Abstract
A lactic acid bacteria (LAB) producing γ-aminobutyric acid (GABA) was isolated from Gat-Kimchi, a Korean vegetable food. The isolate, K285, was identified as Latilactobacillus (formly Lactobacillus) curvatus. The gadB encoding glutamate decarboxylase (GAD) was cloned and an ORF encoding a protein of 451 amino acids was located. K285 GAD was smaller than other LAB GADs, and its amino acid sequence showed less than 80% homology with other LAB GADs, indicating the uniqueness of K285 GAD. The gadC encoding glutamate/GABA antiporter was located 75 bp upstream of gadB, indicating gadCB operon structure. The gadB was overexpressed in Escherichia coli and recombinant GAD was purified. Optimum pH and temperature of recombinant K285 GAD were pH 5.0 and 50 °C, respectively, and the activity was dependent on pyridoxal 5′-phosphate. The Km and Vmax of GAD were 5.35 ± 0.27 mM and 0.041 ± 0.0008 mM/min, respectively. Lb. curvatus K285 might be useful for the production of foods enriched with GABA.
Keywords: GABA, Glutamate decarboxylase, gadB cloning, Latilactobacillus curvatus
Introduction
γ-Aminobutyric acid (GABA) is a non-protein amino acid widely distributed among microorganisms, animals, and plants (Somasundaram et al., 2016). GABA is a major inhibitory neurotransmitter in the mammalian central nervous system, and has several health beneficial effects such as anti-diabetic, anti-hypertension, anti-anxiety, anti-cancer, and tranquilizing effect (Poojary et al., 2017). GABA and the producing microorganisms have been the subjects of recent researches due to its high potential as a bioactive substance (Kook and Cho 2013; Yu et al., 2017). Lactic acid bacteria (LAB) enhance the quality of fermented foods by producing organic acids, peptides, amino acids, flavoring compounds, bacteriocins, and other chemicals (Park and Oh 2007). LAB are also the major source for GABA, and various species with strong GABA producing ability have been isolated from fermented foods (Huang et al., 2007).
GABA is produced by irreversible decarboxylation of L-glutamate, and glutamate decarboxylase (GAD, L-glutamate 1-carboxy-lyase (4-aminobutanoate forming), EC 4.1.1.15) catalyzes the reaction. GAD requires pyridoxal 5′-phosphate (PLP) for the activity, and the activity is induced at low pH as a response against acidic environments (Sarasa et al., 2020). GADs from various LAB have been purified and their biochemical properties have been studied (Yogeswara et al., 2020). Lactobacillus, Lactococcus, Streptococcus, and Enterococcus are the major genera producing GABA. Among them, Lactobacillus is the most important genus, and Lb. brevis (now, Levilactobacillus brevis) is the most important species (Yogeswara et al., 2020). Efforts to isolate novel GABA producers have been continued since novel LAB with strong GABA production ability is desirable not only for the understanding of the roles of GABA and also for the production of diverse fermented foods enriched with GABA.
In this study, Latilactobacillus curvatus K285 was isolated from Gat-Kimchi, one of many Kimchi varieties where Gat (Brassica juncea var. juncea) is the main raw material instead of cabbage or radish. The gadB gene encoding GAD was cloned from Lb. curvatus K285 and overexpressed in Escherichia coli BL21(DE3). Recombinant GAD was purified and the properties were studied. This is the first report on the isolation of a Lb. curvatus strain with high GABA-producing ability, and the characterization of its GAD. The size of GAD from Lb. curvatus K285 (K285 GAD) was smaller than other LAB GADs reported so far. The amino acid sequence of K285 GAD showed lower identity values with those from other GADs, indicating the uniqueness of K285 GAD. Considering its strong GABA production ability, Lb. curvatus K285 and its GAD might be useful as a starter for functional foods enriched with GABA.
Materials and methods
Isolation of GABA-producing LAB from Gat-Kimchi
Gat-Kimchi was purchased at a local market in Sacheon, Gyeongnam, Republic of Korea in June, 2019, and homogenized with 0.1% peptone water by using stomacher®80 (Seward, Worthing, W. Sussex, UK). Serially diluted homogenates were spreaded onto de Man, Rogosa, and Sharpe (MRS, Becton Dickinson Co., Franklin Lakes, NJ, USA) agar plates with 1% CaCO3 and 0.006% bromocresol purple (Kim et al., 2012; Lee et al., 2020). Colonies with yellow color and surrounding clear zone were selected. Each isolate was inoculated into MRS broth (1 ml) supplemented with monosodium glutamate (MSG, 3%, w/v) and incubated at 30 °C for 48 h. Culture was centrifuged (12,000× g, 4 °C, 10 min) and 1 µl of culture supernatant was spotted on a silica gel plate (silica gel 60 F254; Merck Co., Darmstadt, Germany). Thin layer chromatography (TLC) was done using n-butanol/acetic acid/water (4:1:1, v/v/v) as a developing solvent. After separation, the plate was treated with 2% ninhydrin solution and dried at 65 °C for 10 min to visualize spots (Sa et al., 2015).
Identification of K285
An isolate (K285) producing GABA was identified by 16S rRNA and recA genes sequencing (Sa et al., 2015; Sakamoto et al., 2003). 16S rRNA genes were amplified using primer 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-TACGGYTACCTTGTTACGACTT-3′). recA gene was amplified by using a primer pair based on recA sequence of Lb. curvatus KG6 (CP022475.1): curvatus-F (5′-GCGCTCGGTGTAGGTGGGTATCCAC-3′) and curvatus-R (5′-TCTTCGCGTTCTCACGTCCTTGACC-3′). PCR was done using a MJ mini thermal cycler (BioRad, Hercules, CA, USA) under the conditions as described previously (Lee et al., 2020). PCR products were purified using a purification kit (Favorgen, Ping-Tung, Taiwan), and ligated into pGEM-T Easy vector (Promega, Madison, WI, USA). The nucleotide sequences were determined and analyzed by Basic Local Alignment Search Tool (BLAST) at National Center for Biotechnology Information (NCBI, Bethesda, MD, USA).
Cloning of gadB and gadC
gadB from Lb. curvatus K285 was cloned by PCR with a primer pair, which were designed based on the published genome sequence of Lb. curvatus KG6 (CP022475.1): gadB-F (5'-GGGCATATGCTAGAGATGTCGAAAAC-3', NdeI site underlined), gadB-R (5'-CCCTCGAGTTTCTTATTTAAATCGTC-3', XhoI site underlined). gadC from Lb. curvatus K285 was also cloned by using a primer pair designed based on published genome sequence of Lb. curvatus KG6 (CP022475.1): gadC-F (5'-GGTCTATGTCATAAATCAAGCG-3'), and gadC-R (5'-CCAGCCTCTAGGCTACCTAA-3'). Amplified products were ligated into pGEM T-Easy vector (Promega, Madison, WI, USA). The ligation mixture was introduced into E. coli DH5α competent cells by electroporation. Plasmids were prepared from E. coli transformants (TFs) and sequenced at Cosmogenetech (Daejon, Korea).
Overexpression of gadB in E. coli BL21 (DE3) and purification of GAD
gadB was amplified as described above and ligated into pET-26b( +) (Novagen, Madison, WI, USA) at NdeI and XhoI sites. Ligation mixture was introduced into E. coli BL21(DE3) competent cells by electroporation. An E. coli BL21(DE3) TF harboring pETK285 (pET26b( +) with gadB) was grown in LB broth (200 ml, kanamycin, 60 μg/ml) at 37 °C. When culture entered into the exponential growth phase, isopropyl β-d-1-thiogalactopyranoside (IPTG) was added (1 mM) and growth continued overnight at 20 °C. Cells were harvested by centrifugation (12,000 × g, 10 min at 4 °C), washed 3 times with phosphate-buffered saline (PBS, pH 7.4), and resuspended with lysis buffer (50 mM NaH2PO4, 300 mM NaCl, and 10 mM imidazole, pH 7.0). Cells were disrupted by ultra-sonication, and centrifuged (12,000 ×g, 10 min at 4 °C). The resulting pellet (insoluble fraction) and supernatant (soluble fraction) were analyzed by SDS-PAGE. Soluble fraction was loaded onto a Ni–NTA column (GE Healthcare, Uppsala, Sweden), and recombinant GAD was eluted from column by stepwise increase in imidazole concentration (40–500 mM) of elution buffer. SDS-PAGE was done for eluted fractions using a 12% (w/v) acrylamide gel (Lee et al., 2017). Protein concentrations of fractions containing recombinant GAD were determined by the Bradford method with bovine serum albumin as a standard (Zor and Selinger, 1996).
GABase assay
GAD activity was measured as described previously (Hiraga et al., 2008). Enzyme solution (l µg GAD in 0.1 ml of lysis buffer) was mixed with 0.1 ml 4 M ammonium sulfate. After 30 min pre-incubation, 1.3 ml substrate (20 mM MSG, 0.2 mM pyridoxal 5′-phosphate (PLP), 0.2 M pyridine-HCl, pH 4.5) was added, and the mixture was incubated for 1 h. The reaction was stopped by boiling for 5 min. The amount of GABA was measured by GABase assay as described previously (Park et al., 2014). One unit of GAD activity (U) was defined as the amount of enzyme producing 1 µmol GABA per minute.
Properties of recombinant GAD
Recombinant GAD (4 µg) was incubated at different pH (3–9) for 1 h at 45 °C, and GAD activity was measured by GABase assay. GAD was exposed to different temperature (25–65 °C) for 30 min at pH 5.0, and the remaining activity was measured. The effects of PLP (0–1.8 mM) and chemicals (CuSO4, FeCl2, CaCl2, KCI, CoCl2, ZnCl2, MgCl2, AgNO3, MnCl2, 2 mM each) on the GAD activity were determined by incubating GAD (4 µg) for 1.5 h at 50 °C and pH 5.0, and then the activity was measured by GABase assay. The effect of MSG concentration (0–100 mM) on GAD (10 μg) activity was determined by GABase assay as described above. The Michaelis–Menten constant (Km) and maximum velocity (Vmax) were calculated by using the Lineweaver–Burk plot.
Results and discussion
Isolation of a GABA-producing LAB from Gat-Kimchi and its identification
A total of 3000 isolates were obtained from Kimchi and Jeotgal products purchased at traditional markets in Gyeongnam and Jeonnam provinces, Korea. GABA-producers were screened by TLC, and one isolate (K285) from Gat-Kimchi showed a strong GABA spot on a TLC plate (Fig. 1). The intensity of GABA spot from K285 was apparently stronger than those from positive controls, Lactobacillus. zymae (now, Levilactobacillus zymae) GU240 (Park et al., 2014) and Lactobacillus sakei (now, Latilactobacillus sakei) A156 (Sa et al., 2015), which were previously isolated from Kimchi and Myeolchi Joetgal, respectively. BLAST of the 16S rRNA gene sequence of K285 (1348 bp, MW407998) indicated that K285 was 100% identical with Latilactobacillus curvatus MG5246 (MN368279.1), TSGB1195 (MN250808.1), LVP34 (MK659883.1), and Lb. sakei GI59 (MG430201.1) (data not shown). When a phylogenetic tree was constructed based on 16S rRNA gene sequences, K285 was grouped as Lb. curvatus (data not shown). BLAST analysis of recA gene sequence of K285 showed that K285 showed 99.84% identity with Lb. curvatus KG6 (CP022475.1), Lb. curvatus CBA3617 (CP042389.1), Lb. curvatus JCM 1096 (CP026116.1), and 89.50% with Lb. sakei CIP105422 (KC772133.1) and 88.24% with Lb. sakei DS4 (CP025839.1). K285 was identified as Lb. curvatus based on above results.
Fig. 1.
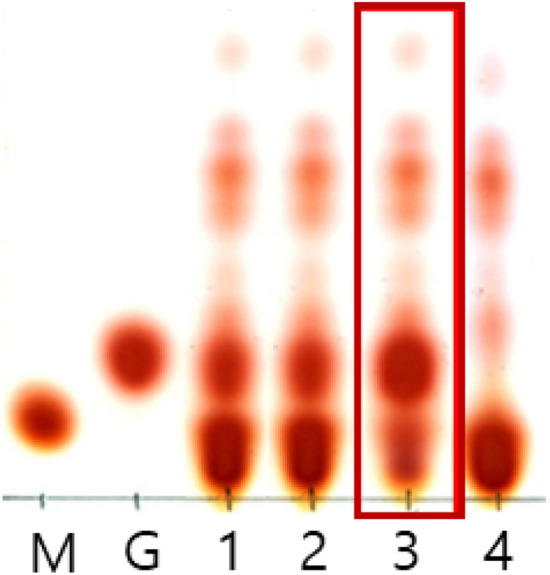
Thin-layer chromatogram showing GABA production by Lb. curvatus K285. M, 0.8 µl of 100 mM MSG; G, 0.8 µl of 100 mM GABA; 1, Lb. zymae GU240 (positive control); 2, Lb. sakei A156 (positive control); 3, Lb. curvatus K285; 04, Leu. mesenteroides ATCC10830 (negative control)
Lb. curvatus K285 grew rapidly in MRS broth at 25–37 °C, and the optimum temperature was 37 °C (data not shown). Lb. curvatus K285 grew best at 0% (w/v) NaCl, and grew slowly in the presence of NaCl, up to maximum 10% (data not shown). Lb. curvatus K285 produced GABA at the concentration of 16.39 ± 0.012 mg per ml culture supernatant as determined by GABase assay (MSG, 20 mM). This value was higher than those from a negative control, Leuconostoc mesenteroides (1.48 ± 0.026 mg GABA), and a positive control, Lb. sakei A156 (15.81 ± 0.098 mg GABA). Considering its high GABA producing ability, Lb. curvatus K285 might be useful as a starter for fermented foods enriched with GABA. This is the first report on the isolation of a GABA-producing Latilactobacillus curvatus from fermented foods, and characterization of the gadB encoding glutamate decarboxylase.
Cloning of gadB and gadC from Lb. curvatus K285
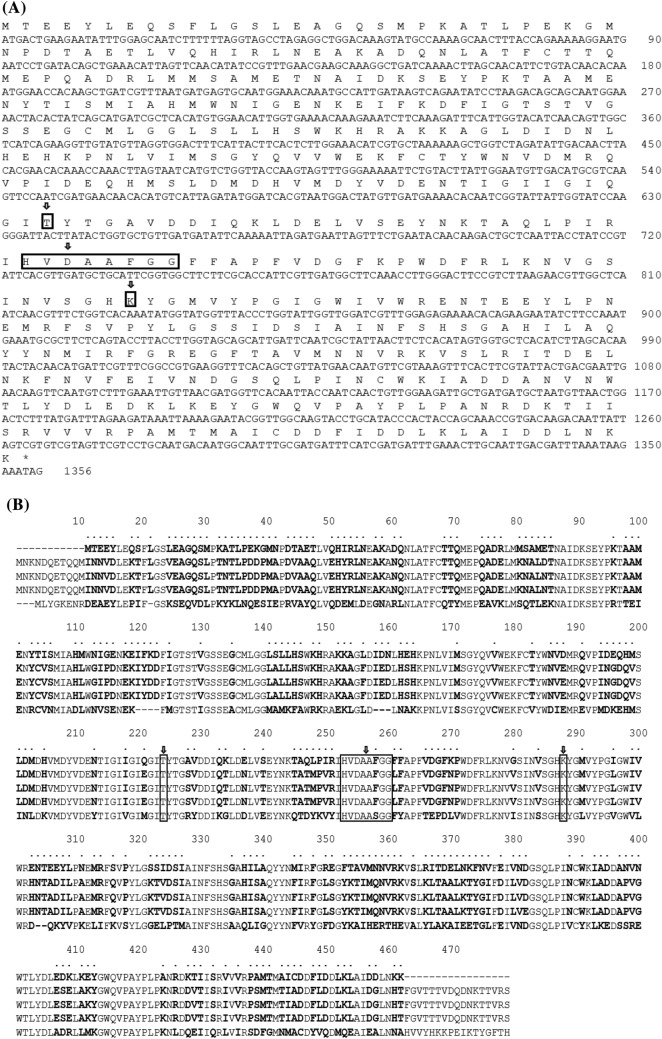
Nucleotide sequence of gadB was determined (MW413809). Sequence analysis located an ORF of 1,356 nucleotides in size, capable of encoding a protein of 451 amino acids (aas) (Fig. 2A). The calculated molecular weight was 51,193.32 Da and isoelectric point (pI) was 4.98. Amino acid sequence of the GAD from Lb. curvatus K285 (K285 GAD) showed the highest identity with GAD from Lb. curvatus KG6 (100%, WP_089542363.1) followed by those from Enterococcus avium G-15 (84.44%, WP_010741191.1), Enterococcus xiangfangensis DSM 105,127 (84.00%, WP_137618973.1), Furfurillactobacillus rossiae DSM 15,814 (79.55%, WP_017262688.1), Lb. plantarum (now, Lactiplantibacillus plantarum) Nizo2801 (79.37%, WP_063488771.1), and Lb. parakefiri (now, Lentilactobacillus parakefiri) JCM 8573 (79.15%, WP_057962091.1). When compared with GADs previously characterized by us, K285 GAD showed 76.80%, 77.03%, 76.80%, and 53.81% identity with GAD from Lb. brevis T118 (Lee et al., 2020), Lb. zymae GU240 (Park et al., 2014), Lb. sakei A156 (Sa et al., 2015), and E. avium M5 (Lee et al., 2017), respectively (Fig. 2B). Considering the degeneracy of genetic codes, homology scores among nucleotide sequences are even lower. For this reason, PCR cloning of gadB from Lb. curvatus K285 was unsuccessful with a primer pair used for the cloning of other gadB genes (Park et al., 2014; Sa et al., 2015; Lee et al., 2017, 2020). This is the first report on the isolation of a Lb. curvatus strain producing GABA, cloning of a functional gadB, and characterization of its GAD. A gadB from Lb. curvatus KG6 (WP_089542363.1) is a putative gene annotated from genome sequence of Lb. curvatus KG6, an isolate from fermented meat products (Jans et al., 2017). Its function has not been studied, and this is the same situation for other putative GADs mentioned above. As shown in this work, data mining of the genomic sequences is useful for the cloning of genes from strains or species where the genome sequences are not available, yet.
Fig. 2.
Nucleotide and translated amino acid sequences of gadB (A) and alignment of K285 GAD with other GADs (B). K285, Lb. curvatus K285; T118, Lb. brevis T118; GU240, Lb. zymae GU240; A156, Lb. sakei A156; M5, E. avium M5. Amino acids showing difference are marked as bold and * at the top. Conserved amino acids are marked by bricks and an arrow at the top
One remarkable point of K285 GAD is its small size. K285 GAD (451 aa) is smaller than other LAB GADs reported so far. LAB GADs consist of 481–459 aas (Cui et al., 2020). The known smallest GAD (459 aas) is that from Streptococcus salivarius subsp. thermophiles Y2 (Lin et al., 2009) and the largest one (481 aas) is that from Lb. paracasei (Komatsuzaki et al., 2008). GADs from Lb. zymae GU240 (Park et al., 2014), Lb. brevis T118 (Lee et al., 2020) and Lb. sakei A156 (Sa et al., 2015) have 479 aas, and the GAD from E. avium M5 has 466 aas (Lee et al., 2017). K285 GAD lacks 11 amino acids at the N-terminus and 17 amino acids at the C-terminus when compared with those lactobacilli GADs, and lacks 3 amino acids at the N-terminus and 17 amino acids at the C-terminus when compared with GAD from E. avium M5 (Fig. 2B). It is unknown that the small size of K285 GAD may affect any property of the enzyme. However, the properties of recombinant K285 GAD were not noticeably different from other lactobacilli GADs (see below). C-terminal part of GAD was suspected to be responsible for the narrow pH range of GAD, usually pH 4.0 –5.5. Mutant GADs were constructed by deleting several amino acids at the C-terminus, and the mutants showed higher activity at neutral pH (Hiraga et al., 2008; Shin et al., 2014). But the pH optimum of K285 GAD was 5.0, same with other GADs such as GAD from Lb. sakei A156. Future studies are necessary on the relationship between the size of GAD and the structure and function. K285 GAD has all the conserved amino acids observed among PLP-dependent decarboxylases such as lysine residue (K277) essential for PLP-binding, and T213 and D240 important for decarboxylation activity (Kim et al., 2007). K285 GAD also has a conserved domain (HVDAAFGG).
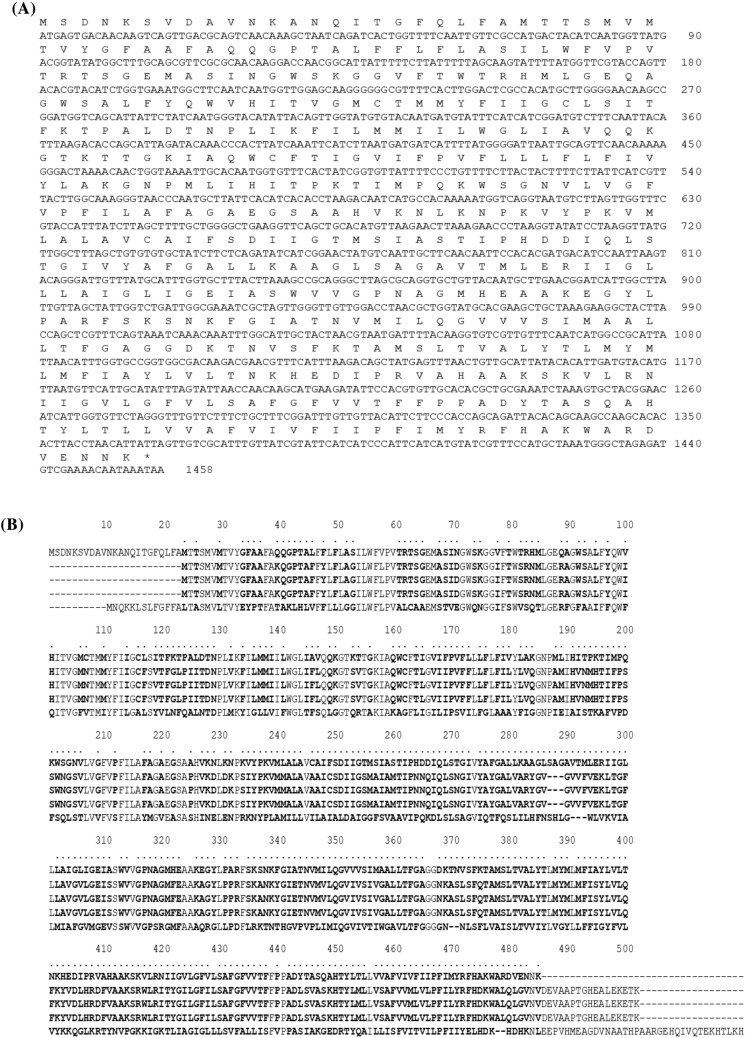
gadC was cloned from Lb. curvatus K285 by using a primer pair as mentioned above, and the nucleotide sequence was determined (MW413810). Sequence analysis located an ORF of 1458 nucleotides in size, capable of encoding a protein of 485 aas with the calculated size of 52.91 kDa and isoelectric point (pI) of 9.58 (Fig. 3A). Translated amino acid sequence of GadC from Lb. curvatus K285 showed significant identities to GadC from E. raffinosus DSM 5633 (75.05%, OJG87346.1), E. malodoratus DSM 20,681 (74.63%, OJG63012.1), E. avium DSM 20,679 (75.05%, OJG21492.1), and Lb. brevis SRCM101174 (72.43%, OJG21492.1). K285 GadC showed 71.58% identity to GadCs from Lb. brevis T`118, Lb. zymae GU240, and Lb. sakei A156. Unlike GAD, GadCs from above 3 lactobacilli (476 aas) were smaller than K285 GadC. GadC from E. avium M5 (503 aas) was larger than K285 GadC and showed 43.83% identity. Figure 3B shows the alignments of K285 GadC with other GadCs reported by us.
Fig. 3.
Nucleotide and translated amino acid sequences of gadC (A) and alignment of K285 GadC with other GadCs (B). K285, Lb. curvatus K285; T118, Lb. brevis T118; GU240, Lb. zymae GU240; A156, Lb. sakei A156; M5, E. avium M5. Amino acids showing difference are marked as bold and * at the top
Unlike GAD, not many studies have been done on GadC, an antiporter which exchanges L-glutamate with GABA. Generally, gadC forms an operon with gadB, but some LAB such as Lb. fermentum has only gadB without gadC (Cui et al., 2020). In Lb. curvatus K285, gadC locates upstream of gadB and promoter-like sequences are located upstream of gadC (data not shown). The start codon of gadB locates 75 nucleotides downstream of the stop codon of gadC, indicating gadCB operon structure in Lb. curvatus K285. The same operon structure was observed in Lb. zymae GU240 (Park et al., 2014), Lb. sakei A156 (Sa et al., 2015) and Lb. brevis T118 (Lee et al., 2020), and proved experimentally in these strains.
Overexpression of gadB in E. coli BL21 (DE3) and purification of GAD
gadB was overexpressed in E. coli BL21 (DE3) by IPTG induction. Recombinant GAD was located from both soluble and insoluble fractions by SDS-PAGE. The band intensity of soluble fraction was stronger than that of insoluble fraction (Fig. 4, lane 1, 2). Recombinant GAD was purified from soluble fraction by using a Ni–NTA column. GAD was eluted from the column at 300 mM imidazole concentration (Fig. 4, lane 5). Purified GAD was 54 kDa in size, which matched with the expected size (52.3 kDa) of recombinant GAD containing extra leucine and glutamic acid (XhoI site) and 6 histidine tag at the C-terminus.
Fig. 4.
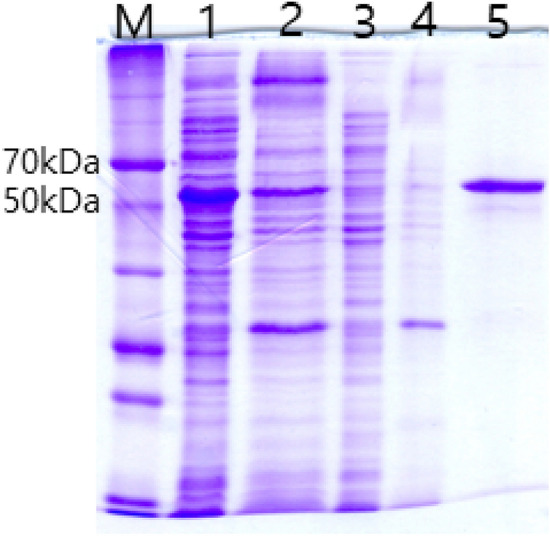
SDS-PAGE of recombinant K285 GAD. M, size marker (Dok-do-mark: Elpis Biotech, Daejon, Korea); 1, soluble fraction from E. coli BL21 (DE3) harboring pETK285; 2, insoluble fraction from E. coli BL21 (DE3) harboring pETK285; 3, soluble fraction from E. coli BL21 (DE3) harboring pET-26b ( +) (negative control); 4, insoluble fraction from E. coli BL21 (DE3) harboring pET-26b ( +) (negative control); 5, purified GAD from soluble fraction eluted at 300 mM imidazole concentration
Properties of recombinant GAD
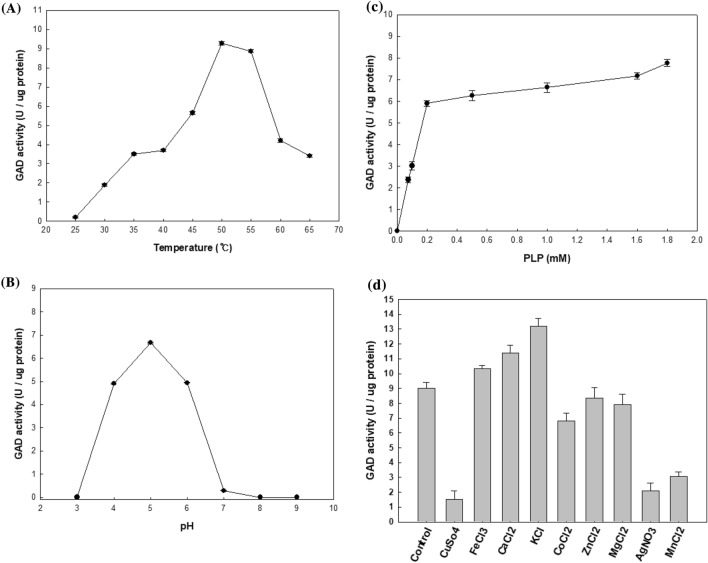
The optimum temperature and pH of recombinant GAD were 50 °C and pH 5, respectively (Fig. 5A and B). The optimum temperature of K285 GAD was higher than that for the growth of Lb. curvatus K285, 37 °C. The optimum temperature and pH of most GADs are 40–55 °C and pH 4.0 –5.0, respectively (Table 1). The major function of microbial GAD is to prevent rapid decrease in pH inside cell, thus GAD shows the highest activity at acidic pHs. This role of GAD is especially important for LAB which produce organic acids during growth, thus causing acidic environments (Kook and Cho, 2013; Yogeswara et al., 2020). K285 GAD showed PLP dependence for its activity like most other GADs. The activity increased rapidly as PLP concentration increased until 0.2 mM. At PLP concentration higher than 0.2 mM, GAD activity increased gradually, but the degree of increase was not significant (Fig. 5C). Among the salts, K+ increased the GAD activity most significantly (159%) followed by Ca+2 (144%) and Fe+2 (122%). Whereas Zn+2 (86%), Mg+2 (74%), Co+2 (45%), and Cu+2 (16%) decreased the GAD activity (Fig. 5D). Km and Vmax of recombinant GAD were 5.35 ± 0.27 mM and 0.041 ± 0.0008 mM/min, respectively when MSG was used as the substrate. Km and Vmax of Lb. zymae GU240 were 1.67 ± 0.05 mM and 0.01 01 ± 0.0001 mM/min, respectively (Park et al., 2014), and those of E. avium M5 were 3.26 ± 0.21 mM and 0.0120 ± 0.0001 mM/min (Lee et al., 2017), respectively. The results showed that K285 GAD had lower affinity to MSG but higher Vmax value than other GADs.
Fig. 5.
GAD activity changes under different conditions. (A) Temperature, (B) pH, (C) pyridoxal 5′-phosphate (PLP) concentration, (D) chemicals. The activity of recombinant GAD was measured at pH 3–9 by GABase assay. To measure the optimal temperature, recombinant GAD was incubated at 20–60 °C for 30 min at pH 5.0. The effects of PLP concentration (0–1.8 mM) and chemicals (2 mM each) were determined at 50 °C and pH 5.0
Table 1.
Characteristics of recombinant glutamate decarboxylases
| Origin | Optimum temperature (°C) | Optimum pH | Km (mM) | Reference |
|---|---|---|---|---|
| Latilactobaciilus curvatus K285* | 50 | 5.0 | 5.35 | This study |
| Latilactobaillus sakei A156* | 55 | 5.0 | 16.0 | (Sa et al., 2015) |
| Lactiplantibacillus plantarum ATCC 14917* | 40 | 4.5 | 22.8 | (Kook et al., 2010) |
| Latilactobacillus sakei OPK2-59 | 30 | 5.0 | NR | (Yu and Oh, 2011) |
| Levilactobacillus brevis CGMCC 1306* | 48 | 4.8 | 10.26 | (Huang et al., 2007) |
| Levilactobacillus brevis 877G* | 45 | 5.2 | 3.6 | (Seo et al., 2013) |
| Levilactobacillus brevis HYE1* | 55 | 4.0 | 4.99 | (Lim et al., 2018) |
| LeviLactobaillus brevis IFO12005 | 30 | 4.2 | 9.3 | (Ueno et al., 1997) |
| LeviLactobaillus zymae GU240* | 41 | 4.5 | 1.7 | (Park et al., 2014) |
| Enterococcus raffinosus TCCC11660 | 45 | 4.6 | NR | (Chang et al., 2017) |
| Enterococcu avium M5* | 55 | 4.5 | 3.26 | (Lee et al., 2017) |
| Enterococcu avium G-15 | NR | 4.4 | NR | (Tamura et al., 2010) |
| Lactococcus lactis | NR | 4.7 | 0.51 | (Nomura et al., 1999) |
|
Streptococcus salivalivarius ssp. thermophilus Y2 |
55 | 4.0 | 2.3 | (Lin et al., 2009) |
NR not reported
*Recombinant protein from E. coli
Lb. curvatus K285 secreting GABA was isolated from Gat-Kimchi, and its gadB gene was cloned and overexpressed in E. coli. The properties of recombinant GAD were examined. So far, many GABA-producing LAB were isolated from diverse fermented foods and their GADs were reported. Different LAB species showed different production yields and the optimum conditions for GABA production were different. Still many GABA-producing LAB species are waiting for their discoveries and optimization of growth conditions for efficient GABA production. This is the first report on the isolation of a GABA-producing Lb. curvatus strain and characterization of its GAD. K285 GAD showed significant differences with other closely related LAB GADs. The size is smaller than other LAB GADs. But K285 GAD still possesses important amino acids conserved among LAB GADs. More studies are necessary on the structure and function relationship of LAB GADs. It is desirable to isolate novel GABA producing LAB strains because of their potentials as starters for functional foods and pharmaceutical products. Considering its strong GABA producing ability, Lb. curvatus K285 might be useful as a starter for functional foods enriched with GABA.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2020R1A2C100826712). Lee SJ, Jeon HS, Yoo JY, and Kim MJ were supported by BK21 Four program from MOE, Korea. Kang YJ and Kim TJ were supported by full-time graduate student scholarship from Gyeongsang National University.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Se Jin Lee, Email: tpwls5151@naver.com.
Hye Sung Jeon, Email: hye4098@naver.com.
Ji Yeon Yoo, Email: u6183@naver.com.
Yun Ji Kang, Email: dbswl4445@naver.com.
Min Jae Kim, Email: alswo6k5twt@naver.com.
Tae Jin Kim, Email: xowlsdl3@naver.com.
Jeong Hwan Kim, Email: jeonghkm@gnu.ac.kr.
References
- Chang C, Zhang J, Ma S, Wang L, Wang D, Zhang J, Gao Q. Purification and characterization of glutamate decarboxylase from Enterococcus raffinosus TCCC11660. Journal of Industrial Microbiology & Biotechnology. 2017;44:817–824. doi: 10.1007/s10295-017-1906-3. [DOI] [PubMed] [Google Scholar]
- Cui Y, Miao K, Niyaphorn S, Qu X. Production of gamma-aminobutyric acid from lactic acid bacteria: a systematic review. International Journal of Molecular Sciences. 2020;21:995. doi: 10.3390/ijms21030995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga K, Ueno Y, Oda K. Glutamate decarboxylase from Lactobacillus brevis: activation by ammonium sulfate. Bioscience Biotechnology and Biochemistry. 72: 1299-1306 (2008) [DOI] [PubMed]
- Huang J, Lehe M, Sheng Q, Shanjing YAO, Dongqiang LIN. Purification and characterization of glutamate decarboxylase of Lactobacillus brevis CGMCC 1306 isolated from fresh milk. Chinese Journal of Chemical Engineering. 2007;15:157–161. doi: 10.1016/S1004-9541(07)60051-2. [DOI] [Google Scholar]
- Jans C, Lagler S, Lacroix C, Meile L, Stevens MJA. Complete genome sequences of Lactobacillus curvatus KG6, L. curvatus MRS6, and Lactobacillus sakei FAM18311, isolated from fermented meat products. Genome Announcements. 5: e00915–17 (2017) [DOI] [PMC free article] [PubMed]
- Kim MJ, Kim KS. Isolation and identification of γ-aminobutyric acid (GABA)-producing lactic acid bacteria from Kimchi. Journal of the Korean Society for Applied Biological Chemistry. 2012;55:777–785. doi: 10.1007/s13765-012-2174-6. [DOI] [Google Scholar]
- Kim SH, Shin BH, Kim YH, Nam SW, Jeon SJ. Cloning and expression of a full-length glutamate decarboxylase gene from Lactobacillus brevis BH2. Biotechnology and Bioprocess Engineering. 2007;12:707–712. doi: 10.1007/BF02931089. [DOI] [Google Scholar]
- Komatsuzaki N, Nakamura T, Kimura T, Shima J. Characterization of glutamate decarboxylase from a high γ-aminobutyric acid (GABA)-producer Lactobacillus Paracasei. Bioscience Biotechnology and Biochemistry. 2008;72:278–285. doi: 10.1271/bbb.70163. [DOI] [PubMed] [Google Scholar]
- Kook MC, Cho SC. Production of GABA (gamma amino butyric acid) by lactic acid bacteria. Food Science of Animal Resource. 2013;33:377–389. doi: 10.5851/kosfa.2013.33.3.377. [DOI] [Google Scholar]
- Kook MC, Seo MJ, Cheigh CI, Lee SJ, Pyun YR, Park H. Enhancement of γ-amminobutyric acid production by Lactobacillus sakei B2–16 expressing glutamate decarboxylase from Lactobacillus plantarum ATCC 14917. Journal of the Korean Society for Applied Biological Chemistry. 2010;53:816–820. doi: 10.3839/jksabc.2010.123. [DOI] [Google Scholar]
- Lee KW, Shim JM, Yao Z, Kim JA, Kim HJ, Kim JH. Characterization of a glutamate decarboxylase (GAD) from Enterococcus avium M5 isolated from Jeotgal, a Korean fermented seafood. Journal of Microbiology and Biotechnology. 2017;27:1216–1222. doi: 10.4014/jmb.1701.01058. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Yao Z, Meng Y, Le HG, Jeon HS, Yoo JY, Kim JH. Isolation of γ-aminobutyric acid producing Lactobacillus brevis T118 from Sun-Tae Jeotgal and its glutamate decarboxylase gene cloning. Journal of Agriculture & Life Science. 2020;54:85–92. doi: 10.14397/jals.2020.54.4.85. [DOI] [Google Scholar]
- Lim HS, Seo DH, Cha IT, Lee H, Nam YD, Seo MJ. Expression and characterization of glutamate decarboxylase from Lactobacillus brevis HYE1 isolated from kimchi. World Journal of Microbiology & Biotechnology. 2018;34:1–10. doi: 10.1007/s11274-018-2427-6. [DOI] [PubMed] [Google Scholar]
- Lin Q, Yang S, Lü F, Lu Z, Bie X, Jiao Y, Zou X. Cloning and expression of glutamate decarboxylase gene from Streptococcus thermophilus Y2. Journal of General and Applied Microbiology. 2009;55:305–310. doi: 10.2323/jgam.55.305. [DOI] [PubMed] [Google Scholar]
- Nomura M, Nakajima I, Fujita Y, Kobayashi M, Kimoto H, Suzuki I, Aso H. Lactococcus lactis contains only one glutamate decarboxylase gene. Microbiology. 1999;145:1375–1380. doi: 10.1099/13500872-145-6-1375. [DOI] [PubMed] [Google Scholar]
- Park KB, Oh SH. Cloning, sequencing and expression of a novel glutamate decarboxylase gene from a newly isolated lactic acid bacterium, Lactobacillus brevis OPK-3. Bioresource Technology. 2007;98:312–319. doi: 10.1016/j.biortech.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Park JY, Jeong SJ, Kim JH. Characterization of a glutamate decarboxylase (GAD) gene from Lactobacillus zymae. Biotechnology Letters. 2014;36:1791–1799. doi: 10.1007/s10529-014-1539-9. [DOI] [PubMed] [Google Scholar]
- Poojary MM, Dellarosa N, Roohinejad S, Koubaa M, Tylewicz U, Gómez-Galindo F, Barba FJ. Influence of innovative processing on γ-aminobutyric acid (GABA) contents in plant food materials. Comprehensive Reviews in Food Science and Food Safety. 2017;16:895–905. doi: 10.1111/1541-4337.12285. [DOI] [PubMed] [Google Scholar]
- Sa HD, Park JY, Jeong SJ, Lee KW, Kim JH. Characterization of glutamate decarboxylase (GAD) from Lactobacillus sakei A156 isolated from Jeot-gal. Journal of Microbiology and Biotechnology. 2015;25:696–703. doi: 10.4014/jmb.1412.12075. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Takeuchi Y, Umeda M, Ishikawa I, Benno Y. Application of terminal RFLP analysis to characterize oral bacterial flora in saliva of healthy subjects and patients with periodontitis. Journal of Medical Microbiology. 2003;52:79–89. doi: 10.1099/jmm.0.04991-0. [DOI] [PubMed] [Google Scholar]
- Sarasa SB, Mahendran R, Muthusamy G, Thankappan B, Selta DRF, Angayarkanni J. A brief review on the non-protein amino acid, gamma-amino butyric acid (GABA): its production and role in microbes. Current Microbiology. 2020;77:534–544. doi: 10.1007/s00284-019-01839-w. [DOI] [PubMed] [Google Scholar]
- Seo MJ, Nam YD, Lee SY, Park SL, Yi SH, Lim SI. Expression and characterization of a glutamate decarboxylase from Lactobacillus brevis 877G producing γ-aminobutyric acid. Bioscience Biotechnology and Biochemistry. 2013;77:853–856. doi: 10.1271/bbb.120785. [DOI] [PubMed] [Google Scholar]
- Shin S, Kim H, Joo Y, Lee S, Lee Y, Lee SJ, Lee DW. Characterization of glutamate decarboxylase from Lactobacillus plantarum and its C-terminal function for the pH-dependence of activity. Journal of Agricultral and Food Chemistry. 2014;62:12186–12193. doi: 10.1021/jf504656h. [DOI] [PubMed] [Google Scholar]
- Somasundaram S, Lee SH, Park SJ, Hong SH. Engineering the intracellular metabolism of Escherichia coli to produce gamma-aminobutyric acid by co-localization of GABA shunt enzymes. Biotechnology Letters. 2016;38:321–327. doi: 10.1007/s10529-015-1982-2. [DOI] [PubMed] [Google Scholar]
- Tamura T, Noda M, Ozaki M, Maruyama M, Matoba Y, Kumagai T, Sugiyama M. Establishment of an efficient fermentation system of gamma-aminobutyric acid by a lactic acid bacterium, Enterococcus avium G-15, isolated from carrot leaves. Biological & Pharmaceutical Bulletin. 2010;33:1673–1679. doi: 10.1248/bpb.33.1673. [DOI] [PubMed] [Google Scholar]
- Ueno Y, Hayakawa K, Takahashi S, Oda K. Purification and characterization of glutamate decarboxylase from Lactobacillus brevis IFO 12005. Bioscience Biotechnology and Biochemistry. 1997;61:1168–1171. doi: 10.1271/bbb.61.1168. [DOI] [PubMed] [Google Scholar]
- Yogeswara IBA, Maneerat S, Haltrich D. Glutamate decarboxylase from lactic acid acteria—a key enzyme in GABA synthesis. Microorganisms. 2020;8:1923. doi: 10.3390/microorganisms8121923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JJ, Oh SH. gamma-Aminobutyric Acid Production and Glutamate Decarboxylase Activity of Lactobacillus sakei OPK2-59 Isolated from Kimchi. Korean Journal of Microbiology. 2011;47:316–322. [Google Scholar]
- Yu HH, Choi JH, Kang KM, Hwang HJ. Potential of a lactic acid bacterial starter culture with gamma-aminobutyric acid (GABA) activity for production of fermented sausage. Food Science and Biotechnology. 2017;26:1333–1341. doi: 10.1007/s10068-017-0161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zor T, Selinger Z. Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Analytical Biochemistry. 1996;236:302–308. doi: 10.1006/abio.1996.0171. [DOI] [PubMed] [Google Scholar]