Highlights
-
•
NaV1.7 is a prized pain target for which few drugs exist.
-
•
Allosteric regulation of NaV1.7 via the interacting protein CRMP2 is antinociceptive.
-
•
Small molecule 194, which blocks CRMP2 SUMOylation, decreases NaV1.7 to achieve antinociception in neuropathic pain models.
-
•
194 reverses and prevents pain chronification in a mouse model of oxaliplatin-induced neuropathic pain.
Abbreviations: CIPN, chemotherapy induced peripheral neuropathy; DRG, dorsal root ganglia; CRMP2, collapsin response mediator protein 2; NaV1.7, voltage-gated sodium channel family 1 isoform 7; TTX, tetrodotoxin; TTX-S, tetrodotoxin-sensitive; TTX-R, tetrodotoxin-resistant; SUMO, smallubiquitin like modifier; Ubc9, E2 SUMO-conjugating enzyme; SNI, spared nerve injury; t-CSM, tat-CRMP2 SUMOylation motif; PWT, paw withdrawal threshold; CRISPR, clustered regularly interspaced short palindromic repeats
Keywords: Oxaliplatin, Chemotherapy, Neuropathy, NaV1.7, CRMP2, SUMOylation
Abstract
Treatment with anti-neoplastic agents can lead to the development of chemotherapy induced peripheral neuropathy (CIPN), which is long lasting and often refractory to treatment. This neuropathic pain develops along dermatomes innervated by peripheral nerves with cell bodies located in the dorsal root ganglia (DRG). The voltage-gated sodium channel NaV1.7 is expressed at high levels in peripheral nerve tissues and has been implicated in the development of CIPN. Efforts to develop novel analgesics directly inhibiting NaV1.7 have been unsuccessful, and our group has pioneered an alternative approach based on indirect modulation of channel trafficking by the accessory protein collapsin response mediator protein 2 (CRMP2). We have recently reported a small molecule, compound 194, that inhibits CRMP2 SUMOylation by the E2 SUMO-conjugating enzyme Ubc9 (Cai et al. , Sci. Transl. Med. 2021 13(6 1 9):eabh1314). Compound 194 is a potent and selective inhibitor of NaV1.7 currents in DRG neurons and reverses mechanical allodynia in models of surgical, inflammatory, and neuropathic pain, including spared nerve injury and paclitaxelinduced peripheral neuropathy. Here we report that, in addition to its reported effects in rats, 194 also reduces mechanical allodynia in male CD-1 mice treated with platinumcomplex agent oxaliplatin. Importantly, treatment with 194 prevented the development of mechanical allodynia when co-administered with oxaliplatin. No effects were observed on the body weight of animals treated with oxaliplatin or 194 throughout the study period. These findings support the notion that 194 is a robust inhibitor of CIPN that reduces established neuropathic pain and prevents the emergence of neuropathic pain during treatment with multiple anti-neoplastic agents in both mice and rats.
Introduction
Therapeutic agents used to treat cancers, especially those belonging to the platinum-complex, taxane, vinca alkaloid, and proteosome inhibitor classes are known to cause chronic peripheral neuropathy that can last for years following treatment (Gadgil et al., 2019, Xiao et al., 2012). These patients are left with a debilitating pain condition, known as chemotherapy induced peripheral neuropathy (CIPN), which is often refractory to treatment with available analgesics (Gordon-Williams and Farquhar-Smith, 2020). Patients that develop CIPN present to the clinic with sensory, motor, and autonomic deficits that develop in a characteristic glove and stocking pattern due to differential toxicity among neurons with longer axons (Starobova and Vetter, 2017). Given the high burden associated with neuropathy following treatment with anti-neoplastic agents, identification of new compounds that can prevent or reverse this type of pain is essential.
Peripheral pain detecting neurons, known as nociceptors, have cell bodies located in the dorsal root ganglia (DRG) with bifurcated projections innervating the skin and the dorsal horn of the spinal cord (Woolf and Ma, 2007). Neurons of the DRG are particularly sensitive to damage following treatment with chemotherapeutic drugs and demonstrate accumulation of these agents following repeated administration (Jimenez-Andrade et al., 2008). This increased exposure of DRG neurons is thought to be a result of significant vascularization of these structures (Cavaletti et al., 2000). The voltage-gated sodium channel NaV1.7 is found primarily in peripheral tissues, including DRG neurons, and its expression is upregulated in multiple pain states (Herzog et al., 2003, Mukai et al., 2014, Sangameswaran et al., 1997, Siqueira et al., 2009, Toledo-Aral et al., 1997). The biophysical properties of NaV1.7 position this channel to amplify subthreshold stimuli and ultimately determine the threshold for action potential generation in nociceptors (Dib-Hajj et al., 2013). This critical role in pain sensation is exemplified by human pain conditions where NaV1.7 gain of function mutations have been linked to severe pain disorders and loss of function mutations are associated with insensitivity to pain (Cox et al., 2006, Yang et al., 2004).
In addition to its involvement in human genetic pain disorders, recent evidence has emerged suggesting that NaV1.7 is also important in the pathophysiology of CIPN. Treatment with the chemotherapeutic agent paclitaxel increases expression of NaV1.7 in rat DRG neurons, which correlated with onset of mechanical allodynia (Wang et al., 2020, Xiao et al., 2016). Cultured rat DRG neurons treated with paclitaxel displayed increased NaV1.7 membrane localization and increased vesicular transport of the channel to distal axonal terminals (Akin et al., 2021). Furthermore, silencing NaV1.7 expression using either a zinc finger protein or a CRISPR construct targeting Scn9a, the gene encoding NaV1.7, resulted in robust reversal of the neuropathic pain phenotype in mice with CIPN (Moreno et al., 2021). Interestingly, another report identified a single nucleotide polymorphism in a Japanese family that predisposed patients to development of CIPN following treatment with drugs in the taxane class (Tanabe et al., 2020). These findings support a crucial role for NaV1.7 in mediating development of sensory neuropathy following treatment with anti-neoplastic agents and suggests targeting NaV1.7 is a viable strategy for ameliorating CIPN associated pain.
While the essential role for NaV1.7 in pain transmission is clear and its involvement in CIPN is emerging, targeting NaV1.7 for analgesia has proven difficult. Many attempts have been made to directly inhibit channel function, leading to the development of small molecule inhibitors, as well as venom derived peptides, that are highly potent (Deuis et al., 2017, McCormack et al., 2013). This approach has produced candidate compounds displaying profound selectivity for human NaV1.7 over other channel isoforms (Alexandrou et al., 2016, Theile et al., 2016). Despite this, clinical trials of direct NaV1.7 inhibiting compounds have largely failed to demonstrate analgesic efficacy (Siebenga et al., 2020). Recent reports have suggested that failure in translation from preclinical studies to the clinic could be related to the homogeneity of pain models used to evaluate novel analgesics, which fail to capture the heterogenous disease mechanisms in patients (Berge, 2011, Eagles et al., 2020). Furthermore, a lack of diversity in the species used to test these candidate compounds in the preclinical stage could also contribute to the high attrition rates observed (Sadler et al., 2021). The failure in direct NaV1.7 inhibitors has led our group to develop a different strategy focused on disruption of protein–protein interaction networks to alter channel trafficking to relieve pain (Chew et al., 2019, Chew and Khanna, 2018).
Exploration of NaV1.7 interacting partners using a mouse expressing an epitope tagged NaV1.7 revealed an interaction between NaV1.7 and the cytosolic phosphoprotein collapsin response mediator protein 2 (CRMP2) (Chew et al., 2019, Kanellopoulos et al., 2018). Previous work from our group suggested that CRMP2 could regulate specific channel populations since knockdown of CRMP2 expression in DRG neurons, using a validated siRNA, reduced TTX-S currents but had no effect on TTX-R currents (Dustrude et al., 2016). We posited that this specificity could arise from precise control of the post-translational modification state of CRMP2. One such modification includes the covalent addition of an ∼ 11 kDa protein known as a small ubiquitin like modifier (SUMO) by the E2 SUMO-conjugating enzyme Ubc9 at target lysine residues (Flotho and Melchior, 2013, Moutal et al., 2019). There is an established role for SUMOylation dependent control of potassium channel inactivation, which indicates that SUMOylation can control ion channel function (Benson et al., 2009, Benson et al., 2007). We have shown that loss of CRMP2 SUMOylation in vitro, by substituting lysine at position 374 with alanine, specifically reduced NaV1.7 currents without affecting currents from other voltage-gated sodium channels (Dustrude et al., 2016, Dustrude et al., 2013). This reduction in sodium current density following loss of CRMP2 SUMOylation was due to clathrin-dependent internalization of the channel via recruitment of an endocytic complex (Dustrude et al., 2016, Gomez et al., 2021, Moutal et al., 2020). In neuropathic pain states, SUMOylation of CRMP2 is increased in the spinal cord, glabrous skin, and sciatic nerve (Moutal et al., 2018). Preventing CRMP2 SUMOylation with a cell penetrant interfering peptide, corresponding to the CRMP2 SUMOylation motif (t-CSM), was sufficient to reverse spared nerve injury (SNI) induced neuropathic pain (François-Moutal et al., 2018a). To further illustrate that SUMOylation was essential for NaV1.7 regulation in vivo we generated a SUMO-null transgenic mouse harboring a knock-in CRMP2K374A/K374A mutation (Moutal et al., 2020). These animals had reduced peak DRG sodium current density, reduced NaV1.7 membrane localization, and were resilient to the development of neuropathic pain. Collectively, these findings led us to conclude that CRMP2 SUMOylation serves as a regulatory node that controls NaV1.7 trafficking, which has implications for the development of novel therapeutics.
2. Methods
2.1. Animals
Pathogen-free adult male CD-1 mice (20–30 g; Envigo, USA) were used. Mice were housed 4–5 per cage in a light- and temperature-controlled room (12:12-h light–dark cycle, lights on at 6:00 am) with food and water available ad libitum. No animals were excluded from this study for any reason. All procedures were approved by the Saint Louis University School of Medicine Institutional Animal Care and Use Committee.
2.2. Chemotherapy induced peripheral neuropathic pain
Male ICR mice were treated daily for five consecutive days with oxaliplatin (3 mg/kg; i.p.) or vehicle (5% dextrose) between experimental days 0–4 and again between days 10–14 (Wahlman et al., 2018). The total cumulative dose was 30 mg/kg per mouse.
2.3. Drug administration
194 was prepared in the appropriate diluent [10% DMSO, (Sigma-Aldrich, St.Louis, MO) 10% tween-80 (Sigma-Aldrich) in saline (Hospira, Inc., Lake Forest, IL)] and administered by oral gavage. For prevention experiments, 194 (2 mg/kg) or vehicle control (10% DMSO, 10% Tween-80, 80% saline) was administered concurrently with oxaliplatin. For the reversal experiments, a single dose of 10 mg/kg 194 or vehicle was administered orally on day 25. An independent experimenter performed dosing to blind the behavioral experimenter to the treatment groups.
2.4. Mechanical allodynia
Mice were placed in elevated chambers (28 X 40 X 35 cm) placed on a wire mesh floor and allowed to acclimate for 30 min prior to behavioral testing. The mechanical paw withdrawal threshold in grams [PWT, (g)] was measured manually with von Frey filaments according to the up and down method (Dixon, 1980) [Stoelting, ranging from 2.36 (0.02 g) to 4.31 (2 g) bending force]. The development of mechanical allodynia is indicated by a significant (p < 0.05) reduction in mean absolute PWT (g) at forces that fail to elicit withdrawal responses before chemotherapy treatment (Day 0). Treatment with chemotherapeutic agents results in bilateral allodynia with no differences between the left and right paw withdrawal thresholds observed, therefore values for both paws were averaged to determine the PWT. Animals receiving chemotherapeutic agents in the presence or absence of 194 did not display signs of any toxicity: i.e., they exhibited normal posture, grooming, locomotor behavior, hair coat was normal, no signs of piloerection or ocular porphyrin discharge, and gained body weight normally, in a fashion that was comparable to vehicle-treated mice.
2.5. Statistical analyses
All data was first tested for a Gaussian distribution using a D’Agostino-Pearson test (Prism 9 Software, Graphpad, San Diego, CA). All data was analyzed using the two-way ANOVA test followed by the Bonferroni test for multiple comparisons. Differences were considered significant if p ≤ 0.05. Error bars in the graphs represent mean ± SD. All data were plotted in Prism 9.
3. Results and discussion
Encouraged by the findings from our studies using the t-CSM peptide (François-Moutal et al., 2018a) and the CRMP2K374A/K374A transgenic mouse (Moutal et al., 2020), we embarked upon a campaign to design a small molecule to disrupt the interaction between CRMP2 and Ubc9. We had previously identified key residues surrounding K374 in CRMP2, specifically R440 and V371, that were critical for interaction with Ubc9 and were therefore suitable for small molecule targeting (François-Moutal et al., 2018b). In silico screening of the CRMP2 interface that facilitates SUMO conjugation by Ubc9 identified a series of compounds that could potentially disrupt this interaction. Subsequent in vitro characterization of the top scoring compounds revealed a benzoylated 2-(4-piperidinyl)-1,3-benzimidazole analog capable of interfering with CRMP2 SUMOylation, which we refer to as compound 194 (Fig. 1A) (Cai et al., 2021, Kingwell, 2021). A recent report from our group outlines the promising characteristics of 194, which shows excellent specificity for inhibiting NaV1.7 currents in cultured DRG neurons (Cai et al., 2021). Treatment with 194 reduced membrane localization of NaV1.7 in a clathrin-dependent manner and reduced presynaptic NaV1.7 in the spinal cord. Furthermore, treatment with compound 194 reversed mechanical allodynia following spared nerve injury (SNI) and after treatment with paclitaxel in male rats (Cai et al., 2021). Since vesicular trafficking and membrane localization of NaV1.7 are increased in CIPN, it is possible that treatment with 194 could normalize this dysregulated trafficking to restore normal levels of nociception.
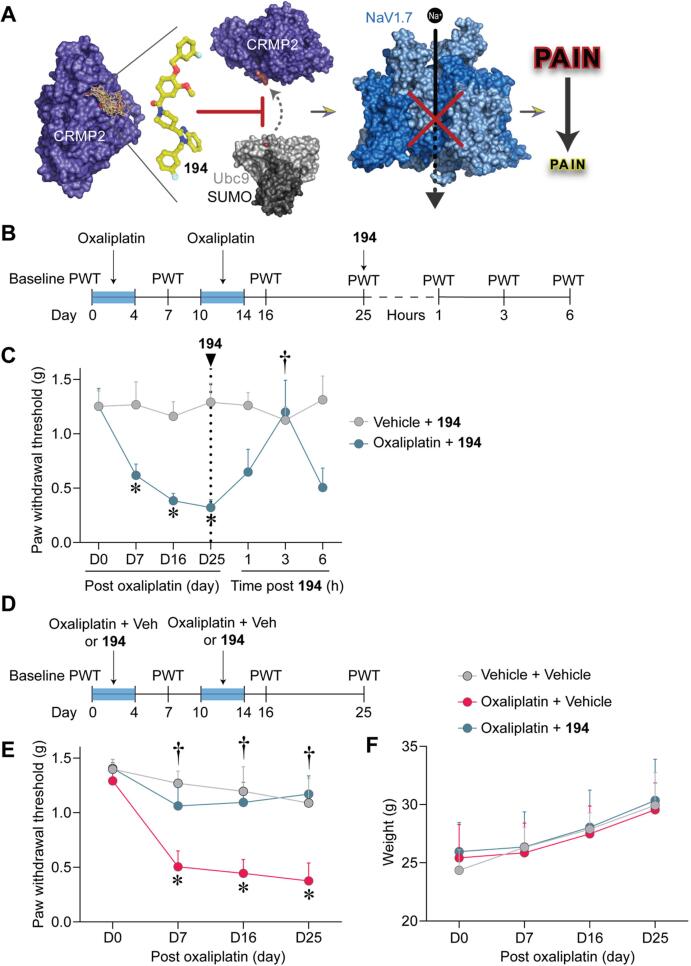
Fig. 1.
194 reduces and prevents the development of mechanical allodynia in male CD-1 mice with oxaliplatin induced peripheral neuropathy. (A) Compound 194 discovery and validation. 194 was designed using the structure–activity relationships of compounds obtained from a virtual screen against a pocket encompassing the SUMOylation target on CRMP2 (PDB 2GSE (Stenmark et al., 2007)). 194 effectively blocks SUMOylation of CRMP2 by the E2 SUMO-conjugating enzyme Ubc9 (PDB 5D2M (Cappadocia et al., 2015)) to reduce cell-surface trafficking of NaV1.7 (PDB 6J8H (Shen et al., 2019)). This results in dramatically reduced sodium currents and amelioration of pain in animal models. Graphic generated with BioRender. (B) Experimental design for behavioral assessment of oral administration of 194 on reversal of oxaliplatin induced CIPN. Baseline paw withdrawal threshold (PWT, in grams) was established at Day 0, followed by induction of CIPN with administration of oxaliplatin between Day 0–4 and Day 10–14 as indicated by the blue box. Mechanical allodynia was evaluated by measuring PWTs at regular intervals as indicated in the timeline. On day 25, mice were orally administered 194 and paw withdrawal thresholds were tested over six hours as indicated. (C) Mice treated with Vehicle and 194 displayed stable PWTs over the experimental time course (gray). Those treated with oxaliplatin developed robust mechanical allodynia that peaked at Day 25 (blue). Treatment with 194 completely reversed the oxaliplatin induced mechanical allodynia to vehicle treated levels 3 h following oral administration. Data are mean ± SD; Two-Way ANOVA with Bonferroni’s multiple comparison *p < 0.05 vs D0; †p < 0.05 vs D25; Oxaliplatin + 194n = 4; Vehicle + 194n = 5. (D) Experimental outline of prevention experiments performed in male CD-1 mice. Baseline PWTs was first established at Day 0, followed by treatment with Vehicle + Vehicle, Oxaliplatin + Vehicle, or Oxaliplatin + 194. PWTs were then assessed at regular intervals as indicated. Blue boxes indicate periods of treatment with oxaliplatin. (E) Mice treated with Vehicle + Vehicle had stable PWTs across the experimental time course (gray). Treatment with Oxaliplatin + Vehicle demonstrated robust mechanical allodynia in male mice (pink). Treatment with Oxaliplatin + 194 prevented the development of mechanical allodynia in these mice (blue). (F) Evaluation of the body weight of mice across the experimental period revealed no significant differences between groups of animals. Data are mean ± SD; Two-Way ANOVA with Bonferroni’s multiple comparison †p < 0.05 vs Oxaliplatin + Vehicle; *p < 0.05 vs D0; Vehicle + Vehicle n = 4; Oxaliplatin n = 5 per group. Experimenters were blinded to the treatment conditions. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Since multiple anti-neoplastic agents are employed in the clinic, it is important to establish that new analgesic candidates are effective in treating neuropathy induced by agents from multiple therapeutic classes. Considering the need for therapies for CIPN, and the importance of cross species validation, we asked whether 194 could reverse mechanical allodynia associated with CIPN in a similar fashion to what we observed previously in paclitaxel treated rats (Cai et al., 2021). Male mice were injected with oxaliplatin, between days 0–4 and 10–14, followed by assessment of paw withdrawal thresholds (Fig. 1B). These mice developed robust mechanical allodynia compared to mice that were not given oxaliplatin. On day 25, both groups of mice were given oral 194, which completely reduced oxaliplatin induced mechanical allodynia 3 h after treatment (Fig. 1C). Treatment with 194 had no effect on animals treated with vehicle instead of oxaliplatin, suggesting no effect of 194 on basal mechanical sensation, which is in line with our previous observations (Cai et al., 2021).
We next asked whether 194 could prevent mechanical allodynia in mice treated with the platinum-complex agent, oxaliplatin. To answer whether 194 could prevent CIPN, male CD-1 mice were treated with oxaliplatin and co-administered oral 194 or a vehicle control followed by assessment of paw withdrawal thresholds (Fig. 1D). Animals co-administered oxaliplatin and vehicle, during days 0–4 and 10–14, developed robust mechanical allodynia, which was prevented in animals co-administered 194 (Fig. 1E). There were no differences observed between groups in the body weight of the animals assessed over the experimental time course (Fig. 1F).
Interpreting these findings in the context of our previous report we can conclude that 194 is a potent inhibitor of mechanical allodynia in preclinical models of CIPN. We have previously shown that 194 reversed mechanical allodynia in rats treated with paclitaxel (Cai et al., 2021). Here, we observed that 194 can reduce mechanical allodynia in male mice treated with oxaliplatin, indicating that this effect is not species specific nor is it restricted to a single class of anti-neoplastic agent. That the reduction lasted three hours can perhaps be attributed to the dose and/or the short half-life of 194. In contrast, we found that 194, when given concurrently with oxaliplatin, prevented the development of mechanical allodynia, which points to a potential role as an adjuvant therapy in the clinic. Patients undergoing treatment for cancer with a neuropathy causing agent could be given 194 to decrease the possibility of developing CIPN. Furthermore, the fact that this compound is effective orally is promising for translation of these findings to the clinic (Yusof and Segall, 2013). It is important to note that we previously assessed the effects of 194 in male rats and here we used male mice. However, it is unlikely that a sex difference exists in response to treatment with 194 because we have previously shown that inhibition of CRMP2 SUMOylation is equally effective in both sexes (Moutal et al., 2020).
A imitation of our study is that we did not evaluate oxaliplatin-induced cold allodynia, which is regarded as an important clinical marker of oxaliplatin neurotoxicity and can be recapitulated in rodents. Among the ion channels contributing to cold perception include TREK1, TRAAK, Kv1.1, NaV1.8, and HCN (Descoeur et al., 2011), while NaV1.7 is thought to be dispensable for cold sensing (MacDonald et al., 2021). However, it remains to be determined whether 194 can block or reduce cold allodynia. This will be evaluated in future studies.
Conclusions
There is an emerging role for NaV1.7 in the CIPN literature supporting dysregulation of this channel in the pathogenesis of CIPN (Akin et al., 2021, Gordon-Williams and Farquhar-Smith, 2020, Li et al., 2018). Our previous work has shown that 194 is highly selective for NaV1.7, which means the effects we observed here are likely mediated by inhibition of NaV1.7 in affected DRG sensory neurons. This evidence adds weight to the conclusion that NaV1.7 is critically involved in CIPN and should be considered in future studies investigating the pathophysiology of CIPN. The findings reported here, as well as those of our prior work, positions 194 to make the transition to the clinic as a novel therapeutic candidate for the prevention and treatment of established CIPN.
Ethics approval and consent to participate
Not applicable.
Ethics approval for use of animals
Saint Louis University School of Medicine Institutional Animal Care and Use Committee sanctioned all experiments.
Consent for publication
Not applicable.
Availability of data and materials
Please contact author for data requests.
Funding
This work is supported by startup funds from St. Louis University to D.S. and grants from the National Institutes of Health awards to R.K. (NINDS (NS098772 and NS120663) and NIDA (DA042852).
Author Contributions
R.K. developed the concept of indirectly targeting NaV1.7.; D.S., and K.B. designed the experiments; K.B. collected and analyzed the data; R.K. provided funding; K.B., H.J.S., D.S., and R.K. wrote the manuscript; and D.S. supervised all experiments. All authors had the opportunity to discuss results and comment on the manuscript.
Declaration of Competing Interest
R. Khanna is the co-founder of Regulonix LLC, a company developing non-opioids drugs for chronic pain. In addition, R. Khanna 481 has patents US10287334 (Non-narcotic CRMP2 peptides targeting sodium channels for chronic 482 pain) and US10441586 (SUMOylation inhibitors and uses thereof) issued to Regulonix LLC. R. Khanna is also a co-founder of ElutheriaTx Inc., a company developing gene therapy approaches for chronic pain.
References
- Akin, E.J., Alsaloum, M., Higerd, G.P., Liu, S., Zhao, P., Dib-Hajj, F.B., Waxman, S.G., Dib-Hajj, S.D., 2021. Paclitaxel increases axonal localization and vesicular trafficking of Nav1.7. Brain. [DOI] [PMC free article] [PubMed]
- Alexandrou A.J., Brown A.R., Chapman M.L., Estacion M., Turner J., Mis M.A., Wilbrey A., Payne E.C., Gutteridge A., Cox P.J., Doyle R., Printzenhoff D., Lin Z., Marron B.E., West C., Swain N.A., Storer R.I., Stupple P.A., Castle N.A., Hounshell J.A., Rivara M., Randall A., Dib-Hajj S.D., Krafte D., Waxman S.G., Patel M.K., Butt R.P., Stevens E.B., Price T.J. Subtype-Selective Small Molecule Inhibitors Reveal a Fundamental Role for Nav1.7 in Nociceptor Electrogenesis, Axonal Conduction and Presynaptic Release. PloS one. 2016;11(4):e0152405. doi: 10.1371/journal.pone.0152405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson, M., Iñiguez-Lluhí, J.A., Martens, J. 2009. SUMO Modification of Ion Channels. In: SUMO Regulation of Cellular Processes. pp. 117-136. Ed. V.G. Wilson. Springer Netherlands: Dordrecht.
- Benson, M.D., Li, Q.-J., Kieckhafer, K., Dudek, D., Whorton, M.R., Sunahara, R.K., Iñiguez-Lluhí, J.A., Martens, J.R., 2007. SUMO Modification Regulates Inactivation of the Voltage-Gated Potassium Channel Kv1.5. Proceedings of the National Academy of Sciences - PNAS 104, 1805-1810. [DOI] [PMC free article] [PubMed]
- Berge, O.-G., 2011. Predictive validity of behavioural animal models for chronic pain. British journal of pharmacology 164, 1195-1206. [DOI] [PMC free article] [PubMed]
- Cai S., Moutal A., Yu J., Chew L.A., Isensee J., Chawla R., Gomez K., Luo S., Zhou Y., Chefdeville A., Madura C., Perez-Miller S., Bellampalli S.S., Dorame A., Scott D.D., François-Moutal L., Shan Z., Woodward T., Gokhale V., Hohmann A.G., Vanderah T.W., Patek M., Khanna M., Hucho T., Khanna R. Selective targeting of NaV1.7 via inhibition of the CRMP2-Ubc9 interaction reduces pain in rodents. Sci. Transl. Med. 2021;13:eabh1314. doi: 10.1126/scitranslmed.abh1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappadocia L., Pichler A., Lima C.D. Structural basis for catalytic activation by the human ZNF451 SUMO E3 ligase. Nat. Struct. Mol. Biol. 2015;22(12):968–975. doi: 10.1038/nsmb.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaletti G., Cavalletti E., Oggioni N., Sottani C., Minoia C., D'Incalci M., Zucchetti M., Marmiroli P., Tredici G. Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicology. 2000;21:389–393. [PubMed] [Google Scholar]
- Chew L.A., Bellampalli S.S., Dustrude E.T., Khanna R. Mining the Nav1.7 interactome: Opportunities for chronic pain therapeutics. Biochem. Pharmacol. 2019;163:9–20. doi: 10.1016/j.bcp.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew, L.A., Khanna, R., 2018. CRMP2 and voltage-gated ion channels: potential roles in neuropathic pain. Neuronal Signal 2, pii: NS20170220. doi: 20170210.20171042/NS20170220. [DOI] [PMC free article] [PubMed]
- Cox J.J., Reimann F., Nicholas A.K., Thornton G., Roberts E., Springell K., Karbani G., Jafri H., Mannan J., Raashid Y., Al-Gazali L., Hamamy H., Valente E.M., Gorman S., Williams R., McHale D.P., Wood J.N., Gribble F.M., Woods C.G. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444(7121):894–898. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuis J.R., Dekan Z., Wingerd J.S., Smith J.J., Munasinghe N.R., Bhola R.F., Imlach W.L., Herzig V., Armstrong D.A., Rosengren K.J., Bosmans F., Waxman S.G., Dib-Hajj S.D., Escoubas P., Minett M.S., Christie M.J., King G.F., Alewood P.F., Lewis R.J., Wood J.N., Vetter I. Pharmacological characterisation of the highly NaV1.7 selective spider venom peptide Pn3a. Scient. Rep. 2017;7:40883. doi: 10.1038/srep40883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoeur J., Pereira V., Pizzoccaro A., Francois A., Ling B., Maffre V., Couette B., Busserolles J., Courteix C., Noel J., Lazdunski M., Eschalier A., Authier N., Bourinet E. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol. Med. 2011;3:266–278. doi: 10.1002/emmm.201100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj S.D., Yang Y., Black J.A., Waxman S.G. The Na(V)1.7 sodium channel: from molecule to man. Nat. Rev. Neurosci. 2013;14(1):49–62. doi: 10.1038/nrn3404. [DOI] [PubMed] [Google Scholar]
- Dixon W.J. Efficient analysis of experimental observations. Ann. Rev. Pharmacol. Toxicol. 1980;20(1):441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Dustrude E.T., Moutal A., Yang X., Wang Y., Khanna M., Khanna R. Hierarchical CRMP2 posttranslational modifications control NaV1.7 function. Proceed. Natl. Acad. Sci. USA. 2016;113(52):E8443–E8452. doi: 10.1073/pnas.1610531113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustrude E.T., Wilson S.M., Ju W., Xiao Y., Khanna R. CRMP2 protein SUMOylation modulates NaV1.7 channel trafficking. J. Biol. Chem. 2013;288(34):24316–24331. doi: 10.1074/jbc.M113.474924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagles D.A., Chow C.Y., King G.F. Fifteen years of NaV1.7 channels as an analgesic target: Why has excellent in vitro pharmacology not translated into in vivo analgesic efficacy? Br. J. Pharmacol. n/a. 2020 doi: 10.1111/bph.15327. [DOI] [PubMed] [Google Scholar]
- Flotho A., Melchior F. Sumoylation: A Regulatory Protein Modification in Health and Disease. Ann. Rev. Biochem. 2013;82(1):357–385. doi: 10.1146/annurev-biochem-061909-093311. [DOI] [PubMed] [Google Scholar]
- François-Moutal L., Dustrude E.T., Wang Y., Brustovetsky T., Dorame A., Ju W., Moutal A., Perez-Miller S., Brustovetsky N., Gokhale V., Khanna M., Khanna R. Inhibition of the Ubc9 E2 SUMO-conjugating enzyme-CRMP2 interaction decreases NaV1.7 currents and reverses experimental neuropathic pain. Pain. 2018;159(10):2115–2127. doi: 10.1097/j.pain.0000000000001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François-Moutal L., Scott D.D., Perez-Miller S., Gokhale V., Khanna M., Khanna R. Chemical shift perturbation mapping of the Ubc9-CRMP2 interface identifies a pocket in CRMP2 amenable for allosteric modulation of Nav1.7 channels. Channels (Austin) 2018;12(1):219–227. doi: 10.1080/19336950.2018.1491244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadgil S., Ergün M., van den Heuvel S.A., van der Wal S.E., Scheffer G.J., Hooijmans C.R., Tang S.-J. A systematic summary and comparison of animal models for chemotherapy induced (peripheral) neuropathy (CIPN) PLoS One. 2019;14(8):e0221787. doi: 10.1371/journal.pone.0221787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez K., Ran D., Madura C.L., Moutal A., Khanna R. Non-SUMOylated CRMP2 decreases Na(V)1.7 currents via the endocytic proteins Numb, Nedd4-2 and Eps15. Molecular Brain. 2021;14:20. doi: 10.1186/s13041-020-00714-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Williams, R., Farquhar-Smith, P., 2020. Recent advances in understanding chemotherapy-induced peripheral neuropathy. F1000Research 9. [DOI] [PMC free article] [PubMed]
- Herzog R.I., Cummins T.R., Ghassemi F., Dib-Hajj S.D., Waxman S.G. Distinct repriming and closed-state inactivation kinetics of Nav1.6 and Nav1.7 sodium channels in mouse spinal sensory neurons. J. Physiol. 2003;551(3):741–750. doi: 10.1113/jphysiol.2003.047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Andrade, J.M., Herrera, M.B., Ghilardi, J.R., Vardanyan, M., Melemedjian, O.K., Mantyh, P.W., 2008. Vascularization of the dorsal root ganglia and peripheral nerve of the mouse: implications for chemical-induced peripheral sensory neuropathies. Mol. Pain 4, 10. [DOI] [PMC free article] [PubMed]
- Kanellopoulos A.H., Koenig J., Huang H., Pyrski M., Millet Q., Lolignier S., Morohashi T., Gossage S.J., Jay M., Linley J.E., Baskozos G., Kessler B.M., Cox J.J., Dolphin A.C., Zufall F., Wood J.N., Zhao J. Mapping protein interactions of sodium channel Na(V)1.7 using epitope-tagged gene-targeted mice. EMBO J. 2018;37(3):427–445. doi: 10.15252/embj.201796692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingwell K. Navigating a new path to Nav1.7 for pain. Nat. Rev. Drug Discov. 2021 doi: 10.1038/d41573-021-00197-2. [DOI] [PubMed] [Google Scholar]
- Li Y., North R.Y., Rhines L.D., Tatsui C.E., Rao G., Edwards D.D., Cassidy R.M., Harrison D.S., Johansson C.A., Zhang H., Dougherty P.M. DRG Voltage-Gated Sodium Channel 1.7 Is Upregulated in Paclitaxel-Induced Neuropathy in Rats and in Humans with Neuropathic Pain. J. Neurosci. 2018;38(5):1124–1136. doi: 10.1523/JNEUROSCI.0899-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack K., Santos S., Chapman M.L., Krafte D.S., Marron B.E., West C.W., Krambis M.J., Antonio B.M., Zellmer S.G., Printzenhoff D., Padilla K.M., Lin Z., Wagoner P.K., Swain N.A., Stupple P.A., de Groot M., Butt R.P., Castle N.A. Voltage sensor interaction site for selective small molecule inhibitors of voltage-gated sodium channels. Proceed. Natl. Acad. Sci. USA. 2013;110(29):E2724–E2732. doi: 10.1073/pnas.1220844110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald D.I., Luiz A.P., Iseppon F., Millet Q., Emery E.C., Wood J.N. Silent cold-sensing neurons contribute to cold allodynia in neuropathic pain. Brain. 2021;144:1711–1726. doi: 10.1093/brain/awab086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A.M., Alemán F., Catroli G.F., Hunt M., Hu M., Dailamy A., Pla A., Woller S.A., Palmer N., Parekh U., McDonald D., Roberts A.J., Goodwill V., Dryden I., Hevner R.F., Delay L., Gonçalves dos Santos G., Yaksh T.L., Mali P. Long-lasting analgesia via targeted in situ repression of Na(V)1.7 in mice. Sci. Transl. Med. 2021;13(584) doi: 10.1126/scitranslmed.aay9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutal A., Cai S., Yu J., Stratton H.J., Chefdeville A., Gomez K., Ran D., Madura C.L., Boinon L., Soto M., Zhou Y., Shan Z., Chew L.A., Rodgers K.E., Khanna R. Studies on CRMP2 SUMOylation-deficient transgenic mice identify sex-specific Nav1.7 regulation in the pathogenesis of chronic neuropathic pain. Pain. 2020;161(11):2629–2651. doi: 10.1097/j.pain.0000000000001951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutal A., Dustrude E.T., Largent-Milnes T.M., Vanderah T.W., Khanna M., Khanna R. Blocking CRMP2 SUMOylation reverses neuropathic pain. Molecular psychiatry. 2018;23(11):2119–2121. doi: 10.1038/mp.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutal A., White K.A., Chefdeville A., Laufmann R.N., Vitiello P.F., Feinstein D., Weimer J.M., Khanna R. Dysregulation of CRMP2 Post-Translational Modifications Drive Its Pathological Functions. Mol. Neurobiol. 2019;56(10):6736–6755. doi: 10.1007/s12035-019-1568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai M., Sakuma Y., Suzuki M., Orita S., Yamauchi K., Inoue G., Aoki Y., Ishikawa T., Miyagi M., Kamoda H., Kubota G., Oikawa Y., Inage K., Sainoh T., Sato J., Nakamura J., Takaso M., Toyone T., Takahashi K., Ohtori S. Evaluation of behavior and expression of NaV1.7 in dorsal root ganglia after sciatic nerve compression and application of nucleus pulposus in rats. Eur. Spine J. Off Publicat. Europ. Spine Society, Eur. Spinal Deform. Soc. Eur. Section Cerv. Spine Res. Soc. 2014;23(2):463–468. doi: 10.1007/s00586-013-3076-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler K.E., Mogil J.S., Stucky C.L. Innovations and advances in modelling and measuring pain in animals. Nat. Rev. Neurosci. 2021 doi: 10.1038/s41583-021-00536-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangameswaran L., Fish L.M., Koch B.D., Rabert D.K., Delgado S.G., Ilnicka M., Jakeman L.B., Novakovic S., Wong K., Sze P., Tzoumaka E., Stewart G.R., Herman R.C., Chan H., Eglen R.M., Hunter J.C. A Novel Tetrodotoxin-sensitive, Voltage-gated Sodium Channel Expressed in Rat and Human Dorsal Root Ganglia. J. Biol. Chem. 1997;272(23):14805–14809. doi: 10.1074/jbc.272.23.14805. [DOI] [PubMed] [Google Scholar]
- Shen H., Liu D., Wu K., Lei J., Yan N. Structures of human Na(v)1.7 channel in complex with auxiliary subunits and animal toxins. Science. 2019;363(6433):1303–1308. doi: 10.1126/science.aaw2493. [DOI] [PubMed] [Google Scholar]
- Siebenga P., Amerongen G., Hay J.L., McDonnell A., Gorman D., Butt R., Groeneveld G.J. Lack of Detection of the Analgesic Properties of PF-05089771, a Selective Na(v) 1.7 Inhibitor, Using a Battery of Pain Models in Healthy Subjects. Clin. Transl. Sci. 2020;13(2):318–324. doi: 10.1111/cts.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira S.R.D.T., Alves B., Malpartida H.M.G., Teixeira M.J., Siqueira J.T.T. Abnormal expression of voltage-gated sodium channels Nav1. 7, Nav1. 3 and Nav1. 8 in trigeminal neuralgia. Neuroscience. 2009;164(2):573–577. doi: 10.1016/j.neuroscience.2009.08.037. [DOI] [PubMed] [Google Scholar]
- Starobova H., Vetter I. Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy. Front. Mol. Neurosci. 2017;10:174. doi: 10.3389/fnmol.2017.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark P., Ogg D., Flodin S., Flores A., Kotenyova T., Nyman T., Nordlund P., Kursula P. The structure of human collapsin response mediator protein 2, a regulator of axonal growth. J. Neurochem. 2007;101(4):906–917. doi: 10.1111/j.1471-4159.2006.04401.x. [DOI] [PubMed] [Google Scholar]
- Tanabe Y., Shiraishi S., Hashimoto K., Ikeda K., Nishizawa D., Hasegawa J., Shimomura A., Ozaki Y., Tamura N., Yunokawa M., Yonemori K., Takano T., Kawabata H., Tamura K., Fujiwara Y., Shimizu C. Taxane-induced sensory peripheral neuropathy is associated with an SCN9A single nucleotide polymorphism in Japanese patients. BMC Cancer. 2020;20:325. doi: 10.1186/s12885-020-06834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile J.W., Fuller M.D., Chapman M.L. The Selective Nav1.7 Inhibitor, PF-05089771, Interacts Equivalently with Fast and Slow Inactivated Nav1.7 Channels. Mol. Pharmacol. 2016;90(5):540–548. doi: 10.1124/mol.116.105437. [DOI] [PubMed] [Google Scholar]
- Toledo-Aral, J.J., Moss, B.L., He, Z.-J., Koszowski, A.G., Whisenand, T., Levinson, S.R., Wolf, J.J., Silos-Santiago, I., Halegoua, S., Mandel, G., 1997. Identification of PN1, a Predominant Voltage-Dependent Sodium Channel Expressed Principally in Peripheral Neurons. Proceed. Natl. Acad. Sci. - PNAS 94, 1527-1532. [DOI] [PMC free article] [PubMed]
- Wahlman C., Doyle T.M., Little J.W., Luongo L., Janes K., Chen Z., Esposito E., Tosh D.K., Cuzzocrea S., Jacobson K.A., Salvemini D. Chemotherapy-induced pain is promoted by enhanced spinal adenosine kinase levels through astrocyte-dependent mechanisms. Pain. 2018;159(6):1025–1034. doi: 10.1097/j.pain.0000000000001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.J., Zhang X.i., Huang L.-D., Xiao Y. Involvement of the Sodium Channel Nav1.7 in Paclitaxel-induced Peripheral Neuropathy through ERK1/2 Signaling in Rats. Curr. Neurovasc. Res. 2020;17(3):267–274. doi: 10.2174/1567202617666200514113441. [DOI] [PubMed] [Google Scholar]
- Woolf C.J., Ma Q. Nociceptors–noxious stimulus detectors. Neuron. 2007;55(3):353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Xiao W.H., Zheng H., Bennett G.J. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience. 2012;203:194–206. doi: 10.1016/j.neuroscience.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Xia Z., Wu Y., Zhao B. Sodium channel Nav1.7 expression is upregulated in the dorsal root ganglia in a rat model of paclitaxel-induced peripheral neuropathy. SpringerPlus. 2016;5:1–7. doi: 10.1186/s40064-016-3351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wang Y., Li S., Xu Z., Li H., Ma L., Fan J., Bu D., Liu B., Fan Z., Wu G., Jin J., Ding B., Zhu X., Shen Y. Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J. Med. Genet. 2004;41:171–174. doi: 10.1136/jmg.2003.012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusof I., Segall M.D. Considering the impact drug-like properties have on the chance of success. Drug Discovery Today. 2013;18(13-14):659–666. doi: 10.1016/j.drudis.2013.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact author for data requests.