Abstract
Microfluidizer is one of the emerging processing technologies which has brought tremendous and desirable changes in food matrix. By generating high cavitation, shear, velocity impact and turbulent forces, microfluidizer brought structural modifications in food which led to significant improvements in physicochemical, functional, nutritional, rheological and sensory properties of food products without affecting their natural flavour. Reduction in particle size and thereby increase in surface area has brought these unique modifications. Microfluidization also improved bioavailability and bioaccessibility of bioactives by making them more exposed. Applications of microfluidizer includes stable emulsion/suspension formation, encapsulation, and nanoparticle production. It has also shown its preservation potential by inactivating enzymes and microbes thus improving food stability. The present review comprehensively discusses the working principle and effect of microfluidizer on dairy products, fruit juices, cereals, starches, egg yolk, emulsions, suspensions, and other novel products formulations. Microfluidization has opened a new channel for developing novel food ingredients non-thermally.
Keywords: Microfluidization, Nano-emulsions, Dairy products, Cereal products, Fruits and Vegetables
Introduction
Homogenization, in general, is referred to as a physical/mechanical process that can convert two immiscible liquids into a relatively homogenous mix (Dhiman & Prabhakar, 2021). Actions, such as mixing, blending, and stirring (agitation) of liquids, solids, and gases, are closely related to homogenization. There are several types of homogenizers like colloidal mills, ultrasonic homogenizers, high shear mixers, membrane homogenizers and high-pressure homogenizer (HPH) which are used in food, pharmaceutical, biotechnology and chemical industries (McClements, 2016). Milk and milk processing industries have been using homogenization at commercial level for more than hundred years. It is commonly used in milk processing in order to disrupt larger droplets into smaller ones to have an advantage of lower creaming rate (Chavan et al., 2016). Disruption of fat globules by homogenization increases its surface area which is then stabilized by mainly casein and skim milk protein (Tobin et al., 2015).
Microfluidization (MF) is a type of non-thermal HPH, which creates a fine emulsion (Chavan et al., 2017). The fluid processing at high-shear through microfluidizer was patented by Cook & Lagace (1985) and Paquin & Giasson (1989) were the first to utilize the technique for food applications. Initially, cosmetic, and pharmaceutical industries utilized the microfluidizer to produce very fine emulsions (Robin et al., 1992) and later became popular in food applications. In microfluidizer, under very high pressure the mechanical energy is transferred to product (Monroy-Rodríguez et al., 2021) and the pressure range used is upto 200 MPa which creates very fine and stable emulsions. Liquid can be passed from the chamber multiple times in order to intensify the exposure time and homogenization effect. Of late, microfluidizer has got increased attention of researchers working for agricultural and food processing sectors including dairy, cereals and fruits & vegetables (Guo et al., 2020; Mert, 2019; Ozturk & Turasan, 2021a). It has solved emulsion instability problems like creaming, sedimentation, flocculence, Ostwald ripening, coalescence and it has also been used for solving cloudiness problem in beverages and for preserving bioactive compounds (Ozturk & Turasan, 2021a). Food commodities are constituted of fat, carbohydrates, proteins and enzymes. Microfluidizer is known to bring in modifications in these constituents depending upon processing parameters such as different pressures and the number of passes. Reduction in particle size, denaturation of protein, formulation of stable nano-emulsions, decrease in syneresis, microbe and enzyme inactivation are some of the modifications observed in food matrix brought by microfluidizer. In addition to this microfluidizer also causes the extension of shelf life of food product and enhancing their rheological, emulsifying, nutritional and sensory properties (Dhiman & Prabhakar, 2021; Ozturk & Turasan, 2021a, 2021b). It has been reported to bring modifications in food macromolecules like starch and fibres, which has led to alterations in its functional properties like solubility, water holding capacity (WHC), oil holding capacity (OHC), porosity and cation exchange capacity (CEC) depending on pressure applied, number of passes, food material properties and its concentration (Guo et al., 2020; Mert, 2019). MF alters the structure of proteins by unfolding them which leads to changes in its functional properties (Monroy-Rodríguez et al., 2021). Using microfluidizer has also been reported to be beneficial for improving bioavailability of phenolic compounds (Ozturk & Turasan, 2021a). Microfluidizer has a huge potential on research and industrial scale. Thus, the objective of this study is to critically review the latest research studies in food processing by using microfluidizer along with its working principle which can provide valuable information to food manufactures and researchers.
Working of microfluidizer
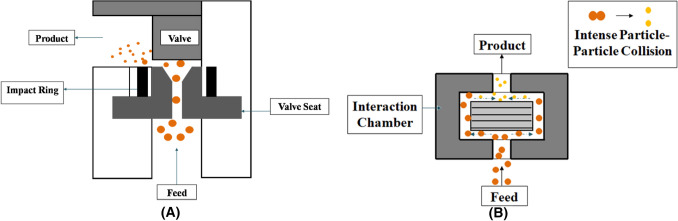
Although microfluidizer is a type of HPH, but is different in terms of working principle and design (Fig. 1). The Microfluidizer M-110P (Microfluidics® high shear fluid processors, USA) equipment is made up of the following major components: Hydraulic Power System, which provides power to the microfluidizer equipment. High pressure single-acting intensifier pump, which multiplies the pressure supplied from the hydraulic power system to an optimal operating level inside the interaction chamber (Ozturk & Turasan, 2021b). Pump oscillates in two strokes as suction and compression, which pressurizes the air upto 276 MPa approx. The interaction chamber is recognized as a continuous micro-channel that is responsible for turbulent mixing, energy dissipation, and is a stabilized geometry that generates a homogeneous pressure profile, thus helps in achieving a narrow size and distribution of particles. The interaction chamber is available in two different types that are Y-type and Z-type (Microfluidics, 2008). Y-type chamber divides the feed into two microstreams where it experiences very high velocity and then these microstreams collide with each other and with the wall, thereby leading to droplet disruption. Z-type chamber has zigzag microchannel through which feed is forced at high pressure where it experiences particle–particle and particle–wall collision thereby causing breakdown of droplets (Mert, 2019). This type of chamber is Equipment also has an optional auxiliary processing module which further processes the product and has the cooling coil which cools the product stream back to ambient temperature as it exits the system. As for the type of pump, a pneumatic pump is used. The fluid is forced in the interaction chamber at very high pressure with high velocity, where it is divided into two streams which collide at 180° in the interaction chamber. Due to a rapid drop in pressure; impact, cavitation, shear and turbulence effect are observed and as a result emulsification occurs (Dhiman & Prabhakar, 2021).
Fig. 1.
Disruption of macro-emulsions into fine droplets by (A) HPH and (B) Microfluidizer (Adapted from: Dhiman & Prabhakar, 2021)
The product stream is acted upon by two primary forces which bring about the desired results. First is the ‘shear force’, which acts between the product streams and walls of the channel at high velocity and second is the ‘impact force’ that is collision that occurs when the high-velocity product stream impinges upon itself. Upon exiting the interaction chamber, a heat exchanger brings the product stream to ambient temperature.
As compared to HPH, microfluidizer can achieve constant pressure with the utilization of an intensifier pump and fixed geometry orifice. The benefit of constant pressure is uniform shear, which can help create very small particles with very tight distributions (Tobin et al., 2015).
Microfluidization advantages and limitations
Microfluidizer produces stable emulsions with uniform particle size distribution and droplet size upto < 0.1 µm. It helps in maintaining consistency in the composition of products. Shearing rate of microfluidizer is much higher than traditional grinders. It maintains greater repeatability than traditional grinders (Dhiman & Prabhakar, 2021). It is scalable and operates in both batch and continuous mode. Product temperature can be controlled here by the cooling jacket. Industrial throughput: up to 20,000 l/h and pressure up to 275 MPa (Mert, 2019; Microfluidics, 2008). Microfluidizer requires high energy and its microchannels are sensitive thus susceptible to wear. Operating it at high pressures causes re-coalescence leading to an increase in droplet size termed as ‘Over Processing’. Beyond a critical point in terms of pressure applied and number of passes used particle size increase has been observed rather than decrease which has been attributed to increase in Brownian motion (Microfluidics, 2008; Ozturk & Turasan, 2021a).
Applications of Microfluidization in different food products
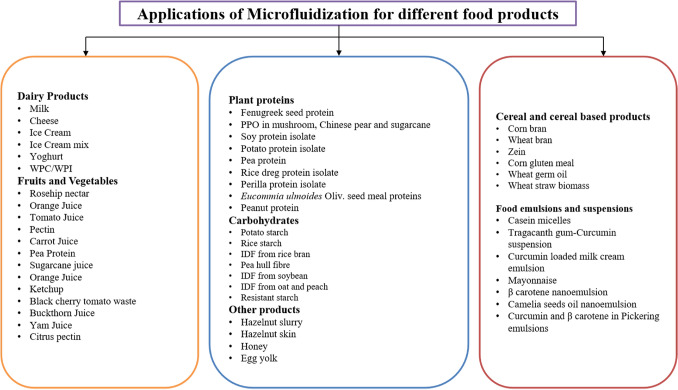
In food processing (Fig. 2) MF has been explored for dairy products, cereals, beverages, proteins/enzymes, polysaccharides, non-starch polysaccharides, dietary fibre, starch, food emulsions, bioactive encapsulation and forming micronized products in order to achieve techno-functional and preservation advantage (Guo et al., 2020; Mert, 2019; Ozturk & Turasan, 2021a, 2021b) which has been discussed in details in below given sections.
Fig. 2.
Microfluidizer as a novel processing technique for different foods
Dairy products
Several researchers have studied the effect of MF treatment over different dairy products i.e. milk, cheese ice-cream, yoghurt, and isolates; which are summarized in Table 1.
Table 1.
Applications of MF in dairy products
| S.no | Product | Pressure (MPa)/Passes | Major outcomes/particle size reduction | Reference |
|---|---|---|---|---|
| 1 | Milk | 35 & 103/1 |
Upto 0.1 µm Inhibition of fat clusters |
(Mccrae, 1994) |
| 2 | Cow milk/Buffalo milk | 17-207/1 |
Reduction in cholesterol content upto 42% in cow milk and 46% in buffalo milk Improved thermal properties, texture profile and digestibility |
(Kumar et al., 2019) |
| 3 | Cheddar cheese | 7–69/1 |
Cheese prepared was whiter in colour Increase in moisture content |
(Lemay et al., 1994) |
| 4 | Mozzarella cheese | 3-172/1 |
Upto 300–400 nm Decrease in melting and flowing properties Decrease in cohesiveness and firmness of cheese on increasing pressure At higher pressure size of fat globule was much smaller |
(Cooke et al., 2000; Van Hekken et al., 2007) |
| 5 | Milk | 14-35/2–5 |
Upto 313 nm Decreased rennet coagulation time and curd firming rate |
(Tosh & Dalgleish, 1998) |
| 6 | Milk | 70-170/2 |
Upto 0.30–0.501 µm Stable emulsion formation Inactivation of alkaline phosphatase Decrease in mesophilic aerobic and psychrophilic milk microflora |
(Bucci et al., 2018) |
| 7 | Ice cream | 50-200/1 |
Reduction in meltdown rate No effect on sensory properties |
(Olson et al., 2003) |
| 8 | Ice cream mix | 50-200/1 | 68% reduction in spores of Bacillus licheniformis | (Feijoo et al., 1997) |
| 9 | Ice cream mix | 220–250/1 | Increase in gumminess, cohesiveness and adhesiveness | (Cavender & Kerr, 2013, 2020) |
| 10 | Yoghurt | 103 & 151/1 |
Shear thinning rheology Reduction in syneresis |
(Chavan et al., 2014) |
| 11 | Yoghurt | 150/1 |
Upto 174 µm Increase in interconnectivity of protein network with fat globules embedded in it Increase in viscosity Increased syneresis in non-fat yoghurt at 150 MPa Increased chalkiness, mouth dryness and astringency in case of non-fat yoghurt |
(Ciron et al., 2010, 2011) |
| 12 | Yoghurt | 50–150/1 |
Increase in gel strength by 171–195% and viscosity by 98–103% Increased matrix density and decreased porosity Improvement in sensory properties Improved flavour attributes, mouthfeel and creaminess |
(Ciron et al., 2012) |
| 13 | WPC | 150/1 |
Stable product against sedimentation Powder manufactured had increased solubility |
(Iordache & Jelen, 2003) |
| 14 | WPI | 138/1 |
Reduction in mean length of fibrils Altered thickening characteristics |
(Koo et al., 2018) |
Milk
Milk microfluidized at 35 and 103 MPa resulted in a fat droplet size of around 0.1 µm. Due to a decrease in fat globule size; casein load was higher at fat/serum interface whereas serum proteins found were lower in amount (about 2–5% of total protein load). At moderate pressure, fat clusters formation was inhibited due to a high protein load (Mccrae, 1994). It was reported by Hardham et al. (2000), that MF of UHT milk was beneficial in terms of reducing extent of fat separation and could keep it stable for 9 months, on the other hand conventional homogenizer was able to keep it stable for 2–3 months. Fat content, protein content, and pressure applied are important factors that are to be considered while optimizing the parameters for microfluidizing milk. Microfluidizing skim, 2% and whole milk reduced the particle size when pressure was increased from 50 to 100 MPa. Above 100 MPa decrease in particle size in skim and 2% milk was not significant in comparison to the pressure at 100 MPa. Whereas, in the case of the whole milk increase in pressure from 100 to 200 MPa and in the case of cream from 100 to 150 MPa, particle size increased, which might be due to formation of homogenization clusters at excessive high pressure. Cluster formation occurs due to a deficiency of coating material available for the fat globule surface (Olson et al., 2004). MF is beneficial for processing milk as it had led to an increase in heat stability of concentrated milk at high pressure and it also does not affect the aroma profile of milk (Paquin, 1999; Van Hekken et al., 2019).
Kumar et al. (2019) studied the effect of MF on cholesterol of milk and concluded that MF was effective in reducing the cholesterol level by 42 and 46% in cow and buffalo milk respectively. MF breaks the membrane of milk fat globules that rupture the bond of cholesterol with protein molecule and cholesterol comes out in an aqueous phase, where the surface area of the cholesterol might be exposed more and higher breakdown occurred during MF. MF also improved in vivo digestibility of proteins, stability of lactose, proteins and melting properties of fat (Kumar et al., 2019).
From the above studies, it could be concluded that MF of milk leads to a lower particle size of fat globules and reduced cream separation effectively without affecting its original quality which also depends on operating parameters i.e. pressure, cycles, and temperature. Apart from this it can also decrease the cholesterol content.
Cheese
Cheddar cheese made from microfluidized milk (7–69 MPa) or cream (14 and 69 MPa) was whiter in colour as compared to control cheese which was attributed to reduction in particle size due to which scattering of light was more. It also had a higher yield and moisture content (37.6–39.3%) which could be due to higher retention of fat in treated cheese (Lemay et al., 1994). For MF of cheese milk, temperature and pressure were found to be very important factors as it affects the melting (hardness, cohesiveness, and springiness) and rheological properties of cheese. Melting and flowing property of mozzarella cheese decreased due to MF (Van Hekken et al., 2007). Melting property of cheese is expressed to the interruption of the protein matrix upon heating with the entrapped melting of fat and the interactions between protein–protein, which impaired and allowed the movement within the protein matrix and the cheese to flow (Lucey et al., 2003). MF might have disrupted the protein matrix and have redistributed lipids throughout the protein matrix which might have decreased its meltability and flowability.
Mozzarella cheese prepared from microfluidized milk (34 and 103 MPa) had a different microstructure when compared with control cheeses, which was observed by Scanning electron microscopy, where homogenization temperature and pressure were the factors altering microstructure. As the physical state of fat gets affected by an increase in temperature and size of the fat globule cavities in cheese decreases with an increase in pressure and temperature, this leads to a difference in microstructure (Cooke et al., 2000). In another study, fat substitutes were prepared by breaking aggregated milk protein into a spherical form so that they mimic the ball-bearing effect of fat particles in food and this thermo-mechanical effect was achieved using MF. Micro-particulation (5–10 µm) of whey protein concentrate and isolate using MF was done to mimic fat and was used to prepare reduced-fat cheddar cheese (15% reduction was achieved) but with same textural properties as of full-fat cheese (Paquin et al., 1993; Paquin, 1999). After MF, the time required for rennet coagulation and curd firming rate decreased, which could be due to the restructuring of casein and changes in properties of curd (Tosh & Dalgleish, 1998).
Raw, pasteurized and thermalized milk was treated at different pressure (125–170 MPa with an inlet temperature of 42–54 °C) to find the best optimum conditions of milk to prepare soft cheese and was compared to control cheese samples (without microfluidizer treatment). Differences in changes were desirable like a decrease in particle size, microbial count, inactivation of alkaline phosphatase and stable emulsion formation which were due to intense pressure, shear, and cavitation experienced by treated milk samples. But it resulted in longer coagulation time and the gel was less in firm. It was found that low pressure and temperature treatment of samples at 75 MPa and 42 °C respectively resulted in properties similar to that of control samples (Bucci et al., 2018).
It can be concluded that low-pressure treatment of milk using MF is effective in cheese processing whereas high pressure treatment can be utilized in the extension of milk’s shelf-life. MF positively influences the melting and flow behaviour properties of cheese as both were decreased and gave desirable characteristics.
Ice-cream
Ice creams prepared from microfluidized non-fat and low-fat milk had slowed down meltdown properties which might be due to protection provided by protein covering. Although it did not affect the sensory properties of ice-cream as compared to control (Olson et al., 2003). Melting properties are dependent on the size of fat globule (Koxholt et al., 2001). Reduction in the size of fat globule might have altered the melting properties of ice cream. Formation of larger chains and aggregates of protein and fat after MF might have been another possible reason for reduction in melting rate (Olson et al., 2003). However, (Cavender & Kerr, 2013) found that sensory properties were influenced by MF. In the study, full fat ice cream mixes were prepared by using xanthan gum and locust bean gum and were processed using microfluidizer. Consumers preferred ice creams made from locust bean gum due to creaminess and firmness also MF influenced individual properties of gums used leading to an increase in gumminess, cohesiveness, and adhesiveness. On microfluidizing full fat ice cream (220–250 MPa) with xanthan or locust bean gum particle size increased slightly. This might have happened due to interaction between protein and polysaccharide components which then aggregate at lipid interface. Microfluidized mixes were softer and had increased shear thinning behaviour (Cavender & Kerr, 2020). MF was also effective in destroying spores of Bacillus licheniformis inoculated in ice cream pre mix upto 68% by using pressure 200 MPa (Feijoo et al., 1997). Shear stresses, sudden pressure drop, torsion, turbulence, high velocity collisions and cavitation shock waves might have ruptured the cell membrane of microbe leading to cell death. In comparison to high pressure processing, microfluidizer uses less pressure for microbial destruction (Dhiman & Prabhakar, 2021).
From the above studies, it was found that MF significantly influenced the characteristics of ice-cream as improved resistance to melting characteristics, the formation of strong gel, inactivation of microbe and provided better sensory properties.
Yoghurt
MF has been found to enhance the rheological, textural and sensory properties of yoghurt along with decreased separation of whey in it. The gel particle size of stirred yoghurt was inversely related with milk’s particle size as MF led to increase in interconnectivity of fat and protein molecules which led to improvement in the rheological behaviour of yoghurt. (Augustin et al., 2008) substituted solid content of milk with microfluidized resistant starch and reported improved viscous and syneresis properties of yoghurt. Addition of microfluidized starch thickens the gel and holds the water which might have reduced syneresis in the yoghurt. Rheological behaviour of microfluidized yoghurt (Chavan et al., 2014; Ciron et al., 2011; Demirkesen et al., 2018) was shear thinning (pseudoplastic), which was similar to the non microfluidized yoghurt (Codin et al., 2016). Shear thinning nature of yoghurt was also proved by Herschel-Bulkley model and power law model. According to (Chavan et al., 2014), the viscosity of microfluidized mango flavoured yoghurt decreased and no change in pH was found with increasing the temperature. Demirkesen et al. (2018) developed novel yoghurt through fermented (Lactic acid bacteria) slurry of microfluidized hazelnut and found that MF improved firmness, viscosity and maintained creamy structure.
Ciron et al. (2010) stated that the texture of microfluidized yoghurt depends on the fat content of milk, heat treatment and different pressure of microfluidizer. Syneresis and texture quality was not good in stirred yoghurt developed from preheated non-fat microfluidized (150 MPa) milk. It was concluded that MF may improve syneresis in fat-containing yoghurt.
Yoghurt prepared from preheated non-fat microfluidized milk had better original flavour but microfluidized yoghurt prepared from non-fat milk had more astringency, mouth dryness and chalkiness taste. Whereas, MF improved the quality such as creaminess, thickness, cohesiveness and smoothness of yoghurt prepared from low-fat preheated milk. It may be due to the strong assimilation networks of fat-protein gel by the uniform microstructure of microfluidized milk (Ciron et al., 2011).
Sensory properties of low fat microfluidized yoghurt were identical as full-fat yoghurt due to improved properties of gel viscosity and gel strength of microfluidized yoghurt (Ciron et al., 2012). Demirkesen et al. (2018) concluded that sensory of yoghurt based on the slurry of microfluidized hazelnut was equivalent to the control sample. Ciron et al. (2010) analysed the sensory properties of low-fat yoghurt prepared from microfluidized milk and claimed that MF may have applications for the development of the best quality of low-fat yoghurt.
According to Swer et al. (2019), microfluidized yoghurt showed better colour (anthocyanin pigment obtained from Sohiong (Prunus nepalensis L.) stability than non-microfluidized sample. This might have happened due to improvement in the texture of yoghurt (prepared from microfluidized milk) which would have kept pigment intact and stable for a longer time.
It was found form the above discussion that MF technique is very effective in improving the texture, rheological, syneresis and sensory properties of yoghurt.
Whey protein concentrate and isolates
Sportsperson consumes whey protein concentrate (WPC) and whey protein isolates (WPI) with milk or fruit beverage as an energy drink. Due to health consciousness among the consumer's consumption of WPC and WPI is increasing. Sedimentation is one of the major quality defects found when whey is mixed in milk, fruit beverage, or other liquid food. In thermally treated beverages, whey protein inclusion is obstructed by heat-induced in solubilisation (Iordache & Jelen, 2003). The concentration of protein, temperature, and pH were found out to be major factors leading to the aggregation of whey proteins (De Lorenzi et al., 1995).
WPC was dissolved in water or fresh acid whey to make whey protein solutions and appearance of WPC solution changed from clear to milky-white upon first heating {90 °C for 10 min at different pH (5.8, 4.8 or 3.8)} but simultaneously increase in sediment volume was observed. To check further heat sensitivity second heating was performed but it did not show any change in sediment value upon reheating. When these heated whey protein solutions were microfluidized (150 MPa) it resulted in non-sedimenting opaque suspension. It could be due to the disintegration of the heat-induced insoluble protein aggregates which would have reduced particle size thereby overcoming the hurdle of sedimentation. It was found that at pH 3.8 solutions were completely resistant to sedimentation. Increasing number of passes did not had any significant effect on sedimentation. Re-aggregation was observed on heating this microfluidized solution and was strong at pH 3.8 due to the exposure of reactive sites of soluble polymer molecules. However, spray and freeze-drying of this heated microfluidized solution to form dry powder, increased solubility, and resistance to sedimentation (Iordache & Jelen, 2003). Similarly, a combination of thermal treatment (85 °C for 20 min) and MF (137.89 MPa) applied to WPI solutions led to reduction in length of fibrils and thereby modulated the functional attributes of globular proteins aggregates along with altering their thickening characteristics (Koo et al., 2018).
It was found form above mentioned studies that MF treatment is very effective in reducing the sedimentation of isolate and concentrates.
Fruits and vegetable products
Different researchers have worked in the field of different fruits and vegetable products, which are mentioned in Table 2. Extraction yield of bioactive compound from food was enhanced by MF along with increasing the juice stability.
Table 2.
Applications of MF in fruit and vegetable products
| S.no | Product | Pressure (MPa)/Passes | Major outcomes/particle size reduction | Reference |
|---|---|---|---|---|
| 15 | Orange juice | 34–138/1–3 |
Upto 23 µm Increase in brightness Increase in juice stability Increase in phenol content and antioxidant properties |
(Yuce, 2011) |
| 16 | Ketchup | 20–120/1 |
Improvement in yield stress, storage modulus, elastic modulus and Bostwick consistency Improved physical stability of samples Increased lycopene content |
(Mert, 2012) |
| 17 | Black cherry tomato waste | 40–160/1 |
Shear thinning rheology Increased average particle size of pectin Increase in degree of esterification from 50.77% to 64.12% Decrease in viscosity and consistency index of pectin |
(Zhang et al., 2017) |
| 18 | Carrot juice | 34–103/1–3 |
Improved carotenoid content (lutein and β carotene) Changes in colour properties Increase in TSS |
(Koley et al., 2020) |
| 19 | Buckthorn Juice | 50–150/1–3 |
Upto 1 µm Over processing at pressure beyond 150 MPa Reduction in turbidity and TSS Improved physical stability and sensory quality |
(Abliz et al., 2021) |
| 20 | Sugarcane Juice | 207/3 |
Reduction in PPO activity Stable physicochemical properties 100% reduction of initial microbial load 56 days shelf life |
(Kohli et al., 2019) |
| 21 | Sugarcane Juice | 50–150/1–7 |
Upto 476 nm Reduction in sedimentation rate |
(Tarafdar & Kaur, 2021) |
| 22 | Sugarcane Juice | 50–200/1–7 |
Reduction in enzymatic activity Increase in chlorophyll content |
(Tarafdar et al., 2021) |
| 23 | Yam Juice | 80–160/2–4 |
Upto 358 nm Change in colour Bactericidal effects on E. Coli and S. Cerevisiae Reduction in non-enzymatic browning, flavonoid, turbidity and TSS by 66.7, 20.7, 86.2 and 35.5% |
(Liu et al., 2021) |
| 24 | Citrus pectin | 152/2 |
Upto 418 nm Reduction in viscosity Decreased this fibrous morphology and degree of branching Increase in DPPH radical scavenging activity Improved encapsulation functionality against UV degradation |
(Wang et al., 2021a, b) |
MF (34–138 MPa and 1–3 pass) brightened the colour of orange juice and reduced its yellowness and redness. Microfluidization significantly reduced the particle size of product upto 23 µm. The stability of the juice was increased; it remained opaque till 14 days at 4 °C when compared with control juice which turned transparent with time. MF also increased phenolic content and thus antioxidant properties of the juice which might have happened due to exposure of these compounds on the surface after MF. With an increase in pressure and cycle, less stable juice was obtained as it could be due to acceleration in the enzymatic activity (Yuce, 2011). Yam juice processed by MF had reduction in non-enzymatic browning, flavonoid content, turbidity and total soluble solids (TSS). MF also had bactericidal effects on Escherichia coli and Saccharomyces cerevisiae. Alteration in the activity of enzymes involved in flavonoid synthesis might be the possible reason for reduction in flavonoid content (Liu et al., 2021). MF was reported to increase the DPPH radical scavenging activity of citrus pectin for which reduction in viscosity was considered to be the possible explanation as it might have led to increase in interaction between free radicals and hydroxyl groups present in pectin. In addition, it also improved the functionality of citrus pectin as an encapsulant against UV degradation. Reduction in viscosity of citrus pectin could had been due to decrease in degree of branching and fibrous morphology (Wang et al., 2021a, b).
In another study, MF increased lycopene content in ketchup and improved its physical stability along with improvement in storage modulus, elastic modulus, Bostwick consistency, and yield stress (Mert, 2012). Improvements in these properties of microfluidized ketchup could be due to the release of soluble and entrapped components into the continuous liquid phase on increasing the MF pressure. The effect on properties (physicochemical, structural characteristics and morphological features) of pectin extracted from black-cherry tomato pomace and potato peel waste after treatment with MF was studied by Zhang et al. (2017). Processing with microfluidizer (40–160 MPa) was effective in modifying the properties of pectin i.e., degree of esterification from 50.77 to 64.12%; from lumpy mass to foamed filiform (viewed by NMR spectrum without altering the main structure); and pectin solution showed shear thinning behaviour (Zhang et al., 2017). As these modifications induce changes in rheological behaviour (viscosity and consistency) and were indicative of their use as a potential additive.
Kohli et al. (2019) worked on microfluidized (3 pass at 206.15–207.53 MPa) sugarcane juice and concluded that MF and natural polypeptides (nisin and polylysine) improved the shelf life of sugarcane juice for 56 days at 5 °C. MF led to 100% reduction of initial microbial load. MF at high pressure and multiple passes converted the bigger particle size into smaller size such that they remain homogeneous throughout the storage period which helped in shelf-life improvement. MF reduces the sedimentation rate of sugarcane juice by reduction in particle size upto 476 nm (Tarafdar & Kaur, 2021). Processing sugarcane juice with microfluidizer was also found to be beneficial in terms of increasing its chlorophyll content which was attributed to the cellular disruption brought by MF (Tarafdar et al., 2021).
In another study it was found that on microfluidizing buckthorn juice (50–150 MPa & 1–3 passes), particle size became smaller than control. On increasing pressure beyond 150 MPa and number of passes for treatment the particle size increased which might have happened either due to over processing or aggregation of proteins at high pressure and temperature or due to van der waals forces between molecules. In addition to this MF also reduced turbidity and TSS of juice which might be attributed to denaturation of enzymes or release of insoluble solid particles. The juice processed had improved physical stability, sensory quality and retained nutritional quality (Abliz et al., 2021).
In a study by Koley et al. (2020), carrot juice was microfluidized at different pressure (34.47–103.42 MPa) with different passes (1–3 passes) and it was found that carotenoids content in juice improved significantly. Available lutein and β-carotene concentration increased 260 and 530% from fresh juice. Impact and shear forces generated inside the chamber might have disrupted the membrane of carotene body and allowed release of pigments such as lutein and β-carotene. Furthermore, MF reduced colour properties significantly which can be attributed to increase in turbidity of carrot juice.
From the above studies, it can be concluded that MF at moderate pressure is effective in increasing the extraction yield, bio-accessibility of different components present in fruits and vegetables juices, or products. It also leads to stability in juice as reduced sedimentation and desired modification in the structure of pectin obtained from fruits and vegetables so that it can be used as additives or stabilizers.
Cereals and cereal-based products
MF improved physicochemical, nutritional and functional properties (Table 3) of cereal or cereal-based products (Mert, 2019). The surface area of corn gluten meals was increased by MF and was used in making gluten-free bread. Modification in structure allowed gas retention thus increasing specific volume (Ozturk & Mert, 2018a). Structural disintegration caused by MF led to exposure of lutein and zeaxanthin. It also increased the WHC and stability of corn gluten meal. Adding citrus fibre and xanthan to the bread had higher springiness and cohesiveness with lower hardness (Ozturk & Mert, 2018b). MF along with the addition of quercetagetin was used to formulate zein nanoparticles. Both treatments improved the particle's thermal stability and also caused changes in its structure which led to reduction in α helix and increase of β-sheets. In addition to it reduction in fluorescence intensity was also observed (Sun et al., 2016). In another study, corn gluten meal was passed through a microfluidizer at varying pH so that modification in particle size, functional and rheological properties could be achieved. The treatment reduced particle size and increased the surface area, enhanced WHC, and lowered sedimentation value. It increased the possibility of using it as an emulsifier and adds value to it, which otherwise is underutilized and used as animal feed (Ozturk & Mert, 2019).
Table 3.
Applications of MF in cereals and cereal based products
| S. no | Product | Pressure (MPa)/Passes | Major outcomes/particle size reduction | Reference |
|---|---|---|---|---|
| 25 | Corn gluten meal | 50–125/3 |
More structural disintegration caused by MF Exposure of lutein and zeaxanthin along with increase in brightness Increased cohesiveness and springiness; decreased hardness Adding microfluidized corn gluten meal in bread led to more gas retention and thereby increasing specific volume |
(Ozturk & Mert, 2018a) |
| 26 | Corn gluten meal | 50–125 |
Upto 1.2 µm Increase in surface area, stability and WHC Exposure of lutein and zeaxanthin Bread formed using microfluidized corn gluten meal as an ingredient had brighter crumb |
(Ozturk & Mert, 2018b) |
| 27 | Zein | 25–100/1–5 |
Upto 20 nm Reduction in fluorescence intensity Improved thermal stability Reduction of α helix and increase of β sheets |
(Sun et al., 2016) |
| 28 | Corn gluten meal | 50–125 |
Upto 1.2 µm Formation of micropores and increase in WHC Shear thinning rheology Reduction in sedimentation rate |
(Ozturk & Mert, 2019) |
| 29 | Corn bran | 159 & 172/1–5 |
Decrease in bulk density Increase in porosity Increase in WHC, OHC, CEC and SC by 90%, 140%, 90% and 140% |
(Wang et al., 2013b) |
| 30 | Corn bran | 124–159/1–5 |
Increase in WHC, OHC and SC Improvement in antioxidant properties |
(He et al., 2016) |
| 31 | Wheat bran | 159 & 172 |
Upto 52.8 µm Decrease in bulk density Increase in specific surface area, OHC, WHC, SC and OHC |
(Wang et al., 2012) |
| 32 | Wheat bran | 159 & 172/1–3 | Increase in antioxidant capacity | (Wang et al., 2013a) |
| 33 | Wheat bran | 150/3 |
Fine separation of fibrous structure Increase in WHC and phenolic content Provided gluten like strength to reduced flour cake batter Affected colour of cake samples and cake had firmer texture Retard moisture loss and slowed staling rate |
(Mert et al., 2014) |
| 34 | Wheat straw biomass | 50–150 |
Alteration in structure of biomass Reduction in lignin content MF was an effective pre-treatment for enzymatic hydrolysis and ethanol yields i.e., sugars |
(Turhan et al., 2015) |
| 35 | Wheat germ oil | 500/1–3 |
Upto 0.2 µm Stable emulsions formed Encapsulation by MF was better than ultrasonics |
(Karadeniz et al., 2018) |
On microfluidizing corn bran, its bulk density decreased along with an increase in porosity, CEC, OHC, swelling capacity and WHC which was attributed to reduction in its particle size along with loosening of its structure. An increase in porosity, surface area and exposure of different functional groups enhances properties of corn bran (Wang et al., 2013b). The technology also leads to improvement in the antioxidant properties of corn bran (He et al., 2016). Similarly, in the case of microfluidized wheat bran which is also a rich source of dietary fibre, an increase in CEC, OHC, swelling capacity and WHC and decrease in bulk density was observed by Wang et al. (2012). In native wheat bran, phenolic compounds are mostly linked to polysaccharides and trapped in the fibre matrix. MF causes an increase in the antioxidant capacity of wheat bran (Wang et al., 2013a), as in the case of corn bran. MF also increased the phenolic content of wheat bran. When microfluidized wheat bran was added to the cake batter, the product formed had a firmer texture than control and required less flour than control. The colour of the cake was also affected. Due to high WHC, staling rate and moisture loss were reduced (Mert et al., 2014).
Due to a reduction in lignin content and change in the structure of wheat straw biomass caused due to MF, its enzymatic hydrolysis was improved and yield of ethanol increased (Turhan et al., 2015). In a study by Yildiz et al. (2016) cookies were produced by incorporating microfluidized hazelnut skin due to which retrogradation slowed down. Retrogradation might have delayed either due to increase in WHC or increase in starch-starch interactions as a result of shredded fibres. Cookies produced had lower water activity and had moisture content compared with control. MF broke large particles into smaller ones and allowed the release of phenolic compounds. This brought changes in textural and rheological properties.
Wheat germ oil was encapsulated using different methods to avoid its oxidation as it is a rich source of α-tocopherol. Among the different methods studied i.e. silent crusher (75,000 rpm for 15 min), MF (500 MPa for 1–3 pass) and ultrasonication (15 min at 40 kHz using 50% pulse) it was found that MF and silent crusher created more stabilized emulsion as compared to ultrasonication as assessed from physicochemical properties and storage stability of the germ oil (Karadeniz et al., 2018).
Different food products
Several researchers have worked in the field of different food products, which are mentioned in Table 4.
Table 4.
Applications of MF in other food products
| S.no | Product | Pressure (MPa)/ Passes |
Major outcomes/particle size | Reference |
|---|---|---|---|---|
| 36 | Resistant starch | 40 & 80/1 & 3 |
Increase in viscosity On adding it to yoghurt, it increased its viscosity Decreased syneresis in yoghurt |
(Augustin et al., 2008) |
| 37 | Hazelnut slurry | 135/1 |
Increased the firmness of yogurt prepared using microfluidized hazelnut Increased colloidal stability Almost similar sensory properties as control yoghurt |
(Demirkesen et al., 2018) |
| 38 | Hazelnut skin | 150/2 |
Breakdown of matrix to flaky and fibrous structure with high WHC Cookies with lower water activity were prepared on using microfluidized hazelnut skin as an ingredient as compared to control having comparable moisture content Released phenolic compounds Slowed down retrogradation rate |
(Yildiz et al., 2016) |
| 39 | Honey | 34–69/1–5 |
Decrease in diastase number, glucose and fructose content Increase in phenolic content and antioxidant activity Reduction in viscosity upto 50% and had Newtonian behaviour |
(Leyva-Daniel et al., 2020) |
| 40 | Egg yolk | 103–207/1 |
Upto 7 µm Altered colour and increased denaturation temperature Increase in viscosity and change of rheology from Newtonian to Shear thinning Improved emulsifying properties |
(Suhag et al., 2021) |
| 41 | Fenugreek seed protein | 69/1 |
Improved emulsion stability and OBC Reduced foam formation and WBC Increase in denaturation temperature Final product lighter in colour |
(Ghanghas et al., 2021) |
| 42 | IDF from oat and peach | 120/1 |
Upto 111 µm (oat IDF) and 204 µm (peach IDF) Improved OHC, WHC and SC Conversion of some IDF to soluble ones Increase in IDF’s postprandial serum glucose ability Increase in α amylase and lipase inhibitory activity |
(Chen et al., 2013) |
| 43 | IDF from soybean | 80–170/3 |
Upto 3 µm Increase in WHC and induced puffed morphology Adding it to rice starch increased viscosity of the paste and decreased breakdown Better for restraining short term retrogradation |
(Liu et al., 2016) |
| 44 | Pea hull fibre | 200/2 |
Increased thermal stability Increase in water retention capacity |
(Morales-Medina et al., 2020) |
| 45 | Sugarcane Juice | 50–200/1–7 |
Reduction in PPO activity by 39–64.7% Reduction in POD activity by 16.4–75% Reduction in activity of sucrose neutral invertase |
(Tarafdar et al., 2021) |
| 46 | Mushroom PPO | 90–150/1–3 |
Increase in activity of PPO Destruction of some secondary structures |
(Liu et al., 2009a) |
| 47 | Chinese pear PPO | 80–180/1 |
Increase in activity of PPO With increase in pressure increase was more |
(Liu et al., 2009b) |
| 48 | Soy protein isolate | 60–140/1 |
Increased gel chewiness, springiness, cohesiveness and hardness Structural changes from α helix, β turns and random coil to β sheets Increased WHC of gel |
(Zheng et al., 2021) |
| 49 | Potato protein isolate | 42–84/1 |
Upto 319 nm Increased solubility, surface charge, foaming and emulsifying properties Disruption of intermolecular hydrogen bonds in secondary structure |
(Hu et al., 2021a, b) |
| 50 | Pea globulin-based emulsion | 50–130 |
Upto 27 µm Reduction in floc size Reduction in oil droplet size Improvement in creaming stability |
(Oliete et al., 2019) |
| 51 | Pea protein | 30–120 |
Upto 17 µm Improved turbidity and solubility Increase in surface hydrophobicity and exposure of free sulfhydryl groups Protein denaturation |
(He et al., 2021) |
| 52 | Rice dreg protein isolate | 40–160 |
Improved solubility and surface hydrophobicity Enzymes used for proteolysis had improved solubility Increase in DPPH radical scavenging activity |
(Zhang et al., 2021) |
| 53 | Perilla protein isolate | 30–150 |
355 nm Increase in surface hydrophobicity and free sulfhydryl content Enhanced thermal stability Improved solubility, foaming and emulsifying capacity |
(Zhao et al., 2021) |
| 54 | IDF from rice bran | 0–150/2 |
Changes in morphology from flaky to fluffy and slightly expanded Increase in total negative charge Increase in Cd (II) absorption capacity |
(Wu et al., 2021) |
| 55 | Eucommia ulmoides Oliv. seed meal proteins | 0–160/1 |
Particle size reduction by 91% Increase in α-helix to β-sheet ratio thereby enhancing bioavailability Increase in solubility, WHC, fat absorption capability, foaming and emulsifying capability |
(Ge et al., 2021) |
| 56 | Rice starch | 50–200 |
165 nm Decreased pasting viscosity and viscoelasticity of starch Increase in enthalpy of gelatinization and relative crystallinity of starch Increase in fraction of resistant starch due to coating provided by bamboo shoot dietary fibre |
(Wang et al., 2021a, b) |
| 57 | Potato starch | 40–160/1 |
Changes in rheological behaviour Reduction in relative crystallinity by 25.46% |
(Chen et al., 2021) |
| 58 | Peanut protein | 120 |
Increase in exposed sulfhydryl groups and surface hydrophobicity Improved emulsion stability and solubility |
(Hu et al., 2021a, b) |
| 59 | Insoluble pea protein | 25–150/1–5 |
0.2 µm Increase in solubility Structural modifications |
(Moll et al., 2021) |
Infant baby food
Fat separation during storage is one of the limiting factors which affect the shelf life of infant baby foods. Thus, homogenization is used to reduce fat separation. It was observed that gelation accelerates in sterilized concentrated infant baby foods due to protein denaturation caused by MF used before sterilization (Pouliot et al., 1990). The results of this study suggest that MF removes casein micelles from the serum phase as they form a tightly bonded fat protein complex. Spreading of casein on fat particles is believed to increase their interaction potential and their availability for the association, which in turn explains the acceleration of age gelation phenomenon.
Honey and egg yolk
Microfluidizing honey led to decrease in fructose and glucose content, diastase number, and increased total phenolic content and antioxidant activity. The negative effect of the treatment was slight increase in hydroxymethylfurfural content but it was in the range of honey international regulations. Turbulence, cavitation and shear stress brought these changes along with favouring Maillard reaction which brought changes in colour, HMF content and antioxidant activity. Microfluidized honey showed Newtonian behaviour and had reduced viscosity upto 50% which can be useful in improving its handling and bottling (Leyva-Daniel et al., 2020). In a study by Suhag et al. (2021) egg yolk was microfluidized at different pressures (103–207 MPa) and it was observed that MF decreased the particle size of egg yolk, affected its colour, increased its denaturation temperature and improved its emulsifying properties. MF pressure increased the viscosity of egg yolk and changed its rheological behaviour from Newtonian to shear thinning. Denaturation of proteins, particle size reduction and molecular rearrangement might have brought these changes in egg yolk.
Starch-based food
Resistant starch plays an important role in human health as they are not digested by the small intestine of a human being. Most of the resistant starches lack functional properties i.e., WHC, thickening, and gel-forming properties. The study was carried out by Augustin et al. (2008) to find the effect of MF (40 and 80 MPa) in modifying the properties of resistant starch. Before MF, high amylose corn starch (resistant starch ingredient) suspension was heated (121 °C for 60 min.) and sheared, which caused a reduction in resistant starch content along with the reduction in apparent molecular weight due to breakdown of starch. MF further reduced its molecular weight. Viscosity of the starch suspension increased due to heating, shearing and MF. On its application in yoghurt, it was found that the heated, sheared and microfluidized suspension was able to enhance the viscosity and decreased the syneresis whereas these effects were not observed in the yoghurt incorporated with heated and sheared starch only (Augustin et al., 2008). Thus, making resistant starch, a potential functional food which can be used to increase water holding properties and viscosity in different food products.
Effect of MF on semi-crystalline structure and digestibility of developed starch-lipid complexes from the starch of lotus seed and six different saturated fatty acids were analysed by Chen et al. (2018) and concluded that MF is an impressive process to develop regular slowly digestible starch by composite with short-chain fatty acid. In the above two experiments disruption in starch matrix and its crystalline regions along with different new complex formation might have led to decline in enzymatic hydrolysis which delayed its digestion. Recently, Wang et al. (2021a, b) used MF and bamboo shoot dietary fibre for rice starch and found changes in the rheology (reduction in viscosity) brought by MF. Along with it enthalpy of gelatinization and relative crystallinity of starch also increased. Combination of bamboo shoot dietary fibre and MF were found to be responsible for rearrangement of molecules which might have brought changes in enthalpy of gelatinization and crytallinity order. On the other hand degree of crytallinity of potato starch was reduced by MF which was attributed to destruction in non covalent linkages, resulting in damaging crystal structure (Chen et al., 2021).
Plant-based proteins
MF of fenugreek seed protein concentrate at 69 MPa was found to be effective in improving its OBC and emulsion stability. Along with it, a product with lighter colour and increased denaturation temperature was obtained. These changes were observed due to conformational and structural changes brought by MF (Ghanghas et al., 2021). MF (30–250 MPa) as a pre-treatment was used to disaggregate peanut protein isolates to develop antihypertensive peptide fractions by Gong et al. (2017). It was found that using pressure of 120 MPa led to maximum disaggregation and beyond that i.e., 150–250 MPa, peanut protein isolates were found to reaggregate. Small peptides contents, degree of hydrolysis of peanut protein hydrolysates and surface hydrophobicity were highest at 120 MPa. The mechanism was discussed by Gong et al. (2019) where it was found that MF altered the polar environment thereby improved promoted surface hydrophobicity and the formation of disulphide bonds, while the free sulfhydryl group content was decreased. The magnitude of these depends upon the pressure treatment which was optimum at 120 MPa. In another study, globulin aggregates of pea (Pisum sativum L.) were microfluidized (130 MPa pressure) and dried (spray drying or freeze-drying). Collisions, shear and turbulence decreased floc size, oil droplet size and modified structure of pea globulin interfacial film. Creaming stability of thermally aggregated pea globulin-based emulsions improved on increasing MF pressure (Oliete et al., 2019). Recently, improvement in rheological properties, functional properties (WHC, OHC, solubility, foaming capacity, emulsifying capacity, emulsion stability, turbidity, surface hydrophobicity) and thermal stability has been reported for soy protein isolate (Zheng et al., 2021), potato protein isolate (Hu et al., 2021a, b), pea protein (He et al., 2021), rice dreg protein isolate (Zhang et al., 2021), perilla protein isolate (Zhao et al., 2021), Eucommia ulmoides Oliv. seed meal proteins (Ge et al., 2021) and peanut protein (Hu et al., 2021a, b). These changes were attributed to structural modications like changes in α-helix to β-sheet ratio, structural changes from α-helix, β-turns and random coil to β-sheets and protein denaturation caused by intense turbulence, cavitation and shear forces generated by microfluidizer.
Insoluble dietary fibre
Size reduction to micron and nano-level by microfluidizer leads to enhancing functional characteristics along with the physicochemical properties of food material and termed as micronization (Dhiman & Prabhakar, 2021). On microfluidizing insoluble dietary fibre (IDF) from oat and peach enhanced changes in physicochemical properties were observed along with reduction in the size of IDF particles. Some composition of fibre was converted into the soluble dietary fibre. OHC, WHC and swelling properties improved. In addition to this MF also caused increase in IDF’s postprandial serum glucose ability and increase in α-amylase and lipase inhibitory activity (Chen et al., 2013). Modification of IDF from soybean was done with microfluidizer, reducing its particle size. On adding modified IDF to rice starch, WHC and viscosity was increased along with paste stability. This was found to be effective in restraining short term retrogradation (Liu et al., 2016). Increase in WHC was attributed to exposure of polar moieties on the surface of particle along with increase in its surface area after size reduction. OHC was improved due to increase in porosity. In another study pea hull fibre was microfluidized and its impact was studied on its microstructure and functionality. It was found that suspensions with smaller particle size were thermally stable and showed thixotropic, pseudoplastic and had viscoelastic behaviour. With decrease in particle size water retention capacity increased. Functionality might have improved due to defibrillation of cellulosic network and disruption of hemicelluloses and pectin (Morales-Medina et al., 2020). In a recent study by Wu et al. (2021), it was found that MF led to increase in Cd (II) absorption capacity of IDF from rice bran. This was attributed to structural disruption of rice bran which led to abundant increase in negatively charged groups leading to more Cd (II) absorption by chemical interaction.
Enzymes
On applying different pressure and number of passes, the effect of MF was studied on mushroom polyphenol oxidase (PPO) activity and it was found that mushroom PPO secondary structure was modified by MF. Loss in α-helix content along with the change in β-turns and β-sheets indicated the modified structure. Relative activity of the mushroom PPO increased along with decrease of the alpha—helix content. These changes might have happened due to increased exposure of these enzymes on surface after MF (Liu et al., 2009a). Pressure-induced enzyme denaturation/activation/inactivation in various foods depends on factors like pH and temperature (Hendrickx et al., 1998). In another study, an increase in activity of PPO was observed on processing P. pyrifolia (Chinese pear) juice using a microfluidizer. The velocity of the reaction catalysed by the PPO was proportional to the enzyme level. Optimum relative velocity was observed at pH 4.5 and 45 °C. Optimum pH varies, depending upon the source of PPO. The relative activity of PPO increased with treatment pressure and the number of passes (Liu et al., 2009b). On the other hand, MF was found to be beneficial for reduction in PPO and peroxidase activity (50–200 MPa) upto 64 and 75% respectively. MF also reduced the activity of sucrose neutral invertase. Reduction in activity was attributed to modification in tertiary and quaternary structure of enzymes due to breakdown of non-covalent linkages (Tarafdar et al., 2021).
Packaging material
Cellulose nano-fibrils have applications in food packaging. Films prepared from these nanocomposites were found to have good barrier, mechanical and protective properties. These films are classified as bio-composites and are fully biodegradable in nature (Ghaderi et al., 2014). In manufacturing cellulose nano-papers, MF (95 and 165 MPa) was used as a processing technique which led to the breakdown of cell wall into nanofibrils. Cellulose nano paper with best mechanical properties was obtained by carboxymethylation pre-treatment which was further disintegrated by MF of wood pulp fibres. Nano-paper manufactured had very high toughness, tensile strength and Young’s modulus (Henriksson et al., 2008).
Food based emulsions and suspensions
Numerous studies have been conducted to prepare micro and nanoemulsions using MF treatment alone as well as with the addition of emulsifiers, surfactants, and enzymes as cited in Table 5.
Table 5.
Applications of MF in food emulsions and suspensions
| S.no | Product | Pressure (MPa)/ Passes |
Major outcomes/particle size | Reference |
|---|---|---|---|---|
| 60 | Casein micelles | 87/6 |
Increased binding affinity for polyphenols Successfully incorporated bioactives in casein micelles |
(Yazdi, 2012) |
| 61 | Tragacanth gum-Curcumin suspension | 100–200/1–12 |
Upto 300 nm Increase in TSS with increase in number of passes Shear thinning behaviour |
(Verma, Tarafdar, & Badgujar, 2021a) |
| 62 | Curcumin loaded milk cream emulsion | 50–200/1–4 |
Upto 358 nm Encapsulation efficiency upto 98% Increased antioxidant capacity by 25% Increased bioaccessibility by 30% |
(Verma, Tarafdar, Mishra, et al., 2021b) |
| 63 | Mayonnaise | 83/3 |
Improved sensory and rheological properties Improvement in texture |
(Kadian et al., 2021) |
| 64 | β carotene nanoemulsion | 120/3 |
Upto 97.2 nm Stable for 5 weeks at 25 °C More stable emulsions formed with WPI |
(Jo & Kwon, 2014) |
| 65 | Camelia seeds oil nanoemulsion | 103–172/15 |
47 nm Stable nanoemulsions formed Antibacterial activity |
(Li et al., 2021) |
| 66 | Curcumin and β carotene in Pickering emulsions | 0–150/2 |
Upto 6.27 µm Improved photothermal stability of curcumin and β carotene on co-encapsulation Enhanced bioaccessibility (< 100 MPa) and promoted lipolysis |
(Wei et al., 2021) |
MF greatly influences the binding affinity of molecules and the stability of an emulsion. Yazdi (2012) reported that MF led to an increase in the binding of bioactive compounds that are curcumin and resveratrol with casein micelles in order to improve the delivery of these bioactive compounds. Due to change in the structure of casein micelles by MF, binding affinity for these polyphenols was increased. Resveratrol was found on the surface of casein micelles whereas curcumin in the hydrophobic core. Presence of these polyphenols’ delays rennet induced gelation time (Yazdi, 2012). Curcumin constituent of Curcuma longa has potential therapeutic benefits and well known for its anti-cancer, anti-inflammatory, and antioxidant activity but has limited bioavailability due to poor solubility in water. Curcumin nanoparticles after MF showed enhanced solubility i.e. 16 times higher than original as well as reduced particle size, higher dispersion rate (Homayouni et al., 2019). In a study by Verma, Tarafdar, & Badgujar (2021), a stable tragacanth gum curcumin suspension was prepared using MF in order to use this gum for delivery of curcumin. With increment in number of passes TSS content of the suspension increased. It was concluded that MF can be used for production of tragacanth gum curcumin suspension to use it as a delivery system of curcumin in nutraceuticals and functional foods. On microfluidizing milk cream emulsion loaded with curcumin, reduction in particle size upto 358 nm and with 98% encapsulation efficiency was observed. MF also increased the antioxidant capacity by 25% along with increasing the bioaccessibility by 30% (Verma, Tarafdar, Mishra, et al., 2021). In another study curcumin and β carotene were co-encapsuled in Pickering emulsions which led to improving there photothermal stability and enhanced bioaccessibility. Improvement in photothermal stability was attributed to dense packing of complexes brought by MF (Wei et al., 2021).
Factors that influence the particle size of emulsions are homogenization pressure, number of passes, type of emulsifier and concentration of emulsifier. MF of mayonnaise was found to be effective in improving its sensory properties (colour, flavour and mouthfeel) and rheological properties (viscosity and spreadability) along with better. Improvement in texture of mayonnaise was attributed to homogeneity brought by MF which aids in binding proteins and oil (Kadian et al., 2021). Jo & Kwon (2014) prepared nanoemulsion of β-carotene with the help of microfluidizer. Emulsifier concentration, number of cycles and pressure were the parameters responsible for decreasing the particle size (416.0–97.2 nm). The optimum parameters were found to be 120 MPa and 3 cycles. At 25 °C, the emulsion was found to be stable for 5 weeks. Cano-Sarmiento et al. (2014) formed α- tocopherol emulsions using different surfactants non-ionic (Tween-20), cationic (lecithin) and anionic (dodecyl sodium sulfate) and observed different average particle size produced by each surfactant. The stability of emulsions produced by surfactants was different and results were best in the case of lecithin. Stability was higher in the case of lecithin and was independent of the concentration of emulsifier and MF cycles. The type of emulsifier plays a very important role in forming a stable emulsion along with MF (McClements, 2016).
MF was also used to prepare nano-emulsion of rosemary essential oil by using surfactants with different hydrophilic-lipophilic balance value. Mean droplet size was dependent on surfactant/ dispersed phase ratio and HLB value. Physically stable nanoemulsions were formulated with 5 and 7.5 g/100 g of rosemary essential oil. This increases the possibility of using these nano-emulsions as a natural food preservative as well as allowing encapsulation of bioactive compounds through MF (Llinares et al., 2018).
Polyunsaturated fatty acids in an emulsion form have therapeutic benefits and its therapeutic efficacy depends on particle size as well as charge. Fish oil emulsion was prepared using microfluidizer and mesquite gum by processing at different pressures (34.5 to 206.8 MPa) and cycles (1–5). It was found that MF reduced the particle size upto 200 nm whereas a very coarse particle size (1.5 μm) was obtained using a rotary mixer. The optimum process parameters were pressure 144 MPa and 2 cycles (García-Márquez et al., 2016).
MF is also suitable for the continuous production of liposomes. Liposomes are colloids created in aqueous solutions by the self-assembly of phospholipids. They are used as a carrier system because they retain hydrophobic substances on their bilayer and hydrophilic substances in its aqueous core i.e., on the polar heads (Takahashi et al., 2007). Liposomes have a much smaller droplet size than that of stable emulsions. They can have hydrophilic substances on both sides of the bilayer. Liposomes safeguard their content from the outside environment while still permitting entry and exit of tiny molecules. They can also be used to delay the release of core material. In the food industry, it is used for entrapping antioxidants, preservatives, enzymes, isolating components, flavour and aroma, water retention and improving absorption of entrapped material (Thompson, 2005).
The size of liposomes can be controlled by optimising the number of passes and pressure generated by the microfluidizer. Thus, MF treatment was used for the production of milk fat coated microcapsules which is prepared by using an emulsifier and milk fat. Addition of the microcapsules to cheese milk helps in retaining 80–90% of the vesicles in cheese. If the material is added in unencapsulated form in cheese, only 2–4% of vesicles are retained. These lipid vesicles can be used as a carrier for enzymes which can help in ripening cheese, as a flavour carrier or as a nutrient carrier (Vuillemard, 1991). Liposomes were also produced from a milk fat globule membrane-derived phospholipid fraction with the help of MF. On increasing the number of passes and pressure droplet diameter reduced upto 95 nm (Thompson & Singh, 2006). In a study by Farhang et al. (2012), nanoliposomes were prepared from MFGM derived phospholipids by using microfluidizer. Liposomes prepared had droplet size upto 100 nm. These nanoliposomes were able to protect encapsulated ascorbic acid for at least 7 weeks which was also dependent on pH conditions.
In a study by Zhao et al. (2010) performance of microfluidizer was compared with piston gap homogenizer for preparing fat emulsions in terms of particle size of the final product. In producing smaller particulate size and narrower size distribution with fewer cycle numbers under medium and high homogenization pressure, microfluidizer (upto 209 µm) was more effective than piston-gap homogenizer (upto 282 µm) (Zhao et al., 2010). On comparing the performance of MF and sonication for preparing liquid–liquid emulsion, it was found that droplet disruption in sonicator was mainly due to cavitation whereas, in the case of microfluidizer combination of crushing and shear forces, cavitation and turbulence leads to droplet disruption. On increasing sonicator intensity, particle size decreased upto 1.1 µm whereas MF reduced the particle size upto 1.8 µm. The emulsifying capacity of both was similar if sonication power supplied is appropriate, but sonication produces a large amount of heat and thus may not be suitable for heat sensitive products. MF produced less thermal stress (Yuh-Fun & Chung, 1999). But in MF on increasing treatment conditions, emulsions become over-processed and droplets start to re-coalescence (Jafari et al., 2007a). Sonication is better in terms of cleaning and operating (Jafari et al., 2006). Ultrasound produces stable emulsions without causing over processing but emulsions prepared had wider distribution (Jafari et al., 2007b). On comparing the stability of oil in water emulsion formed by homogenizer and microfluidizer, more small droplets were formed by microfluidizer. The growth of droplet diameter with time was slow when compared with homogenizer. Emulsifier concentration required for microfluidizer was also less (Pinnamaneni et al., 2003). MF of an emulsion prepared with the help of whey protein as an emulsifier showed better oxidative stability compared to a two-stage valve homogenizer (Horn et al., 2012). In a study by Santos et al. (2020) mandarin essential oil loaded emulsion was developed by microfluidizing it along with two food grade surfactants and guar gum. Physical stability and droplet diameter of emulsion was improved. It was found that physical stability, rheological properties and droplet size was affected by ratio of surfactants used. In preparation for emulsion using MF was found to enhance stability, solubility, bio-availability, and encapsulation. The synergetic effect of treatment was also reported with emulsifiers and surfactants. Moreover, it is more effective as compared to conventional homogenization and ultrasonication treatment.
This review congregates the information about the application of MF in food. MF successfully improved functional, physicochemical, thermal, nutritional, rheological, textural and structural properties of food. Changes in these properties, makes the product as a potential food ingredient in terms of providing techno-functional advantages. Treatment pressure, number of passes, type of food product and properties of food product like concentration, viscosity and molecular size are important parameters affecting the effect of modification. It effectively decreased the particle size of the different components present in food (fat, proteins, starch, dietary fibre) successfully and thus increased their physical stability. Disruptions brought at microstructural level, improved the digestibility of food products. It also enhanced product performance by reducing coagulation time, retrogradation rate, syneresis and inactivating microbes and enzymes non thermally. Inactivation achieved by microfluidizer was at lower pressure when compared with HPP and thus it can be explored further as a new cold pasteurization equipment. Allowed release of bounded antioxidants, enhanced extraction yield, preserved bioactive compounds and increased their bioaccessibility. Produced stable emulsions for long time by reduction in particle size upto micron level thereby leading to reducing clustering, sedimentation rate and flocculation rate. In comparison to other homogenizers, it was found to me more effective in modifying different food products. Interesting application of MF on reducing milk cholesterol can be further explored for products having higher cholesterol content like egg yolk and anhydrous milk fat. In conclusion, MF can be applied to low to medium viscous liquid products without generating thermal stress. Over-processing at very high pressure in a certain case has been found out as frailty of this technology. MF can be explored for the development of novel foods and has huge scope in the industry at the commercial level.
Acknowledgements
The authors thank the National Project Implementation Unit (TEQIP-III) is a unit of Ministry of Human Resource Development, Government of India and AICTE for support through CRS Project: Application ID (1-5746176711) under collaborative Research scheme
Abbreviations
- HPH
High pressure homogenizer
- MF
Microfluidization
- WHC
Water holding capacity
- OHC
Oil holding capacity
- CEC
Cation exchange capacity
- WPC
Whey protein concentrate
- WPI
Whey protein isolates
- IDF
Insoluble dietary fibre
- PPO
Polyphenol oxidase
Declarations
Conflict of interest
Author and co-authors declare no conflict of interest.
Footnotes
Anit Kumar and Atul Dhiman have contributed equally.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rachna Sehrawat, Email: sehrawatrachna2017@gmail.com, Email: sehrawatr@nitrkl.ac.in.
Ashutosh Upadhyay, Email: ashutosh@niftem.ac.in.
References
- Abliz, A., Liu, J., Mao, L., Yuan, F., & Gao, Y. Effect of dynamic high pressure microfluidization treatment on physical stability, microstructure and carotenoids release of sea buckthorn juice. LWT - Food Science and Technology. 135, 110277 (2021)
- Augustin MA, Sanguansri P, Htoon A. Functional performance of a resistant starch ingredient modified using a microfluidizer. Innovative Food Science and Emerging Technologies. 2008;9:224–231. [Google Scholar]
- Bucci AJ, Van Hekken DL, Tunick MH, Renye JA, Tomasula PM. The effects of microfluidization on the physical, microbial, chemical, and coagulation properties of milk. Journal of Dairy Science. 2018;101:6990–7001. doi: 10.3168/jds.2017-13907. [DOI] [PubMed] [Google Scholar]
- Cano-Sarmiento C, Monroy-Villagrana A, Alamilla-Beltrán L, Hernández-Sánchez H, Cornejo-Mazón M, Téllez-Medina DI, Jiménez-Martínez C, Gutiérrez-López GF. Micromorphometric characteristics of α-Tocopherol emulsions obtained by microfluidization. Revista Mexicana De Ingeniería Química. 2014;13(1):201–212. [Google Scholar]
- Cavender, G. A., & Kerr, W. L. Microfluidization of full-fat ice cream mixes: Effects of gum stabilizer choice on physical and sensory changes. Journal of Food Process Engineering. (2013)
- Cavender, G. A., & Kerr, W. L. Microfluidization of full-fat ice cream mixes: Effects on rheology and microstructure. Journal of Food Process Engineering. 43(2) (2020)
- Chavan R, Kumar A, Mishra V, Nema PK. Effect of microfluidization on mango flavoured yoghurt: rheological properties and pH parameter. International Journal of Food and Nutritional Sciences. 2014;3(4):84–90. [Google Scholar]
- Chavan, R. S., Sehrawat R., Mishra, V., & Bhatt, S. Milk Processing, In Encyclopedia of Food Safety and Health (pp. 729-735) Oxford: Academic Press (2016)
- Chavan, R. S., Kumar, A., Sehrawat R., & Nalawade, T. Dairy Engineering: Milk Processing and Milk Products. In Meghwal, M., Goyal, M. R., Chavan, R. S. (Eds.), Dairy Engineering Advanced Technologies and Their Applications (pp. 81-102). Apple Academic Press (2017)
- Chen J, Gao D, Yang L, Gao Y. Effect of microfluidization process on the functional properties of insoluble dietary fiber. Food Research International. 2013;54:1821–1827. [Google Scholar]
- Chen, B., Guo, Z., Miao, S., Zeng, S., Jia, X., Zhang, Y., & Zheng, B. Preparation and characterization of lotus seed starch-fatty acid complexes. Journal of Food Engineering. (2018)
- Chen, L., Dai, Y., Hou, H., Wang, W., Ding, X., Zhang, H., Li, X., & Dong, H. Effect of high pressure microfluidization on the morphology, structure and rheology of sweet potato starch. Food Hydrocolloids. 115(61): 106606 (2021)
- Ciron CIE, Gee VL, Kelly AL, Auty MAE. Comparison of the effects of high-pressure microfluidization and conventional homogenization of milk on particle size, water retention and texture of non-fat and low-fat yoghurts. International Dairy Journal. 2010;20:314–320. [Google Scholar]
- Ciron CIE, Gee VL, Kelly AL, Auty MAE. Effect of microfluidization of heat-treated milk on rheology and sensory properties of reduced fat yoghurt. Food Hydrocolloids. 2011;25:1470–1476. [Google Scholar]
- Ciron CIE, Gee VL, Kelly AL, Auty MAE. Modifying the microstructure of low-fat yoghurt by microfluidization of milk at different pressures to enhance rheological and sensory properties. Food Chemistry. 2012;130:510–519. [Google Scholar]
- Codin GG, Franciuc SG, Mironeasa S. Rheological characteristics and microstructure of milk yogurt as influenced by Quinoa flour addition. Journal of Food Quality. 2016;39:559–566. [Google Scholar]
- Cook, E. J. & Lagace, A. P. (1985). Apparatus for forming emulsions. United States Patent 4533254.
- Cooke PH, Tunick MH, Malin EL, Smith PW, Van Hekken DL. Effect of high pressure microfluidization on microstructure of mozzarella cheese. LWT - Food Science and Technology. 2000;33(8):538–544. [Google Scholar]
- De Lorenzi L, Pricl S, Torriano G. Rheological behaviour of low-fat and full-fat stirred yoghurt. International Dairy Journal. 1995;5:661–671. [Google Scholar]
- Demirkesen, I., Vilgis, T. A., & Mert, B. Effect of microfluidization on the microstructure and physical properties of a novel yoghurt formulation. Journal of Food Engineering. (2018)
- Dhiman, A., & Prabhakar, P. K. Micronization in food processing: A comprehensive review of mechanistic approach, physicochemical, functional properties and self-stability of micronized food materials. Journal of Food Engineering. 292: 110248 (2021)
- Farhang, B., Kakuda, Y., & Corredig, M. Encapsulation of ascorbic acid in liposomes prepared with milk fat globule membrane-derived phospholipids. Dairy Science and Technology. (2012)
- Feijoo SC, Hayes WW, Watson CE, Martin JH. Effects of Microfluidizer® technology on Bacillus licheniformis spores in ice cream mix. Journal of Dairy Science. 1997;80:2184–2187. doi: 10.3168/jds.s0022-0302(97)76166-6. [DOI] [PubMed] [Google Scholar]
- García-Márquez, E., Higuera-Ciapara, I., & Espinosa-Andrews, H. Design of fish oil-in-water nanoemulsion by microfluidization. Innovative Food Science and Emerging Technologies. (2016)
- Ge, Z., Zhang, Y., Jin, X., Wang, W., Wang, X., Liu, M., Zhang, L., & Zong, W. Effects of dynamic high-pressure microfluidization on the physicochemical, structural and functional characteristics of Eucommia ulmoides Oliv. seed meal proteins. Lwt, 138: 110766 (2021)
- Ghaderi M, Mousavi M, Yousefi H, Labbafi M. All-cellulose nanocomposite film made from bagasse cellulose nanofibers for food packaging application. Carbohydrate Polymers. 2014;104:59–65. doi: 10.1016/j.carbpol.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Ghanghas, N., Prabhakar, P. K., Sharma, S., & Mukilan, M. T. Microfluidization of fenugreek (Trigonella foenum graecum) seed protein concentrate: Effects on functional, rheological, thermal and microstructural properties. LWT-Food Science and Technology. 149: 111830 (2021)
- Gong K, Chen L, Xia H, Dai H, Li X, Sun L, Kong W, Liu K. Driving forces of disaggregation and reaggregation of peanut protein isolates in aqueous dispersion induced by high-pressure microfluidization. International Journal of Biological Macromolecules. 2019;130:915–921. doi: 10.1016/j.ijbiomac.2019.02.123. [DOI] [PubMed] [Google Scholar]
- Gong, K., Deng, L., Shi, A., Liu, H., Liu, L., Hu, H., Adhikari, B., & Wang, Q. High-pressure microfluidization pretreatment disaggregate peanut protein isolates to prepare antihypertensive peptide fractions. International Journal of Food Science and Technology. 1-10 (2017)
- Guo X, Chen M, Li Y, Dai T, Shuai X, Chen J, Liu C. Modification of food macromolecules using dynamic high pressure microfluidization: A review. Trends in Food Science and Technology. 2020;100:223–234. [Google Scholar]
- Hardham JF, Imison BW, French HM. Effect of homogenisation and microfluidization on the extent of fat separation during storage of UHT milk. Australian Journal of Dairy Technology. 2000;55(1):16–22. [Google Scholar]
- He, F., Wang, T., Zhu, S., & Chen, G. Modeling the effects of microfluidization conditions on properties of corn bran. Journal of Cereal Science. (2016)
- He, X., Chen, J., He, X., Feng, Z., Li, C., Liu, W., Dai, T., & Liu, C. Industry-scale microfluidization as a potential technique to improve solubility and modify structure of pea protein. Innovative Food Science and Emerging Technologies. 67: 102582 (2021)
- Van Hekken, Diane L., Tunick, M. H., Malin, E. L., & Holsinger, V. H. Rheology and melt characterization of low-fat and full fat Mozzarella cheese made from microfluidized milk. LWT - Food Science and Technology. 40: 89-98 (2007)
- Hendrickx M, Ludikhuyze L, Broeck I, Van Den, Weemaes C. Effects of high pressure on enzymes related to food quality. Trends in Food Science & Technology. 1998;9:197–203. [Google Scholar]
- Henriksson M, Berglund LA, Isaksson P, Lindstro T, Nishino T. Cellulose Nanopaper Structures of High Toughness. Biomacromolecules. 2008;9:1579–1585. doi: 10.1021/bm800038n. [DOI] [PubMed] [Google Scholar]
- Homayouni, A., Amini, M., Sohrabi, M., Varshosaz, J., & Nokhodchi, A. Curcumin nanoparticles containing poloxamer or soluplus tailored by high pressure homogenization using antisolvent crystallization. International Journal of Pharmaceutics. (2019) [DOI] [PubMed]
- Horn AF, Nielsen NS, Jensen LS, Horsewell A, Jacobsen C. The choice of homogenisation equipment affects lipid oxidation in emulsions. Food Chemistry. 2012;134:803–810. doi: 10.1016/j.foodchem.2012.02.184. [DOI] [PubMed] [Google Scholar]
- Hu, C., Xiong, Z., Xiong, H., Chen, L., & Zhang, Z. Effects of dynamic high-pressure microfluidization treatment on the functional and structural properties of potato protein isolate and its complex with chitosan. Food Research International. 140: 109868 (2021) [DOI] [PubMed]
- Hu, X., Amakye, W. K., He, P., Wang, M., & Ren, J. Effects of microfluidization and transglutaminase cross-linking on the conformations and functional properties of arachin and conarachin in peanut. LWT-Food Science and Technology. 146(381): 111438 (2021)
- Iordache M, Jelen P. High pressure microfluidization treatment of heat denatured whey proteins for improved functionality. Innovative Food Science and Emerging Technologies. 2003;4:367–376. [Google Scholar]
- Jafari SM, He Y, Bhandari B. Nano-emulsion production by sonication and microfluidization—A comparison. International Journal of Food Properties. 2006;9(3):475–485. [Google Scholar]
- Jafari SM, He Y, Bhandari B. Optimization of nano-emulsions production by microfluidization. European Food Research and Technology. 2007;225:773–741. [Google Scholar]
- Jafari SM, He Y, Bhandari B. Production of sub-micron emulsions by ultrasound and microfluidization techniques. Journal of Food Engineering. 2007;82(4):478–488. [Google Scholar]
- Jo, Y. J., & Kwon, Y. J. Characterization of β-carotene nanoemulsions prepared by microfluidization technique. In Food Science and Biotechnology (Vol. 23, Issue 1, pp. 107-113) (2014)
- Kadian D, Kumar A, Badgujar PC, Sehrawat R. Effect of homogenization and microfluidization on physicochemical and rheological properties of mayonnaise. Journal of Food Process Engineering. 2021;44(4):1–10. [Google Scholar]
- Karadeniz M, Sahin S, Sumnu G. Enhancement of storage stability of wheat germ oil by encapsulation. Industrial Crops & Products. 2018;114:14–18. [Google Scholar]
- Kohli, G., Jain, G., Bisht, A., Upadhyay, A., Kumar, A., & Dabir, S. Effect of non-thermal hurdles in shelf life enhancement of sugarcane juice. LWT - Food Science and Technology. 112, 108233 (2019)
- Koley, T. K., Nishad, J., Kaur, C., Su, Y., Sethi, S., Saha, S., Sen, S., & Bhatt, B. P. Effect of high-pressure microfluidization on nutritional quality of carrot (Daucus carota L.) juice. Journal of Food Science and Technology. 57(6): 2159-2168 (2020) [DOI] [PMC free article] [PubMed]
- Koo, C. K. W., Chung, C., Ogren, T., Mutilangi, W., & McClements, D. J. Extending protein functionality: Microfluidization of heat denatured whey protein fibrils. Journal of Food Engineering. (2018)
- Koxholt MMR, Eisenmann B, Hinrichs J. Effect of the fat globule sizes on the meltdown of ice cream. Journal of Dairy Science. 2001;84:31–37. doi: 10.3168/jds.S0022-0302(01)74448-7. [DOI] [PubMed] [Google Scholar]
- Kumar, A., Badgujar, P. C., Mishra, V., Sehrawat, R., Babar, O. A., & Upadhyay, A. Effect of microfluidization on cholesterol , thermal properties and in vitro and in vivo protein digestibility of milk. LWT - Food Science and Technology. 116: 108523 (2019)
- Lemay A, Paquin P, Lacroix C. Influence of microfluidization of milk on cheddar cheese composition, color, texture, and yield. Journal of Dairy Science. 1994;77:2870–2879. [Google Scholar]
- Leyva-Daniel, D. E., Alamilla-Beltran, L., Villalobos-Castillejos, F., Monroy-Villagrana, A., Jimenez-Guzman, J., & Welti-Chanes, J. Microfluidization as a honey processing proposal to improve its functional quality. Journal of Food Engineering. 274: 109831 (2020)
- Li, L. W., Chen, X. Y., Liu, L. C., Yang, Y., Wu, Y. J., Chen, G., Zhang, Z. F., & Luo, P. Oil-in-water camellia seeds oil nanoemulsions via high pressure microfluidization: Formation and evaluation. LWT-Food Science and Technology. 140: 110815 (2021)
- Liu W, Liu J, Liu C, Zhong Y, Liu W, Wan J. Activation and conformational changes of mushroom polyphenoloxidase by high pressure microfluidization treatment. Innovative Food Science and Emerging Technologies. 2009;10:142–147. [Google Scholar]
- Liu W, Liu J, Xie M, Liu C, Liu W, Wan J. Characterization and high-pressure microfluidization-induced activation of polyphenoloxidase from chinese pear (Pyrus pyrifolia Nakai) Journal of Agricultural and Food Chemistry. 2009;57:5376–5380. doi: 10.1021/jf9006642. [DOI] [PubMed] [Google Scholar]
- Liu M, Wang R, Li J, Zhang L, Zhang J, Zong W, Mo W. Dynamic high pressure microfluidization (DHPM): Physicochemical properties, nutritional constituents and microorganisms of yam juice. Czech Journal of Food Sciences. 2021;39(3):217–225. [Google Scholar]
- Liu, C. mei, Liang, R. hong, Dai, T. tao, Ye, J. ping, Zeng, Z. cong, Luo, S. jing, & Chen, J. Effect of dynamic high pressure microfluidization modified insoluble dietary fiber on gelatinization and rheology of rice starch. Food Hydrocolloids. 57: 55-61 (2016)
- Llinares R, Santos J, Trujillo-Cayado LA, Ramírez P, Muñoz J. Enhancing rosemary oil-in-water microfluidized nanoemulsion properties through formulation optimization by response surface methodology. LWT - Food Science and Technology. 2018;97:370–375. [Google Scholar]
- Lucey JA, Johnson ME, Horne DS. Invited Review: Perspectives on the basis of the rheology and texture properties of cheese. Journal of Dairy Science. 2003;86:2725–2743. doi: 10.3168/jds.S0022-0302(03)73869-7. [DOI] [PubMed] [Google Scholar]
- McClements, D. J. Emulsion formation. In D. J. McClements (Ed.), Food Emulsions Principles, Practices, and Techniques (pp. 245-284). CRC Press Taylor & Francis Group (2016)
- Mccrae CH. Homogenization of milk emulsions:use of microfluidizer. International Journal of Dairy Technology. 1994;47:28–31. [Google Scholar]
- Mert B. Using high pressure microfluidization to improve physical properties and lycopene content of ketchup type products. Journal of Food Engineering. 2012;109:579–587. [Google Scholar]
- Mert, B., Tekin, A., & Demirkesen, I. Production of microfluidized wheat bran fibers and evaluation as an ingredient in reduced flour bakery product. Food Bioprocess Technology. (2014)
- Mert, I. D. The applications of microfluidization in cereals and cereal-based products: An overview. Critical Reviews in Food Science and Nutrition. 1-18 (2019) [DOI] [PubMed]
- Microfluidics. Microfluidizer processor user guide. 1-11. (2008) In: http://movileanulabsyr.edu/MicrofluidizerProcessorUserGuide.pdf. Accesed on: 07/07/21.
- Moll P, Salminen H, Schmitt C, Weiss J. Impact of microfluidization on colloidal properties of insoluble pea protein fractions. European Food Research and Technology. 2021;247(3):545–554. [Google Scholar]
- Monroy-Rodríguez I, Gutiérrez-López GF, Hernández-Sánchez H, López-Hernández RE, Cornejo-Mazón M, Dorántes-Álvarez L, Alamilla-Beltrán L. Surface roughness and textural image analysis, particle size and stability of microparticles obtained by microfluidization of soy protein isolate aggregates suspensions. Revista Mexicana De Ingeniería Química. 2021;20(2):787–805. [Google Scholar]
- Morales-Medina, R., Dong, D., Schalow, S., & Drusch, S. Impact of microfluidization on the microstructure and functional properties of pea hull fibre. Food Hydrocolloids. 103, 105660 (2020)
- Oliete, B., Potin, F., Cases, E., & Saurel, R. Microfluidization as homogenization technique in pea globulin-based emulsions. Food and Bioprocess Technology. (2019)
- Olson DW, White CH, Watson CE. Properties of frozen dairy desserts processed by microfluidization of their mixes. Journal of Dairy Science. 2003;86:1157–1162. doi: 10.3168/jds.S0022-0302(03)73698-4. [DOI] [PubMed] [Google Scholar]
- Olson DW, White CH, Richter RL. Effect of pressure and fat content on particle sizes in microfluidized milk. Journal of Dairy Science. 2004;87(10):3217–3223. doi: 10.3168/jds.S0022-0302(04)73457-8. [DOI] [PubMed] [Google Scholar]
- Ozturk, O. K., & Turasan, H. Applications of microfluidization in emulsion-based systems, nanoparticle formation, and beverages. Trends in Food Science & Technology. (2021a)
- Ozturk, O. K., & Turasan, H. Latest developments in the applications of microfluidization to modify the structure of macromolecules leading to improved physicochemical and functional properties. Critical Reviews in Food Science and Nutrition. 0(0): 1-23 (2021b) [DOI] [PubMed]
- Ozturk OK, Mert B. The effects of microfluidization on rheological and textural properties of gluten-free corn breads. Food Research International. 2018;105:782–792. doi: 10.1016/j.foodres.2017.12.008. [DOI] [PubMed] [Google Scholar]
- Ozturk OK, Mert B. The use of microfluidization for the production of xanthan and citrus fiber-based gluten-free corn breads. LWT - Food Science and Technology. 2018;96:34–41. [Google Scholar]
- Ozturk OK, Mert B. Characterization and evaluation of emulsifying properties of high pressure microfluidized and pH shifted corn gluten meal. Innovative Food Science and Emerging Technologies. 2019;52:179–188. [Google Scholar]
- Paquin P. Technological properties of high pressure homogenizers: The effect of fat globules, milk proteins, and polysaccharides. International Dairy Journal. 1999;9:329–335. [Google Scholar]
- Paquin P, Giasson J. La. microfluidization comme procédé d’homogénéisation d’une boisson à base de matière grasse laitière. Le Lait. 1989;69(6):491–498. [Google Scholar]
- Paquin, P., Lebeuf, Y., Richard, J. P., & Kalab, M. Microparticulation of milk proteins by high pressure homogenization to produce a fat substitute. International Dairy Federation. 389-396 (1993)
- Pinnamaneni S, Das NG, Das SK. Comparison of oil-in-water emulsions manufactured by microfluidization and homogenization. Pharmazie. 2003;58(8):554–558. [PubMed] [Google Scholar]
- Pouliot Y, Britten M, Latreille B. Effect of high-pressure homogenization on a sterilized infant formula: Microstructure and age gelation. Food Structure. 1990;9:1–8. [Google Scholar]
- Robin, O., Blanchot, V., Vuillemard, J. C., & Paquin, P. Microfluidization of dairy model emulsions. I. Preparation of emulsions and influence of processing and formulation on the size distribution of milk fat globules. Le Lait. 72: 511-531 (1992)
- Santos, J., Calero, N., Trujillo-Cayado, L. A., Martín-Piñero, M. J., & Muñoz, J. Processing and formulation optimization of mandarin essential oil-loaded emulsions developed by microfluidization. Materials. 13(16) (2020) [DOI] [PMC free article] [PubMed]
- Suhag, R., Dhiman, A., Thakur, D., Kumar, A., & Upadhyay, A. Physico-chemical and functional properties of microfluidized egg yolk. Journal of Food Engineering, 294: 110416 (2021)
- Sun, C., Yang, J., Liu, F., Yang, W., Yuan, F., & Gao, Y. Effects of dynamic high-pressure microfluidization treatment and the presence of quercetagetin on the physical, structural, thermal, and morphological characteristics of zein nanoparticles. Food and Bioprocess Technology (2016)
- Swer, T. L., Chauhan, K., Mukhim, C., Bashir, K., & Kumar, A. Application of anthocyanins extracted from Sohiong (Prunus nepalensis L.) in food processing. LWT - Food Science and Technology, 114: 108360 (2019)
- Takahashi M, Inafuku K, Miyagi T, Oku H, Wada K, Imura T, Kitamoto D. Efficient preparation of liposomes encapsulating food materials using lecithins by a mechanochemical method. Journal of Oleo Science. 2007;56(1):35–42. doi: 10.5650/jos.56.35. [DOI] [PubMed] [Google Scholar]
- Tarafdar A, Kaur BP. Sedimentation rate of microfluidized sugarcane juice. Lwt. 2021;145(March):1–5. [Google Scholar]
- Tarafdar A, Kaur BP, Pareek S. Effect of microfluidization on deteriorative enzymes, sugars, chlorophyll, and color of sugarcane juice. Food and Bioprocess Technology. 2021;14(7):1375–1385. [Google Scholar]
- Thompson AK. Structure and properties of liposomes prepared from milk phospholipids. Palmerston North, New Zealand: Massey University; 2005. [Google Scholar]
- Thompson AK, Singh H. Preparation of liposomes from milk fat globule membrane phospholipids using a microfluidizer. Journal of Dairy Science. 2006;89:410–419. doi: 10.3168/jds.S0022-0302(06)72105-1. [DOI] [PubMed] [Google Scholar]
- Tobin, J., Heffernan, S. P., M.Mulvihill, D., Huppertz, T., & Kelly, A. L. Applications of high-pressure homogenization and microfluidization for milk and dairy products. In N. Datta & P. M. Tomasula (Eds.), Emerging Dairy Processing Technologies: Opportunities for the Dairy Industry (First, Vol. 18, p. 93-114) (2015)
- Tosh SM, Dalgleish DG. The physical properties and renneting characteristics of the synthetic membrane on the fat globules of microfluidized milk. Journal of Dairy Science. 1998;81:1840–1847. [Google Scholar]
- Turhan O, Isci A, Mert B, Sakiyan O, Donmez S. Optimization of ethanol production from microfluidized wheat straw by response surface methodology. Preparative Biochemistry and Biotechnology. 2015;45(8):785–795. doi: 10.1080/10826068.2014.958164. [DOI] [PubMed] [Google Scholar]
- Van Hekken DL, Iandola S, Tomasula PM. Short communication: Volatiles in microfluidized raw and heat-treated milk. Journal of Dairy Science. 2019;102(10):8819–8824. doi: 10.3168/jds.2018-15776. [DOI] [PubMed] [Google Scholar]
- Verma, K., Tarafdar, A., & Badgujar, P. C. Microfluidics assisted tragacanth gum based sub-micron curcumin suspension and its characterization. LWT - Food Science and Technology. 135: 110269 (2021a)
- Verma, K., Tarafdar, A., Mishra, V., Dilbaghi, N., Kondepudi, K. K., & Badgujar, P. C. Nanoencapsulated curcumin emulsion utilizing milk cream as a potential vehicle by microfluidization: Bioaccessibility, cytotoxicity and physico-functional properties. Food Research International. 148: 110611 (2021b) [DOI] [PubMed]
- Vuillemard JC. Recent advances in the large-scale production of lipid vesicles for use in food products: microfluidization. Journal of Microencapsulation. 1991;8:547–562. doi: 10.3109/02652049109021878. [DOI] [PubMed] [Google Scholar]
- Wang T, Sun X, Zhou Z, Chen G. Effects of microfluidization process on physicochemical properties of wheat bran. Food Research International. 2012;48(2):742–747. [Google Scholar]
- Wang T, Raddatz J, Chen G. Effects of microfluidization on antioxidant properties of wheat bran. Journal of Cereal Science. 2013;58:380–386. [Google Scholar]
- Wang T, Sun X, Raddatz J, Chen G. Effects of microfluidization on microstructure and physicochemical properties of corn bran. Journal of Cereal Science. 2013;58:355–361. [Google Scholar]
- Wang, N., Wu, L., Huang, S., Zhang, Y., Zhang, F., & Zheng, J. Combination treatment of bamboo shoot dietary fiber and dynamic high-pressure microfluidization on rice starch: Influence on physicochemical, structural, and in vitro digestion properties. Food Chemistry. 350: 128724 (2021) [DOI] [PubMed]
- Wang, W., Feng, Y., Chen, W., Adie, K., Liu, D., & Yin, Y. Citrus pectin modified by microfluidization and ultrasonication: Improved emulsifying and encapsulation properties. Ultrasonics Sonochemistry. 70: 105322 (2021) [DOI] [PMC free article] [PubMed]
- Wei, Y., Wang, C., Liu, X., Mackie, A., Zhang, M., Dai, L., Liu, J., Mao, L., Yuan, F., & Gao, Y. Co-encapsulation of curcumin and β-carotene in pickering emulsions stabilized by complex nanoparticles: Effects of microfluidization and thermal treatment. Food Hydrocolloids. 107064 (2021)
- Wu, Q., Wu, J., Ren, M., Zhang, X., & Wang, L. Modification of insoluble dietary fiber from rice bran with dynamic high pressure microfluidization: Cd(II) adsorption capacity and behavior. Innovative Food Science & Emerging Technologies. 73: 102765 (2021)
- Yazdi, S. R. Changing the structure of casein micelles to improve the delivery of bioactive compounds. Theses & Dissertations, The University of Guelph (2012)
- Yildiz E, Demirkesen I, Mert B. High pressure microfluidization of agro by-product to functionalized dietary fiber and evaluation as a novel bakery ingridient. Journal of Food Quality. 2016;39:599–610. [Google Scholar]
- Yuce, Ö. Application of high dynamic microfluidization to improve some quality parameters and stability of orange juice. Middle East Technical University (2011)
- Yuh-Fun M, Chung CH. Performance of sonication and microfluidization for liquid-liquid emulsification. Pharmaceutical Development and Technology. 1999;4(2):233–240. doi: 10.1081/pdt-100101357. [DOI] [PubMed] [Google Scholar]
- Zhang, W., Xie, F., Lan, X., Gong, S., & Wang, Z. Characteristics of pectin from black cherry tomato waste modified by dynamic high-pressure microfluidization. Journal of Food Engineering. (2017)
- Zhang, L., Chen, X., Wang, Y., Guo, F., Hu, S., Hu, J., Xiong, H., & Zhao, Q. Characteristics of rice dreg protein isolate treated by high-pressure microfluidization with and without proteolysis. Food Chemistry. 358: 129861 (2021) [DOI] [PubMed]
- Zhao N, Liu X, Sun Y, Wang Y, Pu X, Qin Y, Han J, Sun J, He Z. Factors affecting particle size of an intravenous fat emulsions. Asian Journal of Pharmaceutical Sciences. 2010;5(4):161–167. [Google Scholar]
- Zhao, Q., Yan, W., Liu, Y., & Li, J. Modulation of the structural and functional properties of perilla protein isolate from oilseed residues by dynamic high-pressure microfluidization. Food Chemistry. 365: 130497 (2021) [DOI] [PubMed]
- Zheng L, He M, Zhang X, Regenstein JM, Wang Z, Ma Z, Kong Y, Wu C, Teng F, Li Y. Gel properties and structural characteristics of soy protein isolate treated with different salt ions before spray drying combined with dynamic high-pressure micro-fluidization. Food and Bioproducts Processing. 2021;125:68–78. [Google Scholar]