Summary
Background
COVID-19 mRNA vaccines have proven to be highly safe and effective. Myocarditis is an adverse event associated with mRNA vaccination, especially in young male subjects. These events are rare and, in the majority of cases, resolve quickly. As myocarditis can be driven by autoimmune responses, we wanted to determine if the SARS-CoV-2 spike protein antigen encoded in the mRNA COVID vaccines had potential cross-reactivity with auto-antigens previously associated with myocarditis.
Methods
We performed a sequence identity comparison between SARS-CoV-2 spike protein-derived peptides and myocarditis-associated antigens. We also performed a structural analysis of these antigens and the SARS-CoV-2 spike protein to identify potential discontinuous 3-D epitope similarities.
Findings
We found no significant enrichment in the frequency of spike-derived peptides similar to myocarditis-associated antigens as compared to several controls.
Interpretation
Our results do not support the notion that increased occurrence of myocarditis after SARS-CoV-2-spike vaccination is mediated by a cross-reactive adaptive immune response.
Keywords: SARS-CoV-2, Myocarditis, mRNA vaccines, Antigen homology, Autoimmune disease
Research in context.
Evidence before this study
An increase of Myocarditis events has been observed following COVID-19 mRNA vaccination. These adverse events are extremely rare and often not biopsy proven. There is no clear evidence for a mechanism that could lead to these events. One possible mechanism is the triggering of autoimmune reactions, based on cross-reactivity between the SARS-CoV-2 spike protein encoded by the mRNA vaccines and auto-antigens associated with myocarditis.
Added value of this study
We performed a similarity analysis between the SARS-CoV-2 spike protein encoded in the vaccine, and myocarditis-associated antigens reported in the literature. We focused on regions in these proteins that could be the targets of T cell- or B-cell responses. We did not find significant similarity between them, making it doubtful that there is cross-reactive recognition occurring in individuals who developed myocarditis post-COVID-19 mRNA vaccination.
Implications of all the available evidence
Without much evidence of an adaptive cross-reactive response occurring in these individuals, the incidents of post-vaccination myocarditis are unlikely to be T-cell or B-cell mediated and more consistent with an innate response.
Alt-text: Unlabelled box
Introduction
In late 2019, severe acute respiratory coronavirus 2 (SARS-CoV-2) emerged causing a global pandemic of COVID-19 disease resulting in widespread morbidity and mortality. COVID-19 typically presents as a dry cough, sore throat, fever, and loss of taste and smell,1 but more rare complications have arisen as well including heart injury.2 Following the rapid development and approval for emergency use of several different SARS-CoV-2 vaccines, as of December 2021, over eight billion COVID-19 vaccine doses have been administered worldwide.3 Rare occurrences of myocarditis and pericarditis have been reported as associated with COVID-19 vaccination in the context of mRNA,4,5 but only extremely rarely with viral vector-based vaccines which are in turn associated with a different class of adverse event such as increased frequency of blood clots.6 The etiology of these rare side effects is poorly understood, but the possibility of autoimmune adaptive reactions needs to be investigated. As the two currently authorized mRNA vaccines BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna) are both encoding the SARS-CoV-2 spike protein as the vaccine immunogen, we set out to determine if specific sequences contained in the spike protein could lead to a cross-reactive immune response to autoantigens associated with autoimmune myocarditis in particular.7, 8, 9
Methods
Myocarditis associated auto-antigens (cardiac proteins)
To compile a list of myocarditis-associated antigens, we first queried the Immune Epitope Database (IEDB),10 which includes myocarditis-associated epitopes and their respective source antigens. A search for positive assays that included disease entries of “myocarditis” (DOID: 820), “rheumatic myocarditis” (DOID: 8481), and “experimental autoimmune myocarditis” (ONTIE ID: 0003439) revealed 66 human epitopes, which were contained in eight protein antigens. In addition, we reviewed the autoimmune myocarditis literature,7, 8, 9 which provided 23 additional antigens that had known associations with myocarditis and four antigens that were mentioned for potential associations with myocarditis, but either weak or no evidence was noted. In total, we compiled this list of 35 antigens (Table 1) to use for this conservation analysis.
Table 1.
Myocarditis-Associated Cardiac Antigens.
| Protein Name | Gene | UniProt ID | Source |
|---|---|---|---|
| Myosin-6 | MYO6 | Q9UM54 | IEDB |
| Myosin-7 | MYH7 | P12883 | IEDB |
| Muscarinic acetylcholine receptor M2 | CHRM2 | P08172 | IEDB |
| Myosin-binding protein C - cardiac-type | MYBPC3 | Q14896 | IEDB |
| Myosin-binding protein C - fast-type | MYBPC2 | Q14324 | IEDB |
| Beta-2-glycoprotein 1 | APOH | P02749 | IEDB |
| Laminin subunit alpha-1 | LAMA1 | P25391 | IEDB |
| Transmembrane protease serine 4 | TMPRSS4 | Q9NRS4 | IEDB |
| Troponin I | TNNI3 | P19429 | Review Literature |
| Troponin T | TNNT2 | P45379 | Review Literature |
| Beta-1 adrenergic receptor | ADRB1 | P08588 | Review Literature |
| Actin, alpha cardiac muscle 1 | ACTC1 | P68032 | Review Literature |
| Tropomyosin alpha-1 chain | TPM1 | P09493 | Review Literature |
| Tropomyosin beta chain | TPM2 | P07951 | Review Literature |
| Tropomyosin alpha-3 chain | TPM3 | P06753 | Review Literature |
| Cytoplasmic aconitate hydratase | ACO1 | P21399 | Review Literature |
| ADP/ATP translocase 1 | SLC25A4 | P12235 | Review Literature |
| Creatine kinase B-type | CKB | P12277 | Review Literature |
| Creatine kinase S-type, mitochondrial | CKMT2 | P17540 | Review Literature |
| Creatine kinase U-type, mitochondrial | CKMT1A | P12532 | Review Literature |
| Creatine kinase M-type | CKM | P06732 | Review Literature |
| Desmin | DES | P17661 | Review Literature |
| Dihydrolipoyl dehydrogenase, mitochondrial | DLD | P09622 | Review Literature |
| 60 kDa heat shock protein, mitochondrial | HSPD1 | P10809 | Review Literature |
| Heat shock 70 kDa protein 1A | HSPA1A | P0DMV8 | Review Literature |
| Vimentin | VIM | P08670 | Review Literature |
| E3 ubiquitin-protein ligase TRIM21 | TRIM21 | P19474 | Review Literature |
| Lupus La protein | SSB | P05455 | Review Literature |
| Pyruvate kinase | PKLR | P30613 | Review Literature |
| Ubiquinol-cytochrome-c reductase complex assembly factor 1 | UQCC1 | Q9NVA1 | Review Literature |
| Sodium/potassium-transporting ATPase subunit alpha-1 | ATP1A1 | P05023 | Review Literature |
| Natriuretic peptides B | NPPB | P16860 | Review Literature |
| Natriuretic peptides A | NPPA | P01160 | Review Literature |
| Troponin C, slow skeletal and cardiac muscles | TNNC1 | P63316 | Review Literature |
| Transmembrane protein 65 | TMEM65 | Q6PI78 | Review Literature |
Randomized human protein control sets
We compiled 1000 sets of 35 proteins each that were randomly selected from the human proteome (UniProt proteome ID: UP000005640) using custom Python scripts. These sets provide a control on how human proteins not specifically selected to be associated with myocarditis compare to the set described above.
Spike protein-derived peptides and shuffled controls
The SARS-CoV-2 spike protein (UniProtID: P0DTC2) is 1273 amino acids in length. Since cross-reactivity at the level of either CD8+or CD4+ T cells is of potential concern, we considered 9-mers and 15-mers, as these epitope sizes are associated with CD8+or CD4+ T cell epitopes, respectively. To identify possible peptides of relevance, we split the spike protein sequence into all possible 9-mers, overlapping by eight amino acids, and all possible 15-mers, overlapping by 14 amino acids using custom Python scripts. In total, we compiled 1265 9-mers and 1259 15-mers. As a control, we also generated shuffled sequences of all peptides using the Python shuffle function.
Conservation analysis
We considered different levels of sequence identity to identify potentially relevant hits for CD4- and CD8 T cell immune responses. Previous studies11 support the notion that 50% is a conservative identity threshold for cross-reactivity for CD4 T cells, which are typically 15 residues in length. For CD8 epitopes, which are typically 9 residues in length, more than two substitutions are in general non-cross-reactive.12 Both the spike peptide and shuffled peptide sets were searched for matches in the cardiac proteins, as well as the 1000 control sets, using PEPMatch, a tool developed by the IEDB (manuscript in progress; https://github.com/IEDB/PEPMatch). PEPMatch is optimized for short peptide searches, and guarantees finding complete sets of results in contrast to, for example, BLAST13 with default settings.
3-D Structural analysis
To consider the potential for discontinuous 3-D epitope cross-reactivity from B cells, we analysed structural similarities between the SARS-CoV-2 spike protein and the myocarditis-associated antigens using TM-align.14 PDB files for each antigen were extracted from the Protein Data Bank website (https://www.rcsb.org). Where PDB structures were not available for a protein, predicted structures created by AlphaFold15 were used. This analysis was also repeated with 1000 control sets each containing 35 randomly selected proteins from the human proteome (UniProt proteome ID: UP000005640). Since TM-align normalizes its scores based on protein length, the proteins selected for these controls were made to fall within 30% of the average length of the myocarditis-associated proteins.
The solvent-accessible surface area of the residues making up the region of spike that have a TM-align score of 0.5 or above compared with the myocarditis-associated antigens were calculated with the program NACCESS16 using a default probe size of 1.4 Angstroms.
Statistics
Statistics were performed using custom Python scripts with implementation of the SciPy library. For the conservation analyses, we used a Fisher's exact test to determine the association between homology and spike or shuffled peptides.
Role of funders
This work was supported by the National Institutes of Health. The funders had no role in the design, collection of data, statistical analysis, or writing of the paper.
Results
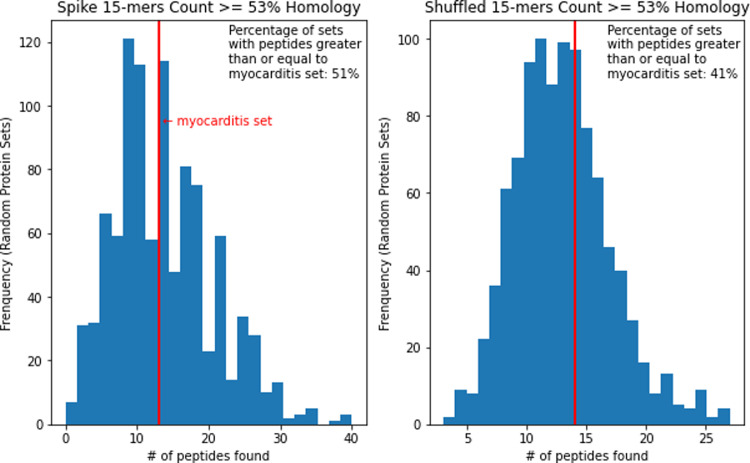
To evaluate the occurrence of peptides in SARS-CoV-2 spike that have high similarity to peptides in proteins associated with cardiac autoimmunity (cardiac proteins for short hereafter), we generated a set of 1259 15-mers overlapping by 14 residues spanning the entire spike protein. 15-mer peptides were considered first, as the typical length of MHC-II restricted CD4 T cell epitopes. We compared these peptides to a set of 35 cardiac proteins associated with cardiac autoimmunity. We found zero peptides in the spike that matched any of these cardiac antigens at a sequence identity of 60% or more. Relaxing the identity threshold further, at 53% homology, we found 13 matches for peptides from the spike protein. However, we also found 14 matches from shuffled peptides, which means there is no statistically significant increased sequence identity of actual spike peptides as compared to shuffled controls at the 53% threshold (p = 1.0, OR=0.928 (Table 2)).
Table 2.
SARS-CoV-2 Spike 15-mers vs. Shuffled 15-mers (Homology >= 53%).
| Match in Cardiac Proteins | No Match in Cardiac Proteins | Total Peptides | |
|---|---|---|---|
| Spike Peptides | 13 | 1246 | 1259 |
| Shuffled Peptides | 14 | 1245 | 1259 |
| Total Peptides | 27 | 2491 | 2518 |
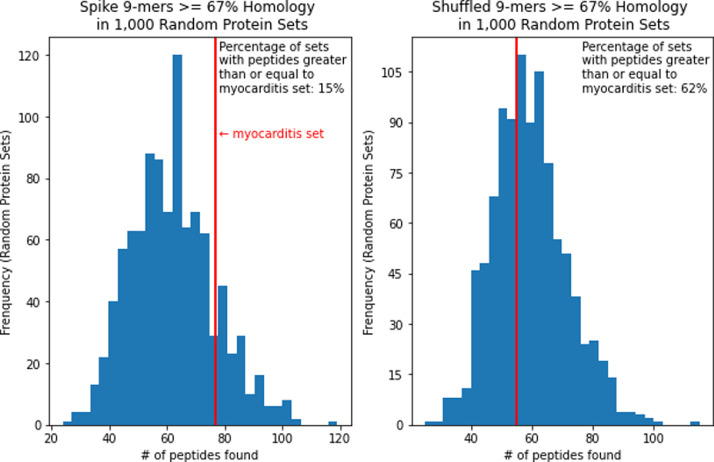
Next, we examined the homology of 9-mer peptide fragments, which is the length of typical MHC-I restricted CD8 T cell epitopes. At 78% homology or more (two substitutions), three spike peptides and one shuffled peptide were found in cardiac proteins, which is not a significant enrichment (p = 0.63). At the 67% homology level, we found 77 homologous peptides from spike and 55 homologous from shuffled peptides (Table 3), which is also not a statistically significant increase (p = 0.06).
Table 3.
SARS-CoV-2 Spike 9-mers vs. Shuffled 9-mers (Homology >= 67%).
| Match in Cardiac Proteins | No Match in Cardiac Proteins | Total Peptides | |
|---|---|---|---|
| Spike Peptides | 77 | 1188 | 1265 |
| Shuffled Peptides | 55 | 1210 | 1265 |
| Total Peptides | 132 | 2398 | 2530 |
While these analyses do show a trend for a higher number of 9-mer peptides in spike that match the cardiac proteins, that enrichment is not statistically significant, and thus does not support the notion that spike protein sequences are significantly enriched in peptides that are potential epitopes with significant sequence identity to human self-proteins associated with autoimmune myocarditis. Conversely, the analysis also identifies 13 15-mer and 77 9-mer peptides that could be further evaluated experimentally for their potential to mediate cross-reactive responses in individuals experiencing post-vaccination myocarditis (Supplemental Table 1).
As an alternative control, we randomly selected sets of human proteins to match the cardiac protein set. We then repeated the peptide match analysis. For 9-mers at the 56% homology level, 89.5% of sets were below the cardiac protein set and 10.5% were at or above it in terms of peptide match frequency. At the 67% homology level, 84.8% were below and 15.2% were at or above the cardiac protein set (Figure 1). This shows a trend for increased hits in cardiac proteins rather than in randomly selected proteins, but as before this trend is not statistically significant at the conventional p = 0.05 cutoff. Only spike 15-mers at the 53% homology level had matches within the cardiac protein set and 48.7% of the randomly selected protein sets were below it and 51.3% were at or above it in terms of peptide match frequency (Figure 2). This is also not considered significant.
Figure 1.
Spike vs shuffled 9-mers >= 67% homology match distribution of 1000 random protein sets.
Figure 2.
Spike vs shuffled 15-mers >= 53% homology match distribution of 1000 random protein sets.
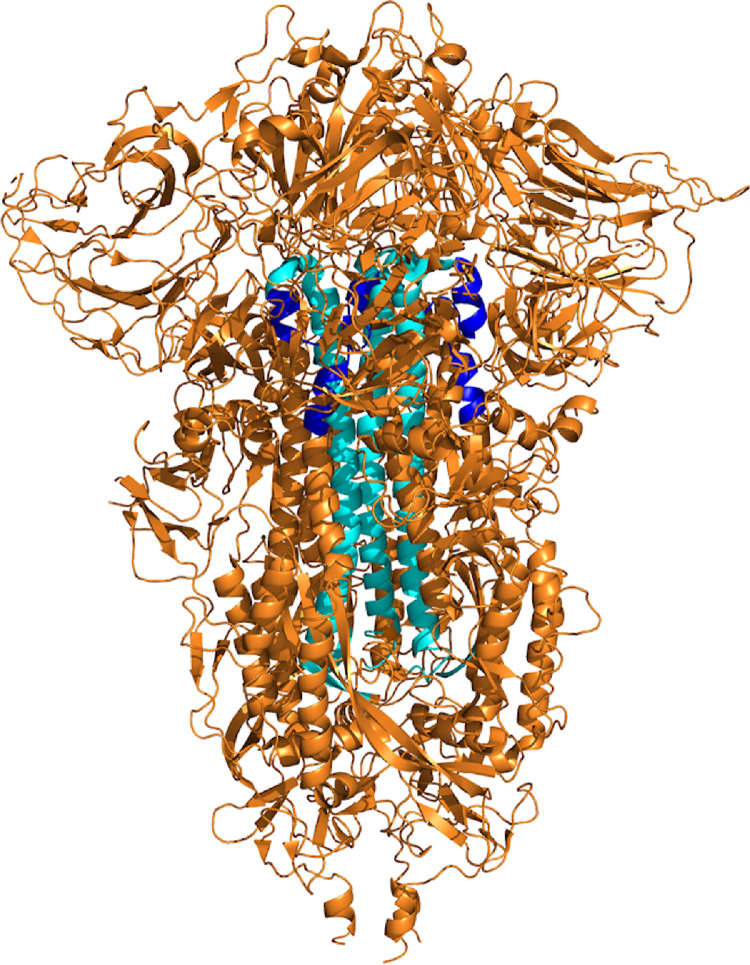
All PDB files for the myocarditis-associated antigens were compared to the SARS-Cov-2 Spike protein using the TM-align program (https://zhanggroup.org/TM-align/) with the structure of the spike protein (PBD ID: 7DDD). TM-align scores are considered significant when greater than or equal to 0.5. Four substructures of these antigens had significant scores (Table 4). Since these are only fragments of the antigen, we mapped their residues onto the 3-D structure of the spike, which shows the location of these regions and their proximity to the surface (Figure 3). Using NACCESS to calculate solvent accessibility, we found that all of these residues had values under 100 square Angstroms. Since only residues with values between 100 and 120 square Angstroms are considered fully exposed, these residues are considered to have a low solvent-accessible surface area.
Table 4.
Significant TM-align scores for antigen fragments compared with spike.
Figure 3.
Regions of the spike protein with significant TM-align scores compared with myocarditis antigens (highlighted in cyan and blue).
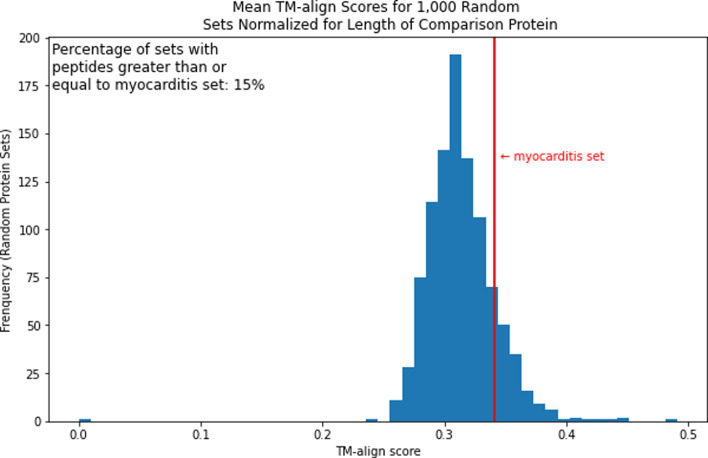
We then repeated this analysis with 1000 random protein sets that have an average length within 30% of the average protein length in the cardiac set. This was to create a distribution of average TM-align scores normalized for the non-spike proteins. We found that 84.5% of sets were below the cardiac protein set and 14.5% were at or above it in terms of mean TM-align score. This would not be considered significant (Figure 4).
Figure 4.
Mean TM-align score distribution of 1000 random protein sets.
Discussion
Myocarditis is an inflammatory disease that affects the muscles of the heart which can be caused by an autoimmune mechanism.8 There have been a number of cases of myocarditis occurring in humans after SARS-CoV-2 infection and with COVID-19 vaccination.4,17 Although these occurrences are extremely rare and often not biopsy proven,18 investigation into a possible adaptive immune response is warranted. Here, we examined the potential for a cross-reactivity link based on sequence similarity of the SARS-CoV-2 spike protein encoded in mRNA COVID-19 vaccines and myocarditis-associated proteins. We did not find statistically significant overlap in terms of linear peptide sequences between cardiac proteins and the spike protein when considering various controls, which would be potential targets of T cell responses. When considering potential 3-D epitope cross-reactivity, which would be targeted by antibodies, we did not find these antigens were significantly higher in structural similarity compared with controls. The antigens that had some structural similarities were similar in spike regions that appear inaccessible, making them unlikely epitope targets of antibody cross-reactivity. This does not support the hypothesis that myocarditis adverse events post-mRNA COVID-19 vaccination are due to cross-reactive reactions of the adaptive immune system. This is further supported by the fact that the median onset of myocarditis incidents occurring post-vaccination was three and a half days and for those hospitalized, the median discharge was two days. By contrast, autoimmune diseases often progress over time through epitope spreading.19 Overall, the incidents of myocarditis post-vaccination may not be T cell-mediated and perhaps are more compatible with a transient innate response.
However, the lack of statistical evidence of similarity between vaccine peptides and autoimmune antigens, in general, does not exclude that, in some individuals, there will be a cross-reactive response. Our analysis does not exclude cross-reactivity as a mechanism for post mRNA COVID-19 vaccine myocarditis, and more evidence is required to elucidate the mechanisms involved. Since median onset of myocarditis seems inconsistent with cross reactive adaptive immunity as a mechanism, future investigations might address additional mechanisms, for example, associated with activation of innate immunity, and employ experimental rather than sequence comparison methodologies.
Declaration of interests
DM has nothing to disclose.
JM has nothing to disclose.
AS has participated in a Moderna Advisory Board.
BP has nothing to disclose.
Acknowledgments
Acknowledgements
We wish to acknowledge the work of the entire IEDB team.
Contributors
Conceptualisation and study design: BP
Data curation: DM and AS
Formal analysis: DM, JM, and BP
Funding acquisition: AS and BP
Methodology: BP
Validation: DM, JM, and BP
Visualization: DM
Writing - original draft: DM, AS, and BP
Writing - review & editing: DM, JM, AS, and BP
Data Sharing Statement
Public data used for this work can be found at UniProt (https://www.uniprot.org/) and the Protein Data Bank (https://www.rcsb.org/). The code used for the analysis will be made available upon request to the corresponding authors.
Footnotes
Funding: This work was funded by 75N93019C00001 from the National Institutes of Health.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103807.
Appendix. Supplementary materials
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han H., Xie L., Liu R., Yang J., Liu F., Wu K., et al. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol. 2020;92(7):819–823. doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Coronavirus (COVID-19) Dashboard [Internet]. [cited 2021 Dec 15]. Available from: https://covid19.who.int
- 4.Diaz G.A., Parsons G.T., Gering S.K., Meier A.R., Hutchinson I.V., Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021 doi: 10.1001/jama.2021.13443. [cited 2021 Sep 2]; Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC . 2020. COVID-19 Vaccination [Internet]. Centers for Disease Control and Prevention.https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html [cited 2021 Sep 13]. Available from. [PubMed] [Google Scholar]
- 6.CDC . 2020. COVID-19 Vaccination [Internet]. Centers for Disease Control and Prevention.https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html [cited 2021 Sep 12]. Available from. [PubMed] [Google Scholar]
- 7.Bracamonte-Baran W., Čiháková D. Cardiac autoimmunity: myocarditis. Adv Exp Med Biol. 2017;1003:187–221. doi: 10.1007/978-3-319-57613-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maisch B. Cardio-immunology of myocarditis: focus on immune mechanisms and treatment options. Front Cardiovasc Med. 2019;6:48. doi: 10.3389/fcvm.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tschöpe C., Ammirati E., Bozkurt B., Caforio A.L.P., Cooper L.T., Felix S.B., et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18(3):169–193. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vita R., Mahajan S., Overton J.A., Dhanda S.K., Martini S., Cantrell J.R., et al. The immune epitope database (IEDB): 2018 update. Nucleic Acids Res. 2019;47(D1):D339–D343. doi: 10.1093/nar/gky1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mateus J., Grifoni A., Tarke A., Sidney J., Ramirez S.I., Dan J.M., et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370(6512):89–94. doi: 10.1126/science.abd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grifoni A., Voic H., Dhanda S.K., Kidd C.K., Brien J.D., Buus S., et al. T Cell responses induced by attenuated flavivirus vaccination are specific and show limited cross-reactivity with other flavivirus species. J Virol. 2020;94(10) doi: 10.1128/JVI.00089-20. e00089-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y., Skolnick J. TM-align: a protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 2005;33(7):2302–2309. doi: 10.1093/nar/gki524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jumper J., Evans R., Pritzel A., Green T., Figurernov M., Ronneberger O., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hubbard S.J. Department of Biochemistry and Molecular Biology; 1993. NACCESS: A Program for Calculating Accessibilities. [Google Scholar]
- 17.Grimaud M., Starck J., Levy M., Marais C., Chareyre J., Khraiche D., et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10(1):69. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144(6):471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuohy V.K., Kinkel R.P. In: Autoimmunity [Internet] Górski A, Krotkiewski H, Zimecki M, editors. Springer Netherlands; Dordrecht: 2001. Epitope spreading: a mechanism for progression of autoimmune disease; pp. 39–48. [cited 2021 Sep 5]Available from. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.