Abstract
Ethnic fermented foods are known for their unique aroma, flavour, taste, texture and other sensory properties preferred by every ethnic community in this world culturally as parts of their eatables. Some beneficial microorganisms associated with fermented foods have several functional properties and health-promoting benefits. Bacteriocins are the secondary metabolites produced by the microorganisms mostly lactic acid bacteria present in the fermented foods which can act as lantibiotics against the pathogen bacteria. Several studies have been conducted regarding the isolation and characterization of potent strains as well as their association with different types of bacteriocins. Collective information regarding the gene organizations responsible for the potent effect of bacteriocins as lantibiotics, mode of action on pathogen bacterial cells is not yet available. This review focuses on the gene organizations, pathways include for bacteriocin and their mode of action for various classes of bacteriocins produced by lactic acid bacteria in some ethnic fermented foods.
Keywords: Novel pathway, Bacteriocin, LAB, Fermented foods, Lactic acid bacteria
Introduction
Fermented foods are mostly ethnic-origin, which are known for their unique aroma, flavour, taste, texture and other sensory properties preferred by every ethnic community in this world culturally as parts of their eatables (Tamang, 2016a, 2020; Voidarou et al., 2021). Some microbial communities associated with fermented foods have functional properties such as production of antimicrobial compounds (Hernández-González et al., 2021), antioxidants (Menezes et al., 2020), probiotics (Marco et al., 2021), immunomodulators (Shahbazi et al., 2021), health-promoting benefits (Garcia-Gonzalez et al., 2021; Melini et al., 2019; Tamang et al., 2016b) and therapeutic uses to combat diseases (Dimidi et al., 2019; Rezac et al., 2018). Many ethnic fermented foods are prepared traditionally by spontaneous or natural fermentation from raw or boiled substrates of plants, animal flesh and milk (Tamang et al., 2020), which facilitate the growth of diverse types of culturable and unculturable microbiota including major domains of bacteria, yeasts, filamentous moulds, virus and archaea (Bhutia et al., 2021; Das and Tamang, 2021; Kharnaior and Tamang, 2021; Leech et al., 2020). Till date, the most commonly reported bacteria from fermented foods are lactic acid bacteria (LAB) for their various fermentative roles imparting functional and other health-promoting benefits mainly in acidic fermented foods including dairy products (Marco et al., 2021; Moh et al., 2021; Tamang et al., 2005, 2008, 2009). LAB belong to phylum Firmicutes are Gram-positive, acid-tolerant and non-spore-formers that bio-synthesize several metabolites and bioactive compounds (Teneva-Angelova et al., 2018). Generally four families of LAB viz. Lactobacillaceae, Streptococcaceae, Streptococcaceae, and Enterococcaceae are reported from fermented foods (Ashaolu and Reale, 2020; Holzapfel and Wood, 2014), which are represented by dominant genera such as Lacticaseibacillus, Lentilactobacillus, Levilactobacillus, Loigolactobacillus, Lactococcus, Leuconostoc, Pediococcus, Enterococcus, Streptococcus, Weissella, Tetragenococcus, Oenococcus, Alkalibacterium, Carnobacterium, Fructobacillus, and Vagococcus (Mathur et al., 2020; Pradhan and Tamang, 2019; Shangpliang and Tamang, 2021; Zheng et al., 2020). Most of the LAB present in the fermented foods are generally regarded as safe (GRAS) food-grade bacteria (Mathur et al., 2020; USFDA, 1988), and also listed in the microbial food cultures (MFC) safety inventory (Bourdichon et al., 2019; Tamang et al., 2021).
Secondary metabolites produced by bacteria are being exploited as useful bioactive molecules (Chaudhary et al., 2021; Pham et al., 2019), since some bacteria are very diverse both phylogenetically and functionally, carrying out complex metabolic transformations (Diether and Willing, 2019). This metabolic versatility can act as a boon for pharmaceutical and food industries. Recently, demand for minimally processed food products with zero chemicals (Dávila-Aviña et al., 2015) is increasing since as the chemical preservatives have some harmful effect to consumers (Chemat et al., 2017; Zhong et al., 2018). One such bio-preservation strategy is the use of bacteriocins or bacteriocins-producing starter cultures to preserve the intended foods (Ng et al., 2020; Silva et al., 2018; Soltani et al., 2021). Importance of bacteriocin produced by LAB have been reviewed earlier (Hernández-González et al. 2021; Kaškonienė et al., 2017; Mokoena, 2017), however, information regarding the gene organizations responsible for potent effect of bacteriocins as lantibiotics, mode of action on pathogen bacterial cells is not yet available. Hence, the present review is aimed to update on the gene organizations, novel pathways for bacteriocin synthesis and their mode of action by lactic acid bacteria isolated from some ethnic fermented foods.
Bacteriocin
Bacteriocin was first discovered by Gratia (1925) namely “colicine” as it showed activity against E. coli. Later in 1953, Jacob et al. (1953) coined the term bacteriocin, showing promises toward the development of microbial antibiotics. Several Gram-positive (Malanovic et al., 2016) and Gram-negative (Duperthuy, 2020) are known to produce substances during their growth, mainly protein or polypeptides that possess antimicrobial activities (Raheem and Straus, 2019), and make up a heterogeneous group of peptides with respect to size, structure, antimicrobial potency, mode of action, immunity mechanism and target cell receptors (Kumar et al., 2018; Lei et al., 2019). Bacteriocins are ribosomal-synthesized antimicrobial peptides produced by bacterial strains (Negash and Tsehai, 2020), which are lethal to closely related species of producer bacteria but being protected by self-immunity (Simons et al., 2020). Bacteriocin plays a major role as competitor, communication molecule among microbial community (Schulz-Bohm et al., 2017) and quorum sensing (Zhao et al., 2020). Some bacteria produce bacteriocin-like compounds but not yet characterized, such bacteriocins are referred as bacteriocin-like inhibitory substances (BLIS) (Choeisoongnern et al., 2020; El-Gendy et al., 2021). The most important bacteriocin class is lantibiotics with an unusual amino acid i.e., lanthionine (Lan) (Islam et al., 2012). More than 25 lantibiotics have been described out of which nisin is widely studied lantibiotic (Garcia-Gutierrez et al., 2020). Nisin is the most utilized antimicrobial peptide produced by Lactococcus lactis strains, due to its excellent features as a food preservative, including high activity against Gram-positive food pathogens (Kitagawa et al., 2019).
Biosynthesis of bacteriocin
Bacteriocins are synthesized in a very specific pathway, which includes the pre-bacteriocin production and subsequently cleavage of the pre-peptide at specific processing site that removes the leader sequence and translocation of the pro-bacteriocin outside the cell membrane (Simons et al., 2020). Specific gene encoding for the synthesis of bacteriocins are clustered in one or two operons, which consist of different components, located on plasmids (chromosome) or in transposons inserted in the chromosome (Drider et al., 2006; Wirawan et al., 2007). First components are the structural gene, which encodes the pre-probacteriocin, that contains an N-terminal (the leader sequence doubleglycine type or peptide signal type sequences type) (Samal, 2013). The two conserved glycines are present at its C-terminus, which is recognized by ABC transporters for processing the leader sequence and helps in the secretion of the mature bacteriocin to the extracellular medium. In all these processes, signal peptide type sequence (SP) plays a significant role in processing and secretion of bacteriocins through the general transport path (GSP). Second components are immunity gene, which is of small proteins consists of 51–154 amino acids. These genes help in protecting the producing strain of the bacteriocin itself. Third components are the genes that encodes protein which is responsible for processing, transport and secretion of the pre-probacteriocin. Fourth components are modification gene, which encodes the enzyme which is responsible for post-translational modifications of the probacteriocin. Fifth components are regulatory gene, which encodes the gene which involved in the regulation of bacteriocin synthesis (Perez et al., 2018; Skaugen et al., 2003). The complete process of bacteriocin production and secretion goes through the signal transduction systems, which consists of three components such as Inductor peptide (IP), response regulatory protein (RR), and sensor histidine protein kinase (HPK) (Fig. 1).
Fig. 1.
Biosynthesis of nisin through the signal transduction systems, which consists of three components such as inductor peptide (IP), response regulatory protein (RR), and sensor histidine protein kinase (HPK) (Fig. 1)
(adopted from Cheigh et al., 2005)
There are two models that explain the induction process, the inducing peptide and signal transduction mechanism (Straume et al., 2007). The IPs (small cationic molecules) that form an amphiphilic α helix and also work as signal of the regulatory systems (or “quorum sensing”) which help in controlling the biosynthesis of certain bacteriocins (Mokoena, 2017). The first model explains that the IP is produced consistently in small amounts, and accumulates progressively during the growth of the cell, and when required it increases the expression of the bacteriocin gene (Lafuente-Rincón et al., 2016). The second model explains the induction process, IP occurs at a level below the required level of self induction, and in certain environmental factors temporarily increases its production, so whenever the required level exceeds, it will induce its own synthesis by expressing the remaining gene from the bacteriocin gene cluster (Lafuente-Rincón et al., 2016).
Classification
Most of the bacteriocins are small, heat stable, cationic, amphiphilic and membrane permeabilizing peptides (Mokoena, 2017). Bacteriocin is classified into four classes on the basis of its amino acid compositions, molecular mass, thermostability, broad spectrum, enzymatic susceptibility genetics, mode of action, producer strains and types of post translational modifications and sized (Kumariya et al., 2019; Negash and Tsehai, 2020; Zimina et al., 2020). Four classes are recognized with their subclasses namely: Class I which represents as lantibiotics, small peptides (< 5 kDa) such as nisin, subtilin, mutacin, etc. (Meade et al., 2020), Class II is non-lantibiotics, small peptides (< 10 kDa) such as enterocins, pediocins, etc. (Song et al., 2014), Class III is large (> 30 kDa) heat liable protein such as helveticin, caseicin, etc. (Mokoena et al., 2017) and Class IV is bacteriocins with non-protein moieties mostly lipids or carbohydrate parts such as plantaricin S, leuconocin S, etc. (Simons et al., 2020).
Class I bacteriocin—Lantibiotics
Lantibiotics are post-translationally modified protein, small peptides (< 5 kDa) make up of 19–50 amino acids, heat stable and containing unusual amino acids residues called lanthionine, β-methyllanthionine, dehydroalanine, labyrinthin and dehydrobutyrine (Kumariya et al., 2019). Based on the biosynthetic enzymes within the operon coding for the production, modification and transport of the lantibiotic, class I bacteriocin is further divided into 5 sub-classes namely: Class Ia (Lantibiotics), Class Ib (labyrinthopeptins), Class1c (Sanctibiotics/Lanthipeptides), ClassId (Lanthipeptides containing Zinc-binding motif) and Class Ie (Lexapeptide) (Simons et al., 2020). Class Ia lantibiotics usually undergo dehydration of threonine and serine and cyclization, which are mainly carried out by the enzyme encoded genes LanB and LanC (Lagedroste et al., 2020). This leads to the formation of screw shaped, elongated, amphipathic peptide molecules producing voltage dependent pores by binding to the receptor lipid II and having mode of action as membrane permeabilization (Dickman et al., 2019). Nisin is one of the most common groups with unique amino acid such as lanthionine and β-methyllanthionine produced by Lactococcus lactis (Saraiva et al., 2020). Class Ib lantibiotics contain enzyme that encoded for lanM gene having the properties of both dehydration and cyclization that lead to the formation of globular, anionic or neutral peptide molecules (Repka et al., 2017). It is a modified post-translational, carbacyclic lantibiotics containing labionin and labyrinthin (Lagedroste et al., 2020). Mutacin II and labyrinthopeptin A1 are produced by Streptococcus mutans T8 (Férir et al., 2013). Class Ic bacteriocin is a sulphur to alpha carbon-containing antibiotics, referred as lanthipeptides or sactibiotics (Alvarez-Sieiro et al., 2016). The formation of lanthipeptides is catalysed by tri-functional enzymes encoded genes, lanKC (that contain lyase, kinase and cyclase) (Repka et al., 2017). Class Id is the lantibiotics such as streptocollin that contains zinc-binding motif in the cyclization domain, produced by Strepotomyces collinus Ti365 (Iftime et al., 2015). Class V is also known as lexapeptide, a novel F42OH2-dependent reductase that catalyse the reduction of dehydroalanine to install D-Ala, is produced by actinomycetes and cyanobacteria (Xu et al., 2020a, 2020b).
Gene organisation, biosynthesis and mode of actions of Lantibiotics
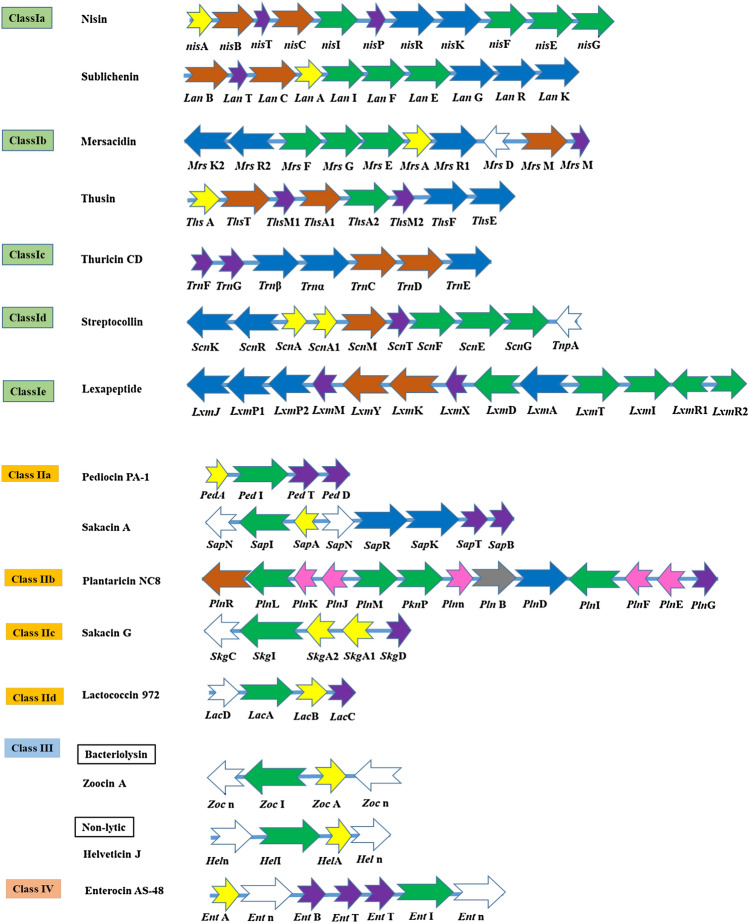
Many genes are involved which form an operon cluster for active production of bacteriocins (Noda et al., 2018). Operons of the lantibiotics have: a) structural genes which consist of leader peptide attach toward the core peptide (LanA); b) biosynthetic enzyme encoding genes: post translation modification of the peptides (LanBC), transporter protein (LanT) in which ABC transporter helps to attach the modification machinery to the cytoplasmic membrane, protease (helps to release the core peptide from leader sequence making peptide active) (LanP); c) immunity proteins: immunity of the peptide (LanIFEG); and d) regulatory unit: regulates the production of peptide (Lan RK) (Lagedroste et al., 2020; Sandiford et al., 2020). Schematic organization of gene clusters involved in the biosynthesis of Class I lantibiotics is shown in Fig. 2. The structural gene and its surrounding regions divergently transcribe and contribute toward the production of lantibiotics, its extracellular translocation, regulation of the lantibiotics synthesis and the immunity of the producers (Lajis et al., 2020; Simons et al., 2020).
Fig. 2.
Schematic representation of gene clusters involved in the biosynthesis of different class of bacteriocins (Class I, Class II, Class III and Class IV). Different colour indicates the different functions of gene responsible for the biosynthesis of lantibiotics: orange arrows indicate the modification gene, purple arrows for transport and processing, green arrows for immunity, blue arrows for regulatory, yellow arrows for structural genes and white and pink arrows is the gene whose function is not known yet
(adopted from Halami, 2019; Iftime et al., 2015; Mathur et al., 2017; Xin et al., 2016; Xu et al., 2020a, 2020b)
Lantibiotics are expressed as pre-propeptides (N-terminal leader sequence and a C-terminal pro-peptide) and post-translationally modified, then biosynthesis of the lantibiotics begin with the enzymatic dehydration of serine and threonine residues that lead to the formation of unusual amino acids 2,3-dehydrobutyrine (Dhb) and 2,3- dehydroalanine (Dha) (Repka et al., 2017). In these amino acids, the thiol group of neighbouring cysteine residue is added, which form the lanthionine from Dha and methyllanthionine from Dhb residues (Jones et al., 2020). The stable thioether-based intramolecular rings determined the 3-D structure of the peptides, which are very much needed for the biological activity (Li et al., 2019). NisB and NisC are modification enzymes that dehydrate and cyclize the pro-peptide and NisT are ABC transporter, which help in the translocation of the pre-peptide into the extracellular space (Repka et al., 2017). Subsequently NisP is the protease that cleaves the leader peptide thereby releasing the active form (mature) nisin, which possess immunity system for the nisA (Montalbán-López et al., 2018). Nisin consists of two component regulation systems: NisK (histidine kinase) and NisR (response regulator), which are responsible for the up-regulation for the nisin gene thereby activating the nisin (Geiger et al., 2017). However, in case of subtilin, the AbrB repressor allows the low-expression of the SpaRK (two-component regulatory system) (Geiger et al., 2017). Subtilin auto induces histidine kinase SpaK, which phosphorylates the SpaR (Response regulator) (Chakicherla et al., 2009), thereby upregulating the transcription of subtilin biosynthesis and immunity genes (Spieß et al., 2015).
Most of the lantibiotics produced by Gram-positive bacteria are active against other species of Gram-positive bacteria (Acedo et al., 2018). Lantibiotics of Gram-positive bacteria are less effective against Gram-negative bacteria which might be due to the outer membrane of Gram-negative bacteria not penetrable by the peptides (Halami, 2019). Lantibiotics are able to destabilize the membrane by forming a pore that will lead to misbalance of the membrane potential which ultimately results in leakage of small metabolites and termination of cellular biosynthetic processes (Barbour et al., 2020). The main target for the cationic bacteriocins is the cytoplasmic membrane of the bacterial cells, having anionic property which is composed of lipopolysaccharide (LPS), cardiolipin (CL), lipotheichoic acid (LTA), phosphatidylethanolamine (PE) phosphatidylglycerol (PG) (Kumariya et al., 2019). Lantibiotics act upon the cytoplasmic membrane of the bacterial cells called membrane bound peptidoglycan precursor lipid II, which will lead to the dissipation of the proton motive force (PMF), which is made up of chemical component and an electrical component (Niamah, 2018). In the membrane, this will drive the synthesis of ATP and lead to the accumulation of ions and other metabolites through PMF-driven transport systems (Pokhrel et al., 2019). Lantibiotics actions will collapse the PMF, resulting in cell death by the stoppage of energy-requiring reactions (Kumariya et al., 2019).
Class II bacteriocin: non lantibiotics
Class II bacteriocins are small (< 10 kDa) peptides, non-modified, ribosomally synthesis peptides, heat stable, glycine content and called non-lantibiotics and further classified into four subtypes listed, which include: Class IIa, Class IIb, Class IIc and Class IId (Cao et al., 2019; Cui et al., 2021; Negash and Tsehai, 2020). Class IIa is a small thermostable peptide, containing N-terminal conservative sequence YGNGV, having mode of action as membrane permeabilization by binding to the receptors mannose permease, and having a strong inhibitory effect on Listeria (Colombo et al., 2018; Wang et al., 2019). These Class IIa bacteriocin is produced by strains of Lactobacillus, Pediococcus, Enterococcus and Leuconostoc (Chen et al., 2018; Hammi et al., 2019; Zommiti et al., 2018). Pediocin PA-1, Lakacin, Leucocin A, Garviecin LG34, Sakacin, Enterocin, Acidocin A, Bavaricin A are the examples of Class IIa (Chen et al., 2018; Cui et al., 2021; Hashim et al., 2019; Hwang et al., 2018; Kassaa et al., 2019; Liu et al., 2019). Class IIb bacteriocin is the unmodified, two-peptides bacteriocins, having the structure of GxxxG motifs (Kumariya et al., 2019; Wang et al., 2019). These include two different peptides which are much needed to form an active poration complex for the membrane permeabilization by binding toward the receptors UppP (undecaprenyl pyrophosphate phosphatise) (Kasuga et al., 2019). Plantaricin JK, lactococcins, lactococin and enterocinX are the common Class IIb bacteriocins produced by Lactiplantibacillus plantarum (= Lactobacillus plantarum), Lactococcus lactis, Lactobacillus johnsonii (Butorac et al., 2020; Xu et al., 2019). Class IIc bacteriocin is a circular bacteriocin in which N and C-termini are covalently linked, having membrane permeabilization as a mode of action in which ABC transporter is the binding receptors. Acidocin B, Gassericin A, Enterocin AS-48, Gassericin A and Garvicin ML are the Class IIc and are produced by Lactobacillus acidophilus, Lb. gasseri, Lb. garvieae, Enterococcus faecalis and Lactococcus lactis (Alizadeh, et al., 2020; Baños et al., 2019; Chi et al., 2018; Garcia-Gutierrez et al., 2020). Class IId bacteriocin is linear, unmodified, leaderless and non-pediocin like bacteriocins having membrane permeabilization as a mode of action by binding to the receptors Metallope-ptidase (Alizadeh et al., 2020). Class IId bacteriocin includes Lacticin Z, Carnobacteriocin A, Enterocin Q, Bactofencin A, LsbB, Weissellicin 110, Thuricin S and Lactococcin A produced by Ligilactobacillus salivarius (= Lactobacillus salivarius), Carnobacterium piscicola, Enterococcus faecium L50 and Weissella cibaria 110 (Nazari and Smith, 2020; O’Connor et al., 2018; Zhang et al., 2019; Zimina et al., 2020).
Gene organisation, biosynthesis and mode of actions of class II bacteriocins
Many genes are involved, which include bacteriocin precursor peptide, immunity protein, ABC transporter complex and two or more operons for the biosynthesis of Class II bacteriocin (Kumariya et al., 2019). In each subtype, the organization of gene is different, such as the location of ABC transporter gene, GG-motifs containing peptides and also the gene encoding an accessory factor (Wang et al., 2019). For example, Pediocin PA-1 consists of four genes pedA, pedI, pedT and pedD (Fig. 2) encoding for pro-peptide, immunity protein, transport and processing. During the export of the peptides, the signal peptide is cleaved off at the C-terminal side of the two glycine residues, which is done by the ATP-binding cassette (Cao et al., 2019). Similarly, plantaricin has a similar operon structure like pediocin PA-1 (Bédard et al., 2018). The class II bacteriocin produces an induction factor that help in activating the transcription of the regulatory genes, hereby play an important role in the biosynthesis of class II bacteriocins (Colombo et al., 2018).
Mode of action of Class II bacteriocins vary from species to species, also it has been reported that it displays narrow spectrum when compared with nisin (Class Ia bacteriocin). Listeria strains are the common pathogens targeted by the class II bacteriocin (Colombo et al., 2018). Many studies have found that Leuconostoc, Lactococcus, Enterococcus, Staphylococcus, Lactobacillus, Pediococcus, Carnobacterium, Micrococcus, Streptococcus, Clostridium and Bacillus and many more are sensitive toward the class II bacteriocin (Negash and Tsehai, 2020). Class II bacteriocin binds to the cytoplasmic membrane thereby inserting the peptides into the membrane, which may cause the formation of pore complex that permeabilizes the membrane leading to the disruption of the PMF which finally cause cell death (Kumariya et al., 2019; Wang et al., 2019).
Class III and IV bacteriocins
Class III bacteriocins are large molecules (> 30 kDa), sensitive to heat and are classified based on the hydrolase activity (Mokoena, 2017). Class IIIa bacteriocins have hydrolase like activity and called as bacteriolysins and Class IIIb bacteriocin is without hydrolase activity and named as non-lytic (Wang et al., 2019). Lysostaphin and Zoocin A are the examples of Class IIIa produced by Staphylococcus simulans and Lactobacillus sp. (Simons et al., 2020). Enterolysin A and Helveticin J, Helveticin M and Lactococcin 972 are the common examples of ClassIIIb bacteriocin and are synthesised by Enterococcus, Lactobacillus and Lactococcus (Li et al., 2021; Meng et al., 2021; Stoyancheva, 2020). Mode of action of Class IIIa bacteriocin is to permeabilize the membrane and to form pores (Simons et al., 2020). Class IV bacteriocins are very complex, circular bacteriocins made up of lipid or carbohydrate moieties. Some examples of Class IV bacteriocins are PediocinN5p, lactocin27 and Enterocin Gr17, Lacstrepsin and Lactocin 27 and are produced by Pediococcus pentosaceus, Lactobacillus helveticus LP27 and LS18, Enterococcus faecalis Gr17 and Streptococcus lactis (Gutiérrez-Cortés et al., 2018; Liu et al., 2019).
Gene organisation, biosynthesis and mode of actions of Class III and IV bacteriocin
Many genes are involved for the production of bacteriocin but in case of Class III bacteriocin, gene involved in the production shows a great diversity in its structural gene, immunity gene, regulatory gene, transport and processing genes (Sun et al., 2018). Figure 2 depicts the gene organization of Class IIIa and Class IIIb bacteriocin, and, the arrangement of gene in Class IV bacteriocin. The main target of Class III bacteriocin in both Gram-negative and Gram-positive bacteria is bacterial cytoplasmic membrane (Negash and Tsehai, 2020). This Class III bacteriocin depolarizes the cell membrane, permits leakage of ATP, thereby increases the membrane impairment (Sun et al., 2018). Class IV circular bacteriocin is ribosomally synthesized peptides, which is post translationally modified in such a way that the first and last amino acids of the peptide (mature) are covalently bonded corresponding to the head to tail ligation (Meade et al., 2020; Vezina et al., 2020). Class IV circular bacteriocin has an inhibitory effect against Gram-positive as well as Gram-negative species (Perez et al., 2018; Wang et al., 2019).
Bacteriocin-producing LAB in fermented food
Bacteriocins produced by the Gram-positive bacteria especially LAB isolated from fermented foods (Goel et al., 2020; Liu et al., 2019; Pei et al., 2020) have been concerning more interest among the researchers because of their biosafety and industrial application most importantly for food preservation (Johnson et al., 2019). Many researchers have isolated the class I bacteriocin such as nisin, subtilin, lactocin as well as class II bacteriocins such as plantaricin, enterocin, pentocin, pediocin, etc. from fermented foods (Table 1). Bacteriocin is reported from many fermented vegetable products such as Korean kimchi (Lee et al., 2002; Liu et al., 2017), Chinese fermented vegetable products (Garcia-Gonzalez et al., 2021; Ullah et al., 2017). Levilactobacillus brevis (= Lactobacillus brevis), Lacticaseibacillus casei (= Lactobacillus casei), Lactiplantibacillus plantarum and Leuconostoc spp. are the most common LAB strains isolated from the fermented vegetables and pickles, which produce class I bacteriocin like subtilin and lactocin as well as class II bacteriocins like plantaricin, leucocin, garviecin (Islam et al., 2020; Kim et al., 2019; Lv et al., 2018; Shi et al., 2016; Zhao et al., 2016). Nisin-like bacteriocin is produced by Lactococcus lactis subsp. lactis A164 isolated from Korean kimchi (Choi et al., 2000). Proline-based cyclic dipeptides is produced by Leuconostoc mesenteroides LBP-K06, isolated from kimchi, which have antimicrobial activities against multidrug-resistant bacteria (Liu et al., 2017).
Table 1.
Bacteriocin-producing lactic acid bacteria isolated from ethnic fermented foods
| Producer strain | Bacteriocin | Class | Fermented foods (Country) | Spectrum | References |
|---|---|---|---|---|---|
| Lactiplantibacillus plantarum strain DHCU70 and Lactiplantibacillus plantarum strain DKP1 | Plantaricin | Class IIa bacteriocin | Dahi fermented yogurt-like milk product and Kinema, fermented soybean food (India) | Kocuria rhizophila ATCC 9341 | Goel et al. (2020) |
| Enterococcus faecalis Gr17 | Enterocin P | class IIa | Suan yu, fermented vegetable pickle (China) | Listeria monocytogenes, Escherichia coli, Staphyococcusaureus, Bacillus subtilis, B. cereus | Liu et al. (2019) |
| Lactobacillus spp. | Bacteriocin | – | Fermented vegetable cucumber & carrot (Bangladesh) | E. coli, S. aureus, B. cereus, B. subtilis, Acetobacter, E. coli, E. fecalis, Salmonella typhi | Islam et al. (2020) |
|
Levilactobacillus brevis DF01 and Pediococcus acidilactici K10 |
BLIS (bacteriocin-like inhibitory substance) | – | Kimchi (Korean fermented vegetable) | S. typhimurium and E. coli | Kim et al. (2019) |
| Lactococcus lactis KC24 | Bacteriocin KC24 | – | Listeria monocytogenes | Han et al. (2013) | |
| Lacticaseibacillus rhamnosus 1.0320 | Bacteriocin 1.0320 | – | Koumiss (Russian fermented milk-kefir grain) | E. coli UB1005 | Xu et al. (2020a, 2020b) |
| Lactiplantibacillus plantarum MXG-68 | Plantaricin MXG-68 | Class IIa |
L. monocytogenes ATCC15313, B. ereus ATCC11788, E. coli ATCC25922, S. typhimurium ATCC14028 |
Man et al. (2019) | |
| Lactiplantibacillus plantarum B21 | Plantacyclin B21AG | Class II | Nem chua (Vietnamese sausage) | L. monocytogene, Clostridium perfringens | Golneshin et al. (2020) |
|
Limosilactobacillus fermentum BZ532 |
Bacteriocin LF-BZ532 | Class II | Bozai (Chinese fermented cereal-based beverage) | E. coli k-12, S. aureus ATCC6538, Salmonella sp. D104 | Rasheed et al. (2020) |
| Lactobacillus pentosus DZ35 | Pentocin DZ1 and pentocin DZ2 | Class II | Pickles and dried cured meat | S. aureus, E. coli | Yi et al. (2020) |
| Lactiplantibacillus plantarum | Bacteriocin- SLG10 | Class IId | Kombucha (Chinese fermented tea) | S. aureus | Pei et al. (2020) |
| Lacticaseibacillus casei KLDS 1.0338 | Bacteriocin | Sourdough (European and American fermented sour bread) | S. aureus, Penicillium sp. | Ma et al. (2020) | |
| Lactiplantibacillus plantarum LPL-1 | Plantaricin LPL-1 | Class IIa | Sausage | L. monocytogenes | Zhang et al. (2020) |
| Lactiplantibacillus plantarum | Bacteriocin-M1-UVs300 | L. monocytogenes, S. aureus, Micrococcus luteus, B. subtilis, Streptococcus thermophiles, Salmonella paratyphi, Shigella dysenteriae, E. coli | An et al. (2017) | ||
| Lactococcus lactis | Nisin Z | Class I | Micrococcus luteus | Saraiva et al. (2020) | |
| Enterococcus hirae ST57ACC | Enterocin | – | Brazilian artisanal cheese | – | Cavicchioli et al. (2019) |
| Pediococcus acidilactici | BLIS | – | Gappal (fermented milk-millet mixture product of Burkina Faso) | E. faecalis ATCC 19,433, M. luteus ATCC 49,732, S. aureus ATCC 2523, L. monocytogenes, B. megaterium, B. sphaericus and B. cereus | Tankoano et al. (2019) |
| Pediococcus pentosaceus LU11 and Lactiplantibacillus plantarum LS6 | Pediocin and Plantaricin | Class IIa | Tungtap (Indian fermented fish) and Tungrymbai (Indian fermented soybean food) | Streptococcus pyogenes, E. faecalis, E. coli, Klebsiella pneumoniae, B. cereus | Biswas et al. (2017) |
| Lacticaseibacillus casei | LiN333 | – | Jiangshui cai (Chinese fermented vegetable) | E. coli, S. aureus | Ullah et al. (2017) |
| Lactobacillus coryniformis MXJ 32 | Lactocin MXJ 32A | Class I | E. coli, S. aureus, Salmonella sp., L. monocytogenes, Cronobacter sakazakii | Lü et al. (2014) | |
| Enterococcus faecalis KT11 | Bacteriocin KT11 | – | Kargı tulum cheese (Turkish goat’ milk cheese) | P. aeruginosa, B. cereus, M. luteus | Abanoz and Kunduhoglu (2018) |
| Lactiplantibacillus plantarum J23 | Bacteriocin Lac-B23 | – | Chinese fermented milk products | L. monocytogenes | Zhang et al. (2018) |
| Lactiplantibacillus plantarum LPL-1 | Plantaricin LPL-1 | Class IIa | Chinese fermented fish products | E. coli, Salmonella sp. | Wang et al. (2018) |
| Lactobacillus alimentarius FM-MM4 | Lactocin MM4 | Class II | Nanx wudl (Chinese fermented meat) | E. coli and Salmonella | Hu et al. (2017) |
| Lactiplantibacillus plantarum Q7 | Plantaricin Q7 | – | Yak yogurt | Ps. fluorescens AS1.1802 | Liu et al. (2016) |
| Lactiplantibacillus plantarum | Plantaricin | Class II | Bekasam (Indonesian fermented meat), tapai (Indonesian fermented rice) and tempoyak (Indonesian fermented durian food) | B. subtilis, Pseudomonas aeroginosa | Sogandi et al. (2019) |
| Lactococcus lactis subsp. Lactis | Nisin Z | Class I | Charqui (Brazilian fermented meat) | L. monocytogenes ScottA | Biscola et al. (2013) |
| Leuconostoc mesenteroides K7 | Leucocin K7 | Class IIa | Fermented pickle | L. Monocytogenes | Shi et al. (2016) |
| Lactiplantibacillus plantarum ZJ5 | Plantaricin ZJ5 | Class II | Fermented mustard | S. aureus | Song et al. (2014) |
| Lactiplantibacillus plantarum JLA-9, | Plantaricin JLA-9 | Class II | Suan-tsai (Chinese fermented cabbage) | B. cereus | Zhao et al. (2016) |
| Lactiplantibacillus plantarum DL3 | Plantaricin DL3 | Pseudomonas aeruginosa | Lv et al. (2018) | ||
| Lactiplantibacillus plantarum KLDS1.0391 | Plantaricin MG | Class II | Jiaoke (Chinese fermented milk product) | L. monocytogenes, S. aureus, Sal. typhimurium, E. coli | Gong et al. (2010) |
| Lactococcus lactis subsp. lactis MA23 | Bacteriocin MA23 | – | Boza (African fermented cereal-based beverage) | Gram-positive bacteria | Akkoc et al. (2011) |
| Lactococcus lactis subsp. lactis BZ | Lactococcin BZ | Class I | Lactobacillus, Enterococcus, Leuconostocs, Listeria, Bacillus, Enterobacter, Escherichia, Rhodococcus, Salmonella, Yersinia, Citrobacter spp. | Şahingil et al. (2011) | |
| Streptococcus infantarius subsp. Infantarius | Bacteriocin | – | Suusac (African fermented camel-milk product) | Other LAB, Listeria | Jans et al. (2012) |
| Lactococcus garvieae BCC 43,578 | Garvieacin Q | Class IIa | Nham (Thai fermented pork meat) | E. faecium, L. monocytogenes | Tosukhowong et al. (2012) |
| Weissella hellenica BCC 7293 | Bacteriocin 7293A and 7293B | – | L. monocytogenes, S. aureus, Sal. typhimurium, E. coli, Pseudomonas aeruginosa, Aeromonas hydrophila | Woraprayote et al. (2015) | |
| Lactobacillus sakei D98 | Sakacin D98a, sakacin D98b and sakacin D98c | Class IIa | Shubo/moto (Japanese starter for sake production) | B. circulans, L. innocua, L. monocytogenes, E.faecalis, L. sakei ssp. sakei | Sawa et al. (2013) |
| Lactococcus garvieae LG34 | Garviecin LG34 | Class IIa | Chinese fermented cucumber | L. delbruleckii subsp. bulgaricus, L. acidophilus, S. aureus, L. monocytogenes, E. coli, Shigella flexneri, L. plantarum L8, L. delbruleckii subsp. delbrueckii, S. thermophilus, Sarcina flava, Salmonella thyphimurium | Gao et al. (2015) |
Lactococcus lactis subsp. lactis strain 63
Lactococcus lactis subsp. lactis strain 63, isolated from Indian dairy products, produces bacteriocin that inhibits Listeria monocytogenes, Bacillus cereus, E. coli, Klebsiella, Enterobacter, Citrobacter, Proteus and Serratia strains (Goyal et al., 2018). Several LAB species isolated from Romanian traditional fermented fruits and vegetables have antimicrobial activity against L. monocytogenes, E. coli, Salmonella, and Bacillus (Gaggia et al., 2011).
Fermented milk and milk products are considered as the vital sources for isolation of bacteriocin producing LAB. Lactiplantibacillus plantarum strain DHCU70 isolated from dahi, a yogurt-like Indian fermented milk product produces class II preservative called plantaricin and found to exhibit cell-wall inhibiting mechanism to the target cells (Goel et al., 2020). Lactobacillus rhamnosus and Lactiplantibacillu plantarum, isolated from koumiss a fermented milk-kefir grain beverage, have shown the antimicrobial effect against E-coli and Listeria monocytogenes due to production of bacteriocin such as plantaricin (Man et al., 2019; Xu et al., 2020a, 2020b). Bacteriocin-producing LAB have been reported from yogurt and cheese (Gutiérrez-Cortés et al., 2018; Niederhäusern et al., 2020; Silva et al., 2018), fermented milk products of Egypt (Refay et al., 2020), African fermented milk (Maleke et al., 2021). Bacteriocin-producing LAB isolated from many fermented cereal-based products may be utilized for the bio-preservation of foods (Ma et al., 2020; Pei et al., 2020; Rasheed et al., 2020). Enterocin P producing Enterococcus faecalis Gr17, isolated from the traditional Chinese fermented fish called suan yu, shows the antimicrobial effects against the L. momocytogenes, E. coli, S. aureus and B. cereus (Liu et al., 2019).
Ethnic fermented foods dock several species of functional and beneficial microorganisms, which produce bacteriocin as one of the secondary metabolites. Due to the differences in its gene coding for the production, secretion and immunity, each bacteriocins have unique structures and different modes of activity thus making them ideal candidate having different properties. Thereby, many investigators shifted their focus on bacteriocin from food preservation to the treatment of infection and antibiotic-resistant diseases causing bacteria. Also with the latest technique in gene mining, synthesis and protein expression study, it became promising to look forward with the novel bacteriocin having advanced applications.
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abanoz HS, Kunduhoglu B. Antimicrobial activity of a bacteriocin produced by Enterococcus faecalis KT11 against some pathogens and antibiotic-resistant bacteria. Food Science of Animal Resources. 2018;38(5):1064–1079. doi: 10.5851/kosfa.2018.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acedo JZ, Chiorean S, Vederas JC, van Belkum MJ. The expanding structural variety among bacteriocins from Gram-positive bacteria. FEMS Microbiology Reviews. 2018;42:805–828. doi: 10.1093/femsre/fuy033. [DOI] [PubMed] [Google Scholar]
- Akkoc N, Ghamat A, Akcelik M. Optimisation of bacteriocin production of Lactococcus lactis subsp. lactis MA23, a strain isolated from Boza. International Journal of Dairy Technology. 2011;64:425–32. [Google Scholar]
- Alizadeh AM, Hashempour-Baltork F, Alizadeh-Sani M, Maleki M, Azizi-Lalabad M, Khosravi-Darani K. Inhibition of Clostridium (C.) botulinum and its toxins by probiotic bacteria and their metabolites: an update review. Quality Assurance and Safety of Crops & Foods. 2020;12:59–68. [Google Scholar]
- Alvarez-Sieiro P, Montalbán-López M, Mu D, Kuipers OP. Bacteriocins of lactic acid bacteria: extending the family. Applied Microbiology and Biotechnology. 2016;100:2939–2951. doi: 10.1007/s00253-016-7343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Y, Wang Y, Liang X, Yi H, Zuo Z, Xu X, Zhang D, Yu C, Han X. Purification and partial characterization of M1-UVs300, a novel bacteriocin produced by Lactobacillus plantarum isolated from fermented sausage. Food Control. 2017;81:211–217. [Google Scholar]
- Ashaolu TJ, Reale A. A holistic review on Euro-Asian lactic acid bacteria fermented cereals and vegetables. Microorganisms. 2020;8(8):1176. doi: 10.3390/microorganisms8081176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baños A, Ariza JJ, Nuñez C, Gil-Martínez L, García-López JD, Martínez-Bueno M, Valdivia E. Effects of Enterococcus faecalis UGRA10 and the enterocin AS-48 against the fish pathogen Lactococcus garvieae. Studies in vitro and in vivo. Food Microbiology. 2019;77:69–77. doi: 10.1016/j.fm.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Barbour A, Wescombe P, Smith L. Evolution of lantibiotic salivaricins: New weapons to fight infectious diseases. Trends in Microbiology. 2020;28:578–593. doi: 10.1016/j.tim.2020.03.001. [DOI] [PubMed] [Google Scholar]
- Bédard F, Hammami R, Zirah S, Rebuffat S, Fliss I, Biron E. Synthesis, antimicrobial activity and conformational analysis of the class IIa bacteriocin pediocin PA-1 and analogs thereof. Scientific Reports. 2018;8:9029. doi: 10.1038/s41598-018-27225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutia MO, Thapa N, Shangpliang HNK, Tamang JP. Metataxonomic profiling of bacterial communities and their predictive functional profiles in traditionally preserved meat products of Sikkim state in India. Food Research International. 2021;140:110002. doi: 10.1016/j.foodres.2020.110002. [DOI] [PubMed] [Google Scholar]
- Biscola V, Todorov SD, Capuano VS, Abriouel H, Gálvez A, Franco BD. Isolation and characterization of a nisin-like bacteriocin produced by a Lactococcus lactis strain isolated from charqui, a Brazilian fermented, salted and dried meat product. Meat Science. 2013;93:607–613. doi: 10.1016/j.meatsci.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Biswas K, Upadhayay S, Rapsang GF, Joshi SR. Antibacterial and synergistic activity against β-Lactamase-producing nosocomial bacteria by bacteriocin of LAB isolated from lesser known traditionally fermented products of India. HAYATI Journal of Biosciences. 2017;24(2):87–95. [Google Scholar]
- Bourdichon F, Laulund S, Tenning P. Inventory of microbial species with a rationale: a comparison of the IDF/EFFCA inventory of microbial food cultures with the EFSA Biohazard Panel qualified presumption of safety. FEMS Microbiology Letters. 2019;366(5):1–6. doi: 10.1093/femsle/fnz048. [DOI] [PubMed] [Google Scholar]
- Butorac K, Banić M, Novak J, Pavunc AL, Uroić K, Durgo K, Oršolić N, Kukolj M, Radović S, Scalabrin S, Žučko J, Starčević A, Šušković J, Kos B. The functional capacity of plantaricin-producing Lactobacillus plantarum SF9C and S-layer-carrying Lactobacillus brevis SF9B to withstand gastrointestinal transit. Microbial Cell Factories. 2020;19:106. doi: 10.1186/s12934-020-01365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Du R, Zhao F, Xiao H, Han Y, Zhou Z. The mode of action of bacteriocin CHQS, a high antibacterial activity bacteriocin produced by Enterococcus faecalis TG2. Food Control. 2019;96:470–478. [Google Scholar]
- Cavicchioli VQ, Todorov SD, Iliev I, Ivanova I, Drider D, Nero LA. Physiological and molecular insights of bacteriocin production by Enterococcus hirae ST57ACC from Brazilian artisanal cheese. Brazilian Journal of Microbiology. 2019;50(2):369–377. doi: 10.1007/s42770-019-00068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakicherla A, Ecale Zhou CL, Dang ML, Rodriguez V, Hansen JN, Zemla A. SpaK/SpaR two-component system characterized by a structure-driven domain-fusion method and in vitro phosphorylation studies. PLOS Computational Biology. 2009;5(6):e1000401. doi: 10.1371/journal.pcbi.1000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary A, Bhalla S, Patiyal S, Raghava GPS, Sahni G. FermFooDb: A database of bioactive peptides derived from fermented foods. Heliyon. 2021;7(4):e06668. doi: 10.1016/j.heliyon.2021.e06668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheigh CI, Pyun YR. Nisin biosynthesis and its properties. Biotechnology Letters. 2005;27(21):1641–1648. doi: 10.1007/s10529-005-2721-x. [DOI] [PubMed] [Google Scholar]
- Chemat F, Rombaut N, Meullemiestre A, Turk M, Perino S, Fabiano-Tixier AS, Abert-Vian M. Review of green food processing techniques. Preservation, transformation, and extraction. Innovative Food Science and Emerging Technologies. 2017;41:357–77. [Google Scholar]
- Chen YS, Wu HC, Kuo CY, Chen YW, Ho S, Yanagida F. Leucocin C-607, a novel bacteriocin from the multiple-bacteriocin-producing Leuconostoc pseudomesenteroides 607 isolated from persimmon. Probiotics and Antimicrobial Proteins. 2018;10(2):148–156. doi: 10.1007/s12602-017-9359-6. [DOI] [PubMed] [Google Scholar]
- Chi H, Holo H. Synergistic antimicrobial activity between the broad spectrum bacteriocin garvicin KS and nisin, farnesol and polymyxin B against Gram-positive and Gram-negative bacteria. Current Microbiology. 2018;75(3):272–277. doi: 10.1007/s00284-017-1375-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choeisoongnern T, Sivamaruthi BS, Sirilun S, Peerajan S, Choiset Y, Rabesona H, Haertlé T, Chaiyasut C. Screening and identification of bacteriocin-like inhibitory substances producing lactic acid bacteria from fermented products. Food Science and Technology. 2020;40(3):571–579. [Google Scholar]
- Choi HJ, Cheigh CI, Kim SB, Pyun YR. Production of a nisin-like bacteriocin by Lactococcus lactis subsp. lactis A164 isolated from Kimchi. Journal of Applied Microbiology. 2000;88(4):563–571. doi: 10.1046/j.1365-2672.2000.00976.x. [DOI] [PubMed] [Google Scholar]
- Colombo NS, Chalón MC, Navarro SA, Bellomio A. Pediocin-like bacteriocins: new perspectives on mechanism of action and immunity. Current Genetics. 2018;64(2):345–351. doi: 10.1007/s00294-017-0757-9. [DOI] [PubMed] [Google Scholar]
- Cui Y, Luo L, Wang X, Lu Y, Yi Y, Shan Y, Liu B, Zhou Y, Lü X. Mining, heterologous expression, purification, antibactericidal mechanism, and application of bacteriocins: A review. Comprehensive Reviews in Food Science and Food Safety. 2021;20(1):863–899. doi: 10.1111/1541-4337.12658. [DOI] [PubMed] [Google Scholar]
- Das S, Tamang JP. Changes in microbial communities and their predictive functionalities during fermentation of toddy, an alcoholic beverage of India. Microbiological Research. 2021;248:126769. doi: 10.1016/j.micres.2021.126769. [DOI] [PubMed] [Google Scholar]
- Dávila-Aviña JE, Solís-Soto LY, Rojas-Verde G, Salas NA. Sustainability and challenges of minimally processed foods. In: Siddiqui M, Rahman M, editors. Minimally Processed foods Food Engineering Series. Cambridge: Springer; 2015. [Google Scholar]
- Dickman R, Mitchell SA, Figueiredo AM, Hansen DF, Tabor AB. Molecular recognition of lipid II by lantibiotics: synthesis and conformational studies of analogues of nisin and mutacin rings A and B. The Journal of Organic Chemistry. 2019;84(18):11493–11512. doi: 10.1021/acs.joc.9b01253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diether NE, Willing BP. Microbial fermentation of dietary protein: an important factor in diet (-) microbe (-) host interaction. Microorganisms. 2019;7(1):19. doi: 10.3390/microorganisms7010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimidi E, Cox SR, Rossi M, Whelan K. Fermented foods: definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients. 2019;11(8):1806. doi: 10.3390/nu11081806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drider D, Fimland G, Héchard Y, McMullen LM, Prévost H. The continuing story of class IIa bacteriocins. Microbiology and Molecular Biology Reviews. 2006;70(2):564–582. doi: 10.1128/MMBR.00016-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duperthuy M. Antimicrobial peptides: virulence and resistance modulation in Gram-negative bacteria. Microorganisms. 2020;8(2):280. doi: 10.3390/microorganisms8020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gendy AO, Brede DA, Essam TM, Amin MA, Ahmed SH, Holo H, Nes IF, Shamikh YI. Purification and characterization of bacteriocins-like inhibitory substances from food isolated Enterococcus faecalis OS13 with activity against nosocomial enterococci. Scientific Reports. 2021;11:3795. doi: 10.1038/s41598-021-83357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Férir G, Petrova MI, Andrei G, Huskens D, Hoorelbeke B, Snoeck R, Vanderleyden J, Balzarini J, Bartoschek S, Brönstrup M, Süssmuth RD. The lantibiotic peptide labyrinthopeptin A1 demonstrates broad anti-HIV and anti-HSV activity with potential for microbicidal applications. PloS One. 2013;8(5):e64010. doi: 10.1371/journal.pone.0064010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggia F, Di Gioia D, Baffoni L, Biavati B. The role of protective and probiotic cultures in food and feed and their impact in food safety. Trends in Food Science and Technology. 2011;22:58–66. [Google Scholar]
- Gao Y, Li D, Liu S, Zhang L. Garviecin LG34, a novel bacteriocin produced by Lactococcus garvieae isolated from traditional Chinese fermented cucumber. Food Control. 2015;50:896–900. [Google Scholar]
- Garcia-Gonzalez N, Battista N, Prete R, Corsetti A. Health-promoting role of Lactiplantibacillus plantarum isolated from fermented foods. Microorganisms. 2021;9(2):349. doi: 10.3390/microorganisms9020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gutierrez E, O’Connor PM, Saalbach G, Walsh CJ, Hegarty JW, Guinane CM, Mayer MJ, Narbad A, Cotter PD. First evidence of production of the lantibiotic nisin P. Scientific Report. 2020;10:3738. doi: 10.1038/s41598-020-60623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger C, Spieß T, Korn SM, Kötter P, Entian KD. Specificity of subtilin-mediated activation of histidine kinase SpaK. Applied and Environmental Microbiology. 2017;83(18):e00781–e817. doi: 10.1128/AEM.00781-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Halami PM, Tamang JP. Genome analysis of Lactobacillus plantarum isolated from some Indian fermented foods for bacteriocin production and probiotic marker genes. Frontiers in Microbiology. 2020;11:40. doi: 10.3389/fmicb.2020.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golneshin A, Gor MC, Williamson N, Vezina B, Van TT, May BK, Smith AT. Discovery and characterisation of circular bacteriocin plantacyclin B21AG from Lactiplantibacillus plantarum B21. Heliyon. 2020;6(8):e04715. doi: 10.1016/j.heliyon.2020.e04715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong HS, Meng XC, Wang H. Plantaricin MG active against Gram-negative bacteria produced by Lactobacillus plantarum KLDS1 0391 isolated from “Jiaoke”, a traditional fermented cream from China. Food Control. 2010;21(1):89–96. [Google Scholar]
- Goyal C, Malik RK, Pradhan D. Purification and characterization of a broad spectrum bacteriocin produced by a selected Lactococcus lactis strain 63 isolated from Indian dairy products. Journal of Food Science and Technology. 2018;55(9):3683–3692. doi: 10.1007/s13197-018-3298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratia A. Sur un remarquable exemple d'antagonisme entre deux souches de coilbacille. Comptes Rendus Des Seances De La Societe De Biologie Et De Ses Filiales. 1925;93:1040–1042. [Google Scholar]
- Gutiérrez-Cortés C, Suarez H, Buitrago G, Nero LA, Todorov SD. Characterization of bacteriocins produced by strains of Pediococcus pentosaceus isolated from Minas cheese. Annals of Microbiology. 2018;68:383–398. [Google Scholar]
- Halami PM. Sublichenin, a new subtilin-like lantibiotics of probiotic bacterium Bacillus licheniformis MCC 2512T with antibacterial activity. Microbial Pathogenesis. 2019;128:139–146. doi: 10.1016/j.micpath.2018.12.044. [DOI] [PubMed] [Google Scholar]
- Hammi I, Amensag K, Ennahar S, Delalande F, Marchioni E, Cianferani S, Belkhou R. Native production of pediocin PA-1 by Enterococcus faecium E16 isolated from goats' cheese. Journal of Food & Nutrition Research. 2019;58:1–8. [Google Scholar]
- Han EJ, Lee NK, Choi SY, Paik HD. Bacteriocin KC24 produced by Lactococcus lactis KC24 from kimchi and its antilisterial effect in UHT milk. Journal of Dairy Science. 2013;96(1):101–104. doi: 10.3168/jds.2012-5884. [DOI] [PubMed] [Google Scholar]
- Hashim H, Sikandar S, Khan MA, Qurashi AW. Bacteriocin: the avenues of innovation towards applied microbiology. Pure and Applied Biology (PAB). 2019;8(1):460–78. [Google Scholar]
- Hernández-González JC, Martínez-Tapia A, Lazcano-Hernández G, García-Pérez BE, Castrejón-Jiménez NS. Bacteriocins from lactic acid bacteria: a powerful alternative as antimicrobials, probiotics, and immunomodulators in veterinary medicine. Animals. 2021;11(4):979. doi: 10.3390/ani11040979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel WH, Wood BJB. Lactic Acid Bacteria: Biodiversity and Taxonomy. Wiley-Blackwell. ISBN-10 : 9781444333831 (2014)
- Hu Y, Liu X, Shan C, Xia X, Wang Y, Dong M, Zhou J. Novel bacteriocin produced by Lactobacillus alimentarius FM-MM4 from a traditional Chinese fermented meat Nanx Wudl: Purification, identification and antimicrobial characteristics. Food Control. 2017;77:290–297. [Google Scholar]
- Hwang IC, Oh JK, Kim SH, Oh S, Kang DK. Isolation and characterization of an anti-listerial bacteriocin from Leuconostoc lactis SD501. Korean Journal for Food Science of Animal Resources. 2018;38(5):1008. doi: 10.5851/kosfa.2018.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftime D, Jasyk M, Kulik A, Imhoff JF, Stegmann E, Wohlleben W, Süssmuth RD, Weber T. Streptocollin, a type IV lanthipeptide produced by Streptomyces collinus Tü 365. ChemBioChem. 2015;16(18):2615–2623. doi: 10.1002/cbic.201500377. [DOI] [PubMed] [Google Scholar]
- Islam MR, Nagao JI, Zendo T, Sonomoto K. Antimicrobial mechanism of lantibiotics. Biochemical Society Transactions. 2012;40(6):1528–1533. doi: 10.1042/BST20120190. [DOI] [PubMed] [Google Scholar]
- Islam R, Hossain MN, Alam MK, Uddin ME, Rony MH, Imran MA, Alam MF. Antibacterial activity of lactic acid bacteria and extraction of bacteriocin protein. Advances in Bioscience and Biotechnology. 2020;11(2):49–59. [Google Scholar]
- Jacob F, Lwoff A, Siminovitch A, Wollman E. Definition of some terms relative to lysogeny. Annales De L'institut Pasteur. 1953;84:222–224. [PubMed] [Google Scholar]
- Jans C, Bugnard J, Njage PM, Lacroix C, Meile L. Lactic acid bacteria diversity of African raw and fermented camel milk products reveals a highly competitive, potentially health-threatening predominant microflora. LWT-Food Science and Technology. 2012;47(2):371–379. [Google Scholar]
- Johnson E, Jung YG, Jin Y, Jayabalan R, Yang SH, Suh JW. Bacteriocins as food preservatives: challenges and emerging horizons. Critical Reviews in Food Science and Nutrition. 2019;58:2743–2767. doi: 10.1080/10408398.2017.1340870. [DOI] [PubMed] [Google Scholar]
- Jones L. Dehydroamino acid chemical biology: an example of functional group interconversion on proteins. RSC Chemical Biology. 2020;1:298–304. doi: 10.1039/d0cb00174k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaškonienė V, Stankevičius M, Bimbiraitė-Survilienė K, Naujokaitytė G, Šernienė L, Mulkytė K, Malakauskas M, Maruška A. Current state of purification, isolation and analysis of bacteriocins produced by lactic acid bacteria. Applied Microbiology and Biotechnology. 2017;101:1323–1335. doi: 10.1007/s00253-017-8088-9. [DOI] [PubMed] [Google Scholar]
- Kassaa IA, Rafei R, Moukhtar M, Zaylaa M, Gharsallaoui A, Asehraou A, El Omari K, Shahin A, Hamze M, Chihib NE. LABiocin database: a new database designed specifically for Lactic Acid Bacteria bacteriocins. International Journal of Antimicrobial Agents. 2019;54(6):771–779. doi: 10.1016/j.ijantimicag.2019.07.012. [DOI] [PubMed] [Google Scholar]
- Kasuga G, Tanaka M, Harada Y, Nagashima H, Yamato T, Wakimoto A, Arakawa K, Kawai Y, Kok J, Masuda T. Homologous expression and characterization of gassericin T and gassericin S, a novel class IIb bacteriocin produced by Lactobacillus gasseri LA327. Applied and Environmental Microbiology. 2019;85(6):e02815–e2818. doi: 10.1128/AEM.02815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharnaior P, Tamang JP. Bacterial and fungal communities and their predictive functional profiles in kinema, a naturally fermented soybean food of India. Nepal and Bhutan. Food Research International. 2021;140:110055. doi: 10.1016/j.foodres.2020.110055. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kim WJ, Kang SS. Inhibitory effect of bacteriocin-producing Lactobacillus brevis DF01 and Pediococcus acidilactici K10 isolated from kimchi on enteropathogenic bacterial adhesion. Food Bioscience. 2019;30:100425. doi: 10.1016/j.fbio.2019.100425. [DOI] [Google Scholar]
- Kitagawa N, Otani T, Inai T. Nisin, a food preservative produced by Lactococcus lactis, affects the localization pattern of intermediate filament protein in HaCaT cells. Anatomical Science International. 2019;94:163–171. doi: 10.1007/s12565-018-0462-x. [DOI] [PubMed] [Google Scholar]
- Kumar P, Kizhakkedathu JN, Straus SK. Antimicrobial peptides: diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules. 2018;8(1):4. doi: 10.3390/biom8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumariya R, Garsa AK, Rajput YS, Sood SK, Akhtar N, Patel S. Bacteriocins: classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microbial Pathogenesis. 2019;128:171–177. doi: 10.1016/j.micpath.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Lafuente-Rincón DF, Chávez TEV, Norma M. Bacteriocins of Gram-positive bacteria: Features and biotherapeutic approach. African Journal of Microbiology Research. 2016;10(45):1873–1879. [Google Scholar]
- Lagedroste M, Reiners J, Knospe CV, Smits SHJ, Schmitt L. A structural view on the maturation of lanthipeptides. Frontiers in Microbiology. 2020;9(11):1183. doi: 10.3389/fmicb.2020.01183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajis AFB. Biomanufacturing process for the production of bacteriocins from Bacillaceae family. Bioresource and Bioprocessing. 2020;7:8. doi: 10.1186/s40643-020-0295-z. [DOI] [Google Scholar]
- Lee KH, Moon GS, An JY, Lee HJ, Chang H, Chung D, Lee JH, Kim J. Isolation of a nisin-producing Lactococcus lactis strain from Kimchi and characterization of its nisZ gene. Journal of Microbiology and Biotechnology. 2002;12:389–397. [Google Scholar]
- Leech J, Cabrera-Rubio R, Walsh AM, Macori G, Walsh CJ, Barton W, Finnegan L, Crispie F, O’Sullivan O, Claesson MJ, Cotter PD. Fermented-food metagenomics reveals substrate-associated differences in taxonomy and health-associated and antibiotic resistance determinants. mSystems. 2020;5(6):e00522–20. doi: 10.1128/mSystems.00522-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J, Sun L, Huang S, Zhu C, Li P, He J, Mackey V, Coy DH, He Q. The antimicrobial peptides and their potential clinical applications. American Journal of Translational Research. 2019;11(7):3919–3931. [PMC free article] [PubMed] [Google Scholar]
- Li Q, Montalban-Lopez M, Kuipers OP. Feasibility of introducing a thioether ring in vasopressin by nisBTC co-expression in Lactococcus lactis. Frontiers in Microbiology. 2019;10:1508. doi: 10.3389/fmicb.2019.01508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Song Q, Wang M, Ren J, Liu S, Zhao S. Comparative genomics analysis of Pediococcus acidilactici species. Journal of Microbiology. 2021;59:573–583. doi: 10.1007/s12275-021-0618-6. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang L, Yi H, Han X, Chi C. Identification and characterization of plantaricin Q7, a novel plantaricin produced by Lactobacillus plantarum Q7. LWT - Food Science and Technology. 2016;71:386–390. [Google Scholar]
- Liu R, Kim AH, Kwak MK, Kang SO. Proline-based cyclic dipeptides from Korean fermented vegetable kimchi and from Leuconostoc mesenteroides LBP-K06 have activities against multidrug-resistant bacteria. Frontiers in Microbiology. 2017;8:761. doi: 10.3389/fmicb.2017.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Wang Y, Li X, Hao X, Xu D, Zhou Y, Mehmood A, Wang C. Genetic and biochemical evidence that Enterococcus faecalis Gr17 produces a novel and sec-dependent bacteriocin, Enterocin Gr17. Frontiers in Microbiology. 2019;10:1806. doi: 10.3389/fmicb.2019.01806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü X, Yi L, Dang J, Dang Y, Liu B. Purification of novel bacteriocin produced by Lactobacillus coryniformis MXJ 32 for inhibiting bacterial foodborne pathogens including antibiotic-resistant microorganisms. Food Control. 2014;46:264–271. [Google Scholar]
- Lv X, Lin Y, Jie Y, Sun M, Zhang B, Bai F, Zhao H, Li J. Purification, characterization, and action mechanism of plantaricin DL3, a novel bacteriocin against Pseudomonas aeruginosa produced by Lactobacillus plantarum DL3 from Chinese Suan-Tsai. European Food Research and Technology. 2018;244(2):323–331. [Google Scholar]
- Ma J, Yu W, Hou J, Han X, Shao H, Liu Y. Characterization and production optimization of a broad-spectrum bacteriocin produced by Lactobacillus casei KLDS 1.0338 and its application in soybean milk biopreservation. International Journal of Food Properties. 2020;23(1):677–92. [Google Scholar]
- Malanovic N, Lohner K. Antimicrobial peptides targeting Gram-positive bacteria. Pharmaceuticals (basel). 2016;9(3):59. doi: 10.3390/ph9030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleke MS, Adefisoye MA, Doorsamy W, Adebo OA. Processing, nutritional composition and microbiology of amasi: A Southern African fermented milk product. Scientific African. 2021;12:e00795. doi: 10.1016/j.sciaf.2021.e00795. [DOI] [Google Scholar]
- Man LL, Xiang DJ. Characterization of a broad spectrum bacteriocin produced by Lactobacillus plantarum MXG-68 from Inner Mongolia traditional fermented koumiss. Folia Microbiologica. 2019;64(6):821–834. doi: 10.1007/s12223-019-00697-0. [DOI] [PubMed] [Google Scholar]
- Marco ML, Sanders ME, Gänzle M, Arrieta MC, Cotter PD, Vuyst DL, Hill C, Holzapfel W, Lebeer S, Merenstein D, Reid G. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nature Reviews Gastroenterology & Hepatology. 2021;18:196–208. doi: 10.1038/s41575-020-00390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur H, Fallico V, O’Connor PM, Rea MC, Cotter PD, Hill C, Ross RP. Insights into the mode of action of the sactibiotic thuricin CD. Frontiers in Microbiology. 2017;8:696. doi: 10.3389/fmicb.2017.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur H, Beresford TP, Cotter PD. Health benefits of lactic acid bacteria (LAB) fermentates. Nutrients. 2020;12:1679. doi: 10.3390/nu12061679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade E, Slattery MA, Garvey M. Bacteriocins, potent antimicrobial peptides and the fight against multi drug resistant species: resistance is futile? Antibiotics (basel). 2020;9(1):32. doi: 10.3390/antibiotics9010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melini F, Melini V, Luziatelli F, Ficca AG, Ruzzi M. Health-promoting components in fermented foods: an up-to-date systematic review. Nutrients. 2019;11(5):1189. doi: 10.3390/nu11051189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes AGT, Ramos CL, Cenzi G, Melo DS, Dias DR, Schwan RF. Probiotic potential, antioxidant activity, and phytase production of indigenous yeasts isolated from indigenous fermented foods. Probiotics and Antimicrobial Proteins. 2020;12:280–288. doi: 10.1007/s12602-019-9518-z. [DOI] [PubMed] [Google Scholar]
- Meng F, Zhu X, Zhao H, Nie T, Lu F, Lu Z, Lu Y. A class III bacteriocin with broad-spectrum antibacterial activity from Lactobacillus acidophilus NX2-6 and its preservation in milk and cheese. Food Control. 2021;121:107597. doi: 10.1016/j.foodcont.2020.107597. [DOI] [Google Scholar]
- Moh LG, Etienne PT, Jules-Roger K. Seasonal diversity of lactic acid bacteria in artisanal yoghurt and their antibiotic susceptibility pattern. International Journal of Food Science. 2021 doi: 10.1155/2021/6674644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokoena MP. Lactic acid bacteria and their bacteriocins: classification, biosynthesis and applications against uropathogens: a mini-review. Molecules. 2017;22(8):1255. doi: 10.3390/molecules22081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalbán-López M, Deng J, van Heel AJ, Kuipers OP. Specificity and application of the lantibiotic protease NisP. Frontiers in Microbiology. 2018;9:160. doi: 10.3389/fmicb.2018.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari M, Smith DL. A PGPR-produced bacteriocin for sustainable agriculture: a review of thuricin 17 characteristics and applications. Frontiers in Plant Science. 2020;20:11. doi: 10.3389/fpls.2020.00916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negash AW, Tsehai BA. Current applications of bacteriocin. International Journal of Microbiology. 2020 doi: 10.1155/2020/4374891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng ZJ, Zarin MA, Lee CK, Tan JS. Application of bacteriocins in food preservation and infectious disease treatment for humans and livestock: a review. RSC Advances. 2020;10:38937–38964. doi: 10.1039/d0ra06161a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niamah AK. Structure, mode of action and application of pediocin natural antimicrobial food preservative: a review. Basrah Journal of Agricultural Sciences. 2018;31(1):59–69. [Google Scholar]
- Niamah AK. Structure, mode of action and application of pediocin natural antimicrobial food preservative: a review. Basrah Journal of Agricultural Sciences. 2020;31(1):59–69. [Google Scholar]
- Niederhäusern Sd, Camellini S, Sabia C, Iseppi R, Bondi M, Messi P. Antilisterial activity of bacteriocins produced by lactic bacteria isolated from dairy products. Foods. 2020;9(12):1757. doi: 10.3390/foods9121757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Miyauchi R, Danshiitsoodol N, Matoba Y, Kumagai T, Sugiyama M. Expression of genes involved in bacteriocin production and self-resistance in Lactobacillus brevis 174A is mediated by two regulatory proteins. Applied and Environmental Microbiology. 2018;84(7):e02707–e2717. doi: 10.1128/AEM.02707-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor PM, O’Shea EF, Cotter PD, Hill C, Ross RP. The potency of the broad spectrum bacteriocin, bactofencin A, against Staphylococci is highly dependent on primary structure, N-terminal charge and disulphide formation. Scientific Reports. 2018;8(1):1–8. doi: 10.1038/s41598-018-30271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei J, Jin W, Abd El-Aty AM, Baranenko DA, Gou X, Zhang H, Geng J, Jiang L, Chen D, Yue T. Isolation, purification, and structural identification of a new bacteriocin made by Lactobacillus plantarum found in conventional kombucha. Food Control. 2020;110:106923. doi: 10.1016/j.foodcont.2019.106923. [DOI] [Google Scholar]
- Perez RH, Zendo T. Sonomoto K (2018) Circular and leaderless bacteriocins: biosynthesis, mode of action, applications, and prospects. Frontiers in Microbiology. 2018;9:2085. doi: 10.3389/fmicb.2018.02085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham JV, Yilma MA, Feliz A, Majid MT, Maffetone N, Walker JR, Kim E, Cho HJ, Reynolds JM, Song MC, Park SR, Yoon YJ. A review of the microbial production of bioactive natural products and biologics. Frontiers in Microbiology. 2019;10:1404. doi: 10.3389/fmicb.2019.01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokhrel R, Bhattarai N, Baral P, Gerstman BS, Park JH, Handfield M, Chapagain PP. Molecular mechanisms of pore formation and membrane disruption by the antimicrobial lantibiotic peptide Mutacin 1140. Physical Chemistry Chemical Physics. 2019;21(23):12530–9. doi: 10.1039/c9cp01558b. [DOI] [PubMed] [Google Scholar]
- Pradhan P, Tamang JP. Phenotypic and genotypic identification of bacteria isolated from traditionally prepared dry starters of the Eastern Himalayas. Frontiers in Microbiology. 2019;10:2526. doi: 10.3389/fmicb.2019.02526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raheem N, Straus SK. Mechanisms of action for antimicrobial peptides with antibacterial and antibiofilm functions. Frontiers in Microbiology. 2019;10:2866. doi: 10.3389/fmicb.2019.02866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed HA, Tuoheti T, Zhang Y, Azi F, Tekliye M, Dong M. Purification and partial characterization of a novel bacteriocin produced by bacteriocinogenic Lactobacillus fermentum BZ532 isolated from Chinese fermented cereal beverage (Bozai) LWT-Food Science and Technology. 2020;124:109113. doi: 10.1016/j.lwt.2020.109113. [DOI] [Google Scholar]
- Refay RM, Abushady HM, Amer SA, Mailam MA. Determination of bacteriocin-encoding genes of lactic acid bacteria isolated from traditional dairy products of Luxor province. Egypt. Future Journal of Pharmaceutical Sciences. 2020;6:22. doi: 10.1186/s43094-020-00031-3. [DOI] [Google Scholar]
- Repka LM, Chekan JR, Nair SK, van der Donk WA. Mechanistic understanding of lanthipeptide biosynthetic enzymes. Chemical Reviews. 2017;117(8):5457–5520. doi: 10.1021/acs.chemrev.6b00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezac S, Kok CR, Heermann M, Hutkins R. Fermented foods as a dietary source of live organisms. Frontiers in Microbiology. 2018;9:1785. doi: 10.3389/fmicb.2018.01785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şahingil D, İşleroğlu H, Yildirim Z, Akcelik M, Yildirim M. Characterization of lactococcin BZ produced by Lactococcus lactis subsp. lactis BZ isolated from boza. Turkish Journal of Biology. 2011;35(1):21–33. [Google Scholar]
- Samal SK. Leader Sequence. Brenner's Encyclopedia of Genetics. 2013 doi: 10.1016/B978-0-12-374984-0.00850-0. [DOI] [Google Scholar]
- Sandiford SK. An overview of lantibiotic biosynthetic machinery promiscuity and its impact on antimicrobial discovery. Expert Opinion on Drug Discovery. 2020;15(3):373–382. doi: 10.1080/17460441.2020.1699530. [DOI] [PubMed] [Google Scholar]
- Saraiva MAF, Jirata Birri D, Anders Brede D, Baracat-Pereira MC, de Queiroz MV, Nes IF, de Moraes CA. Nisin Z production by wild strains of Lactococcus lactis isolated from Brazilian (Italian Type) fermented sausage. International Journal of Microbiology. 2020;2020:9309628. doi: 10.1155/2020/9309628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa N, Koga S, Okamura K, Ishibashi N, Zendo T, Sonomoto K. Identification and characterization of novel multiple bacteriocins produced by L actobacillus sakei D98. Journal of Applied Microbiology. 2013;115(1):61–69. doi: 10.1111/jam.12226. [DOI] [PubMed] [Google Scholar]
- Schulz-Bohm K, Martín-Sánchez L, Garbeva P. Microbial volatiles: small molecules with an important role in intra- and inter-kingdom interactions. Frontiers in Microbiology. 2017;8:2484. doi: 10.3389/fmicb.2017.02484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi R, Sharifzad F, Bagheri R, Alsadi N, Yasavoli-Sharahi H, Matar C. Anti-inflammatory and immunomodulatory properties of fermented plant foods. Nutrients. 2021;13(5):1516. doi: 10.3390/nu13051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shangpliang HNK, Tamang JP. Phenotypic and genotypic characterizations of lactic acid bacteria isolated from exotic naturally fermented milk (cow and yak) products of Arunachal Pradesh. India. International Dairy Journal. 2021;118:105038. doi: 10.1016/j.idairyj.2021.105038. [DOI] [Google Scholar]
- Shi F, Wang Y, Li Y, Wang X. Mode of action of leucocin K7 produced by Leuconostoc mesenteroides K7 against Listeria monocytogenes and its potential in milk preservation. Biotechnology Letters. 2016;38(9):1551–1557. doi: 10.1007/s10529-016-2127-y. [DOI] [PubMed] [Google Scholar]
- Silva CCG, Silva SPM, Ribeiro SC. Application of bacteriocins and protective cultures in dairy food preservation. Frontiers in Microbiology. 2018;9(9):594. doi: 10.3389/fmicb.2018.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons A, Alhanout K, Duval RE. Bacteriocins, antimicrobial peptides from bacterial origin: overview of their biology and their impact against multidrug-resistant bacteria. Microorganisms. 2020;8(5):639. doi: 10.3390/microorganisms8050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaugen M, Cintas LM, Nes IF. Genetics of bacteriocin production in lactic acid bacteria. In Genetics of lactic acid bacteria, Springer, Boston, MA, 225-260 (2003)
- Sogandi S, Mustopa AZ, Artika IM. The characterization of bacteriocins produced by Lactobacillus plantarum strains isolated from traditional fermented foods in Indonesia and the detection of its plantaricin-encoding genes. Indonesian Journal of Biotechnology. 2019;24(1):1–7. [Google Scholar]
- Soltani S, Hammami R, Cotter PD, Rebuffat S, Said LB, Gaudreau H, Bédard F, Biron E, Drider D, Fliss I. Bacteriocins as a new generation of antimicrobials: toxicity aspects and regulations. FEMS Microbiology Reviews. 2021 doi: 10.1093/femsre/fuaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DF, Zhu MY, Gu Q. Purification and characterization of plantaricin ZJ5, a new bacteriocin produced by Lactobacillus plantarum ZJ5. PLoS One. 2014;9(8):e105549. doi: 10.1371/journal.pone.0105549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieß T, Korn SM, Kötter P, Entian KD. Autoinduction specificities of the lantibiotics subtilin and nisin. Applied and Environmental Microbiology. 2015;81(22):7914–23. doi: 10.1371/journal.pone.0105549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoyancheva G. Study of helveticin gene in Lactobacillus crispatus strains and evaluation of its use as a phylogenetic marker. Archives of Microbiology. 2020;202(1):205–208. doi: 10.1007/s00203-019-01711-2. [DOI] [PubMed] [Google Scholar]
- Straume D, Kjos M, Nes IF, Diep DB. Quorum-sensing based bacteriocin production is down-regulated by N-terminally truncated species of gene activators. Molecular Genetics and Genomics. 2007;278(3):283–293. doi: 10.1007/s00438-007-0251-z. [DOI] [PubMed] [Google Scholar]
- Sun Z, Wang X, Zhang X, Wu H, Zou Y, Li P, Sun C, Xu W, Liu F, Wang D. Class III bacteriocin Helveticin-M causes sublethal damage on target cells through impairment of cell wall and membrane. Industrial Microbiology and Biotechnology. 2018;45(3):213–227. doi: 10.1007/s10295-018-2008-6. [DOI] [PubMed] [Google Scholar]
- Tamang JP, Tamang B, Schillinger U, Franz CMAP, Gores M, Holzapfel WH. Identification of predominant lactic acid bacteria isolated from traditionally fermented vegetable products of the Eastern Himalayas. International Journal of Food Microbiology. 2005;105:347–356. doi: 10.1016/j.ijfoodmicro.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Tamang B, Tamang JP, Schillinger U, Franz CMAP, Gores M, Holzapfel WH. Phenotypic and genotypic identification of lactic acid bacteria isolated from ethnic fermented tender bamboo shoots of North East India. International Journal of Food Microbiology. 2008;121:35–40. doi: 10.1016/j.ijfoodmicro.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Tamang JP, Tamang B, Schillinger U, Guigas C, Holzapfel WH. Functional properties of lactic acid bacteria isolated from ethnic fermented vegetables of the Himalayas. International Journal of Food Microbiology. 2009;135(1):28–33. doi: 10.1016/j.ijfoodmicro.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Tamang JP, Holzapfel WH, Watanabe K. Diversity of microorganisms in global fermented foods and beverages. Frontiers in Microbiology. 2016;7:377. doi: 10.3389/fmicb.2016.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamang JP, Shin DH, Jung SJ, Chae SW. Functional properties of microorganisms in fermented foods. Frontiers in Microbiology. 2016;7:578. doi: 10.3389/fmicb.2016.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamang JP, Watanabe K, Holzapfel WH. Diversity of microorganisms in global fermented foods and beverages. Frontiers in Microbiology. 2016;7:377. doi: 10.3389/fmicb.2016.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamang JP, Cotter P, Endo A, Han NS, Kort R, Liu SQ, Mayo B, Westerik N, Hutkins R. Fermented foods in a global age: east meets west. Comprehensive Reviews in Food Science and Food Safety. 2020;19:184–217. doi: 10.1111/1541-4337.12520. [DOI] [PubMed] [Google Scholar]
- Tamang JP, Jeyaram K, Rai AK, Mukherjee PK. Diversity of beneficial microorganisms and their functionalities in community-specific ethnic fermented foods of the Eastern Himalayas. Food Research International. 2021;148:110633. doi: 10.1016/j.foodres.2021.110633. [DOI] [PubMed] [Google Scholar]
- Tankoano A, Diop MB, Sawadogo-Lingani H, Mbengue M, Kaboré D, Traoré Y, Savadogo A. Isolation and characterization of lactic acid bacteria producing bacteriocin like inhibitory substance (BLIS) from “Gappal”, a dairy product from Burkina Faso. Advances in Applied Microbiology. 2019;9(4):343–358. [Google Scholar]
- Teneva-Angelova T, Hristova I, Pavlov A, Beshkova, D. Chapter 4 - Lactic Acid Bacteria—from nature through food to health. Editor(s): Holban AM, Grumezescu AM, In Handbook of Food Bioengineering, Advances in Biotechnology for Food Industry, Academic Press, pp. 91-133 (2018)
- Tosukhowong A, Zendo T, Visessanguan W, Roytrakul S, Pumpuang L, Jaresitthikunchai J, Sonomoto K, Garvieacin Q. a novel class II bacteriocin from Lactococcus garvieae BCC 43578. Applied and Environmental Microbiology. 2012;78(5):1619–1623. doi: 10.1128/AEM.06891-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Federal Register (U.S. Food and Drug Administration): Nisin preparation: affirmation of GRAS status as a direct human food ingredient. 21 CFR Part 184, US Federal Register. 53: 11247-11251 (1988)
- Ullah N, Wang X, Wu J, Guo Y, Ge H, Li T, Khan S, Li Z, Feng X. Purification and primary characterization of a novel bacteriocin, LiN333, from Lactobacillus casei, an isolate from a Chinese fermented food. LWT-Food Science and Technology. 2017;84:867–875. [Google Scholar]
- Vezina B, Rehm BHA, Smith AT. Bioinformatic prospecting and phylogenetic analysis reveals 94 undescribed circular bacteriocins and key motifs. BMC Microbiology. 2020;20:77. doi: 10.1186/s12866-020-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voidarou C, Antoniadou Μ, Rozos G, Tzora A, Skoufos I, Varzakas T, Lagiou A, Bezirtzoglou E. Fermentative foods: microbiology, biochemistry, potential human health benefits and public health issues. Foods (basel, Switzerland). 2021;10(1):69. doi: 10.3390/foods10010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shang N, Qin Y, Zhang Y, Zhang J, Li P. The complete genome sequence of Lactobacillus plantarum LPL-1, a novel antibacterial probiotic producing class IIa bacteriocin. Journal of Biotechnology. 2018;266:84–88. doi: 10.1016/j.jbiotec.2017.12.006. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang S, Ouyang Y, Li R. Current developments of bacteriocins, screening methods and their application in aquaculture and aquatic products. Biocatalysis and Agricultural Biotechnology. 2019;22:101395. doi: 10.1016/j.bcab.2019.101395. [DOI] [Google Scholar]
- Wirawan RE, Swanson KM, Kleffmann T, Jack RW, Tagg JR. Uberolysin: a novel cyclic bacteriocin produced by Streptococcus uberis. Microbiology. 2007;153(5):1619–1630. doi: 10.1099/mic.0.2006/005967-0. [DOI] [PubMed] [Google Scholar]
- Woraprayote W, Pumpuang L, Tosukhowong A, Roytrakul S, Perez RH, Zendo T, Sonomoto K, Benjakul S, Visessanguan W. Two putatively novel bacteriocins active against Gram-negative food borne pathogens produced by Weissella hellenica BCC 7293. Food Control. 2015;55:176–184. [Google Scholar]
- Xin B, Zheng J, Liu H, Li J, Ruan L, Peng D, Sajid M, Sun M. Thusin, a novel two-component lantibiotic with potent antimicrobial activity against several Gram-positive pathogens. Frontiers in Microbiology. 2016;7:1115. doi: 10.3389/fmicb.2016.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Yang L, Li P, Gu Q. Heterologous expression of Class IIb bacteriocin plantaricin JK in Lactococcus lactis. Protein Expression and Purification. 2019;159:10–16. doi: 10.1016/j.pep.2019.02.013. [DOI] [PubMed] [Google Scholar]
- Xu C, Fu Y, Liu F, Liu Z, Ma J, Jiang R, Song C, Jiang JT, Hou J. Purification and antimicrobial mechanism of a novel bacteriocin produced by Lactobacillus rhamnosus 1.0320. LWT-Food Science and Technology. 2020;137:110338. doi: 10.1016/j.lwt.2020.110338. [DOI] [Google Scholar]
- Xu M, Zhang F, Cheng Z, Bashiri G, Wang J, Hong J, Wang Y, Xu L, Chen X, Huang SX, Lin S. Functional genome mining reveals a class v lanthipeptide containing ad-amino acid introduced by an F420H2-dependent reductase. Angewandte Chemie. 2020;59(41):18029–18035. doi: 10.1002/anie.202008035. [DOI] [PubMed] [Google Scholar]
- Yi L, Qi T, Hong Y, Deng L, Zeng K. Screening of bacteriocin-producing lactic acid bacteria in Chinese homemade pickle and dry-cured meat, and bacteriocin identification by genome sequencing. LWT-Food Science and Technology. 2020;125:109177. doi: 10.1016/j.lwt.2020.109177. [DOI] [Google Scholar]
- Zhang J, Yang Y, Yang H, Bu Y, Yi H, Zhang L, Han X, Ai L. Purification and partial characterization of bacteriocin Lac-B23, a novel bacteriocin production by Lactobacillus plantarum J23, isolated from Chinese traditional fermented milk. Frontiers in Microbiology. 2018;9:2165. doi: 10.3389/fmicb.2018.02165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Gänzle M, Yang X. Complementary antibacterial effects of bacteriocins and organic acids as revealed by comparative analysis of Carnobacterium spp. from meat. Applied and Environmental Microbiology. 2019;85(20):e01227–19. doi: 10.1128/AEM.01227-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Qin Y, Wang Y, Huang Y, Li P, Li P. Lactobacillus plantarum LPL-1, a bacteriocin producing strain, changed the bacterial community composition and improved the safety of low-salt fermented sausages. LWT-Food Science and Technology. 2020;128:109385. doi: 10.1016/j.lwt.2020.109385. [DOI] [Google Scholar]
- Zhao S, Han J, Bie X, Lu Z, Zhang C, Lv F. Purification and characterization of plantaricin JLA-9: a novel bacteriocin against Bacillus spp. produced by Lactobacillus plantarum JLA-9 from Suan-Tsai, a traditional Chinese fermented cabbage. Journal of Agricultural and Food Chemistry. 2016;64(13):2754–2764. doi: 10.1021/acs.jafc.5b05717. [DOI] [PubMed] [Google Scholar]
- Zhao X, Yu Z, Ding T. Quorum-sensing regulation of antimicrobial resistance in bacteria. Microorganisms. 2020;8(3):425. doi: 10.3390/microorganisms8030425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Wittouck S, Salvetti E, Franz CMAP, Harris HMB, Mattarelli P, et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. International Journal of Systematic and Evolutionary Microbiology. 2020;70(4):2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Wu L, Chen X, Huang Z, Hu W. Effects of food-additive-information on consumers' willingness to accept food with additives. International Journal of Environmental Research and Public Health. 2018;15(11):2394. doi: 10.3390/ijerph15112394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimina M, Babich O, Prosekov A, Sukhikh S, Ivanova S, Shevchenko M, Noskova S. Overview of global trends in classification, methods of preparation and application of bacteriocins. Antibiotics. 2020;9(9):553. doi: 10.3390/antibiotics9090553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zommiti M, Bouffartigues E, Maillot O, Barreau M, Szunerits S, Sebei K, Feuilloley M, Connil N, Ferchichi M. In vitro assessment of the probiotic properties and bacteriocinogenic potential of Pediococcus pentosaceus MZF16 isolated from artisanal Tunisian meat “Dried Ossban”. Frontiers in Microbiology. 2018;9:2607. doi: 10.3389/fmicb.2018.02607. [DOI] [PMC free article] [PubMed] [Google Scholar]