Abstract
Aim
Diabetes and aging are both well-established risk factors for insomnia. Therefore, we investigated the changes in subjective sleep quality in relation to clinical backgrounds and age in patients with type 2 diabetes mellitus (T2DM).
Methods
This cross-sectional study included 380 participants with T2DM who were between 18 and 79 years of age from our outpatient clinics. Individuals with any symptoms and medical histories associated with obstructive sleep apnea (OSA) were excluded from the interview and analyses. Data were collected using self-administered questionnaires, namely the Pittsburgh Sleep Quality Index (PSQI) and the Morning-Evening Questionnaire (MEQ), as well as medical records and blood samples. We performed stratified analyses according to age decades.
Results
The number of patients in the age groups (in years) was as follows: < 50 (n = 69), 50–60 (n = 52), 60–70 (n = 138), and 70–80 (n = 121). PSQI score was highest in the < 50 group (4.99 ± 2.40), and significantly decreased with age (p < 0.05). Body mass index (BMI) was also highest in the < 50 group (25.5 ± 4.8 kg/m2), and markedly decreased with age (p < 0.01). Interestingly, BMI was significantly correlated with the PSQI score (rs = 0.157, p < 0.05). We also found that younger patients had shorter sleep duration, stronger daytime sleepiness, and a tendency for the evening type.
Conclusion
Younger T2DM patients had poorer sleep quality and higher BMI. Our findings suggest that insomnia should be accounted for as a potential comorbidity when examining or treating patients with T2DM and obesity even in the younger population.
Keywords: Type 2 diabetes, Aging, Sleep quality, Insomnia, Obstructive sleep apnea
Introduction
The prevalence of insomnia has been increasing on a global scale in modern society [1]. In Japan, it has been reported that one in five adults exhibit symptoms associated with such disorders [2]; they are also well known to cause loss of work productivity, impaired cognitive function, mental deficits, and reduced quality of life [3].
Recent epidemiologic [4] and laboratory [4, 5] studies have revealed that chronic insomnia is an additional factor contributing to abnormal glucose tolerance development [6]. Consequently, adding to social and psychological problems, insomnia has piqued interest as a potential factor underpinning the onset of type 2 diabetes mellitus (T2DM).
At the same time, patients with diabetes are known to have poor sleep quality due to the presence of comorbidities and changes in the balance of hormones and in the activity of sympathetic tones [6–9], and the prevalence rate of insomnia is 2–4 times higher than that in patients without diabetes [10, 11]. However, accumulating evidence strongly suggests an association between diabetes and insomnia. In general, aging has been identified as the most significant factor affecting sleep, followed by physiological changes in sleep architecture, unemployment, lack of habitual exercise, poor perceived health, and psychological stress [12].
However, to date, only a limited number of reports have focused on the relationship between age and sleep quality in patients with T2DM. Therefore, in the present study, we aimed to investigate the changes in subjective sleep quality according to age and clinical background in patients with T2DM using validated questionnaires.
Materials and methods
Study design and population
We conducted a cross-sectional study using self-administered questionnaires between November 2015 and December 2016, and between August and October 2019 in our outpatient clinics in Japan. The study was registered with the University Hospital Medical Information Network Clinical Trial Registry (UMIN000019329), a non-profit organization in Japan that meets the requirements of the International Committee of Medical Journal Editors. The study was approved by the Medical Ethics Committee of Toho University Omori Medical Center, Japan (Approval no. #M19093; Approval date: August 29, 2019). This study was conducted according to the Declaration of Helsinki and current legal regulations in Japan. Prior to enrollment in the survey, all eligible participants were informed about this study in written form, and the answer to the questionnaire was considered consent to enrollment.
Participants included in the study were individuals with T2DM who were between 18 and 79 years of age. Exclusion criteria were as follows: known obstructive sleep apnea (OSA); night shift work; use of any sleep or psychiatric agents in the past 12 months; history of a coexisting disorder likely to disturb the quality of sleep; history of type 1 diabetes or secondary forms of diabetes; ketoacidosis; coma; myocardial infarction, unstable angina, or stroke in the past 6 months; severe infection; perioperative period; severe trauma; and considered unsuitable to participate in the study for medical reasons. We screened 2450 patients with T2DM in our outpatient clinics and selected individuals who met the inclusion criteria based on their medical records. Eligible patients (n = 480) were distributed two types of validated questionnaires from their physicians: the Pittsburgh Sleep Quality Index (PSQI) and the Morning-Evening Questionnaire (MEQ). The PSQI is a previously validated tool containing seven components: (1) sleep quality, (2) sleep latency, (3) sleep duration, (4) sleep efficiency, (5) sleep disturbance, (6) use of sleep medication, and (7) daytime dysfunction [13, 14]. Each component was scored on a range of 0–3, with a possible total score range of 0–21. A higher score indicated poorer sleep quality, and a score of > 5 was considered the threshold for poor sleep quality [13, 14]. MEQ is used for the assessment of circadian typology. It is composed of five items with a total score range of 4–25. Individuals are assigned to one of the three possible circadian typologies: 4–11 for the evening type, 12–17 for the neither type, and 18–25 for the morning type [15]. The questionnaires were distributed by the physicians at each outpatient visit, and patients answered either at the clinic or at home (n = 472). Biological data were collected and assayed at local hospital laboratories, and anthropometric data were measured by nurses at the time of distributing the questionnaire. Other clinical information such as treatment, medical history, and social background were derived from medical records.
Statistical analysis
In the stratified analysis, eligible participants (n = 380) were categorized by age into four groups according to patients who were (1) less than 50 years (< 50), (2) between 50 and 60 years, (3) between 60 and 70 years, and (4) between 70 and 80 years. Analyses were performed using Statcel (OMS, Saitama, Japan) and JMP® 15 (SAS Institute Inc., Cary, NC, USA). Differences in continuous variables except for sleep parameters were evaluated using one-way ANOVA, and differences in categorical variables were assessed using the χ2 test. Subjective sleep parameters were assessed using ANCOVA. The model was adjusted for the following covariates that could have potentially impacted the results: sex, duration of diabetes, and glycated hemoglobin (Hb)A1c. Associations between values were assessed using the Spearman’s rank correlation. Data are shown as mean ± standard deviation. Statistical significance was defined as a P value < 0.05.
Results
Demographics
As shown in Fig. 1, 2450 patients with T2DM were screened in our outpatient clinics, and 480 were selected for the study; among them 472 returned the filled questionnaire. Participants who answered insufficiently (n = 66) or met the exclusion criteria (n = 26) were excluded from the analysis. Consequently, the final number of eligible participants was 380; they were divided into four age groups by decade: < 50 years (n = 69), 50–60 years (n = 52), 60–70 years (n = 138), and 70–80 years (n = 121). Table 1 summarizes the general characteristics of all participants and that after stratification by age decade.
Fig. 1.
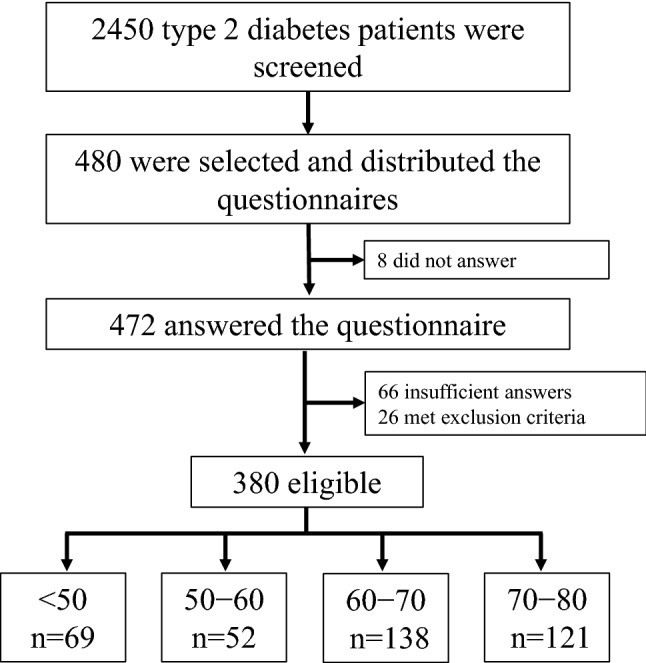
Flow diagram of patient population and final age groups (in years)
Table 1.
Demographic characteristics of all participants stratified by age
| All (n = 380) | Age group (in years) | |||||
|---|---|---|---|---|---|---|
| < 50 (n = 69) | 50–60 (n = 52) | 60–70 (n = 138) | 70–80 (n = 121) | P | ||
| Female, n (%) | 127 (33.3) | 12 (17.4) | 18 (34.6) | 50 (36.2) | 47 (38.8) | 0.018a |
| Age, years | 62.4 ± 11.6 | 42.9 ± 6.5 | 54.9 ± 2.8 | 64.9 ± 2.8 | 73.7 ± 2.7 | < 0.01b |
| Duration of diabetes, years | 12.6 ± 11.0 | 7.6 ± 8.6 | 9.2 ± 8.0 | 12.5 ± 9.5 | 17.0 ± 13.2 | < 0.01b |
| BMI, kg/m2 | 25.5 ± 4.8 | 28.4 ± 5.9 | 26.3 ± 3.4 | 25.1 ± 4.2 | 23.9 ± 4.4 | < 0.01b |
| HbA1c, % | 7.19 ± 0.97 | 7.41 ± 1.44 | 7.26 ± 0.92 | 7.10 ± 0.86 | 7.11 ± 0.69 | NSb |
| Plasma glucose, mg/dL | 148.3 ± 43.2 | 148.2 ± 39.9 | 151.4 ± 50.8 | 143.6 ± 41.4 | 152.2 ± 43.4 | NSb |
| Retinopathy | ||||||
| Non-diabetic retinopathy, n (%) | 244 (64.2) | 53 (76.8) | 29 (55.8) | 85 (61.6) | 77 (63.6) | |
| Simple diabetic retinopathy, n (%) | 56 (14.7) | 4 (5.8) | 9 (17.3) | 29 (21.0) | 14 (11.6) | |
| Pre-proliferative diabetic retinopathy, n (%) | 18 (4.7) | 1 (1.4) | 6 (11.5) | 5 (3.6) | 6 (5.0) | |
| Proliferative diabetic retinopathy, n (%) | 23 (6.1) | 6 (8.7) | 5 (9.6) | 6 (4.3) | 6 (5.0) | |
| Nephropathy | ||||||
| Stage 1, n (%) | 224 (58.9) | 46 (66.7) | 32 (61.5) | 80 (58.0) | 66 (54.5) | |
| Stage 2, n (%) | 89 (23.4) | 17 (24.6) | 8 (15.4) | 32 (23.2) | 32 (26.4) | |
| Stage 3, n (%) | 38 (10.0) | 4 (5.8) | 6 (11.5) | 11 (8.0) | 17 (14.0) | |
| Stage 4, n (%) | 11 (2.9) | 1 (1.4) | 1 (1.9) | 6 (4.3) | 3 (2.5) | |
| Stage 5, n (%) | 14 (3.7) | 1 (1.4) | 4 (7.7) | 8 (5.8) | 1 (0.8) | |
Data are presented as mean ± SD. P values for differences among age groups were calculated by aχ2 test for categorical variables or bone-way ANOVA for continuous variables
BMI body mass index; HbA1c glycated hemoglobin A1c; NS not significant
Questionnaire results
Table 2 and Fig. 2 show the results of the PSQI and MEQ. The PSQI score was highest in patients < 50 years (4.99 ± 2.40), and significantly decreased with age to 4.15 ± 2.34 in the group of 70–80 years (P < 0.05). The number of patients with poor sleep quality (PSQI score > 5) was similar among groups.
Table 2.
Questionnaire results of all participants and age groups
| All (n = 380) | Age group (in years) | |||||
|---|---|---|---|---|---|---|
| < 50 (n = 69) | 50–60 (n = 52) | 60–70 (n = 138) | 70–80 (n = 121) | P | ||
| PSQI score | 4.36 ± 2.31 | 4.99 ± 2.40 | 4.71 ± 2.08 | 4.12 ± 2.27 | 4.15 ± 2.34 | 0.0453 |
| Male | 4.36 ± 2.24 | 5.02 ± 2.41 | 4.59 ± 2.02 | 4.15 ± 2.23 | 4.00 ± 2.24 | |
| Female | 4.00 ± 2.24 | 4.83 ± 2.44 | 4.94 ± 2.24 | 4.08 ± 2.37 | 4.38 ± 2.63 | |
| PSQI > 5, n (%) | 98 (25.8) | 23 (33.3) | 17 (32.7) | 29 (21.0) | 29 (24.0) | NS |
| Sleep quality | 1.00 ± 0.60 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.28 ± 0.58 | 1.00 ± 0.00 | NS |
| Sleep latency | 0.78 ± 0.86 | 0.88 ± 0.88 | 0.79 ± 0.87 | 0.69 ± 0.85 | 0.81 ± 0.85 | NS |
| Sleep duration | 0.94 ± 0.87 | 1.12 ± 0.95 | 1.17 ± 0.83 | 0.91 ± 0.84 | 0.71 ± 0.80 | < 0.01 |
| Sleep efficiency | 0.38 ± 0.77 | 0.45 ± 0.85 | 0.19 ± 0.52 | 0.33 ± 0.71 | 0.48 ± 0.87 | NS |
| Sleep disturbance | 0.77 ± 0.53 | 0.77 ± 0.53 | 0.94 ± 0.50 | 0.77 ± 0.49 | 0.70 ± 0.57 | NS |
| Use of sleep medication | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | NS |
| Daytime dysfunction | 0.47 ± 0.64 | 0.65 ± 0.76 | 0.58 ± 0.64 | 0.41 ± 0.59 | 0.41 ± 0.59 | < 0.002 |
| MEQ | 58.1 ± 7.56 | 54.1 ± 7.74 | 58.3 ± 6.41 | 58.7 ± 7.41 | 59.8 ± 7.36 | < 0.001 |
Data are presented as mean ± SD. Data were adjusted for sex, duration of diabetes, and glycated hemoglobin A1c. P values were different among the age–decade groups. PSQI > 5 was considered the threshold for poor sleep quality
PSQI Pittsburgh Sleep Quality Index; MEQ Morning-Evening Questionnaire; NS not significant
Fig. 2.
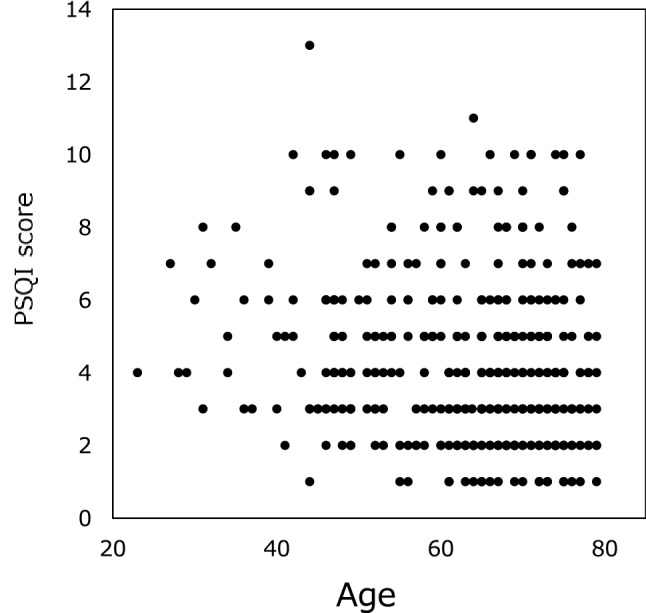
PSQI scores relative to age. Average PSQI score decreased with age from 4.99 ± 2.40 in patients < 50 years of age to 4.15 ± 2.34 in those aged 70 − 80 years. PSQI Pittsburgh Sleep Quality Index
We next investigated the detailed sleep parameters by analyzing the seven components of the PSQI and circadian typology (Table 2). Our findings show that patients < 50 years had the most severe perception of short sleep duration and daytime dysfunction, which was comparable to PSQI scores and improved with age. Patients within this group could be categorized as the evening type; however, we found no substantial difference in circadian typology, which was evaluated by MEQ, between the groups (data not shown). All other parameters were not significantly different between groups.
Association between clinical characteristics and sleep parameters by age
BMI was the highest in patients who were < 50 years of age (28.4 ± 5.9 kg/m2), and it decreased significantly with age in patients who were in the 70–80-year age group (23.9 ± 4.4 kg/m2; P < 0.01). We next sought to determine a potential correlation between BMI and sleep parameters. Indeed, our observations demonstrate that the PSQI score was significantly associated with BMI (rs = 0.157, P < 0.01) (Fig. 3).
Fig. 3.
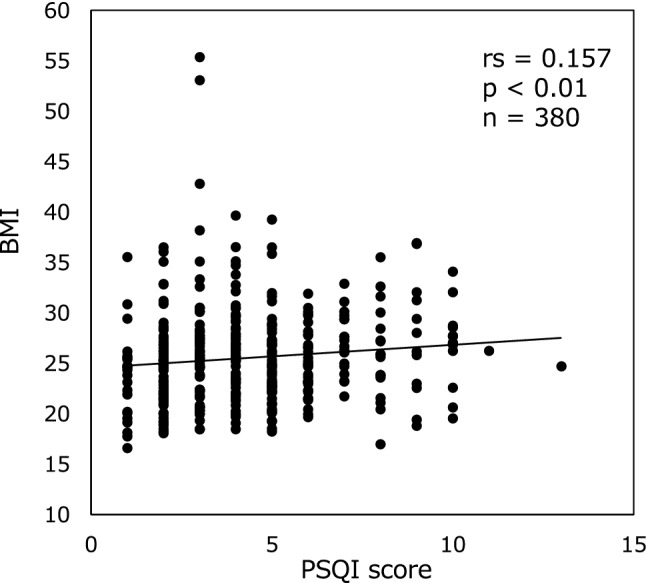
Correlation between PSQI score and BMI. The PSQI score was significantly correlated with the BMI. The P value indicates statistical significance using Spearman’s correlation analysis. PSQI Pittsburgh Sleep Quality Index; BMI body mass index
Discussion
We performed a cross-sectional study to investigate the changes in subjective sleep parameters among patients with T2DM in association with age and clinical backgrounds. Our findings demonstrate that the PSQI score, which is an indicator of poor sleep quality, was highest in the youngest patients and significantly decreased with age. The analysis of clinical characteristics revealed that lower age was also associated with a higher BMI, indicating a relationship between sleep quality and BMI in patients with T2DM.
Aging has been identified as the most significant factor contributing to the development of insomnia [16]. The prevalence of this disease has been well known to increase with age [2, 16]. Changes in sleep architecture have been suggested as a potential reason, as older people have considerably reduced amounts of deep sleep, also known as slow-wave sleep [9, 12, 17]. Conversely, a decrease in the duration and frequency of deep sleep contribute to a change in sleep architecture and disturb sleep maintenance in older people. [12]. In addition to alterations in sleep architecture, an exacerbation of health conditions and psychological stress are known to contribute to a deterioration in sleep quality, which in turn can lead to insomnia among older people. Contrary to previous reports, we found that younger patients had poorer sleep quality than older ones (Fig. 2). In relation to the discordance, we noted that younger patients had higher BMIs. It is well known that obesity strongly contributes to incident diabetes, especially in Asian populations [18, 19]. In this regard, the association between high BMI and diabetes was reported to be stronger among younger patients as compared to older ones [18, 20]. Moreover, the average BMI in patients with T2DM was shown to be higher among younger individuals [20], which is consistent with our results (Table 1). Interestingly, we found that the PSQI score was significantly correlated with BMI. Obesity is a well-established risk factor for OSA [21]. Recent reports have demonstrated that the prevalence of OSA increases with an increase in BMI [22]. In particular, the Asian population has been known to have a stronger relationship between BMI and OSA than the European population due to the difference in maxillofacial anatomy [21]. Consequently, compared to Europeans, even a mild increase in body weight may have a stronger impact on the incidence of OSA. In addition, patients with T2DM have a high prevalence rate of OSA (40–60%) [22]. In support of these reports, our patients had a strong perception of short sleep duration and daytime dysfunction (Table 2), and these symptoms are in accordance with those of OSA [22]. Thus, even though our study excluded patients diagnosed with OSA, the markedly poor sleep quality in young participants may indicate the presence of such sleep problems.
Although obesity is strongly associated with the onset of OSA and diabetes, it is known that OSA itself may contribute to insulin resistance by initiating systematic inflammation [23], which in turn facilitates the progression of glucose abnormality [1, 24]. Moreover, poor sleep habits have been suggested to induce hyperphagia and obesity by changing the balance of appetite-regulating hormones [25, 26]. The link between T2DM, OSA, and obesity highlights the importance of a healthy lifestyle including regular sleep habits, a balanced diet, and exercise.
Our study has several limitations. First, we only enrolled a small number of Japanese participants from a single university hospital. Accordingly, our findings may not be applicable to all patients with T2DM. Second, we excluded participants who used sleep agents for insomnia and those who were over 80 years of age or could not answer the questionnaire due to severe dementia. As mentioned above, a strong relationship between insomnia and aging or dementia is well established. Thus, there is a possibility that the results of our study may have been altered due to the exclusion of the highest-risk group. Third, we derived data associated with treatment, medical history, and social backgrounds from medical records; however, we could not exclude unknown dementia, psychiatric conditions, diabetic neuropathy, or social perspectives that may have affected sleep quality. Fourth, sleep quality, which is represented by PSQI scores, has been suggested to be influenced by sleep duration, which changes with lifestyle, job, sex, and other factors. In this study, patients in the youngest group had a significantly shorter sleep duration, which could have contributed to the marked increase in the PSQI scores. Therefore, the influence of a shorter sleep duration, especially in younger patients, might not have been assessed while evaluating inherent sleep quality. Moreover, since the PSQI score is influenced by many factors, a multivariate analysis and an analysis separated by sex are necessitated. Fifth, sleep measures were assessed using only subjective tools; no objective tools such as polysomnography or actigraphy were used. Therefore, we were unable to objectively evaluate the presence of OSA. To address these limitations, future studies with a larger sample size and a multicenter approach with objective sleep assessments are needed.
Conclusions
Our stratified analysis demonstrated an improvement in sleep quality with age in patients with T2DM. Therefore, when examining or treating patients with T2DM and obesity, insomnia should be accounted for as a potential comorbidity even in the young population.
Acknowledgements
We would like to thank Shuki Usui, Hiroshi Yoshino, and Ken Kanazawa, as well as all the staff of the outpatient clinic for their help in collecting data for this study. We also thank Editage (www.editage.com) for English language editing.
Funding
This work was supported by JSPS KAKENHI, Grant Number 18K07481.
Declarations
Conflict of interest
T. Hirose received research funds from Nippon Boehringer Ingelheim Co., Ltd., AstraZeneca K.K., Mitsubishi Tanabe Pharma Corporation, Novo Nordisk Pharma Ltd.; and received lecture fees from Sanofi K.K., Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd., Takeda Pharmaceutical Company Limited, MSD K.K., Sumitomo Dainippon Pharma Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Ono Pharmaceutical Co., Ltd., AstraZeneca K.K., Mitsubishi Tanabe Pharma Corporation, Kowa Company, Limited, Kissei Pharmaceutical Co., Ltd. N. Kumashiro received research funds from Boehringer Ingelheim Pharmaceuticals, Inc., and lecture fees from Takeda Pharmaceutical Company Limited, Sanofi K.K., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd. None of the funding agencies had any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Human and animal rights
The study was approved by the Medical Ethics Committee of Toho University Omori Medical Center (Approval no. #M19093; Approval date: August 29, 2019). This study was conducted according to the Declaration of Helsinki and current legal regulations in Japan.
Informed consent
Prior to enrollment in the survey, all eligible participants were informed about this study in written form, and the answer to the questionnaire was considered consent to enrollment.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Matsumoto T, Murase K, Tabara Y, Gozal D, Smith D, Minami T, Tachikawa R, Tanizawa K, Oga T, Nagashima S, Wakamura T, Komenami N, Setoh K, Kawaguchi T, Tsutsumi T, Takahashi Y, Nakayama T, Hirai T, Matsuda F, Chin K. Impact of sleep characteristics and obesity on diabetes and hypertension across genders and menopausal status: the Nagahama study. Sleep. 2018 doi: 10.1093/sleep/zsy071. [DOI] [PubMed] [Google Scholar]
- 2.Ministry of Health Labour and Welfare. Summary results of the national health and nutrition survey Japan; 2018. https://www.mhlw.go.jp/content/10900000/000688863.pdf. (Accessed 8 May 2021).
- 3.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–1484. doi: 10.1001/jama.1989.03430110069030. [DOI] [PubMed] [Google Scholar]
- 4.Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768–1774. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- 5.Yoda K, Inaba M, Hamamoto K, Yoda M, Tsuda A, Mori K, Imanishi Y, Emoto M, Yamada S. Association between poor glycemic control, impaired sleep quality, and increased arterial thickening in type 2 diabetic patients. PLoS ONE. 2015;10:e0122521. doi: 10.1371/journal.pone.0122521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leproult R, Deliens G, Gilson M, Peigneux P. Beneficial impact of sleep extension on fasting insulin sensitivity in adults with habitual sleep restriction. Sleep. 2015;38:707–715. doi: 10.5665/sleep.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruehl H, Rueger M, Dziobek I, Sweat V, Tirsi A, Javier E, Arentoft A, Wolf OT, Convit A. Hypothalamic-pituitary-adrenal axis dysregulation and memory impairments in type 2 diabetes. J Clin Endocrinol Metab. 2007;92:2439–2445. doi: 10.1210/jc.2006-2540. [DOI] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am. 2002;31:15–36. doi: 10.1016/S0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- 9.Pallayova M, Donic V, Gresova S, Peregrim I, Tomori Z. Do differences in sleep architecture exist between persons with type 2 diabetes and nondiabetic controls? J Diabetes Sci Technol. 2010;4:344–352. doi: 10.1177/193229681000400215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haba-Rubio J, Marques-Vidal P, Andries D, Tobback N, Preisig M, Vollenweider P, Waeber G, Luca G, Tafti M, Heinzer R. Objective sleep structure and cardiovascular risk factors in the general population: the HypnoLaus Study. Sleep. 2015;38:391–400. doi: 10.5665/sleep.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sridhar GR, Madhu K. Prevalence of sleep disturbances in diabetes mellitus. Diabetes Res Clin Pract. 1994;23:183–186. doi: 10.1016/0168-8227(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 12.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 13.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 14.Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 15.Antúnez JM. Circadian typology is related to emotion regulation, metacognitive beliefs and assertiveness in healthy adults. PLoS ONE. 2020;15:e0230169. doi: 10.1371/journal.pone.0230169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim K, Uchiyama M, Okawa M, Liu X, Ogihara R. An epidemiological study of insomnia among the Japanese general population. Sleep. 2000;23:41–47. doi: 10.1093/sleep/23.1.1a. [DOI] [PubMed] [Google Scholar]
- 17.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boffetta P, McLerran D, Chen Y, Inoue M, Sinha R, He J, Gupta PC, Tsugane S, Irie F, Tamakoshi A, Gao Y-T, Shu X-O, Wang R, Tsuji I, Kuriyama S, Matsuo K, Satoh H, Chen C-J, Yuan J-M, Yoo K-Y, Ahsan H, Pan W-H, Gu D, Pednekar MS, Sasazuki S, Sairenchi T, Yang G, Xiang Y-B, Nagai M, Tanaka H, Nishino Y, You S-L, Koh W-P, Park SK, Shen C-Y, Thornquist M, Kang D, Rolland B, Feng Z, Zheng W, Potter JD. Body mass index and diabetes in Asia: a cross-sectional pooled analysis of 900000 individuals in the Asia cohort consortium. PLoS ONE. 2011;6:e19930. doi: 10.1371/journal.pone.0019930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Someya Y, Tamura Y, Kohmura Y, Aoki K, Kawai S, Daida H, Naito H. A body mass index over 22 kg/m2 at college age is a risk factor for future diabetes in Japanese men. PLoS ONE. 2019;14:e0211067. doi: 10.1371/journal.pone.0211067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasai H, Sairenchi T, Iso H, Irie F, Otaka E, Tanaka K, Ota H, Muto T. Relationship between obesity and incident diabetes in middle-aged and older Japanese adults: the Ibaraki Prefectural Health Study. Mayo Clin Proc. 2010;85:36–40. doi: 10.4065/mcp.2009.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higurashi N, Kikuchi M, Miyazaki S, Itasaka Y. Comparison of Ricketts analysis and downs-Northwestern analysis for the evaluation of obstructive sleep apnea cephalograms. Psychiatry Clin Neurosci. 2001;55:259–260. doi: 10.1046/j.1440-1819.2001.00850.x. [DOI] [PubMed] [Google Scholar]
- 22.Feher M, Hinton W, Munro N, de Lusignan S. Obstructive sleep apnoea in type 2 diabetes mellitus: increased risk for overweight as well as obese people included in a national primary care database analysis. Diabet Med. 2019;36:1304–1311. doi: 10.1111/dme.13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aurora RN, Punjabi NM. Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. Lancet Respir Med. 2013;1:329–338. doi: 10.1016/S2213-2600(13)70039-0. [DOI] [PubMed] [Google Scholar]
- 24.Cizza G, Piaggi P, Lucassen EA, de Jonge L, Walter M, Mattingly MS, Kalish H, Csako G, Rother KI, Sleep Extension Study Group Obstructive sleep apnea is a predictor of abnormal glucose metabolism in chronically sleep deprived obese adults. PLoS ONE. 2013;8:e65400. doi: 10.1371/journal.pone.0065400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cedernaes J, Schiöth HB, Benedict C. Determinants of shortened, disrupted, and mistimed sleep and associated metabolic health consequences in healthy humans. Diabetes. 2015;64:1073–1080. doi: 10.2337/db14-1475. [DOI] [PubMed] [Google Scholar]
- 26.Ford ES, Li C, Wheaton AG, Chapman DP, Perry GS, Croft JB. Sleep duration and body mass index and waist circumference among US adults. Obesity (Silver Spring) 2014;22:598–607. doi: 10.1002/oby.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]


