Abstract
Introduction
Electrolyzed hydrogen-rich water (EHW) is known to have suppressive effects on oxidative stress (OS). However, its benefit in type 2 diabetes mellitus (T2DM) remains unclear. This study aimed to investigate the effect of EHW on T2DM.
Methods
This was a multicenter, prospective, double-blind, randomized controlled trial of 50 patients with T2DM who were assigned to the EHW or filtered water (FW) groups. The primary endpoint was changes in insulin resistance (IR) evaluated using the homeostasis model assessment of insulin resistance (HOMA-IR). OS markers such as urinary 8-hydroxy-2′-deoxyguanosine excretion (8-OHdG), plasma diacron-reactive oxygen metabolites (d-ROM), and plasma biological antioxidant potential (BAP) and other clinical data, including serum lactate concentration (lactate), were evaluated.
Results
There were no significant differences in the changes in HOMA-IR between the EHW and FW groups. However, lactate levels decreased significantly in the EHW group, and this decrease was significantly correlated with a reduction in HOMA-IR, fasting plasma glucose, and fasting plasma insulin level. Serum lactate level also significantly correlated to decreased insulin bolus secretion after 90 min with glucose loading in the EHW subjects with HOMA-IR > 1.73. No EHW treatment-related adverse effects were observed.
Conclusion
There were no significant effect of EHW in the change in HOMA-IR in this study; larger-scale and longer-term study are needed to verify the effects of EHW in T2DM patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13340-021-00524-3.
Keywords: Electrolyzed hydrogen-rich water, Gluconeogenesis, Insulin resistance, Oxidative stress
Introduction
The incidence of type 2 diabetes mellitus (T2DM) is continuously increasing. Although many drugs have been developed to improve glycemic control, most patients require multidrug treatment that results in high treatment cost. Since T2DM is a genetic disease [1], it would be useful to establish a generalized method that can be used by T2DM patients to prevent the onset of T2DM and avoid drug treatment. The basic pathologies of T2DM are increased insulin resistance (IR) and impaired insulin secretion [2]. Meanwhile, increased oxidative stress (OS) is associated with increased IR [3]. Thus, it is assumed that IR can be suppressed if OS can be suppressed. However, this effect has not been confirmed.
Electrolyzed hydrogen-rich water (EHW) is a type of water in which molecular hydrogen (H2) are dissolved in the cathode during electrolysis [4, 5]. H2, as an antioxidant, can selectively reduce hydroxyl radicals and peroxynitrite anions to exert therapeutic antioxidant activity [6]. However, although H2 appears to be an important biological molecule with antioxidant effects, the exact mechanisms of action remain elusive. H2 is administered by EHW ingestion. EHW could activate nuclear-factor-(erythroid-derived 2)-like 2 (Nrf2), thus reducing OS in mammals [7].
EHW has been reported to have various anti-diabetic benefits [8]. Research has shown that drinking EHW improves glucose tolerance in insulin-deficient diabetes [8]. In a previous preliminary study, drinking EHW improved fasting plasma glucose (FPG) levels (unpublished data). Based on the results of our preliminary study and the suppressive effects of EHW, we hypothesized that drinking EHW may improve IR, and this could be possibly due to a reduction in glucose supply to the blood while fasting, that is, gluconeogenesis. We suspected the contribution of Nrf2-mediated lactate synthesis from the relationship between changes in lactate and oxidative stress. We presumed that EHW-mediated OS suppression also leads to the suppression of lactate synthesis, thereby causing a reduction in gluconeogenesis from lactate [9] (Figure S1).
Given that OS is involved in increasing IR, IR may be improved by drinking EHW. However, this effect of EHW has not been scientifically verified. Establishing the IR-suppressive effects of EHW will help clarify its preventive benefit for T2DM and, ultimately, establish its usefulness as a generalized modality for the management of individuals with T2DM. Thus, this randomized control trial aimed to investigate the influence of EHW on BG and IR in patients with T2DM with adequate insulin secretion capabilities. Towards this goal, we compared the changes in fasting serum lactate level and the presence or absence of IR improvement effects between T2DM patients assigned to the EHW and filtered water (FW) groups.
Materials and methods
Study design and subjects
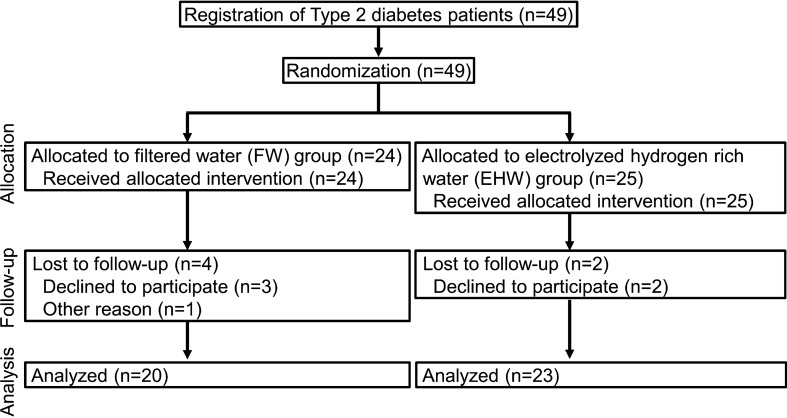
This was a multicenter prospective double-blind randomized control trial carried out jointly with Nihon Trim Co., Ltd. (Osaka, Japan). This trial was registered with the University Hospital Medical Information Network as “Electrolysis hydrogen water improves insulin resistance in type 2 diabetes” (UMIN000019032) (Fig. 1, Figure S2). This study was approved by the Medical Ethics Committee of Tohoku University, Tohoku Central Hospital, and Iwate Prefectural Takata Hospital (Approval no. 2015-2-120-1) and was conducted according to the tenets of the 1964 Helsinki Declaration and its later amendments. All participants provided written informed consent.
Fig. 1.
CONSORT flow diagram. Flow diagram of the progress through the phases of a parallel randomized trial of two groups: enrollment, intervention allocation, follow-up, and data analysis
Given that the primary condition for enrollment in the study was an adequately maintained insulin secretory capability, we recruited Japanese patients with T2DM who had not undergone insulin therapy. The inclusion criteria were as follows: age between 20 and 80 years, no history of insulin treatment, no experience of drinking EHW, no possibility of pregnancy or lactation, and no diseases that impose water-drinking restrictions such as heart failure or renal insufficiency. A power analysis was performed based on the results of our preliminary study and previous research (unpublished data) and with α = 0.05 and a detection capability of 80%, the number of target patients was calculated as 18 for each group. Taking into consideration the huge variations and the possible dropouts anticipated mid-way through the study, we established the target number of patients as 25 for each group, totaling to an overall study population of 50 subjects.
Randomization and masking
Randomization and double-blinding of the subjects were carried out extremely rigorously (Online Appendix 1). The physicians in charge, the subjects, installation operators/engineers, and persons in charge of analysis were blinded to group allocation until the test ended and the allocations were revealed. Randomization, installation of the devices, data collection, and analysis were all implemented and rigorously managed by a third-party institution (SRD Co., Ltd., Tokyo, Japan). The devices were installed in every subject’s household, making sure not to have the model type identifiable and were made such that the subjects could drink the water every day without failure (Figure S3). The devices for producing EHW and FW all had identical outward appearances and could not be differentiated; neither the subjects nor the installation operators had any information about the model.
Trial protocol
EHW was produced using TRIM ION HYPER EHW apparatus (manufacturer: Nihon Trim Co., Ltd.; Osaka, Japan). The subjects were asked to drink 1,500–2000 mL of water every day, write their drinking status daily, and confirm it during the test period. The amount of water consumed per day was monitored rigorously (Online Appendix 2, Figure S3, Figure S4). The level of electrolysis increased gradually. The level was set as level 1 (pH = 7.5–9.0) in the first 3 days, level 2 (pH = 8.0–9.5) during the 4th to 7th day, and level 3 (pH = 8.5–9.9) after the 8th day.
Because changes in meals, exercise, and treatment drugs are likely to have marked effects on the test results, we prohibited any such changes or interventions during both test and observation periods. The subjects were individuals who had relatively favorable glycemic control (Table 1); complied with the rules on meals, exercise, and drug dosing; and required no changes in their treatment method during the test period. The trial period was set at 3 months to allow evaluation of hemoglobin A1c (HbA1c). All patients were treated according to the Japanese clinical practice guidelines for diabetes. Immediately before starting the test (drinking the test water) and at the time of completing the test, the subjects had their fasting blood and first morning urine collected. Concurrently, a 75-g OGTT was performed to assess the subjects’ glucose tolerance. The collected blood and urine samples were used to evaluate clinical parameters.
Table 1.
Clinicodemographic subjects characteristics in the overall cohort and by treatment group
| All subjects | FW | EHW | p | |
|---|---|---|---|---|
| Age (y) | 68.0 (63.0, 75.0) | 68.0 (57.5, 75.0) | 66.0 (64.5, 72.5) | 0.802 |
| Sex [M/F (Male %)] | 25/20 (55.6) | 12/10 (54.5) | 13/10 (56.5) | 1.000 |
| BMI (kg/m2) | 26.8 + 4.4 | 27.7 + 5.0 | 25.9 + 3.8 | 0.193 |
| HbA1c (mmol/mol) | 45.8 + 4.9 | 44.9 + 4.9 | 46.6 + 4.9 | 0.264 |
| HbA1c (%) | 6.41 + 0.50 | 6.33 + 0.52 | 6.50 + 0.48 | 0.264 |
| Duration of diabetes (y) | 10 (7, 20) | 10 (5, 18) | 12 (10, 21) | 0.093 |
| Systolic BP (mmHg) | 133.6 + 16.7 | 134.5 + 15.1 | 132.8 + 18.3 | 0.733 |
| Diastolic BP (mmHg) | 76.6 + 11.9 | 76.2 + 12.3 | 76.9 + 11.7 | 0.859 |
| HR (BPM) | 78.8 + 13.5 | 76.9 + 15.2 | 80.5 + 11.8 | 0.380 |
| HOMA-IR | 1.49 (0.99, 2.48) | 1.61 (1.33, 2.53) | 1.06 (0.89, 2.07) | 0.171 |
| HOMA-β | 44.3 (27.8, 65.2) | 46.2 (27.9, 68.5) | 42.7 (27.8, 59.0) | 0.708 |
| OGTT-G-0 (mmol/L) | 5.8 (5.4, 6.8) | 5.9 (5.4, 6.9) | 5.8 (5.3, 6.6) | 0.708 |
| OGTT-G-120 (mmol/L) | 13.3 + 4.4 | 13.4 + 3.9 | 13.2 + 4.9 | 0.919 |
| OGTT-G-AUC | 24.1 + 4.8 | 24.1 + 4.6 | 24.2 + 5.0 | 0.943 |
| OGTT-I-0 (pmol/L) | 40.3 (25.0, 58.3) | 44.4 (35.6, 64.9) | 29.9 (24.0, 55.9) | 0.146 |
| OGTT-I-120 (pmol/L) | 209.7 (113.2, 340.3) | 192.4 (121.2, 373.1) | 216.0 (109.7, 295.5) | 0.768 |
| OGTT-I-AUC | 296.6 (191.7, 454.2) | 333.1 (191.6, 457.7) | 289.3 (213.1, 427.4) | 0.744 |
| OGTT-[GxI]-AUC | 6932 (4746, 9852) | 7256 (4792, 9483) | 6932 (4518, 9916) | 0.831 |
| Glycoalbumin (%) | 15.9 + 2.0 | 15.7 + 1.5 | 16.1 + 2.3 | 0.421 |
| Total cholesterol (mmol/L) | 5.03 + 0.79 | 4.75 + 0.66 | 5.30 + 0.83 | 0.018 |
| HDL cholesterol (mmol/L) | 1.29 (1.14, 1.60) | 1.25 (1.09, 1.64) | 1.34 (1.16, 1.59) | 0.625 |
| Triglyceride (mmol/L) | 1.18 + 0.46 | 1.15 + 0.51 | 1.21 + 0.42 | 0.684 |
| S-uric acid (µmol/L) | 339 (274, 404) | 366 (293, 422) | 303 (268, 348) | 0.084 |
| U-uric acid (mmol/mol Cre) | 339 + 120 | 374 + 114 | 305 + 117 | 0.049 |
| S-lactate (mmol/L) | 1.21 + 0.44 | 1.25 + 0.39 | 1.17 + 0.48 | 0.539 |
| S-pyruvate (µmol/L) | 70.4 (57.9, 88.6) | 74.4 (66.4, 88.6) | 60.2 (55.6, 87.4) | 0.280 |
| S-MG (nmol/L) | 143 (112, 199) | 133 (114, 161) | 144 (111, 210) | 0.638 |
| U-MG (µmol/mol Cre) | 107 (89, 139) | 107 (91, 138) | 108 (88, 142) | 0.981 |
| U-pH | 6.0 (5.5, 7.0) | 6.0 (5.5, 6.9) | 6.0 (5.5, 6.8) | 0.935 |
| U-8-OHdG (μmol/mol Cre) | 3.0 (2.3, 3.5) | 3.1 (2.3, 3.7) | 3.0 (2.4, 3.3) | 0.790 |
| d-ROMs (U CARR) | 291.4 + 39.8 | 296.1 + 37.6 | 287.0 + 42.2 | 0.453 |
| BAP (μmol/L) | 2077 + 171 | 2124 + 141 | 2034 + 187 | 0.078 |
| S-creatinine (µmol/L) | 62.8 (55.7, 78.7) | 62.3 (56.4, 75.8) | 71.6 (56.6, 88.0) | 0.352 |
| eGFR (mL min-1 [1.73 m]−2) | 67.5 + 19.9 | 70.5 + 19.2 | 65.1 + 20.6 | 0.441 |
| ACR (g/mol Cre) | 0.82 (0.48, 1.95) | 0.88 (0.54, 2.82) | 0.66 (0.40, 1.43) | 0.481 |
| BUN (mmol/L) | 5.39 (4.39, 6.96) | 5.60 (4.42, 6.78) | 5.28 (4.41, 7.07) | 0.973 |
| UUN (mol/mol Cre) | 31.9 (29.5, 37.9) | 33.7 (30.1, 40.0) | 31.1 (26.8, 36.3) | 0.284 |
| BNP (ng/L) | 20.4 (11.1, 49.3) | 30.4 (10.6, 51.8) | 19.6 (11.9, 37.2) | 0.549 |
| S-Na+ (mmol/L) | 141 + 2 | 141 + 2 | 141 + 1 | 0.170 |
| S-K+ (mmol/L) | 4.3 (4.2, 4.8) | 4.3 (4.2, 4.8) | 4.3 (4.2, 4.7) | 0.945 |
| S-Cl− (mmol/L) | 104.3 + 2.5 | 105.0 + 2.4 | 103.7 + 2.5 | 0.083 |
| U-Na+ (mol/mol Cre) | 19.1 (11.9, 32.1) | 18.4 (9.3, 38.2) | 19.1 (13.0, 28.5) | 0.991 |
| U-K+ (mol/mol Cre) | 4.04 (3.37, 5.87) | 4.68 (3.51, 6.33) | 3.86 (3.14, 5.60) | 0.194 |
| U-Cl− (mol/mol Cre) | 18.0 (11.8, 31.7) | 17.5 (12.0, 39.2) | 19.6 (12.5, 28.2) | 1.000 |
| Red blood cells (1012/L) | 4.47 + 0.47 | 4.51 + 0.56 | 4.43 + 0.38 | 0.592 |
| Hematocrit (%) | 42.1 + 4.0 | 42.4 + 4.5 | 41.7 + 3.6 | 0.569 |
| Hemoglobin (g/L) | 138 + 15 | 139 + 17 | 136 + 13 | 0.550 |
| White blood cell (109/L) | 5.60 + 1.36 | 5.28 + 1.23 | 5.91 + 1.43 | 0.119 |
| Platelet (109/L) | 242 (205, 275) | 225 (195, 265) | 267 (213, 327) | 0.061 |
| ALT (µkat/L) | 0.33 (0.23, 0.42) | 0.39 (0.30, 0.59) | 0.30 (0.23, 0.33) | 0.011 |
The values are expressed as the mean + SD or median (interquartile range)
FW filtered water, EHW electrolyzed hydrogen-rich water, HR heart rate, BPM beats per minute, HOMA-IR HOMA-Insulin resistance, HOMA-β HOMA beta-cell, OGTT oral glucose tolerance test, G glucose, 0 at 0 min, 120 at 120 min, I insulin, S serum, U urinary, MG methylglyoxal, 8-OHdG 8-hydroxy-2′-deoxyguanosine, d-ROMs diacron-reactive oxygen metabolites, U CARR Carratelli units, BAP biological antioxidant potential, ACR albumin creatinine ratio, BUN blood urea nitrogen, UUN urine urea nitrogen, ALT alanine transaminase
Assessments and endpoints
The primary study endpoint was the HOMA-IR, while the secondary endpoints were changes in the following: (glucose AUC in 75-g OGTT) × (insulin AUC in 75-g OGTT), plasma glucose, insulin, HbA1c, glycoalbumin, serum uric acid levels, urinary uric acid excretion volume, serum lactate levels (lactate), serum pyruvic acid levels, plasma methylglyoxal, urinary methylglyoxal excretion, urinary pH (U-pH), urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) excretion, plasma diacron-reactive oxygen metabolites (d-ROM), plasma biological antioxidant potential (BAP), urinary albumin creatinine ratio, blood urea nitrogen, urinary urea nitrogen, serum sodium, urinary sodium, serum chloride, urinary chloride, peripheral complete blood cell count, and BMI. We analyzed all the data that we could collected. However, some urinary 8-OHdG values in the subjects were lacking, resulting in a reduced graph data.
Statistical analysis
The normality of the numerical figures was validated using the Shapiro–Wilk test. Parametric factors are shown as mean ± SD, and non-parametric factors as median (interquartile range, IQR). Pre- and post-treatment comparisons were performed using Student’s paired two-tailed t test for parametric factors and Wilcoxon signed rank test for non-parametric factors. Meanwhile, intergroup comparisons using the Student’s unpaired two-tailed t test for parametric factors and the Mann–Whitney U test for non-parametric factors. Single correlations were evaluated using Pearson product-moment correlation. The chi-squared test was used to compare the rate of changes.
The changes in values were compared by separating them into the inside or outside of the reference range group based on the baseline values of HOMA-IR, FPG, d-ROM, and BAP. Stratification criteria were set as 1.73 for HOMA-IR [10], 6.1 mM for FPG [11], 300 UCARR for d-ROM [12], and 2200 μmol/L for BAP [12] based on previous reports.
All statistical analyses were performed using SigmaPlot 12.3 (Systat Software Inc., San Jose, CA). A p value of < 0.05 was considered statistically significant.
Results
Subject characteristics
In total, 49 subjects were recruited and randomly allocated to the EHW group (n = 25) and the FW group (n = 24). Two subjects from the EHW group and four from the FW group dropped out. In the end, there were 23 subjects in the EHW group and 20 in the FW group (Fig. 1).
Table 1 shows the baseline results of various measurement factors in the study subjects obtained immediately before they started drinking the test water. Compared with the FW group, the EHW group showed significantly higher values of serum total cholesterol concentration (p = 0.018), but also significantly lower values of urinary uric acid excretion volume (p = 0.049) and alanine aminotransferase (ALT) (p = 0.011). No other factors showed significant differences between the two groups.
Primary and secondary endpoints
HOMA-IR did not change in either the EHW group or the FW group. There were also no differences in the degree or rate of change between the two groups (Table 2 and Figure S5A). With respect to the secondary endpoints, the EHW group showed a significant reduction in lactate (Δchange from baseline; − 0.14 (− 0.51, 0.11) mmol/L, p = 0.038) (Figure S6A) and an increase in U-pH (Table 2, Figure S7), which FW group did not show. Meanwhile, the FW group showed more significant increases in white blood cells and platelets (Table 2) than did the EHW group. No changes were seen in any other factors, including the results of the 75-g OGTT (Table S1).
Table 2.
Comparison of changes in parameters during the water ingestion trial over 3 months
| Δ change | % change | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FW | EHW | FW | EHW | |||||||
| Δ change | p1 | Δ change | p2 | p3 | % change | p1 | % change | p2 | p3 | |
| HOMA-IR | 0.04 (− 0.51, 0.75) | 0.850 | − 0.27 (− 0.50, 0.15) | 0.105 | 0.282 | 3 (− 33, 33) | 0.784 | − 21 (− 28, 19) | 0.422 | 0.638 |
| HOMA-β | 7.5 (− 14.6, 20.8) | 0.571 | − 2.9 (− 12.8, 12.9) | 0.896 | 0.587 | − 0 (− 32, 62) | 0.386 | − 11 (− 27, 30) | 0.800 | 0.569 |
| OGTT-G-0 (mmol/L) | − 0.02 + 0.94 | 0.927 | − 0.21 + 0.83 | 0.246 | 0.498 | 1.0 + 15.7 | 0.787 | − 1.6 + 12.1 | 0.539 | 0.561 |
| OGTT-G-120 (mmol/L) | − 0.31 (− 1.99, 0.97) | 0.417 | − 0.44 (− 1.00, 1.50) | 0.820 | 0.706 | − 2.5 (− 11.2, 8.4) | 0.622 | − 2.9 (− 7.6, 14.1) | 0.996 | 0.709 |
| OGTT-G-AUC (mmol L−1 h) | − 0.80 + 4.68 | 0.451 | − 0.35 + 3.51 | 0.637 | 0.724 | − 1.1 + 19.3 | 0.801 | 0.0 + 14.1 | 0.988 | 0.827 |
| OGTT-I-0 (pmol/L) | − 0.83 + 26.88 | 0.896 | − 4.23 + 15.18 | 0.195 | 0.374 | 7 (− 31, 35) | 0.596 | − 14 (− 23, 8) | 0.452 | 0.462 |
| OGTT-I-120 (pmol/L) | 13 (− 52, 83) | 0.249 | − 2 (− 33, 50) | 0.601 | 0.971 | 19.6 + 63.5 | 0.183 | 15.3 + 54.5 | 0.192 | 0.812 |
| OGTT-I-AUC | 20 (− 24, 186) | 0.035 | 5 (− 25, 71) | 0.315 | 0.587 | 9.4 (− 9.8, 47.3) | 0.028 | 1.7 (− 9.4, 24.5) | 0.200 | 0.523 |
| OGTT-[GxI]-AUC | 513 (− 1162, 2594) | 0.108 | − 252 (− 872, 1785) | 0.360 | 0.800 | 19.3 + 47.5 | 0.086 | 13.8 + 39.9 | 0.112 | 0.800 |
| BMI (kg/m2) | 0.0 (− 0.4, 0.0) | 0.283 | 0.0 (0.0, 0.2) | 0.683 | 0.258 | 0.0 (− 1.5, 0.0) | 0.363 | 0.0 (0.0, 0.6) | 0.689 | 0.338 |
| HbA1c (mmol/mol) | 0.0 (− 2.0, 1.0) | 0.452 | 0.0 (− 1.0, 1.0) | 0.274 | 0.883 | − 1.02 + 5.94 | 0.428 | − 0.72 + 4.52 | 0.300 | 0.769 |
| HbA1c (%) | 0.0 (− 0.2, 0.1) | 0.452 | 0.0 (− 0.1, 0.1) | 0.274 | 0.883 | − 0.70 + 3.84 | 0.428 | − 0.68 + 3.08 | 0.300 | 0.769 |
| Glycoalbumin (%) | − 0.12 + 1.04 | 0.625 | 0.01 + 0.66 | 0.926 | 0.638 | − 0.81 + 6.66 | 0.594 | 0.24 + 3.98 | 0.775 | 0.670 |
| Total cholesterol (mmol/L) | − 0.12 (− 0.31, 0.11) | 0.287 | 0.03 (− 0.39, 0.30) | 0.615 | 0.567 | − 1.6 + 13.8 | 0.605 | − 0.3 + 9.9 | 0.893 | 0.720 |
| HDL cholesterol (mmol/L) | − 0.03 + 0.22 | 0.563 | 0.02 + 0.18 | 0.549 | 0.408 | − 2.4 + 17.5 | 0.550 | 2.4 + 11.7 | 0.336 | 0.308 |
| Triglyceride (mmol/L) | 0.07 (− 0.10, 0.25) | 0.775 | 0.01 (− 0.22, 0.18) | 0.574 | 0.737 | 10.5 (− 9.8, 35.7) | 0.159 | 0.9 (− 17.7, 18.4) | 0.988 | 0.462 |
| S-uric acid (µmol/L) | − 3.0 (− 26.8, 14.9) | 0.601 | 17.8 (− 20.8, 41.6) | 0.498 | 0.294 | − 1.1 (− 9.5, 5.7) | 0.632 | 5.2 (− 8.1, 13.9) | 0.300 | 0.342 |
| U-uric acid (mmol/mol Cre) | − 11.8 (− 63.5, 48.5) | 0.870 | 24.1 (8.1, 78.3) | 0.012 | 0.096 | − 3.0 (− 18.3, 15.8) | 0.956 | 12.8 (3.2, 32.3) | 0.004 | 0.049 |
| S-lactate (mmol/L) | − 0.02 (− 0.28, 0.17) | 0.644 | − 0.14 (− 0.51, 0.11) | 0.038 | 0.273 | − 3.4 + 29.3 | 0.606 | − 7.2 + 36.7 | 0.360 | 0.714 |
| S-pyruvate (µmol/L) | − 1.1 (− 11.9, 27.3) | 0.847 | − 5.7 (− 13.1, 4.5) | 0.211 | 0.348 | − 2 (− 14, 40) | 0.546 | − 10 (− 16, 9) | 0.475 | 0.401 |
| S-MG (nmol/L) | − 15.5 (− 47.3, 3.0) | 0.117 | 2.0 (− 24.5, 28.5) | 0.867 | 0.210 | − 12.9 (− 27.0, 4.2) | 0.261 | 1.8 (− 13.7, 23.7) | 0.544 | 0.195 |
| U-MG (μmol/mol Cre) | − 7.4 (− 19.3, 18.6) | 0.701 | -0.7 (− 20.5, 35.5) | 0.800 | 0.763 | 3.1 + 39.6 | 0.730 | 10.8 + 40.5 | 0.212 | 0.531 |
| U-pH | 0.00 (− 0.13, 0.50) | 0.383 | 0.00 (0.00, 0.50) | 0.044 | 0.434 | 0.0 (− 1.9, 6.8) | 0.505 | 0.0 (0.0, 9.1) | 0.023 | 0.299 |
| U-8-OHdG (μmol/mol Cre) | − 0.35 + 0.83 | 0.240 | − 0.87 + 1.11 | 0.061 | 0.295 | − 9.4 + 33.1 | 0.419 | − 25.8 + 32.5 | 0.059 | 0.318 |
| d-ROMs (U CARR) | 4.7 + 41.4 | 0.618 | − 3.4 + 18.1 | 0.379 | 0.184 | 2.5 + 12.6 | 0.382 | − 0.6 + 6.0 | 0.620 | 0.316 |
| BAP (μmol/L) | 47.6 + 148.9 | 0.169 | 56.7 + 193.8 | 0.174 | 0.862 | 2.4 + 7.0 | 0.152 | 3.3 + 9.8 | 0.115 | 0.699 |
| S-creatinine (mmol/L) | − 1.8 (− 4.4, 3.5) | 0.627 | − 0.9 (− 4.4, 3.1) | 0.563 | 1.000 | − 2.8 (− 6.3, 5.7) | 0.596 | − 1.4 (− 4.7, 3.6) | 0.687 | 0.782 |
| eGFR (mL min−1 [1.73 m]−2) | 2.3 (− 3.0, 4.5) | 0.626 | 1.7 (− 1.4, 4.0) | 0.234 | 0.413 | 3.1 (− 5.3, 6.9) | 0.583 | 2.8 (− 2.6, 5.7) | 0.211 | 0.886 |
| ACR (g/mol Cre) | − 0.07 (− 0.29, 0.42) | 1.000 | 0.07 (− 0.06, 0.42) | 0.094 | 0.358 | − 10 (− 35, 33) | 0.956 | 14 (− 14, 31) | 0.186 | 0.375 |
| BUN (mmol/L) | − 0.05 (− 1.17, 1.04) | 0.803 | 0.00 (− 0.91, 0.21) | 0.426 | 0.527 | − 0.8 (− 18.3, 24.1) | 0.784 | 0.0 (− 17.5, 4.0) | 0.363 | 0.352 |
| UUN (mol/mol Cre) | 0.51 + 8.99 | 0.804 | 0.12 + 6.47 | 0.930 | 0.874 | 4.4 + 27.0 | 0.472 | 2.7 + 19.9 | 0.526 | 0.813 |
| BNP (ng/L) | 1.9 (− 6.4, 10.9) | 0.330 | − 0.7 (− 5.2, 7.0) | 0.595 | 0.688 | 28 (− 7, 60) | 0.048 | − 1 (− 22, 44) | 0.410 | 0.462 |
| S-Na+ (mmol/L) | 0.5 (− 1.0, 2.0) | 0.537 | 0.0 (− 1.5, 1.0) | 0.707 | 0.491 | 0.4 (− 0.7, 1.4) | 0.384 | 0.0 (− 1.1, 0.7) | 0.931 | 0.487 |
| S-K+ (mmol/L) | 0.00 (− 0.13, 0.40) | 0.459 | 0.10 (− 0.10, 0.30) | 0.130 | 0.779 | 0.0 (− 2.9, 9.5) | 0.384 | 2.3 (− 2.2, 7.2) | 0.077 | 0.592 |
| S-Cl− (mmol/L) | 0.0 (− 2.0, 2.0) | 1.000 | 1.0 (− 2.0, 2.5) | 0.559 | 0.924 | 0.0 (− 1.9, 1.9) | 0.977 | 1.0 (− 1.9, 2.4) | 0.521 | 0.917 |
| U-Na+ (mol/mol Cre) | 2.43 + 17.67 | 0.547 | 1.50 + 8.34 | 0.399 | 0.831 | 23.3 (− 38.1, 86.4) | 0.177 | 6.4 (− 8.4, 34.3) | 0.200 | 0.800 |
| U-K+ (mol/mol Cre) | − 0.60 (− 1.06, 0.65) | 0.498 | 0.11 (− 0.56, 1.23) | 0.393 | 0.212 | − 13.1 (− 25.3, 18.4) | 0.596 | 2.8 (− 11.6, 33.2) | 0.345 | 0.178 |
| U-Cl− (mol/mol Cre) | 1.17 + 17.39 | 0.767 | 0.93 + 7.64 | 0.567 | 0.954 | 8.8 (− 40.6, 60.3) | 0.475 | 0.1 (− 9.0, 36.1) | 0.286 | 0.838 |
| Red blood cells (1012/L) | − 0.03 + 0.21 | 0.513 | − 0.02 + 0.23 | 0.648 | 0.891 | − 0.79 + 4.74 | 0.464 | − 0.64 + 5.43 | 0.579 | 0.921 |
| Hematocrit (%) | − 0.27 + 1.79 | 0.515 | -0.10 + 2.27 | 0.828 | 0.797 | − 0.73 + 4.36 | 0.461 | − 0.29 + 5.70 | 0.812 | 0.772 |
| Hemoglobin (g/L) | − 1.7 + 6.5 | 0.257 | − 1.9 + 8.6 | 0.296 | 0.927 | − 1.4 + 4.8 | 0.216 | − 1.5 + 6.4 | 0.267 | 0.925 |
| White blood cell (109/L) | 0.61 + 1.17 | 0.031 | − 0.12 + 0.90 | 0.522 | 0.029 | 3.8 (− 2.7, 21.6) | 0.051 | 0.0 (− 12.0, 10.1) | 0.929 | 0.046 |
| Platelet (109/L) | 20.9 + 27.0 | 0.003 | 0.5 + 34.7 | 0.943 | 0.037 | 10.0 (2.6, 17.1) | 0.003 | 0.5 (− 6.4, 4.3) | 0.988 | 0.027 |
| ALT (µkat/L) | − 0.1 (− 0.1, 0.00) | 0.068 | 0.0 (− 0.0, 0.1) | 0.378 | 0.032 | − 10.7 (− 20.0, 0.0) | 0.076 | 7.1 (− 9.7, 31.7) | 0.162 | 0.051 |
The values are expressed as the mean + SD or median (interquartile range)
p1 0 vs 3 M in the FW group, p2 0 vs 3 M in the EHW group, p3 FW group vs. EHW group
FW filtered water, EHW electrolyzed hydrogen-rich water, HOMA-IR HOMA-insulin resistance, HOMA-β HOMA beta-cell, OGTT oral glucose tolerance test, G glucose, 0 at 0 min, 120 at 120 min, I insulin, S serum, U urinary, MG methylglyoxal, 8-OHdG 8-hydroxy-2′-deoxyguanosine, d-ROMs diacron-reactive oxygen metabolites, U CARR Carratelli units, BAP biological antioxidant potential, ACR albumin creatinine ratio, BUN blood urea nitrogen, UUN urine urea nitrogen, ALT alanine transaminase
Subanalysis
With respect to blood glucose level, if the value of HOMA-IR was within the normal range, there was a possibility that HOMA-IR would not drop any further. We, therefore, performed an analysis by dividing the subjects according to an increased HOMA-IR (≥ 1.73) and a non-increased HOMA-IR (< 1.73) at baseline [10].
Figure S5 shows the changes in HOMA-IR in the overall subjects (A) and in the EHW and FW groups with HOMA-IR values of ≥ 1.73 (B) and < 1.73 (C). Among the overall subjects and the group with HOMA-IR < 1.73, there were no differences in the changes in HOMA-IR between the EHW and FW groups. Meanwhile, in the subjects with HOMA-IR ≥ 1.73, the HOMA-IR of those in the EHW group decreased more sharply than did the HOMA-IR of those in the FW group (Figure S5B). This indicated that EHW did not lower the HOMA-IR in the normal range any further and that it reduced IR only when the HOMA-IR had increased.
Lactate also did not change in the FW group, whereas it decreased significantly in the EHW group (Table 2, Figure S6A). Figure S6B shows the relationship between serum lactate level and urinary 8-OHdG excretion amount at the baseline period. The serum lactate level showed a significant positive correlation with urinary 8-OHdG excretion (r = 0.382, p = 0.049). Figure S6C-E shows the changes in lactate and the correlation between the changes in 8-OHdG and the changes in lactate in the overall subjects (C) and in the EHW (E) and FW groups (D). The changes in 8-OHdG levels were positively correlated with changes in lactate levels only in the overall population and the EHW group. Both the amount (r = 0.778, p = 0.023, Figure S6E) and rate (r = 0.742, p = 0.035) of change of lactate correlated positively with the amount and rate of change of 8-OHdG. In the EHW group, those whose 8-OHdG levels were lower had lower lactate levels. This indicated that OS is suppressed by EHW and that this suppression of OS, in turn, suppresses lactate.
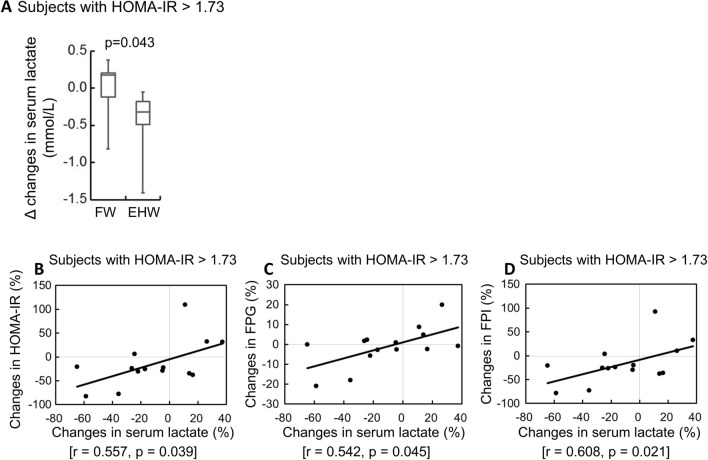
To identify the relationship between improvements in HOMA-IR and lactate levels, we analyzed the lactate levels of subjects with HOMA-IR > 1.73, which indicate improvements in HOMA-IR values that were attributable to EHW. Comparison of the amount of change in lactate in subjects with HOMA-IR > 1.73 between the EHW and FW groups (Fig. 2A) showed that it was significantly larger in the EHW group that in the EHW group [1.55 (− 1.08, 1.83) vs − 2.90 (− 4.40, − 1.63); p = 0.043, Mann–Whitney rank sum test]. Further, the lactate level also decreased in EHW group. Among the subjects with HOMA-IR ≥ 1.73 in the EHW group, the rate of change in lactate showed a significant positive correlation with the rate of change in HOMA-IR (r = 0.557, p = 0.039) (Fig. 2B), the rate of change in fasting plasma glucose concentration (FPG) (r = 0.542, p = 0.045) (Fig. 2C), and the rate of change in fasting plasma insulin concentration (FPI) (r = 0.608, p = 0.021) (Fig. 2D). This indicated that if the HOMA-IR was ≥ 1.73, the FPG and FPI decreased and the HOMA-IR improved in those whose lactate levels decreased due to drinking EHW.
Fig. 2.
Effects of EHW on serum lactate and correlation between serum lactate and insulin resistance (IR) in the subjects whose IR deteriorated. Comparison of the amount of change in serum lactate concentration between the subjects in the EHW and FW groups with HOMA-IR > 1.73 (A) A correlation diagram between the rate of changes in serum lactate concentration and the rate of change in HOMA-IR (B), fasting plasma glucose concentration (FPG) (C), and fasting plasma insulin concentration (FPI) (D)
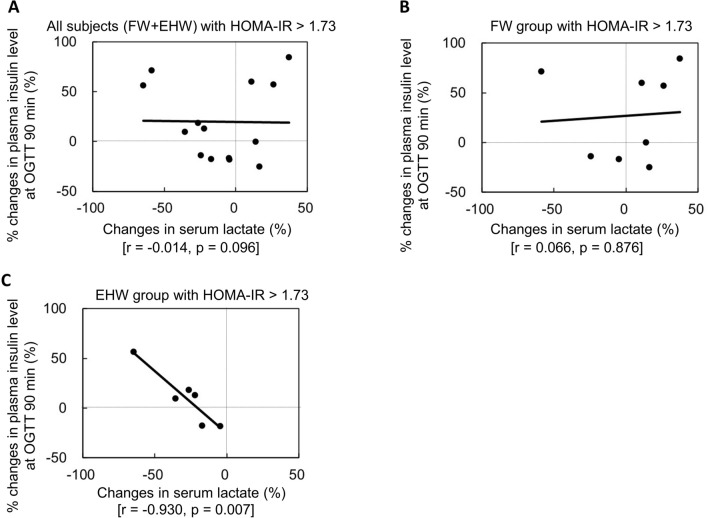
Figure 3 shows the correlation between the rate of changes in lactate and the rate of changes in insulin concentration 90 min after glucose loading in 75-g OGTT in the subjects with HOMA-IR > 1.73 in overall population (A), the FW group (B), and the EHW group (C). The changes in both the amount and rate of lactate correlated negatively with the changes in both the amount and rate of insulin concentration 90 min after glucose loading in the 75-g OGTT. The rate of changes in serum lactate concentration showed a significant negative correlation with the plasma insulin concentration at 90 min on OGTT in the EHW group (r = − 0.930, p = 0.007), but not in the whole patient group and the FW group. Meanwhile, the EHW group showed no changes in the plasma glucose concentration 90 min after glucose loading in the 75-g OGTT (r = 0.047, p = 0.831). Among the subjects whose fasting lactate decreased, the insulin secretion increased 90 min after glucose loading in 75-g OGTT. It is, therefore, possible that these changes in either lactate or OS influence the secretion of insulin (Fig. 3C).
Fig. 3.
Correlation between the changes in the amount (A) and rate (B) of serum lactate concentration and the changes in the amount and rates of insulin values 90 min after 75-g OGTT in the subjects with HOMA-IR > 1.73 in the EHW group. The changes in serum lactate concentration correlated negatively with the changes in insulin values 90 min after the 75-g OGTT. The higher the fasting serum lactate concentration, the higher the increase in insulin secretion after 90 min
The rate of change in lactate correlated positively with the rate of change in FPG in the FW group (r = 0.453, p = 0.045) independent of the HOMA-IR level. This indicated that the FPG increased as the lactate level increased. Even in subjects with HOMA-IR > 1.73 in the FW group, the amount (r = 0.721, p = 0.043) and rate of change (r = 0.707, p = 0.050) in lactate correlated positively with FPG, showing that FPG was significantly influenced by changes in lactate. The rate of change in lactate among the subjects with HOMA-IR > 1.73 in the FW group correlated positively with the rate of change in plasma glucose concentration 60 min after glucose loading in 75-g OGTT (r = 0.715, p = 0.046). This shows that an increase in fasting lactate affects not only the increase in FPG, but also the increase in blood glucose after glucose loading.
Considering the possibility that, as with HOMA-IR, FPG may decreased only with high values (> 6.1 mM = 110 mg/dL), we divided the subjects into two groups at a cutoff of baseline FPG 6.1 mM. In the FPG ≥ 6.1 mM group, those in whom the basal insulin secretion was decreased did not show changes in the FPG if they drank the EHW, although the fasting plasma insulin (FPI) dropped significantly from 49 (26, 61) to 39 (19, 45) pmol/L (p = 0.048). In the analysis of subjects with FPG < 6.1 mM who maintained basal insulin secretion, there were no significant changes in FPG in both the EHW and FW groups (Table S2).
Drinking EHW induced no significant changes in HOMA-IR or OS markers such as 8-OHdG, d-ROM, or BAP in all subjects. As with HOMA-IR, we considered that EHW did not suppress OS to any greater extent in subjects with non-OS exacerbation. We, therefore, investigated the changes by dividing the subjects into those with high d-ROM (≥ 300) and BAP (> 2200) and those with low d-ROM (< 300) and BAP (≤ 2200) [7]. Among those with high d-ROM, d-ROM was suppressed in the subjects in the EHW group but not in those in the FW group. Among those with low d-ROM, d-ROM was unchanged in the subjects in the EHW group, whereas it was significantly increased in the subjects in the FW group. BAP is a marker of anti-OS capability increased in the subjects in the EHW group whose BAP had decreased to ≤ 2200. This was not observed in the FW group (Figure S8). The EHW group demonstrated OS suppression action only when OS was increased.
No EHW-related adverse effects including changes in electrolytes were observed in this study (Table S3).
Discussion
The benefit of EHW in T2DM is yet to be clarified to date. In this study, the primary endpoint of improvement in IR in T2DM was not observed. However, the pathology of T2DM differs in every patient: some have aggravated IR, while others do not [13]. Although the subjects whose IR was not aggravated (HOMA-IR < 1.73) showed no IR improvement from drinking EHW, those whose IR had aggravated (HOMA-IR ≥ 1.73) did show improvements. Although we excluded T2DM patients with impaired insulin secretion because they require insulin injections, some study subjects were still diagnosed with T2DM despite having a HOMA-IR value below 1.73. This shows that a reduction in insulin secretion was their dominant pathology, rather than an increase in IR. It seems that IR was not improved in the subjects of the non-insulin resistant type but such impaired insulin secretion type. Furthermore, in subjects with FPG > 6.1 mM (110 mg/dL), the insulin level significantly decreased as a result of drinking EHW, although the BG levels did not show any significant changes. A high FPG is believed to indicate that basal insulin at the time was being secreted to its limit. FPG should have declined if IR had improved, with insulin secretion remaining unchanged. However, no such decline occurred. This indicates that, as blood glucose supply (gluconeogenesis, in case of a fasting state) decreased, the amount of insulin needed decreased, making it possible to maintain the same FPG at lower levels of insulin secretion.
An important item of knowledge obtained from this study is the improvement of insulin resistance (HOMA-IR) due to oxidative stress (OS) having been suppressed by electrolyzed hydrogen-rich water (EHW). Another important fact is that this suppression of OS was seen only when OS had been exacerbated, and that the improvement of IR (HOMA-IR) was seen only when suppression of OS was observed. Increased OS, therefore, appears to affect either fasting blood glucose (FBG) values or insulin sensitivity. We previously reported that the uptake of glucose in the skeletal muscles improved as a result of OS suppression [14]. It is also reported that diabetics show an increase in phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphatase (G6Pase), which are gluconeogenic enzymes of the kidney, and that gluconeogenesis is exacerbated as a result of the kidney’s G6P and glucose having increased as a result of blood glucose control [15]. OS is believed to aggravate IR because of the increased supply of glucose to the blood by gluconeogenesis, and the suppression of glucose uptake in the skeletal muscle [16]. It appears, however, that this increase in IR due to OS cannot continue indefinitely. This is because nuclear-factor-(erythroid-derived 2)-like related factor 2 (Nrf2), which is an antioxidative stress transcription factor that is induced by the increase in OS, seems to suppress gluconeogenesis [17, 18]. As Nrf2 is activated, glutamine, which is a gluconeogenic substrate, is consumed to fuel glutathione synthesis and lactate production, making it no longer so readily available for gluconeogenesis [9]. Instead, lactate production increases. However, since PEPCK activity is also suppressed because of Nrf2, lactate cannot be used for gluconeogenesis, so it continues to accumulate. Our study showed that, because OS was suppressed because of EHW, gluconeogenesis that had increased because of OS, as well as glucose uptake in the skeletal muscles, was now seen to decrease. Nrf2, previously induced by OS, was also suppressed, decreasing the synthesis and accumulation of lactate as a result. The fact that a relationship was seen between the reduction of lactate and improvement of FBG may be attributable to this. There is a possibility, therefore, that EHW improves IR by suppressing OS in situations where OS has slightly increased. This means, therefore, that even if healthy individuals who see little or no increases in OS drink EHW, they will not develop hypoglycemia. Our study included many subjects with low OS but relatively favorable blood glucose control. This was assumed to have been why no significant changes were seen overall. As a candidate to benefit from EHW drinking, we investigated the background of subjects who has both high IR and OS. Table S4 compares the baseline parameters of patients with and without increased both IR and OS. Patients with increased IR (HOMA-IR > 1.73) and OS (d-ROM > 300 U CARR or BAP < 2200 μmol/L) was younger, had lower urinary Na+ and Cl−excretion, had higher BMI, HbA1c, diastolic blood pressure, HOMA-β, FPS, fasting plasma insulin, insulin secretion during 75-g-OGTT, triglyceride, serum pyruvate, red blood cell, hematocrit, hemoglobin, and ALT activity during baseline period.
Our concern was that the risk of drinking EHW might increase the level of ionized hydrogen (H+) in the blood and in turn elevate the concentration of K+. However, in our previous preliminary study and in our clinical practice, we did not encounter any patient who developed hyperkalemia or acidosis from drinking EHW. However, as precaution, we decided to exclude renal failure patients and monitored the changes in the subjects’ blood K+ levels every month. The results showed no changes in K+ in either the EHW group or the FW group (Table S5). Molecular hydrogen (H2), rather than H + , could be increased in the blood as a result of drinking EHW, supporting that EHW is not an acidic water, but rather alkaline (pH = 7.5–9.9) water [19]. WHO recommends a pH of 6.5–9.5 for drinking water, and the EHW has slightly higher, but similar pH [20]. Since it has been reported that ingestion of bicarbonate improves insulin resistance, the effects of drinking alkaline water need to be further investigated [21]. In addition, no adverse effects have been reported in this study after taking bicarbonate for one year.
In this study, 16 of the 20 (80%) subjects in the FW group and 17 of the 23 (74%) subjects in the EHW have received biguanide treatment. Although biguanide could affect gluconeogenesis, no significant difference was observed in changes in fasting plasma glucose, fasting plasma insulin, serum lactate, urinary, and serum uric acid levels during the experimental period between subjects who did and did not receive biguanide treatment (Table S6). Therefore, the changes observed in this study caused by EHW appear to be dependent on hydrogen dissolved in EHW rather than on medicine treatment. Further, EHW suppressed only OS that had increased inappropriately and decreased only IR that had increased unphysiologically. Therefore, there are no contraindications to EHW for individuals with normal IR, and drinking EHW can be expected to have beneficial effects to individuals with T2DM in who IR has increased.
Limitations
We did not find significant differences in the delta change (p = 0.213) and %change (p = 0.222) of HOMA-IR between the FW and EHW groups, and this may be due to the small sample size. We also did not evaluate Nrf2 or gluconeogenesis. Further studies in pre-diabetes patients with high HOMA-IR are needed. Japan has fewer severely obese diabetes individuals than Europe or the US [13]. None of the subjects in our study were morbidly obese. We, therefore, found no subjects whose IR had markedly increased. Moreover, there is a possibility that the subjects had already undergone extensive treatments, and thus their insulin secretion may have already been considerably modified. We cannot rule out the possibility that these factors may have influenced our results. The favorable blood glucose control of the subjects, with their HbA1c remaining within 6.4–6.5%, may also have made its influence on BG difficult to observe. Larger-scale and longer-term studies are needed to verify the effects of EHW in T2DM patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the clinical support of the nurses for their help with preparing the 75-g OGTT and for their expert assistance in the preparation of the sampling and preservation of the blood and urine. We also wish to thank all the participants for their efforts in drinking the water and sampling. We also thank all members of the staff who helped us in this study. YO and SK are employees of Nihon Trim Co., Ltd. (Osaka, Japan). The authors declare that there are no other conflicts of interest.
Author contributions
SO had full access to all the data in this study and made the decision to submit for publication. SO, MS, KN, MO, KT, and YT contributed to participant recruitment. SO wrote the manuscript and researched the data; MS, KN, and MO contributed to the discussion and researched the data. SI reviewed and edited the manuscript. The references and the EHW and placebo apparatus used were prepared by SK and YO.
Funding
This study was funded by the Nihon Trim Co. Ltd. The funder was not involved in the study design; collection, analysis, and interpretation of data; and the decision to submit the article for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rich SS. Mapping genes in diabetes. Genetic epidemiological perspective. Diabetes. 1990;39(11):1315–1319. doi: 10.2337/diab.39.11.1315. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furukawa S, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamasaki T, et al. Electrochemically reduced water exerts superior reactive oxygen species scavenging activity in HT1080 cells than the equivalent level of hydrogen-dissolved water. PLoS ONE. 2017;12(2):e0171192. doi: 10.1371/journal.pone.0171192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, et al. Monolayered Platinum Nanoparticles as Efficient Electrocatalysts for the Mass Production of Electrolyzed Hydrogen Water. Sci Rep. 2020;10(1):10126. doi: 10.1038/s41598-020-67107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shirahata S, et al. Electrolyzed-reduced water scavenges active oxygen species and protects DNA from oxidative damage. Biochem Biophys Res Commun. 1997;234(1):269–274. doi: 10.1006/bbrc.1997.6622. [DOI] [PubMed] [Google Scholar]
- 7.Zhu WJ, et al. Amelioration of cardio-renal injury with aging in dahl salt-sensitive rats by H2-enriched electrolyzed water. Med Gas Res. 2013;3(1):26. doi: 10.1186/2045-9912-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim MJ, Kim HK. Anti-diabetic effects of electrolyzed reduced water in streptozotocin-induced and genetic diabetic mice. Life Sci. 2006;79(24):2288–2292. doi: 10.1016/j.lfs.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Mitsuishi Y, et al. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22(1):66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Ohnishi H, et al. Relationship between insulin resistance and accumulation of coronary risk factors. Diabetes Obes Metab. 2002;4(6):388–393. doi: 10.1046/j.1463-1326.2002.00232.x. [DOI] [PubMed] [Google Scholar]
- 11.Haneda M, et al. Japanese clinical practice guideline for diabetes 2016. J Diabetes Investig. 2018;9(1):1–45. doi: 10.1111/jdi.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen E, Ruskovska T. Serum biomarkers of (anti)oxidant status for epidemiological studies. Int J Mol Sci. 2015;16(11):27378–27390. doi: 10.3390/ijms161126032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kodama K, et al. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36(6):1789–1796. doi: 10.2337/dc12-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Q, et al. Methylglyoxal contributes to the development of insulin resistance and salt sensitivity in Sprague-Dawley rats. J Hypertens. 2009;27(8):1664–1671. doi: 10.1097/HJH.0b013e32832c419a. [DOI] [PubMed] [Google Scholar]
- 15.Tojo A, et al. Angiotensin receptor blocker telmisartan suppresses renal gluconeogenesis during starvation. Diabetes Metab Syndr Obes. 2015;8:103–113. doi: 10.2147/DMSO.S78771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swe MT, et al. Molecular signaling mechanisms of renal gluconeogenesis in nondiabetic and diabetic conditions. J Cell Physiol. 2019;234(6):8134–8151. doi: 10.1002/jcp.27598. [DOI] [PubMed] [Google Scholar]
- 17.de Oliveira MC, et al. Tibolone impairs glucose and fatty acid metabolism and induces oxidative stress in livers from female rats. Eur J Pharmacol. 2011;668(1–2):248–256. doi: 10.1016/j.ejphar.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 18.Harmon RC, et al. Time-dependent effect of p-aminophenol (PAP) toxicity in renal slices and development of oxidative stress. Toxicol Appl Pharmacol. 2005;209(1):86–94. doi: 10.1016/j.taap.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Shirahata SH, Teruya K. Advanced research on the health benefit of reduced water. Trends Food Sci Technol. 2012;23(2):124–131. doi: 10.1016/j.tifs.2011.10.009. [DOI] [Google Scholar]
- 20.Mousa HA. Health effects of alkaline diet and water, reduction of digestive-tract bacterial load, and earthing. Altern Ther Health Med. 2016;22(Suppl 1):24–33. [PubMed] [Google Scholar]
- 21.Bellasi A, et al. Correction of metabolic acidosis improves insulin resistance in chronic kidney disease. BMC Nephrol. 2016;17(1):158. doi: 10.1186/s12882-016-0372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.