin monotherapy, but only after 26 weeks of treatment [25]. Notably, in the current study, changes in HbA1c levels were achieved through exercise alone without any change to diet or pharmaceutical intervention. For patients trying to manage their diabetes, it may be beneficial to know that exercise intervention can improve diabetic parameters. This may provide physicians with the option to prescribe exercise as an initial intervention before introducing or changing antidiabetic medication.
Overall, exercise was well tolerated; however, discontinuation rates were slightly higher in the supervised exercise group compared with the standard therapy group, with adverse events contributing to the difference between groups. Exercise-related TEAEs such as muscle strain or pain, back pain, and osteoarthritis were expected as patients in the supervised exercise group were more likely to injure themselves or have muscle pain compared with patients who were not exercising. Scheduling issues were also a contributor to the discontinuation rate in the supervised exercise group as some people were not able to attend the gym 3 times a week. Low or no discontinuations have been reported in a number of randomized controlled studies of supervised exercise intervention conducted in non-Japanese [19, 21, 26] and Japanese patients [13, 14]. A systematic review assessing structured exercise versus activity advice only showed that structured exercise programs had a dropout rate of < 20% [7], which is similar to what was observed in this study. In addition, structured exercise is associated with a more pronounced HbA1c reduction compared with advice only [3, 6, 7], suggesting that exercise prescription should involve a detailed exercise program for maximum benefit in diabetes management. In Japan, adherence to exercise prescription is low, with < 50% of patients implementing prescribed exercise programs regularly and 30% not implementing prescribed exercise programs at all [11, 27]. The most common reasons for discontinuing exercise were lack of time or interest and insufficient understanding of the exercise prescription [11, 27]. Similarly, lack of time and inconvenience were some of the reasons for discontinuation in this study. Current diabetes treatment guidelines in Japan specify that a combination of aerobic and resistance exercise should be performed for ≥ 150 min per week; however, the guidelines do not provide detail of the exact combination or type of exercise for maximum benefit, resulting in patients having to develop their own exercise programs and be responsible for their own training [8, 9]. The current study has provided a detailed exercise regimen that significantly improved glycemic control. Furthermore, the exercises were conducted under the supervision and guidance of a trainer, which possibly contributed to the low discontinuation rate.
Baseline characteristics were similar to other exercise intervention studies conducted in Japan [13, 14]. Most patients were male, overweight, and had average HbA1c levels of 7.7%, but were slightly younger (54.6–55.7 years) compared with other exercise intervention studies conducted in Japan (61.9–69.8 years) [13, 14]. Approximately 15% of the Japanese population older than 40 years are strongly suspected of having diabetes [28] and, according to the Japan Diabetes Clinical Data Management Study Group, the mean age for patients with T2DM in 2018 was 66.82 years [29]. The age range in this study is similar to 2 large cohort studies (Health Benefits of Aerobic and Resistance Training in individuals with type 2 diabetes [HART-D] and Diabetes Aerobic and Resistance Exercise [DARE]) assessing structured, combined exercise intervention in predominantly Caucasian patients [19, 20]. Therefore, the findings from this study may be generalizable to other patients with T2DM who have similar characteristics (e.g., BMI of approximately 26 kg/m2, somewhat higher than the average BMI of 24.8 kg/m2 in Japanese patients with T2DM [29]; no insulin or sulfonylurea treatment).
Strengths of this study include the emphasis on efficacy, focusing solely on the impact of exercise on diabetes parameters, and the use of a tightly controlled exercise regimen, with all exercise completed at a gym with supervision from a trainer. Exercise completion and target achievement were also recorded by the trainer after one-on-one sessions with the patient. Discontinuation rates were low, indicating that the exercise program was well tolerated. This study is also strengthened by the number of patients included. Previous studies conducted in Japan have assessed only 10–40 patients [13, 14], whereas this study has assessed a larger cohort, similar to Western studies [19, 20]. Although the exercise program significantly lowered HbA1c and other laboratory measures, whether patients would be able to continue the set exercise program for the long term, in the absence of a sports gym or the guidance of a trainer, should be further investigated. As noted above, the participants in this study were relatively young compared with the average Japanese outpatient population (66.82 years [29]), possibly due to the study design requirement of attending a sports gym, and therefore, the application of these findings to older patients needs further research. Furthermore, this was a relatively short study, and the long-term effect of continued exercise on diabetes parameters needs to be examined. Finally, given the short time frame of the study, changes in cardiopulmonary function, renal function, or physical ability (e.g., 6-min walking test) were not assessed. In conclusion, among Japanese patients with T2DM, a structured program combining aerobic and resistance exercise improved HbA1c levels and was well accepted and safe.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank all study participants, medical staff, and exercise trainers. We also acknowledge the contribution of Motohiro Kanayama (Astellas Pharma Inc.) in the design of the exercise program and assistance with conducting the trial.
This study was funded by Astellas Pharma Inc. Medical writing assistance was provided by Rebecca Lew, PhD, CMPP, and Joanna Best, PhD, of ProScribe-Envision Pharma Group, funded by Astellas Pharma Inc. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3).
Author contributions
All authors participated in the study design, analysis, and interpretation of study results, and in the drafting, critical revision, and approval of the final version of the manuscript. Tetsushi Takada collected the data. All authors agree to be accountable for all aspects of the work.
Funding
Astellas Pharma Inc. was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Data accessibility and materials
Researchers may request access to anonymized participant level data, trial level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Declarations
Conflicts of interest
Yasuo Terauchi has received lecture fees from Astellas Pharma Inc., AstraZeneca K.K., Daiichi Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Eli Lilly Japan K.K., Merck Sharp & Dohme K.K., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanofi K.K., Sanwa Kagaku Kenkyusho Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd., and has received research donations from AstraZeneca K.K., Daiichi Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Eli Lilly Japan K.K., Merck Sharp & Dohme K.K., Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanofi K.K., and Takeda Pharmaceutical Co., Ltd. Tetsushi Takada is an employee of Astellas Pharma Inc. Satoshi Yoshida is an employee of Astellas Pharma Inc.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and/or with the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from all patients for being included in the study.
Human rights statement
This research involves human participants. The study protocol was approved by the local ethics committee at each medical institution, the Medical Corporation Toukeikai Kitamachi Clinic Ethical Review Board (approval number AST05921, approved June 28, 2018), or the Joint Ethical Review Board (approval number 14000050.20180817-4654, approved August 17, 2018).
Informed consent
All patients provided written informed consent before participating.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286(10):1218–1227. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 2.Gordon BA, Benson AC, Bird SR, Fraser SF. Resistance training improves metabolic health in type 2 diabetes: a systematic review. Diabetes Res Clin Pract. 2009;83(2):157–175. doi: 10.1016/j.diabres.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 3.Sampath Kumar A, Maiya AG, Shastry BA, Vaishali K, Ravishankar N, Hazari A, et al. Exercise and insulin resistance in type 2 diabetes mellitus: a systematic review and meta-analysis. Ann Phys Rehabil Med. 2019;62(2):98–103. doi: 10.1016/j.rehab.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Snowling NJ, Hopkins WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care. 2006;29(11):2518–2527. doi: 10.2337/dc06-1317. [DOI] [PubMed] [Google Scholar]
- 5.Nery C, Moraes SRA, Novaes KA, Bezerra MA, Silveira PVC, Lemos A. Effectiveness of resistance exercise compared to aerobic exercise without insulin therapy in patients with type 2 diabetes mellitus: a meta-analysis. Braz J Phys Ther. 2017;21(6):400–415. doi: 10.1016/j.bjpt.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan B, Ge L, Xun YQ, Chen YJ, Gao CY, Han X, et al. Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Int J Behav Nutr Phys Act. 2018;15(1):72. doi: 10.1186/s12966-018-0703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Umpierre D, Ribeiro PA, Kramer CK, Leitão CB, Zucatti AT, Azevedo MJ, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305(17):1790–1799. doi: 10.1001/jama.2011.576. [DOI] [PubMed] [Google Scholar]
- 8.Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int. 2020;11(3):165–223. doi: 10.1007/s13340-020-00439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Japan Diabetes Society: Treatment guide for diabetes 2016–2017. http://www.fa.kyorin.co.jp/jds/uploads/Treatment_Guide_for_Diabetes_2016-2017.pdf. Accessed September 8, 2020.
- 10.Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065–2079. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamiya A, Ohsawa I, Fujii T, Nagai M, Yamanouchi K, Oshida Y, et al. A clinical survey on the compliance of exercise therapy for diabetic outpatients. Diabetes Res Clin Pract. 1995;27(2):141–145. doi: 10.1016/0168-8227(95)01032-9. [DOI] [PubMed] [Google Scholar]
- 12.Arakawa S, Watanabe T, Sone H, Tamura Y, Kobayashi M, Kawamori R, et al. The factors that affect exercise therapy for patients with type 2 diabetes in Japan: a nationwide survey. Diabetol Int. 2015;6:19–25. doi: 10.1007/s13340-014-0166-y. [DOI] [Google Scholar]
- 13.Okada S, Hiuge A, Makino H, Nagumo A, Takaki H, Konishi H, et al. Effect of exercise intervention on endothelial function and incidence of cardiovascular disease in patients with type 2 diabetes. J Atheroscler Thromb. 2010;17(8):828–833. doi: 10.5551/jat.3798. [DOI] [PubMed] [Google Scholar]
- 14.Takenami E, Iwamoto S, Shiraishi N, Kato A, Watanabe Y, Yamada Y, et al. Effects of low-intensity resistance training on muscular function and glycemic control in older adults with type 2 diabetes. J Diabetes Investig. 2019;10(2):331–338. doi: 10.1111/jdi.12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes Association Standards of Medical Care in Diabetes 3. Foundations of care and comprehensive medical evaluation. Diabetes Care. 2016;39(Suppl 1):S23–S35. doi: 10.2337/dc16-S006. [DOI] [PubMed] [Google Scholar]
- 16.Haneda M, Noda M, Origasa H, Noto H, Yabe D, Fujita Y, et al. Japanese clinical practice guideline for diabetes 2016. J Diabetes Investig. 2018;9(3):657–697. doi: 10.1111/jdi.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American College of Sports Medicine . ACSM's guidelines for exercise testing and prescription (10th edition) Philadelphia: Wolters Kluwer; 2018. [DOI] [PubMed] [Google Scholar]
- 18.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33(12):e147–e167. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010;304(20):2253–2262. doi: 10.1001/jama.2010.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud'homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(6):357–369. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 21.Vinetti G, Mozzini C, Desenzani P, Boni E, Bulla L, Lorenzetti I, et al. Supervised exercise training reduces oxidative stress and cardiometabolic risk in adults with type 2 diabetes: a randomized controlled trial. Sci Rep. 2015;5:9238. doi: 10.1038/srep09238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umpierrez G, Tofé Povedano S, Pérez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3) Diabetes Care. 2014;37(8):2168–2176. doi: 10.2337/dc13-2759. [DOI] [PubMed] [Google Scholar]
- 24.Seino Y, Hiroi S, Hirayama M, Kaku K. Efficacy and safety of alogliptin added to sulfonylurea in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label, long-term extension study. J Diabetes Investig. 2012;3(6):517–525. doi: 10.1111/j.2040-1124.2012.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeFronzo RA, Fleck PR, Wilson CA, Mekki Q, Alogliptin Study 010 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care. 2008;31(12):2315–2317. doi: 10.2337/dc08-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balducci S, Leonetti F, Di Mario U, Fallucca F. Is a long-term aerobic plus resistance training program feasible for and effective on metabolic profiles in type 2 diabetic patients? Diabetes Care. 2004;27(3):841–842. doi: 10.2337/diacare.27.3.841. [DOI] [PubMed] [Google Scholar]
- 27.Sato Y, Sone H, Kobayashi M, Kawamori R, Atsumi Y, Oshida Y. Current situation of exercise therapy in patients with diabetes mellitus in Japan (Report No. 2): a nationwide survey to patients using the questionnaires. J Japan Diab Soc. 2015;2015(11):850–859. doi: 10.11213/tonyobyo.58.850. [DOI] [Google Scholar]
- 28.Ministry of Health Labour and Welfare: 2018 National Health and Nutrition Survey Report. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/eiyou/h30-houkoku_00001.html. Accessed September 8, 2020.
- 29.Japan Diabetes Clinical Data Management Study Group. 2018. http://jddm.jp/data/index-2018. Accessed 10 July 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Researchers may request access to anonymized participant level data, trial level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.