Abstract
Correlation between vaccine reactogenicity and immunogenicity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is unclear. Thus, we investigated to determine whether the reactogenicity after coronavirus disease 2019 vaccination is associated with antibody (Ab) titers and T cell responses. This study was prospective cohort study done with 131 healthcare workers at tertiary center in Seoul, South Korea. The degrees of the local reactions after the 1st and 2nd doses of ChAdOx1 nCov-19 (ChAdOx1) vaccination were significantly associated with the S1-specific IgG Ab titers (p=0.003 and 0.01, respectively) and neutralizing Ab (p=0.04 and 0.10, respectively) in age- and sex-adjusted multivariate analysis, whereas those after the BNT162b2 vaccination did not show significant associations. T cell responses did not show significant associations with the degree of reactogenicity after the ChAdOx1 vaccination or the BNT162b2 vaccination. Thus, high degree of local reactogenicity after the ChAdOx1 vaccine may be used as an indicator of strong humoral immune responses against SARS-CoV-2.
Keywords: COVID-19, Vaccine, Antibody response, Neutralizing antibody, Injection site reaction
INTRODUCTION
Local and systemic adverse reactions have been reported to frequently occur after coronavirus disease 2019 (COVID-19) vaccinations (1). Inflammation presenting as reactogenicity symptoms usually reflects the activation of the innate immune system, and may trigger subsequent adaptive immune response (2) ; as such, local injection site reaction has long been considered as a surrogate marker for immune response in vaccination (3,4,5). We thus examined whether local or systemic reactogenicity is associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific humoral and cell-mediated immune responses in healthcare workers (HCWs) who received the first and second doses of the ChAdOx1 nCovV-19 vaccine (ChAdOx1) or the BNT162b2 mRNA vaccine (BNT162b2).
MATERIALS AND METHODS
Study design and participants
We enrolled HCWs at Asan Medical Center (Seoul, South Korea) who did not have a history of SARS-CoV-2 infection confirmed with negative SARS-CoV-2 S1-specific antibody (Ab) by ELISA at baseline sampling before vaccination. They received the ChAdOx1 or BNT162b2 vaccine between March 5 and June 22, 2021. According to the policy of the Korean government, the BNT162b2 vaccine was assigned to high-risk HCWs in direct contact with COVID-19 patients, and the ChAdOx1 vaccine was assigned to those involved in general patient care. The second dose of ChAdOx1 was given 12 weeks after the the first dose of ChAdOx1, and the second dose of BNT162b2 was given 3 weeks after the first dose of BNT162b2, according to the recommendation of the Korean government. Recruitment of study participants was done by spontaneous contact to the research team from vaccination candidates who checked the intranet post and notices. Prophylactic use of antipyretics was not recommended but allowed. Blood sampling was performed right before and 3 weeks after the 1st dose vaccination and right before and 2 weeks after the 2nd dose vaccination. Blood sampling within 24 hours after the 1st dose vaccination was allowed, but blood sampling as baseline of the 2nd dose vaccination was strictly complied with before vaccination. The study was reviewed and approved by the Institutional Review Board of Asan Medical Center (#2021-0170).
Collection of data on baseline characteristics and reactogenicity after vaccination
Data on the baseline demographic information and the local and systemic reactions during the 7-day period post-vaccination were obtained through a questionnaire-based electronic-diary released before vaccination (Appendix 1). Text messages were sent daily to remind participants to record adverse effects on e-diary. HCWs completed the questionnaire every day during the 0–7-day period. The reactogenicity was presented as the sum of symptom score, which was calculated as the sum of each symptom for 7 days after vaccination by assigning a score according to the severity as follows: none=0, mild=1, moderate=2, severe=3, life-threatening=4 (6).
Measurement of Ab and T cell response
An in-house-developed ELISA was used to measure the titer of anti-SARS-CoV2 S1 specific IgG Ab and the data are presented as relative OD values based on a 1:100 dilution factor at 450 nm. We measured the mean values and SD of the OD values from negative control plasma, and calculated the cut-off value of 0.4 by calculating the mean OD plus 3 folds of the SD values (7,8,9).
We also measured plasma levels of neutralizing Abs using a microneutralization assay. Briefly, a 100 tissue culture infective dose 50 of SARS-CoV-2 (βCoV/Korea/KCDC/2020 NCCP43326) was mixed with an equal volume of diluted plasma specimen, incubated at 37°C for 30 min, and added to Vero cells. After 96 hours, the cytopathic effect of SARS-CoV-2 on the infected cells was measured. Neutralization Ab titer was calculated as the reciprocal of the highest dilution of test plasma giving 50% neutralization (ID50).
SARS-CoV-2-specific T cell response was measured by interferon-gamma ELISPOT assay measured from isolated PBMCs. T cells were stimulated using SARS-CoV-2 spike-overlapping peptides (Miltenyi Biotec, Bergisch Gladbach, Germany) and the number of spot-forming cells per 5.0 × 105 PBMCs was counted with an automated ELISPOT reader (AID iSPOT; Autoimmun Diagnostika GmbH, Strassberg, Germany).
Statistical analysis
We used the χ2 test or Fisher's exact test for the analysis of categorical variables. Mann Whitney U test for continuous variables and Kruskal-Wallis test for continuous variables of multiple groups were used. Spearman's rank correlation was used for the analysis of the correlation and multiple linear regression model was used to adjust for confounding factors. The dependent variables, that is, both S1-specific IgG and neutralizing Ab titer were log-transformed in linear regression for the normality. Two-tailed p-values <0.05 were considered statistically significant. R version 4.1.1 (R Project for Statistical Computing, Vienna, Austria) and Graphpad Prism version 8.0 (GraphPad Software, San Diego, CA, USA) were used for the analysis and graph plotting of the results.
RESULTS
Baseline characteristics of study participants
The study flow chart is shown in Supplementary Fig. 1. Of the 131 HCWs enrolled in this study, 96 (73%) received the 1st dose of the ChAdOx1 vaccine, of whom 88 (92%) completed the 2nd dose of ChAdOx1 12 weeks after the 1st dose and followed-up. The remaining 35 (27%) received the 1st and 2nd doses of the BNT162b2 vaccine at 3-week intervals (Table 1). The median age was significantly higher in the ChAdOx1 group than in the BNT162b2 group (36.0 vs. 32.0 years; p=0.002) and the distribution of sex showed no significant difference between the 2 groups (p=0.17).
Table 1. Baseline characteristics of the study participants.
| Variables | ChAdOx1 1st dose (n=96) | ChAdOx1 2nd dose (n=88)* | BNT162b2 1st and 2nd doses (n=35) | p-value | |
|---|---|---|---|---|---|
| Age at vaccination (yr) | 35.5 (21.0–64.0) | 36.0 (29.5–42.5) | 32.0 (26.0–35.0) | 0.002 | |
| Age range | 0.027 | ||||
| 20s | 26 (27.0) | 22 (25.0) | 17 (48.6) | ||
| 30s | 37 (39.0) | 35 (39.8) | 15 (42.9) | ||
| 40s | 23 (24.0) | 21 (23.9) | 2 (5.7) | ||
| 50s | 8 (8.0) | 8 (9.1) | 1 (2.9) | ||
| 60s | 2 (2.1) | 2 (2.3) | 0 (0.0) | ||
| Sex | 0.17 | ||||
| Female | 76 (79.0) | 70 (79.5) | 23 (65.7) | ||
| Male | 20 (21.0) | 18 (20.5) | 12 (34.3) | ||
| Occupation | 0.001 | ||||
| Doctor | 26 (27.0) | 25 (28.7) | 11 (31.4) | ||
| Nurse | 38 (40.0) | 34 (39.1) | 24 (68.6) | ||
| Paramedic | 6 (6.3) | 4 (4.6) | 0 (0.0) | ||
| Office worker | 26 (27.0) | 24 (27.6) | 0 (0.0) | ||
Data represent median (interquartile range) or number (%) unless indicated otherwise.
*Of the 96 healthcare workers who received the first dose of ChAdOx1, 88 (92%) completed the second dose of ChAdOx1 12 weeks after the first dose and followed-up.
Reactogenicity and immune responses after vaccination
All participants were confirmed as SARS-CoV-2 S1-specific IgG Ab negative by ELISA. The proportion of HCWs reporting at least one adverse reaction during the 7-day period post-vaccination after the 1st and 2nd doses were 96% and 92%, respectively in the ChAdOx1 group and 77% and 92%, respectively in the BNT162b2 group. Local reactogenicity in terms of the severity grade afte the 1st and 2nd doses did not show significant difference between ChAdOx1 and BNT162b2 group. In contrast, systemic reactogenicity after the 1st and 2nd doses were significantly higher in the ChAdOx1 group than in the BNT162b2 group (both p<0.01, Supplementary Table 1).
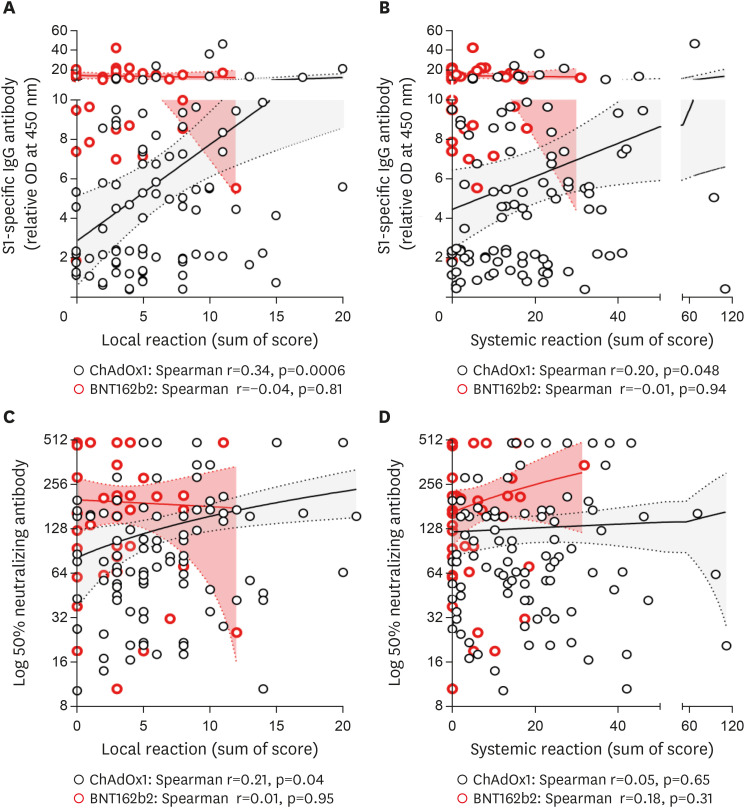
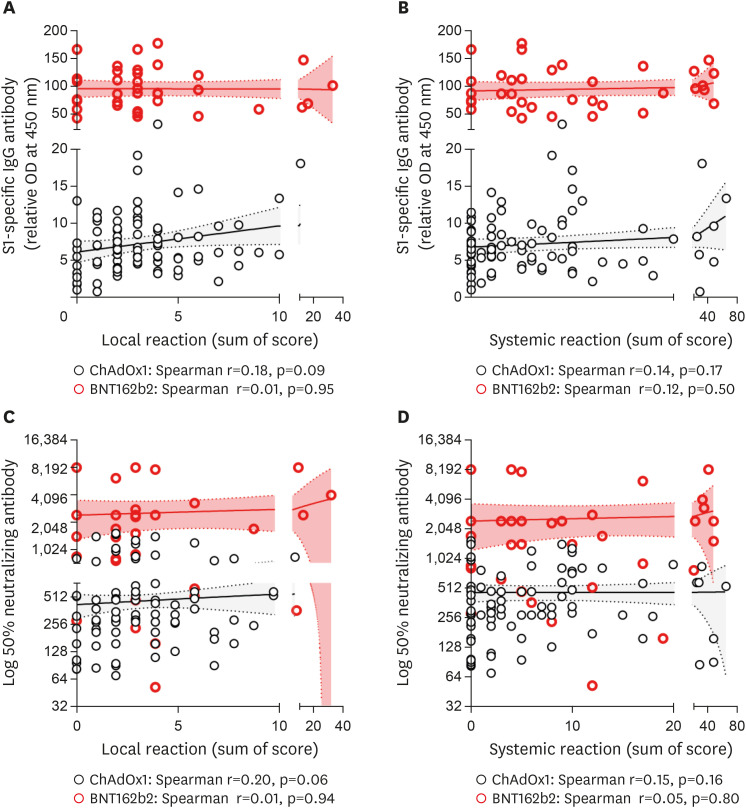
In the ChAdOx1 group, the degree of local reactogenicity was significantly correlated with the SARS-CoV-2 S1-specific Ab titer and neutralizing Ab at 3 weeks after the 1st dose (p=0.0006 and p=0.04, respectively; Fig. 1) but not at 2 weeks after the 2nd dose (p=0.09 and p=0.06, respectively; Fig. 2). Age- and sex-adjusted multivariate analysis revealed a significant positive correlation between local reaction and S1-specific IgG Ab titer after both 1st and 2nd doses (p=0.003 and p=0.01, respectively; Table 2). The degree of systemic reactogenicity did not show significant association with the S1-specific IgG titer and neutralizing Ab at 3 weeks after the 1st dose (p=0.048 and p=0.65, respectively; Fig. 1) and 2 weeks after the 2nd dose vaccination (p=0.17 and p=0.16, respectively; Fig. 2). Age- and sex-adjusted multivariate analysis also did not show significant association of systemic reaction with S1-specific IgG and neutralizing Ab after 1st dose (p=0.47 and p=0.81, respectively; Table 2) and 2nd dose (p=0.20 and p=0.98, respectively; Table 2).
Figure 1. Correlation of reactogenicity and immunogenicity of COVID-19 vaccine after 1st dose. Solid lines denote the mean and dotted lines denote the 95% confidential intervals. (A) SARS-CoV-2 S1-specific IgG and local reaction. (B) SARS-CoV-2 S1-specific IgG and systemic reaction. (C) Neutralizing Ab and local reaction. (D) Neutralizing Ab and systemic reaction.
Figure 2. Correlation of reactogenicity and immunogenicity of COVID-19 vaccine after 2nd dose. Solid lines denote the mean and dotted lines denote the 95% confidential intervals. (A). SARS-CoV-2 S1-specific IgG and local reaction. (B) SARS-CoV-2 S1-specific IgG and systemic reaction. (C) Neutralizing Ab and local reaction. (D) Neutralizing Ab and systemic reaction.
Table 2. Variables associated with S1-specific IgG and neutralizing Ab of ChAdOx1 nCoV-19 vaccine in multivariate analysis.
| Variables | ChAdOx1 nCoV-19 1st dose (n=96) | ChAdOx1 nCoV-19 2nd dose (n=88) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standardized β | p-value | Standardized β | p-value | R2 | Standardized β | p-value | Standardized β | p-value | R2 | |||
| Log S1-specific IgG | ||||||||||||
| Age | 0.006 | 0.96 | −0.03 | 0.77 | 0.05 | 0.69 | −0.01 | 0.94 | ||||
| Female Sex | −0.10 | 0.40 | −0.13 | 0.29 | −0.02 | 0.91 | 0.01 | 0.92 | ||||
| Occupation | ||||||||||||
| Office worker | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Doctor | 0.08 | 0.56 | 0.09 | 0.52 | 0.11 | 0.45 | 0.11 | 0.44 | ||||
| Nurse | 0.29 | 0.02 | 0.35 | 0.007 | 0.09 | 0.50 | 0.09 | 0.50 | ||||
| Paramedic | 0.20 | 0.05 | 0.21 | 0.05 | 0.20 | 0.09 | 0.20 | 0.10 | ||||
| Local reaction after each dose (sum of symptom score) | 0.30 | 0.003 | - | - | 0.19 | 0.29 | 0.01 | - | - | 0.11 | ||
| Systemic reaction after each dose (sum of symptom score) | - | - | 0.08 | 0.47 | 0.11 | - | - | 0.14 | 0.20 | 0.05 | ||
| Log 50% neutralizing Ab | ||||||||||||
| Age | −0.02 | 0.84 | −0.07 | 0.53 | 0.12 | 0.33 | 0.09 | 0.48 | ||||
| Female Sex | −0.06 | 0.64 | −0.07 | 0.58 | 0.07 | 0.60 | 0.11 | 0.44 | ||||
| Occupation | ||||||||||||
| Office worker | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Doctor | 0.21 | 0.12 | 0.22 | 0.11 | 0.07 | 0.64 | 0.07 | 0.63 | ||||
| Nurse | 0.17 | 0.18 | 0.22 | 0.09 | 0.05 | 0.71 | 0.05 | 0.73 | ||||
| Paramedic | 0.18 | 0.11 | 0.18 | 0.11 | −0.05 | 0.66 | −0.07 | 0.56 | ||||
| Local reaction after each dose (sum of symptom score) | 0.22 | 0.04 | - | - | 0.10 | 0.19 | 0.10 | - | - | 0.05 | ||
| Systemic reaction after each dose (sum of symptom score) | - | - | −0.03 | 0.81 | 0.06 | - | - | −0.003 | 0.98 | 0.02 | ||
Ref, reference value.
In contrast, the local and systemic reactogenicity in the BNT162b2 group did not show significant associations with both S1-specific IgG Ab and neutralizing Ab at 3 weeks after 1st and 2 weeks after 2nd dose vaccination (Figs. 1 and 2, respectively). The age- and sex-adjusted multivariate analyses are shown in Table 3.
Table 3. Variables associated with S1-specific IgG and neutralizing Ab of BNT162b2 mRNA vaccine in multivariate analysis.
| Variables | BNT162b2 mRNA 1st dose (n=35) | BNT162b2 mRNA 2nd dose (n=35) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standardized β | p-value | Standardized β | p-value | R2 | Standardized β | p-value | Standardized β | p-value | R2 | |||
| Log S1-specific IgG | ||||||||||||
| Age | −0.08 | 0.69 | −0.07 | 0.74 | 0.12 | 0.56 | 0.16 | 0.42 | ||||
| Female sex | 0.08 | 0.68 | 0.08 | 0.71 | 0.07 | 0.75 | 0.04 | 0.86 | ||||
| Occupation | ||||||||||||
| Doctor | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Nurse | −0.08 | 0.71 | −0.09 | 0.66 | −0.10 | 0.62 | −0.08 | 0.68 | ||||
| Local reaction after each dose (sum of symptom score) | −0.06 | 0.77 | - | - | 0.02 | 0.03 | 0.89 | - | - | 0.03 | ||
| Systemic reaction after each dose (sum of symptom score) | - | - | 0.02 | 0.91 | 0.01 | - | - | 0.19 | 0.33 | 0.06 | ||
| Log 50% neutralizing Ab | ||||||||||||
| Age | −0.02 | 0.93 | 0.03 | 0.87 | −0.15 | 0.45 | −0.14 | 0.49 | ||||
| Female sex | 0.04 | 0.86 | 0.009 | 0.96 | 0.16 | 0.45 | 0.16 | 0.45 | ||||
| Occupation | ||||||||||||
| Doctor | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Nurse | 0.05 | 0.82 | −0.01 | 0.96 | 0.05 | 0.81 | 0.05 | 0.80 | ||||
| Local reaction after each dose (sum of symptom score) | −0.10 | 0.60 | - | - | 0.01 | 0.03 | 0.88 | - | - | 0.08 | ||
| Systemic reaction after each dose (sum of symptom score) | - | - | 0.18 | 0.35 | 0.03 | - | - | 0.05 | 0.81 | 0.08 | ||
Ref, reference value.
We performed an additional analysis comparing Ab response according to the severity grade of reactogenicity by grade 0–4. There were no significant differences in S1-specific IgG Ab and neutralizing Ab according to the severity grade at 3 weeks after the 1st dose vaccination in both ChAdOx1 and BNT162b2 (Supplementary Figs. 2 and 3). However, age- and sex-adjusted analysis revealed significantly higher Ab titer in ChAdOx1 with more than a moderate grade of local reaction in both S1-specific IgG and neutralizing Ab (Supplementary Table 2) but not after the 2nd dose of BNT162b2 (Supplementary Table 3). The S1-specific IgG Ab titer at 2 weeks after the 2nd dose of ChAdOx1 was significantly higher with more than moderate grade local reaction which indicated limitation in daily activity (p=0.02; Supplementary Fig. 4), but not after the 2nd dose of BNT162b2 (p=0.34; Supplementary Fig. 4). Also, neutralizing Ab titer at 2 weeks after the 2nd dose of ChAdOx1 and BNT162b2 did not show significant difference according to the severity grade (Supplementary Fig. 5). However, age- and sex-adjusted analysis revealed significantly higher Ab titer with more than a moderate grade of local reaction in both S1-specific IgG and neutralizing Ab after the 2nd dose of ChAdOx1 (p=0.00007 and p=0.007, respectively, Supplementary Table 2) but not after the 2nd dose of BNT162b2 (Supplementary Table 3). The S1-specific IgG titer after 1st dose was not significantly associated with age or sex in both groups (Appendix 2).
T cell responses at 3 weeks after vaccination were measured in 33 (34%) HCWs in the ChAdOx1 group and all HCWs in the BNT162b2 group. The degrees of local and systemic reactogenicity in terms of sum of symptom score did not show significant associations with the T cell responses in both the ChAdOx1 group (p=0.71 and p=0.99; Supplementary Fig. 6) and the BNT162b2 group (p=0.57 and p=0.60; Supplementary Fig. 6). Age- and sex-adjusted multivariate analysis also did not reach statistical significance in both group (Supplementary Table 4). Neither age nor sex was significantly associated with the T cell responses (Appendix 3). The S1-specific IgG used in this study had a moderate or good correlation with the neutralizing Ab assay using authenic SARS-CoV-2 (Appendix 4).
DISCUSSION
We observed that the degree of local reaction after the ChAdOx1 vaccination was significantly associated with the level of SARS-CoV-2-specific Ab titer at 3 weeks after the 1st dose (including neutralizing Ab) and at 2 weeks after the 2nd dose vaccination in age- and sex-adjusted multivariate analysis. Adenoviral vectors harbor adjuvant properties that stimulate the innate immune responses by inducing the secretion of type I IFN through TLR-9 from Ag presenting cell including dendritic cells at the injection site (10). Vaccine-derived type 1 IFN is known to provide second signal for activating differentiation of helper T cells that promote B cell differentiation to Ab-secreting plasma cells. In addition, the type 1 IFN induced by antigen presenting cells entered with adenoviral vector deliver signal to T cells in lymph nodes that drain the injection site, which might partially explain the local injection site pain, redness and swelling. Thus, we presumed that the reactogenicity representing innate immunity by adenoviral vector might correlates to subsequent high Ab responses. Therefore, the reactogenicity of adenovirus-based vaccines such as the ChAdOx1 nCoV-19 may translate into subsequent high Ab responses. The previous study also showed the association between Ab response and adverse events after the 1st dose of ChAdOx1 (11). However, our findings are in contrast with a recent study in which the reactogenicity after the 1st dose of ChAdOx1 vaccine did not show a significant association with anti-S1 IgG levels (12), which may be due to the relatively small number of participants (n=42). Interestingly, we found that there was a significant difference in Ab titer both after the 1st and 2nd doses of ChAdOx1 nCov-19 (ChAdOx1) using a similar analysis with a previous study (12) (Supplementary Tables 2). In contrast, our study participants who received the BNT162b2 mRNA vaccine did not show a significant association between reactogenicity and humoral or cellular immune responses. This may be due to the fact that the currently used mRNA-based COVID-19 vaccines have modifications in the nucleotides that reduce the binding to TLR, which leads to limited production of type 1 interferon and its inhibitory function on cellular translation (10). However, other factors such as IL-6, IL-1β, TNF-α, and prostaglandin E2 may be associated with the adverse effects. Furthermore, it is unclear why local reactogenicity, not systemic reactogenicity, was only correlated with Ab response in ChAdOx1-vaccinated individuals. Further studies are needed on the follow-up study for our observation and the immunologic mechanism for this phenomenon.
It is worth noting that age was not significantly associated with the SARS-CoV-2 Ab response after both vaccines. A previous study reported that Ab responses after BNT162b2 decreased with age (13). This discrepancy may be largely due to the distribution of the age of the participants between the 2 studies, as while less than 10% of the participants in our study were over 50 years of age, about half of the participants in the previous study were older than 80 years.
Our findings may have been affected by those with severe reactogenicity who used antipyretics or non-steroidal anti-inflammatory drugs, which are known to affect the immune responses after vaccination. Moreover, the relatively small number of BNT162b2 vaccinees may limit the interpretation of our analysis. Despite these limitations, our study may provide an answer to the common question evoked by the general population on whether people who experience less reactogenicity after COVID-19 vaccination have less immune response against SARS-CoV-2. Our findings offer an important piece of scientific evidence that there was no association of SARS-CoV-2-specific immune response with the reactogenicity of the 1st or 2nd dose of the BNT162b2 vaccine. In contrast, there was a significant association of SARS-CoV-2-specific Ab response with the local reactogenicity of the 1st and 2nd doses of the ChAdOx1 vaccine. Accordingly, our findings suggest that a high degree of local reactogenicity after the ChAdOx1 vaccine may be used as a surrogate marker of strong humoral immune responses including the titer of neutralizing Ab against SARS-CoV-2.
ACKNOWLEDGEMENTS
This study was supported by grants from the National Research Foundation of Korea (NRF), which is funded by the Ministry of Science and ICT, Republic of Korea (grant No. MSIT, No 2020M3H8A1115041 and 2017M3A9G6068254).
Abbreviations
- COVID-19
coronavirus disease 2019
- HCW
healthcare worker
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
Appendix 1
Questionnaire sheet for adverse events after vaccination
This is a questionnaire for monitoring local and systemic adverse reactions after COVID-19 vaccination. To understand the adverse reactions after receiving the coronavirus vaccine, we are going to collect and use personal information for monitoring all employees as follows. All information will be anonymized and fully encrypted before analysis. The survey collects employee ID code, sex, age, body weight, height, and adverse reactions.
✓ I agree to the collection and use of personal information.
① Yes ② No
(Question 1) Please give us some information about yourself.
1-1. Please fill in your employee number. ____________.
1-2. Sex ① Male ② Female
1-3. Age ___________.
1-4. Body weight ___________.
1-5. Height ___________.
(Question 2) Did you experience any adverse reactions (shown below) after vaccination?
① No
② Yes (please check the specific items below)
(Question 3) Symptom checklist with severity scores. You only need to check the items that you experienced. Unchecked items are considered symptom-free. Please grade the severity of adverse events as following criteria: mild (transient or mild discomfort, no interference with daily activity, and no requirement of medical intervention or therapy), moderate (mild-to-moderate limitation in daily activity, and no or minimal requirement of medical intervention or therapy), severe (substantial limitation in daily activity and requirement of medical intervention or therapy), or potentially life-threatening (requires assessment in an emergency department or hospital admission).
3-1. Pain at the injection site ① mild ② moderate ③ severe ④ potentially life-threatening
3-2. Redness at the injection site (except for minor redness of less than 2cm)
① 2.0 to 5.0 cm in diameter; larger than the size of a coin
② >5.0 to 10.0 cm in diameter; larger than the size of an egg
③ >10.0 cm in diameter; larger than the size of a fist
④ necrosis or exfoliative dermatitis
3-3. Swelling at the injection site (except for minor redness of less than 2cm)
① 2.0 to 5.0 cm in diameter; larger than the size of a coin
② >5.0 to 10.0 cm in diameter; larger than the size of an egg
③ >10.0 cm in diameter; larger than the size of a fist
④ necrosis
3-4. Vomiting ① 1-2 times within 24h ② more than 2 times within 24h ③ needs fluid therapy ④ potentially life-threatening
3-5. Diarrhea ① 2-3 times within 24h ② 4-5 times within 24h ③ more than 5 times ④ potentially life-threatening
3-6. Headache ① mild ② moderate ③ severe ④ potentially life-threatening
3-7. Fatigue/tiredness ① mild ② moderate ③ severe ④ potentially life-threatening
3-8. Chills ① mild ② moderate ③ severe ④ potentially life-threatening
3-9. Muscle pain ① mild ② moderate ③ severe ④ potentially life-threatening
3-10. Arthralgia ① mild ② moderate ③ severe ④ potentially life-threatening
3-11. Fever ① 38.0–38.4°C ② 38.5–38.9°C ③ 39.0–40.0°C ④ >40.0°C
Appendix 2
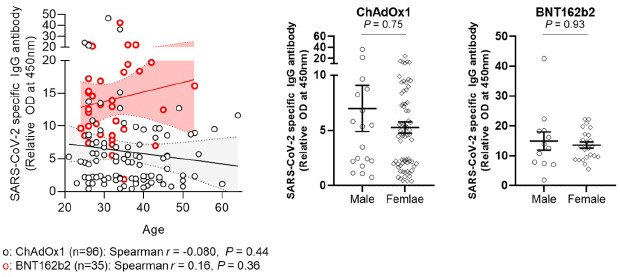
Association between Ab response against SARS-CoV-2 and age (left panel) or sex (right panel) after 1st dose vaccination. Solid lines denote the mean and dotted lines denote the 95% confidential intervals (left panel). Horizontal bars denote the mean values and error bars denote the standard error of the mean values (right panel).
Appendix 3
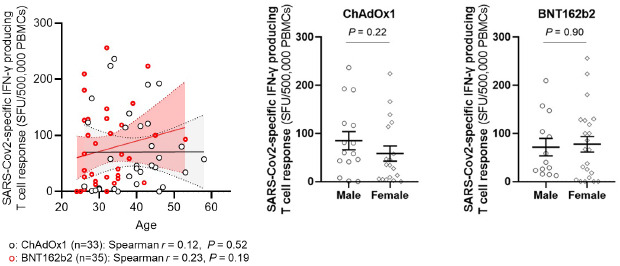
Association between T cell response against SARS-CoV-2 and age (left panel) or sex (right panel). Solid lines denote the mean and dotted lines denote the 95% confidential intervals (left panel). Horizontal bars denote the mean values and error bars denote the standard error of the mean values (right panel).
Appendix 4
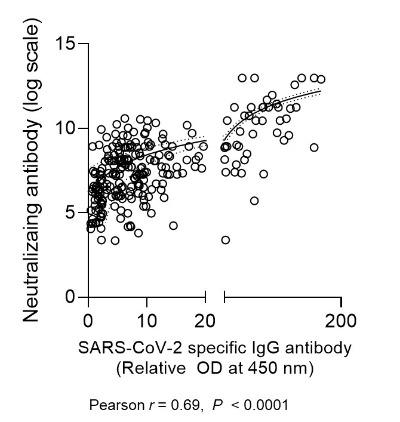
The correlation of SARS-CoV-2 S1-specific IgG ELISA and neutralizing Ab (microneutralization assay).
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Kim SH, Jee Y, Bae S, Jung J.
- Data curation: Lim SY, Kwon JS, Park JY, Cha HH, Suh MH, Lee HJ.
- Formal analysis: Lim SY, Kim JY, Park S, Kwon JS, Cha HH, Lee N, Kim K.
- Funding acquisition: Kim SH, Jee Y.
- Investigation: Lim SY, Kim JY, Park S, Kwon JS, Cha HH.
- Methodology: Lim SY, Lim JS, Kim SH.
- Project administration: Shum D, Kim SH, Jee Y.
- Software: Lim SY, Kim JY, Kwon JS.
- Supervision: Kim JY, Shum D, Jee Y, Kim SH.
- Validation: Kim SH, Jee Y, Shum D.
- Visulization: Lim SY.
- Writing - original draft: Lim SY, Kim JY, Kwon JS, Kim SH.
- Writing - review & editing: Lim SY, Kim JY, Park S, Kwon JS, Park JY, Cha HH, Suh MH, Lee HJ, Lim JS, Bae S, Jung J, Lee N, Kim K, Shum D, Jee Y, Kim SH.
SUPPLEMENTARY MATERIALS
Reactogenicity and immunogenicity of study participants by type of vaccine
Variables associated with S1-specific IgG and neutralizing Ab of ChAdOx1 nCoV-19 vaccine in multivariate analysis
Variables associated with S1-specific IgG and neutralizing Ab of BNT162b2 mRNA vaccine in multivariate analysis
Variables associated with SARS-CoV-2-specific INF-γ producing T cell in ChAdOx1 and BNT162b2 vaccines in multivariate analysis
Flowchart of the study participants.
Reactogenicity and S1-specific IgG Ab after 3 weeks of 1st dose vaccination. Horizontal lines denote the mean and error bars denote the standard error of the mean. Kruskal-Wallis test was used for the comparison of mean value by grade. (A) SARS-CoV-2 S1-specific IgG and local reaction after 1st dose ChAdOx1. (B) SARS-CoV-2 S1-specific IgG and systemic reaction after 1st dose ChAdOx1. (C) SARS-CoV-2 S1-specific IgG and local reaction after 1st dose BNT162b2. (D) SARS-CoV-2 S1-specific IgG and systemic reaction after 1st dose BNT162b2.
Reactogenicity and neutralizing Ab after 3 weeks of 1st dose vaccination. Horizontal lines denote the mean and error bars denote the standard error of the mean. Kruskal-Wallis test was used for the comparison of mean value by grade. (A) Neutralizing Ab and local reaction after 1st dose ChAdOx1. (B) Neutralizing Ab and systemic reaction after 1st dose ChAdOx1. (C) Neutralizing Ab and local reaction after 1st dose BNT162b2. (D) Neutralizing Ab and systemic reaction after 1st dose BNT162b2.
Reactogenicity and S1-specific IgG Ab after 2 weeks of 2nd dose vaccination. Horizontal lines denote the mean and error bars denote the standard error of the mean. Kruskal-Wallis test was used for the comparison of mean value by grade. (A) SARS-CoV-2 S1-specific IgG and local reaction after 2nd dose ChAdOx1. (B) SARS-CoV-2 S1-specific IgG and systemic reaction after 2nd dose ChAdOx1. (C) SARS-CoV-2 S1-specific IgG and local reaction after 2nd dose BNT162b2. (D) SARS-CoV-2 S1-specific IgG and systemic reaction after 2nd dose BNT162b2.
Reactogenicity and neutralizing Ab after 2 weeks of 2nd dose vaccination. Horizontal lines denote the mean and error bars denote the standard error of the mean. Kruskal-Wallis test was used for the comparison of mean value by grade. (A) Neutralizing Ab and local reaction after the 2nd dose ChAdOx1. (B) Neutralizing Ab and systemic reaction after 2nd dose ChAdOx1. (C) Neutralizing Ab and local reaction after 2nd dose BNT162b2. (D) Neutralizing Ab and systemic reaction after 2nd dose BNT162b2.
Correlation of reactogenicity and T cell response after the 1st doses of the ChAdOx1 and BNT162b2 vaccines. Solid lines denote the mean and dotted lines denote the 95% confidential intervals. (A) T cell responses and local reaction. (B) T cell responses and systemic reaction.
References
- 1.Bae S, Lee YW, Lim SY, Lee JH, Lim JS, Lee S, Park S, Kim SK, Lim YJ, Kim EO, et al. Adverse reactions following the first dose of Chadox1 nCov-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021;36:e115. doi: 10.3346/jkms.2021.36.e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hervé C, Laupèze B, Del Giudice G, Didierlaurent AM, Tavares Da Silva F. The how's and what's of vaccine reactogenicity. NPJ Vaccines. 2019;4:39. doi: 10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SH, Bang JW, Park KH, Park WB, Kim HB, Kim NJ, Jee Y, Cho H, Oh MD, Choe KW. Prediction of residual immunity to smallpox, by means of an intradermal skin test with inactivated vaccinia virus. J Infect Dis. 2006;194:377–384. doi: 10.1086/505505. [DOI] [PubMed] [Google Scholar]
- 4.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. Geneva: World Health Organization; 1988. [Google Scholar]
- 5.Wulff H, Chin TD, Wenner HA. Serologic responses of children after primary vaccination and revaccination against smallpox. Am J Epidemiol. 1969;90:312–318. doi: 10.1093/oxfordjournals.aje.a121075. [DOI] [PubMed] [Google Scholar]
- 6.Center for Biologics Evaluation and Research. Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Silver Spring, MD: U.S. Department of Health and Human Services FDA; 2007. [Google Scholar]
- 7.Classen DC, Morningstar JM, Shanley JD. Detection of antibody to murine cytomegalovirus by enzyme-linked immunosorbent and indirect immunofluorescence assays. J Clin Microbiol. 1987;25:600–604. doi: 10.1128/jcm.25.4.600-604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lardeux F, Torrico G, Aliaga C. Calculation of the ELISA's cut-off based on the change-point analysis method for detection of Trypanosoma cruzi infection in Bolivian dogs in the absence of controls. Mem Inst Oswaldo Cruz. 2016;111:501–504. doi: 10.1590/0074-02760160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JY, Kwon JS, Bae S, Cha HH, Lim JS, Kim MC, Chung JW, Park SY, Lee MJ, Kim BN, et al. SARS-CoV-2-specific antibody and T cell response kinetics according to symptom severity. Am J Trop Med Hyg. 2021;105:395–400. doi: 10.4269/ajtmh.20-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JY, Choi SH, Chung JW, Hwang MH, Kim MC. Systemic adverse events and use of antipyretics predict the neutralizing antibody positivity early after the first dose of chadox1 coronavirus disease vaccine. J Clin Med. 2021;10:2844. doi: 10.3390/jcm10132844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang YH, Song KH, Choi Y, Go S, Choi SJ, Jung J, Kang CK, Choe PG, Kim NJ, Park WB, et al. Can reactogenicity predict immunogenicity after COVID-19 vaccination? Korean J Intern Med. 2021;36:1486–1491. doi: 10.3904/kjim.2021.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller L, Andrée M, Moskorz W, Drexler I, Walotka L, Grothmann R, Ptok J, Hillebrandt J, Ritchie A, Rabl D, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reactogenicity and immunogenicity of study participants by type of vaccine
Variables associated with S1-specific IgG and neutralizing Ab of ChAdOx1 nCoV-19 vaccine in multivariate analysis
Variables associated with S1-specific IgG and neutralizing Ab of BNT162b2 mRNA vaccine in multivariate analysis
Variables associated with SARS-CoV-2-specific INF-γ producing T cell in ChAdOx1 and BNT162b2 vaccines in multivariate analysis
Flowchart of the study participants.
Reactogenicity and S1-specific IgG Ab after 3 weeks of 1st dose vaccination. Horizontal lines denote the mean and error bars denote the standard error of the mean. Kruskal-Wallis test was used for the comparison of mean value by grade. (A) SARS-CoV-2 S1-specific IgG and local reaction after 1st dose ChAdOx1. (B) SARS-CoV-2 S1-specific IgG and systemic reaction after 1st dose ChAdOx1. (C) SARS-CoV-2 S1-specific IgG and local reaction after 1st dose BNT162b2. (D) SARS-CoV-2 S1-specific IgG and systemic reaction after 1st dose BNT162b2.
Reactogenicity and neutralizing Ab after 3 weeks of 1st dose vaccination. Horizontal lines denote the mean and error bars denote the standard error of the mean. Kruskal-Wallis test was used for the comparison of mean value by grade. (A) Neutralizing Ab and local reaction after 1st dose ChAdOx1. (B) Neutralizing Ab and systemic reaction after 1st dose ChAdOx1. (C) Neutralizing Ab and local reaction after 1st dose BNT162b2. (D) Neutralizing Ab and systemic reaction after 1st dose BNT162b2.
Reactogenicity and S1-specific IgG Ab after 2 weeks of 2nd dose vaccination. Horizontal lines denote the mean and error bars denote the standard error of the mean. Kruskal-Wallis test was used for the comparison of mean value by grade. (A) SARS-CoV-2 S1-specific IgG and local reaction after 2nd dose ChAdOx1. (B) SARS-CoV-2 S1-specific IgG and systemic reaction after 2nd dose ChAdOx1. (C) SARS-CoV-2 S1-specific IgG and local reaction after 2nd dose BNT162b2. (D) SARS-CoV-2 S1-specific IgG and systemic reaction after 2nd dose BNT162b2.
Reactogenicity and neutralizing Ab after 2 weeks of 2nd dose vaccination. Horizontal lines denote the mean and error bars denote the standard error of the mean. Kruskal-Wallis test was used for the comparison of mean value by grade. (A) Neutralizing Ab and local reaction after the 2nd dose ChAdOx1. (B) Neutralizing Ab and systemic reaction after 2nd dose ChAdOx1. (C) Neutralizing Ab and local reaction after 2nd dose BNT162b2. (D) Neutralizing Ab and systemic reaction after 2nd dose BNT162b2.
Correlation of reactogenicity and T cell response after the 1st doses of the ChAdOx1 and BNT162b2 vaccines. Solid lines denote the mean and dotted lines denote the 95% confidential intervals. (A) T cell responses and local reaction. (B) T cell responses and systemic reaction.