Abstract
Birth by Caesarean-section (C-section), which increases the risk for metabolic and immune disorders, disrupts the normal initial microbial colonisation of the gut, in addition to preventing early priming of the stress and immune-systems.. Animal studies have shown there are enduring psychological processes in C-section born mice. However, the long-term impact of microbiota-gut-brain axis disruptions due to birth by C-section on psychological processes in humans is unknown. Forty age matched healthy young male university students born vaginally and 36 C-section delivered male students were recruited. Participants underwent an acute stressor, the Trier social stress test (TSST), during a term-time study visit. A subset of participants also completed a study visit during the university exam period, representing a naturalistic stressor. Participants completed a battery of cognitive tests and self-report measures assessing mood, anxiety, and perceived stress. Saliva, blood, and stool samples were collected for analysis of cortisol, peripheral immune profile, and the gut microbiota. Young adults born by C-section exhibit increased psychological vulnerability to acute stress and a prolonged period of exam-related stress. They did not exhibit an altered salivary cortisol awakening response to the TSST, but their measures of positive affect were significantly lower than controls throughout the procedure. Both C-section and vaginally-delivered participants performed equally well on cognitive assessments. Most of the initial effects of delivery mode on the gut microbiome did not persist into adulthood as the gut microbiota profile showed modest changes in composition in adult vaginally-delivered and C-sectioned delivered subjects. From an immune perspective, concentrations of IL-1β and 1L-10 were higher in C-section participants. These data confirm that there is a potential enduring effect of delivery mode on the psychological responses to acute stress during early adulthood. The mental health implications of these observations require further study regarding policies on C-section use.
Keywords: Stress, Microbiome, Cognition, Caesarean section
Highlights
-
•
Adults born by C-section display increased vulnerability to acute stress.
-
•
C-section adults have a greater psychological stress response to a naturalistic stressor.
-
•
Cognitive performance and microbiome composition are similar in vaginal birth and C-section.
1. Introduction
Caesarean section can be lifesaving and vital for the safe delivery of a baby and the health and welfare of the mother. However, there is a worrying trend of increased numbers of elective but non-medically indicated C-sections across the globe, which is associated with high costs to healthcare systems as well as increased risk to the mother (Betran et al., 2016, World Health Organization Human Reproduction Programme, 2015). Furthermore, birth by C-section is associated with an increased risk of the baby developing immunological and metabolic disorders later in childhood (Keag et al., 2018). Therefore, understanding the long-term impacts of C-section is important to develop evidence-based interventions for the prevention of potential negative health effects. Likewise, new evidence will help inform policy surrounding the rise of non-medically indicated C-section procedures.
One clear difference in infants born by C-section is with regard to the resultant gut microbial profile present. The gut microbiota is initially colonised during parturition, whereby there is vertical transmission of microbiota from the mother to the baby. The resulting infant gut microbiome closely resembles that of the maternal vaginal microbiome (Dominguez-Bello et al., 2010). In contrast, the gut microbiome of infants born by C-section is very different and closely resembles that of the skin, including that of non-maternal sources, in addition to the environment of the delivery suite (Dominguez-Bello et al., 2010). Intrapartum antibiotic prophylaxis can also impact on the gut microbiome profile of the infant (Azad et al., 2016; Hill et al., 2017). Infants born by C-section have decreased diversity and richness of the gut microbiota, as well as decreased Bifidobacterium, Bacteroides, and Lactobacillus (Biasucci et al., 2010; Penders et al., 2006). Furthermore, recent evidence has shown a high colonisation of opportunistic pathogens, many containing antibiotic resistance genes, in babies born by C-section (Shao et al., 2019). In addition to differences in the bacteriome, differences in the virome have also been described, with a higher diversity of the gut virome present in vaginally-delivered infants at one year of age (McCann et al., 2018). It should be noted though, that differences in the composition of the C-section microbiome are largely confined to early life, but it is important to consider the effect of disrupting the microbiome during such a critical window can have on the brain and behaviour.
With time, differences in the bacteriome between C-section and naturally delivered infants diminish, with studies describing the effects of birth mode on gut microbiota to be absent from 6 to 8 weeks (Chu et al., 2017; Hill et al., 2017) to 2 years after birth (Bokulich et al., 2016; Jakobsson et al., 2014; Palmer et al., 2007). There are also a variety of other factors which impact on the gut microbiota in early life, including prenatal antibiotic use, breastfeeding, growth in the early years of childhood and geography (Vatanen et al., 2019). However, it is apparent that the functional effects of differential colonisation of the gut during this critical neurodevelopmental period may endure. Perturbations in early life by C-section birth or antibiotic use have been linked to increased risk for developing childhood obesity (Cho and Norman, 2013; Li et al., 2013; Mueller et al., 2015; Yuan et al., 2016) and childhood asthma (Darabi et al., 2019; Metsala et al., 2015). Indeed, the gut microbiome significantly influences the development and maturation of the immune system in early life (Cebra, 1999; Cho and Norman, 2013; Schlinzig et al., 2017). It is of note that the initial seeding of the gut microbiota also occurs at the same time as a critical period in the development of the core stress axis, namely the hypothalamic-pituitary-adrenal (HPA) axis (Ng, 2000). Birth by C-section bypasses activation of the HPA axis which would otherwise occur during passage through the birth canal and this may hamper the typical development of the stress system (Lagercrantz, 2016; Lagercrantz and Slotkin, 1986). Moreover, we have recently shown enduring effects of C-section across the entire microbiota-gut-brain axis in a mouse model (Morais et al., 2020).
There is increasing evidence to indicate that stress, both chronic and acute together with the related psychological disorders anxiety and depression are regulated by multiple factors including the gut microbial profile (Dinan et al., 2018; Kennedy et al., 2014). To date there has been limited investigation on the long-term effects of an initially altered gut microbial profile on psychological and physiological responses to stress, especially in human cohorts. To fill this knowledge gap, a cohort of young adult volunteers of known birth mode underwent a Trier Social Stress Test (during a non-exam period) to investigate the HPA axis, as well as assessments of cognition, inflammatory and psychological response to psychosocial stress. In addition, participants also underwent similar assessments during an exam period.
2. Methods
2.1. Study population
The study protocol (APC050) and all procedures were approved by the Cork Teaching Hospitals Ethics Committee and conducted in accordance with the ICH Guidelines on Good Clinical Practice, and the Declaration of Helsinki. Study participants were all male between 18 and 25 years of age, recruited via advertisement from the student population of University College Cork, (Table 1). Forty vaginally delivered participants and 36 participants born by C-section (18-planned C-section; 15-emergency C-section; 3-unknown) were enrolled. Participants were matched based on age, BMI and average units of alcohol consumed per week. Exclusion criteria included having a BMI ≥30, formal psychiatric diagnosis of major depression, anxiety disorder, bipolar spectrum disorder, schizophrenia, or other DSM-IV Axis-I disorder, use of psychoactive medication(s) (anxiolytics, antipsychotics, antidepressants, corticosteroids, and opioid pain relievers), regular use of non-steroidal anti-inflammatory medications or antibiotic use in the previous 4 weeks. In addition, participants were excluded if they reported a history of chronic physical illness. Based on previous findings from our laboratory (Kennedy et al., 2014) we powered our study to detect between group differences in the salivary cortisol response with a medium effect size (f = 0.25). At an alpha of 0.05 and obtaining a power of 0.08, a total sample size of 74 was required. This calculation is based on a repeated measures ANOVA, between factors, using G*Power software (Faul et al., 2007).
Table 1.
Participant Characteristics. Participants were grouped (Vaginally-delivered or delivered by C-section) according to their birth mode, and they matched for body mass index (BMI); years of education; average of units of alcohol intake per week; habitual smoking status; socioeconomic (SE) status (as classified from parental SE status. Data is represented as the mean ± S.E.M.
| Vaginally-delivered | C-section | p-value | ||
|---|---|---|---|---|
| Age [years ± S.E.M] | 20.22 ± 0.24 | 20.34 ± 0.22 | 0.72 | |
| BMI [kg/m2± S.E.M] | 23.66 ± 0.46 | 24.58 ± 0.63 | 0.21 | |
| Years of Education [years ± S.E.M] | 16.03 ± 0.22 | 15.83 ± 0.19 | 0.5 | |
| Alcohol consumption [units per week ± S.E.M] | 7.42 ± 1.04 | 9.96 ± 1.53 | 0.17 | |
| Cigarette smoking status [N (%)] | 4 (10) | 2 (5.6) | 0.473 | |
| SE Status (%) | 1 (Professional) | 18 (45) | 22 (61.1) | 0.232 |
| 2 (Managerial/Technical) | 6 (15) | 1 (2.8) | ||
| 3 (Non-Manual) | 3 (7.5) | 6 (16.7) | ||
| 4 (Skilled Manual) | 3 (7.5) | 3 (8.3) | ||
| 5 (Semi-Skilled) | 4 (10) | 1 (2.8) | ||
| 6 (Unskilled) | 5 (12.5) | 3 (8.3) | ||
| 7 (Other gainfully occupied & unknown) | 1 (2.5) | 0 (0) | ||
2.2. Study procedures
Participants were screened to check suitability for study inclusion and were subsequently scheduled to attend one or two study visits at the Clinical Research Facility in Mercy University Hospital, Cork City. All participants provided full written informed consent before any experimental procedures commenced. All participants attended one visit during term-time (Non-Stress/TSST visit), but not within ±6 weeks of a formal end of term examination period, and a subset of 38 vaginally delivered and 32 C-section participants completed another visit carried out during the participants university examination period (Exam Stress visit) (See Table 1 for detailed description of participants demographics). Order of visits was counterbalanced so that roughly half of each group completed the Non-Stress visit first and the other half completed the Exam Stress visit first. At the Exam Stress and Non-Stress visit, participants completed a battery of cognitive tests and completed self-report measures assessing mood, anxiety, and perceived stress (see Self-Report Measures). In addition, during the Non-Stress visit participants completed the Trier Social Stress Test.
2.3. Trier Social Stress Test
The TSST procedure began between 1300 and 14.30 h for each participant to control for diurnal fluctuations in cortisol levels (Allen et al., 2014). Participants were instructed to abstain from alcohol and strenuous physical exercise for 24 h prior to visits. In addition, they were asked not to consume any caffeine containing products on the day of their Non-Stress study visit, and to consume only water for 2 h prior to the TSST procedure. After collection of the first saliva sample for measuring salivary cortisol, participants rested for a 45-min baseline period. Participants were then given standardized written instructions, introducing the TSST which was carried out as previously described (Allen et al., 2014). Participants were then led to a separate room, equipped with a video camera and microphone and two desks. After reiterating the task instructions, participants were then given a 3-min speech preparation period after which, participants were required to perform a 5-min speech outlining their suitability for an ideal job of their choice, followed by a 5-min mental arithmetic task in which they serially subtracted 17 from 2023. Task were performed in front of two committee members (one male and one female), wearing white laboratory coats and introduced as being experts in identifying non-verbal aspects of behaviour. Participants were also informed that their speech would be both audio and video recorded for later behavioural analysis. Saliva samples and self-report measures of mood and stress were collected at a number of time-points pre- and post-the TSST procedure (See Below). Participants were fully debriefed following collection of the last sample.
2.4. Self-report measures
2.4.1. TSST mood & stress measures
Mood was assessed using the Positive and Negative Affect Schedule (PANAS; (Watson et al., 1988)), and psychological stress was measured using a visual analogue scale (VAS), ranging from 0 (‘not stressed at all’) to 100 (‘As stressed as I could possibly imagine’).
2.4.2. State Trait Anxiety Inventory (STAI)
The STAI is a self-report measure consisting of two subscales each with 20 items, one measuring trait anxiety and the other measuring state anxiety (Spielberger, 1983)). Participants rate how they feel either right now (state) or generally (trait), in response to each item on a 4-point scale from ‘not at all’ to ‘very much.’ The range of scores for each sub-scale is 20–80 with higher scores indicating greater anxiety.
2.5. Other self-report measures
2.5.1. Perceived Stress Scale (PSS)
The PSS is a self-report measure in which participants rate, on a 5 point scale ranging from 0 (never) to 4 (very often), how often they have particular thoughts or feelings described by each of the 10 items (Cohen et al., 1983). Scores range from 0 to 40 with higher scores indicating greater stress over the previous month.
2.5.2. Beck depression inventory (BDI)-II
The BDI-II is a self-report measure consisting of 21 items rated on a 4-point scale from 0 (absence of symptom) to 3 (severe manifestation of symptom (Beck et al., 1996). Scores range from 0 to 63. Cut-off scores indicating clinically relevant levels of depression have been determined as 0–13 (minimal); 14–19 (mild); 20–28 (moderate); 29–63 (severe).
2.6. Other questionnaires
Dietary intake was quantified using a food frequency questionnaire (Harrington et al., 2011). Nutrient intakes were calculated using the FETA software (v2.53) to calculate macro- and micronutrient intakes. All study participants completed the short version of the Childhood Trauma Questionnaire (CTQ), which is an eight-item, self-report questionnaire that identifies the presence or absence of childhood traumatic events (Bernstein et al., 2003).
2.7. Proinflammatory cytokine sampling & analysis
To determine the immune response to acute psychosocial stress 5–10 ml of whole blood was collected in EDTA tubes at 4 time-points throughout the TSST: time(t) −45, t+20, t+50, t+80 min. To determine the effect of exam-stress on immune function 10–15 ml of whole blood was collected in EDTA tubes during each study visit (Non-Stress/Exam Stress). Samples were centrifuged immediately at 1500×g for 10 min and aliquoted plasma samples were frozen at −80 °C until analysis. Plasma levels of IL-10, IL-1β, IL-6, IL-8, and TNF-α were assayed in duplicate using high sensitivity commercially available electrochemiluminescence MULTI-SPOT® Meso Scale Discovery kits (MSD, Rockville, MD, USA) as per manufacturer's instructions. The median lower limits of detection for each cytokine are IL-6- 0.06 pg/ml, IL-8- 0.04 pg/ml, TNF-α- 0.04 pg/ml and IFN-γ- 0.2 pg/ml.
2.8. HPA axis response
Saliva samples were obtained using Salivette® devices (Sarstedt, Ireland). Participants were instructed to roll the synthetic bud around their mouths while chewing lightly for 1.5 min. To determine the HPA axis response to acute psychosocial stress, saliva samples were collected at seven time-points throughout the TSST: t −45, t0, t+20, t+35, t+50, t+65, t+80. Samples were kept chilled at ∼4 °C until the end of the experimental protocol. To determine the effect of exam stress on HPA axis function, the salivary cortisol awakening response (CAR) was measured. On the morning prior to each study visit (Non-Stress/Exam Stress) participants were instructed to collect 4 saliva samples (upon wakening, 30 min post-wakening (t+30), 45 min post-wakening (t+45), and 60 min post-wakening (t+60).
Saliva samples were centrifuged for 5 min at 1000 g and saliva was collected and stored at −80 °C until analysis. Cortisol concentrations were determined using the Cortisol Enzyme Immunoassay Kit as per manufacturers’ instruction (Enzo® Life Sciences, Exeter, UK). Assay detection limit was 0.16 nmol/L. Inter and intra-assay % C.Vs were 11.24% and 8.2% respectively.
2.9. Cognitive assessment: tests from the CANTAB battery
(http://www.cambridgecognition.com/)
CANTAB tests were presented on a high-resolution touch-screen monitor under computer control. Participants interact with the system by touching the touch screen whilst a test administrator provides verbal instructions from a standardised script, and specific verbal prompts and encouragement when needed. The test administrator had full control of a keyboard used to start, pause or abort each test. Participants were assessed on the following tests from the battery:
2.9.1. IED: attentional flexibility and reversal learning
The IED is a test of executive function and assesses rule acquisition and reversal, attentional set formation, maintenance and shifting. The outcome measures assessed to determine reversal learning performance were the errors made on stage 2, 5, 7 and 9, and to determine attentional flexibility performance the errors made on stage 6 and 8.
2.10. Stop Signal Task (SST): response inhibition
The SST assesses participant's ability to inhibit a prepotent response. The outcome measure assessed was the stop signal reaction time (SSRT; calculated for last 20 sub-blocks).
2.10.1. Paired Associates Learning (PAL): visuospatial memory
The PAL assesses conditional learning of pattern-location associations and gives an index of visuospatial memory. The outcome measure assessed to determine visuospatial memory was the PAL total errors adjusted.
2.11. DNA isolation from faecal samples
Total bacterial DNA was extracted from the faecal samples using the QIAamp DNA Stool Mini Kit (Qiagen, Sussex, UK) according to manufacturer's instructions, coupled with an initial bead-beating step.
2.12. 16S rRNA gene amplicon sequencing of stool samples
DNA was prepared for 16S sequencing. The V3–V4 regions of the 16S rRNA gene were amplified and prepared for sequencing according to the 16S Metagenomic Sequencing Library Protocol. The DNA was first amplified using primers specific to the V3–V4 regions of the 16S rRNA gene:
(Forward primer 5′TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG;
Reverse primer 5′GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC).
Each reaction contained 2.5 μl genomic DNA, 5 μl forward primer (1 μm), 5 μl reverse primer (1 μm) and 12.5 μl 2X Kapa HiFi Hotstart ReadyMix. PCR amplification was carried out using the following program: 95 °C × 3 mins, 25 cycles of 95 °C × 30s, 55 °C × 30s, 72 °C × 30s, 72 °C × 5 mins and held at 4 °C. PCR products were visualised using gel electrophoresis and then purified using AMPure XP beads. Following this, a second PCR reaction was carried out on the purified DNA using two indexing primers per sample. Each reaction contained 5 μl purified DNA, 5 μl index 1 primer (N7xx), 5 μl index 2 primer (S5xx), 25 μl 2x Kapa HiFi Hot Start Ready mix and 10 μl PCR grade water. PCR amplification was completed using the previous program but with only 8 amplification cycles instead of 25. PCR products were visualised and purified as described above. Samples were quantified using the Qubit™ 3.0 Fluorometer along with the high sensitivity DNA quantification assay kit and then pooled in an equimolar fashion (20 nM). The sample pool was prepared following Illumina guidelines and sequenced on the MiSeq sequencing platform in Teagasc Moorepark, Fermoy using standard Illumina sequencing protocols.
Raw sequences were merged using Flash (with a minimum overlap of 30 bp and minimum read length of 460bp). The forward primer was removed using cutadapt. The reads were quality checked to a Phred quality score of Q20 or better using the split_libraries_fastq.py script (with default parameters) from the QIIME package version 1.9.1. The reverse primers were removed using truncate_reverse_primer.py from the QIIME package. Reads were clustered into OTUs with 97% sequence identity threshold and chimeras and singletons removed with the 64-bit version of USEARCH (version 8.1). Subsequently, OTUs were aligned to the SILVA rRNA specific database (version 128) to remove remaining chimeras. The read counts were rarefied to a depth of 44673 reads for alpha and beta diversity analysis. A phylogenetic tree for diversity calculations was generated, followed by alpha and beta diversity computation, all within QIIME. Taxonomy to genus level was assigned using the Mothur program (version 11.4) using the RDP database (version 11.4). Species level classification was performed using SPINGO (version 1.3) using the RDP database (version 11.2). The read counts for testing differential abundance of taxa were normalised to proportions of each sample.
2.13. Statistical analysis
Independent sample t-tests were used to explore differences in group characteristics (age, BMI, years of education and units of alcohol per week). To allow for repeated measures analysis and to avoid bias that may be introduced by using list-wise deletion of incomplete cases, missing data analysis was performed on physiological, psychological and cognitive variables subject to repeated measures analysis. In total 5.95% of data was missing and determined to be missing completely at random (MCAR) using Littles MCAR test; χ2 (688) = 703.356, p = 0.334)). Missing values were imputed by assigning the group mean for that variable. All analyses were carried out with missing data excluded (data not shown) and missing data imputed, which showed that imputing values using this method did not significantly change the nature of our results. Participants with four or more missing data points for salivary cortisol were excluded from this analysis. If a participant had missing data at all time-points for a given variable (during the TSST or Exam Stress/Non Stress visit) missing values were not imputed and the participant was excluded from this analysis. When the GG (Greenhouse-Geisser) or HF (Huynh-Feldt) adjustments were made under conditions where the test of sphericity was significant, adjusted df were used. Following data imputation, normality checks were performed using the Shapiro-Wilk test and visual inspection of histograms. Outliers were checked using box and whisker plots and only extreme outliers (3rd quartile + 3*interquartile range and 1st quartile – 3*interquartile range) were considered for exclusion from analysis. Salivary cortisol, PANAS, STAI, BDI and CANTAB PAL (Visuospatial Memory) data were not normally distributed and transformed using a natural log transformation (ln); VAS psychological stress data was transformed using a square-root transformation; CANTAB IED (Attentional Flexibility/Reversal Learning) data was not normally distributed, but no transformations improved normality, so we proceeded with parametric analysis but with caution in interpreting the analysis. PSS and CANTAB SST data (stop signal response time last 20) were normally distributed and no transformations were performed. Following data imputation and transformation (if needed) to improve normality, repeated measures analysis of variance (ANOVA) with mode of delivery as between-subjects factor and change across time points due to exam stress in each variable (salivary cortisol, PANAS Positive/Negative, VAS psychological stress, STAI State and Trait, BDI, PSS, PAL Total Errors Adj., SSRT last 20, IED Attentional Flexibility, IED Reversal Learning) as the within-subjects factor. Significant main effects were followed by post-hoc comparisons using a Bonferroni correction for multiple comparisons as appropriate. Where Mauchly's test of sphericity was significant, the Greenhouse-Geisser or Huynh-Feldt correction was applied. Our primary outcome variable was salivary cortisol output in response to the TSST. Non-transformed data are presented as mean ± standard error of the mean (SEM). Effect sizes are reported as partial Eta squared ηp2). All statistical analyses were carried out using IBM SPSS Statistics 22.0 for Windows software package. Statistical differences for microbiota composition based on alpha diversity and for differentially abundant taxa were calculated using the compareGroups package in R. Taxa below 0.5% sample abundance and the unclassified taxa were grouped into the “Other” category.
3. Results
C-section and vaginally delivered participants did not significantly differ in relation to age, BMI, years of education, units of alcohol consumed per week, socioeconomic status, or early life stress (child abuse, data not shown), (Table 1), or with respect to nutritional intake (Table 2). Self-reported race and ethnicity for vaginally delivered participants was 38 Caucasian and 2 Asian, and for C-section participants was 34 Caucasian and 2 Asian. As a representation of the Irish population this compares to the most recent national census where the largest group is “White Irish” with 3,854,226 (82.2%) followed by “any other white background” (9.5%), non-Chinese Asian (1.7%) and “other including mixed background” (1.5%), (Ireland, 2016). The indication for C-sections were, planned (18/36), emergency (10/36) and unknown (8/36).
Table 2.
Comparison of nutrient intake between groups at each visit Participants matched with regard to intake of macronutrients at each visit, although differences in the micronutrients riboflavin and iodine were observed in the vaginally delivered cohort in the Exam Stress visit when compared with the Non-Stress visit.
|
Vaginally Delivered |
C-section |
||||||
|---|---|---|---|---|---|---|---|
| Nutrient | Recommended daily intakea | Non-Stressed | Stressed | Non-stressed | Stressed | VD vs. CS non-stressed | VD vs. CS stressed |
| Kilocalories | 2000–2400 (males; depending on activity level) | 2329 ± 140 | 2452 ± 188 | 2427 ± 187 | 2266 ± 135 | NS | NS |
| Protein (g) | 10–35% of total energy | 127 ± 11 (22%) | 125 ± 12 (20%) | 114 ± 9 (19%) | 116 ± 10 (20%) | NS | NS |
| Fat (g) | 20–35% of total calories | 92 ± 7 (36%) | 98 ± 9 (36%) | 96 ± 8.5 (35%) | 93 ± 7 (37%) | NS | NS |
| Carbohydrate (g) | 45–65% of total calories | 256 ± 14 (46%) | 273 ± 19 (45%) | 277 ± 22 (43%) | 245 ± 13 (43%) | NS | NS |
| Alcohol (ml) | 21 standard drinks (1/2 pint of beer, small glass of wine, one measure of spirits) | 4.6 ± 0.7 | 5.6 ± 1.1 | 9.1 ± 2.4 | 6.6 ± 1.4 | NS | NS |
| Monounsaturated fatty acids (g) | >12% of total energy | 31 ± 2.5 (12%) | 33 ± 3.2 (12%) | 33 ± 3.1 (13%) | 32 ± 2.7 (13%) | NS | NS |
| Polyunsaturated fatty acids (g) | >6% of total energy | 15 ± 1.2 (5.8%) | 19 ± 2 (7%) | 18 ± 1.8 (6.5%) | 16 ± 1.4 (6%) | NS | NS |
| Saturated fatty acids (g) | <10% of total energy | 33 ± 2.9 (12.7%) | 32 ± 3 (12%) | 32 ± 2.7 (11.5%) | 32 ± 2.7 (13%) | NS | NS |
| Cholesterol (mg) | 300 mg | 420 ± 45 | 402 ± 47 | 386 ± 38 | 432 ± 50 | NS | NS |
| Total sugar (g) | <10% of total energy | 99 ± 7 (17%) | 98 ± 9 (16%) | 98 ± 9.7 (16%) | 90 ± 7 (16%) | NS | NS |
| Starch (g) | 154 ± 9.7 | 173 ± 12 | 177 ± 15 | 152 ± 8.3 | NS | NS | |
| Fibre (g) | >25 g | 26 ± 1.8 | 28 ± 2.4 | 28 ± 2.8 | 24 ± 2.2 | NS | NS |
| Retinol Equiv. (μg) | 800 μg | 989 ± 9.88 | 876 ± 102 | 844 ± 93 | 991 ± 158 | NS | NS |
| Thiamine (mg) | 1.1 mg | 1.9 ± 0.1 | 2.0 ± 0.2 | 1.8 ± 0.2 | 1.7 ± 0.1 | NS | NS |
| Riboflavin (mg) | 1.4 mg | 2.0 ± 0.2 | 1.6 ± 0.1 | 1.5 ± 0.1 | 1.6 ± 0.1 | 0.03 | NS |
| Niacin (mg) | 16 mg | 35 ± 3.2 | 37 ± 3.8 | 32 ± 2.6 | 32 ± 3.3 | NS | NS |
| Vitamin B6 (mg) | 1.4 mg | 3.5 ± 0.3 | 3.5 ± 0.3 | 3.2 ± 0.3 | 3.1 ± 0.2 | NS | NS |
| Vitamin B12 (μg) | 2.5 μg | 5.9 ± 0.6 | 4.6 ± 0.5* | 4.9 ± 0.5 | 5.5 ± 0.6 | NS | NS |
| Folate (μg) | 200 μg | 347 ± 29 | 333 ± 33 | 320 ± 31 | 293 ± 21 | NS | NS |
| Vitamin C (mg) | 80 mg | 197 ± 25 | 181 ± 27 | 143 ± 20 | 125 ± 15 | NS | NS |
| Vitamin D (μg) | 5 μg | 3.8 ± 0.4 | 3.6 ± 0.4 | 3.7 ± 0.6 | 4.1 ± 0.5 | NS | NS |
| Vitamin E (mg) | 12 mg | 8.1 ± 0.7 | 8.7 ± 0.9 | 8.3 ± 0.9 | 7.5 ± 0.7 | NS | NS |
| Phosphorous (mg) | 700 mg | 1812 ± 133 | 1685 ± 144 | 1614 ± 127 | 1557 ± 111 | NS | NS |
| Calcium (mg) | 1000 mg | 893 ± 93 | 661 ± 60* | 693 ± 61 | 652 ± 53 | NS | NS |
| Iron (mg) | 7 mg | 14 ± 0.8 | 14 ± 1.1 | 13 ± 1.1 | 13 ± 0.9 | NS | NS |
| Selenium (μg) | 55 μg | 66 ± 5.7 | 646.2 | 60 ± 5.4 | 61 ± 5.7 | NS | NS |
| Zinc (mg) | 10 mg | 14 ± 1.1 | 13 ± 1.1 | 13 ± 1.0 | 13 ± 0.9 | NS | NS |
| Sodium (mg) | 1600 mg | 3246 ± 203 | 3515 ± 252 | 3323 ± 264 | 3245 ± 221 | NS | NS |
| Potassium (mg) | 2000 mg | 4220 ± 299 | 4079 ± 356 | 3785 ± 341 | 3556 ± 225 | NS | NS |
| Magnesium (mg) | 375 mg | 384 ± 27 | 388 ± 33 | 375 ± 33 | 338 ± 22 | NS | NS |
| Copper (mg) | 1 mg | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 | NS | NS |
| Chloride (mg) | 800 mg | 5020 ± 322 | 5280 ± 379 | 5014 ± 429 | 4812 ± 355 | NS | NS |
| Manganese (mg) | 2 mg | 3.7 ± 0.3 | 4.1 ± 0.3 | 3.9 ± 03 | 3.5 ± 0.3 | NS | NS |
| Iodine (μg) | 15 μg | 170 ± 19 | 131 ± 15* | 114 ± 11 | 127 ± 13 | 0.02 | NS |
NS – Not Significant.
Source: Food Safety Authority of Ireland; *p < 0.05.
3.1. C-section participants have a greater psychological stress response to an acute stressor
Participants born by C-section reported greater psychological stress in response to the TSST when compared to vaginally delivered participants at T2 immediately post-stressor but were comparable with vaginally delivered participants at T3 (Fig. 1a). However, C-section participants did not exhibit a differential salivary cortisol response to the TSST (Fig. 1b). Measures of positive affect were significantly lower throughout the procedure in C-section participants (Fig. 1b), although negative affect was not (Fig. 1c). Concentrations of IL-1β (Fig. 1e) and IL-10 (Fig. 1f), but not IL-6, IL-8 or TNFα (Supplementary Fig. 1), were significantly higher in C-Section participants compared with vaginally delivered participants throughout the testing period, although this was due to elevated baseline levels and not acute stress.
Fig. 1.
Heightened psychological response to an acute stress in C-section participants in young adulthood, and elevated inflammatory markers in C-section participants
A) Self-reported psychological stress response (Stress (F(2, 146) = 92.53, p < 0.001, ηp2 = 0.56); Delivery method (F(1, 73) = 4.72, p = 0.033, ηp2 = 0.061); Stress x Delivery method (F(2, 146) = 1.17, p = 0.31, ηp2 = 0.016; n = 40 [VB], n = 35 [C-section]), to the TSST procedure; B) Salivary cortisol response (Stress (F(3.19, 220.01) = 73.26, p = 0.001, ηp2 = 0.52); Delivery method (F(1, 69) = 0.14, p = 0.7, ηp2 = 0.002); Stress x Delivery method (F(3.19, 220.01) = 0.36, p = 0.79, ηp2 = 0.005; n = 3 [VB], n = 32 [C-section]); C) Positive Affect response as measured using the Positive and Negative Affect Schedule (Stress (F(2, 144) = 6.74, p = 0.02, ηp2 = 0.086); Delivery method (F(1, 72) = 5.78, p = 0.019, ηp2 = 0.074); Stress x Delivery method (F(2, 144) = 0.17, p = 0.85, ηp2 = 0.002; n = 40 [VB], n = 34 [C-section]). D) Negative Affect response to the TSST procedure as measured using the Positive and Negative Affect Schedule; (Stress (F(2, 144) = 101.12, p < 0.001, ηp2 = 0.58); Delivery method (F(1, 72) = 1.71, p = 0.19, ηp2 = 0.023); Stress x Delivery method (F(2, 144) = 0.86, p = 0.43, ηp2 = 0.012; n = 35 [VB], n = 31 [C-section]); E) Peripheral IL-1β response (Stress (F(3, 120) = 3.143, p = 0.026, ηp2 = 0.043); Delivery method (F(1, 70) = 6.584, p = 0.012, ηp2 = 0.086); Stress x Delivery method (F(3,120) = 5.92, p = 0.62, ηp2 = 0.008; n = 39 [VB], n = 33 [C-section]); F) Blood IL-10 levels are elevated in C-Section participants from baseline and across time-points (Stress (F(2.492, 176.96) = 0.521, p = 0.634, ηp2 = 0.007); Delivery method (F(1, 71) = 4.24, p = 0.043, ηp2 = 0.056); Stress x Delivery method (F(2.492, 176.96) = 0.615, p = 0.58, ηp2 = 0.009; n = 39 [VB], n = 34 [C-section]).; Post hoc comparisons using Bonferroni correction: *p > 0.05; **p < 0.01. Data are presented as mean ± standard error of the mean (S.E.M.).
3.2. C-section participants have a greater psychological stress response to a naturalistic stressor
To further examine the effect of stress on individuals born by C-section, a subset of participants attended two experimental days, one of which took place during their end-of-term university examination period (Exam Stress; after their 1st but prior to their last exam, and not on the same day as an exam) and one took place during term-time, but at least 4–6 weeks before or after their end-of-term examination period (Non-Stress). When comparing psychological distress levels during the Non-Stress and Exam-Stress periods, participants born by C-section reported significantly greater levels of perceived stress at the Exam-Stress period (Fig. 2a). However, HPA axis function, as measured by the cortisol awakening response during the Non-Stress and Exam-Stress periods, was not significantly different between C-section and vaginally delivered participants (Fig. 2b). Furthermore, state (Fig. 2c) and trait anxiety (Fig. 2a), was elevated in participants born by C-section when compared with vaginally delivered participants, during the Exam Stress period but not during the Non-Stress period. The anti-inflammatory cytokine IL-10 was significantly elevated during the Exam-Stress period in C-Section participants (Fig. 2f). However, there was no group difference in IL-1β (Fig. 2e), TNF-α, IL-6 or IL-8 (Fig. 2).
Fig. 2.
Heightened response to a naturalistic stressor, exam stress, in C-section participants in young adulthood.
A) Psychological stress as measured using the Perceived Stress Scale (Stress (F(1, 68) = 5.12,p = 0.027, ηp2 = 0.07); Delivery method (F(1, 68) = 5.45, p = 0.023, ηp2 = 0.074); Stress x Delivery method (F(1, 68) = 2.98, p = 0.089, ηp2 = 0.07; n = 38 [Vaginally born], n = 32 [C-section]), during a Non-Stress and Exam Stress period.; B) Salivary cortisol awakening response (Stress (F(1, 56) = 2.692, p = 0.11, ηp2 = 0.046); Delivery method (F(1, 56) = 0.146, p = 0.704, ηp2 = 0.003); Stress x Delivery method (F(1, 56) = 3.711, p = 0.059, ηp2 = 0.062; n = 33 [VB], n = 35 [C-section]). C) State anxiety levels as measured using the State Trait Anxiety Inventory (Stress (F(1, 67) = 35.48, p < 0.001, ηp2 = 0.35); Delivery method (F(1, 67) = 5.21, p = 0.026, ηp2 = 0.072); Stress x Delivery method (F(1, 67) = 0.59, p = 0.59, ηp2 = 0.45; n = 38 [VB], n = 31 [C-section]); D) Trait anxiety levels as measured using the State Trait Anxiety Inventory (Stress(F1,68) = 6.372,p = 0.14, ηp2 = 0.086); (Delivery method (F(1, 68) = 6.638, p = 0.012, ηp2 = 0.089); Stress x Delivery method = (F(1,68) = 8.335, p = 0.005, ηp2 = 0.109; n = 38 [VB], n = 31 [C-section]); E) Exam Stress TNF-α, Stress: (F(1, 65) = 30.448, p < 0.001, ηp2 = 0.319), Delivery: F(1,65) = 4.513, p = 0.037, ηp2 = 0.065, Stress x Delivery: (F(1, 65) = 0.006, p = 0.936, ηp2 < 0.001); F) Exam Stress IL-6, Stress: (F(1, 64) = 0.434, p = 0.512, ηp2 = 0.007), Delivery: F(1,61) = 2.304, p = 0.134, ηp2 = 0.035, Stress x Delivery: (F(1, 64) = 0.825, p = 0.367, ηp2 = 0.013); G) Exam stress IL-10, Stress: (F(1, 66) = 0.853, p = 0.359, = 0.013), Delivery: (F(1,66) = 6.044, p = 0.017, ηp2 = 0.084), Stress x Delivery: (F(1, 66) = 0.22, p = 0.884, ηp2 < 0.001); H) Exam Stress IL-1β,Stress: (F(1, 61) = 7.878, p = 0.007, ηp2 = 0.114), Delivery: F(1,61) = 2.934, p = 0.092, ηp2 = 0.046, Stress x Delivery: (F(1, 61) = 0.57, p = 0.453, ηp2 = 0.009); I) Exam Stress IL-8, Stress: (F(1, 65) = 11.557, p = 0.001, ηp2 = 0.151), Delivery: F(1,65) = 0.027, p = 0.87, ηp2 < 0.001, Stress x Delivery: (F(1, 65) = 0.25, p = 0.619, ηp2 = 0.004).
3.3. Cognitive performance was the same for both C-section and vaginally delivered participants
Interestingly, we did not identify any difference in cognitive performance on tests of visuospatial memory (Fig. 3a), response inhibition (Fig. 3b) attentional flexibility Fig. 3c) or reversal learning (Fig. 3d) between C-section and vaginally delivered participants during the Non-Stress or Exam Stress period.
Fig. 3.
No differences in cognition between participants, born either by C-section or naturally delivered, in young adulthood.
A) Visuospatial memory as measured by the Paired Associates Learning (PAL) test, Total Errors Adjusted (Adj) (Stress (F(1, 67) = 0.02, p = 0.89, ηp2 < 0.001); Delivery method (F(1, 67) = 0.462, p = 0.499, ηp2 < 0.007); Stress x Delivery method (F(1, 67) = 0.076, p = 0.783, ηp2 = 0.001; n = 37 [VB], n = 32 [C-section]). B) Response inhibition as measured by the Stop Signal Task (SST), stop signal reaction time (SSRT; calculated for last 20 sub-blocks) (Stress (F(1, 67) = 1.44, p = 0.24, ηp2 = 0.021); Delivery method (F(1, 67) = 0.494, p = 0.484, ηp2 = 0.007); Stress x Delivery method (F(1, 67) = 1.37, p = 0.25, ηp2 = 0.02; n = 37 [VB], n = 32 [C-section]). C) Attentional Flexibility as measured by the Intra-Extra Dimensional Set Shift (IED), total errors made on stage 6 and 8 (Stress (F(1, 67) = 0.28, p = 0.598, ηp2 = 0.004); Delivery method (F(1, 67) = 0.016, p = 0.9, ηp2 = 0.001); Stress x Delivery method (F(1, 67) = 1.82, p = 0.182, ηp2 = 0.026; n = 37 [VB], n = 32 [C-section]). D) Reversal Learning as measured by the IED, total errors on stage 2, 5, 7 and 9 (Stress (F(1, 67) = 0.795, p = 0.376, ηp2 = 0.012); Delivery method (F(1, 67) = 0.009, p = 0.93, ηp2 = 0.001); Stress x Delivery method (F(1, 67) = 1.81, p = 0.183, ηp2 = 0.026; n = 37 [VB], n = 32 [C-section]). Data are presented as mean ± standard error of the mean (S.E.M.).
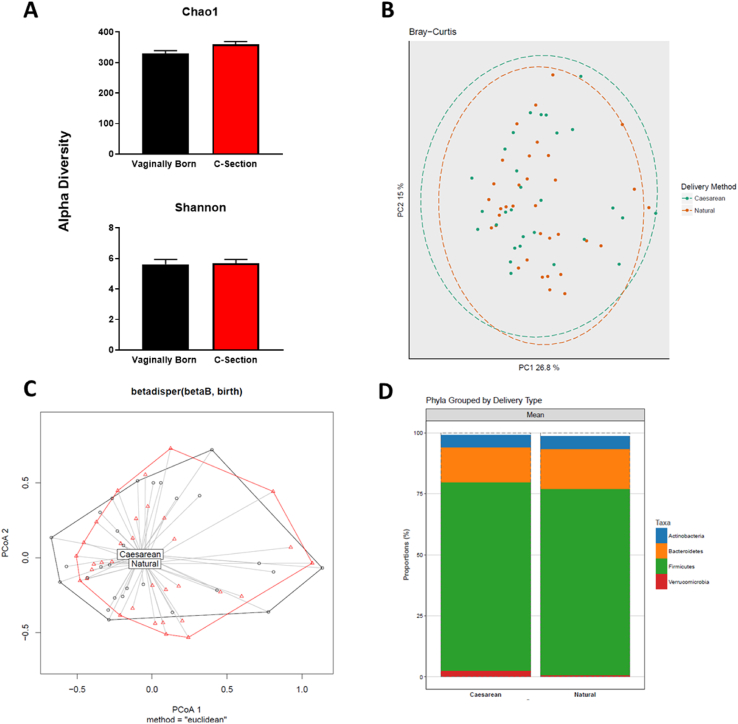
3.4. Faecal 16S microbiome sequencing shows modest differences between C-section and vaginally delivered participants by adulthood
With regard to the microbiome composition, beta-diversity was assessed using unweighted UniFrac distances from the averaged rarefied 16S rRNA gene dataset determined that the microbiota data from C-section delivered young adult participants did not separate from vaginally delivered young adult participants (Fig. 4, Table 3). Likewise, there were no differences in alpha diversity while a small number of differences in relative phylum abundance in vaginally delivered and C-section born adults (Fig. 4) were present. When we examined specific differential abundance, we saw an increase in the phylum Verrucomicrobia in faeces from vaginally born participants, similarly at the family level, Verrucomicrobiaceae was increased in the vaginally born group while at the genus level, Akkermansia was significantly increased compared to participants born by C-section (Table 4). There was no significant correlation between any of the other parameters measured and the modest relative abundance changes seen in Table 4.
Fig. 4.
Similar microbiome compositions in participants, born either by C-section or naturally delivered, in young adulthood. Alpha diversity estimation in Natural vs. Caesarean born patients. Chao1 and Shannon indices are shown (Chao1 p = 0.08, Shannon p = 0.36). The full dataset is tested, and the p-value refers to a nonparametric test on the median. B) Principle Coordinates Analysis (PCoA) plots of beta diversity metrics Bray-Curtis. The first and second axes are shown with the variance explained by each. There was no significant clustering for any metric, (r = −0.018, p = 0.76). C) PCoA dispersion plots for Bray-Curtis. The grouping labels are located at the centroid of the shapes for each group. Natural, red; Caesarean, black. D) Stacked barplots showing the taxonomic classification for each phylogenetic level: Phylum. Mean proportions of a sample are shown on the y-axis with grouping by birth mode on the x-axis. Natural birth N = 34 Caesarean Section = 29. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 3.
Beta-diversity statistics.
| Beta-diversity metric | R statistic | Significance |
|---|---|---|
| Unweighted UniFrac | −0.01752 | 0.758 |
| Weighted UniFrac | −0.01796 | 0.812 |
| Bray-Curtis | −0.01367 | 0.705 |
Table 4.
Differentially abundance taxa between VB and CS groups in adults.
| Taxa | Taxonomic Level | VB | CS | P-value |
|---|---|---|---|---|
| Actinobacteria | p | 4.29 [2.95; 5.87] | 4.06 [2.52; 6.64] | 0.847 |
| Bacteroidetes | p | 13.7 [7.31; 19.7] | 15.7 [9.64; 22.0] | 0.448 |
| Firmicutes | p | 77.4 [71.5; 81.8] | 77.3 [72.1; 82.9] | 0.934 |
| Proteobacteria | p | 0.30 [0.20; 0.61] | 0.33 [0.15; 0.66] | 0.815 |
| Verrucomicrobia | p | 0.62 [0.02;2.16] | 0.02 [0.00;0.33] | 0.027 |
| Actinomycetaceae | f | 0.01 [0.00; 0.01] | 0.01 [0.01; 0.02] | 0.098 |
| Bifidobacteriaceae | f | 2.73 [1.98; 3.70] | 2.71 [1.30; 3.96] | 0.945 |
| Coriobacteriaceae | f | 1.55 [0.87; 2.15] | 1.13 [0.83; 2.28] | 0.491 |
| Bacteroidaceae | f | 5.45 [3.91; 11.0] | 9.19 [5.74; 14.0] | 0.092 |
| Porphyromonadaceae | f | 1.05 [0.52; 2.07] | 0.92 [0.53; 1.46] | 0.424 |
| Prevotellaceae | f | 0.46 [0.01; 4.30] | 0.12 [0.01; 1.82] | 0.624 |
| Rikenellaceae | f | 0.96 [0.52; 1.55] | 1.04 [0.37; 1.86] | 0.836 |
| Enterococcaceae | f | 0.01 [0.00; 0.03] | 0.01 [0.00; 0.02] | 0.195 |
| Lactobacillaceae | f | 0.01 [0.00; 0.09] | 0.01 [0.01; 0.03] | 0.689 |
| Streptococcaceae | f | 0.34 [0.13; 0.66] | 0.37 [0.16; 0.73] | 0.526 |
| Clostridiaceae_1 | f | 0.57 [0.21; 1.12] | 0.44 [0.11; 1.09] | 0.629 |
| Eubacteriaceae | f | 0.01 [0.01; 0.02] | 0.02 [0.01; 0.03] | 0.572 |
| Lachnospiraceae | f | 32.1 [27.5; 42.0] | 33.3 [29.6; 39.0] | 0.424 |
| Peptostreptococcaceae | f | 1.19 [0.62; 2.39] | 1.54 [0.60; 2.06] | 0.879 |
| Ruminococcaceae | f | 28.6 [24.7; 32.2] | 29.5 [23.7; 34.8] | 0.679 |
| Erysipelotrichaceae | f | 1.07 [0.54; 2.52] | 1.67 [0.84; 2.23] | 0.356 |
| Acidaminococcaceae | f | 0.07 [0.01; 1.15] | 0.47 [0.01; 1.52] | 0.244 |
| Veillonellaceae | f | 3.74 [1.38; 5.49] | 1.54 [0.18; 5.44] | 0.19 |
| Sutterellaceae | f | 0.10 [0.02; 0.22] | 0.05 [0.01; 0.18] | 0.436 |
| Desulfovibrionaceae | f | 0.07 [0.03; 0.12] | 0.04 [0.02; 0.15] | 0.639 |
| Enterobacteriaceae | f | 0.01 [0.00; 0.06] | 0.02 [0.01; 0.09] | 0.25 |
| Pasteurellaceae | f | 0.01 [0.00; 0.03] | 0.02 [0.00; 0.07] | 0.33 |
| Verrucomicrobiaceae | f | 0.62 [0.02;2.16] | 0.02 [0.00;0.33] | 0.027 |
| Actinomyces | g | 0.01 [0.00; 0.02] | 0.01 [0.01; 0.02] | 0.136 |
| Akkermansia | g | 0.65 [0.02;2.18] | 0.02 [0.00;0.34] | 0.025 |
| Alistipes | g | 0.96 [0.54; 1.56] | 1.06 [0.40; 1.89] | 0.858 |
| Anaerostipes | g | 1.26 [0.58; 1.59] | 1.14 [0.59; 2.03] | 0.689 |
| Asaccharobacter | g | 0.10 [0.04; 0.17] | 0.05 [0.02; 0.13] | 0.209 |
| Bacteroides | g | 5.90 [4.00; 11.0] | 9.67 [6.02; 15.6] | 0.098 |
| Barnesiella | g | 0.23 [0.12; 0.69] | 0.23 [0.15; 0.48] | 0.684 |
| Bifidobacterium | g | 2.75 [1.99; 3.76] | 2.85 [1.31; 3.99] | 0.912 |
| Bilophila | g | 0.05 [0.01; 0.09] | 0.03 [0.01; 0.08] | 0.535 |
| Blautia | g | 5.58 [4.52; 9.30] | 6.10 [4.48; 8.48] | 0.793 |
| Butyricicoccus | g | 0.43 [0.31; 0.59] | 0.41 [0.28; 0.66] | 0.783 |
| Butyricimonas | g | 0.02 [0.00; 0.04] | 0.02 [0.00; 0.05] | 0.792 |
| Clostridium_IV | g | 0.50 [0.23; 1.42] | 0.40 [0.26; 0.89] | 0.6 |
| Clostridium_sensu_stricto | g | 0.58 [0.21; 1.13] | 0.45 [0.11; 1.09] | 0.62 |
| Clostridium_XI | g | 1.19 [0.63; 2.43] | 1.55 [0.61; 2.10] | 0.858 |
| Clostridium_XlVa | g | 1.06 [0.77; 1.94] | 1.26 [0.92; 1.67] | 0.408 |
| Clostridium_XlVb | g | 0.05 [0.02; 0.17] | 0.04 [0.01; 0.14] | 0.517 |
| Clostridium_XVIII | g | 0.53 [0.18; 0.92] | 0.74 [0.27; 1.24] | 0.16 |
| Collinsella | g | 0.90 [0.63; 1.83] | 0.84 [0.50; 1.62] | 0.416 |
| Coprococcus | g | 3.32 [1.20; 6.18] | 2.13 [1.07; 3.76] | 0.195 |
| Dialister | g | 1.98 [0.74; 4.11] | 0.64 [0.01; 3.40] | 0.067 |
| Dorea | g | 1.41 [0.81; 1.96] | 1.64 [0.94; 2.56] | 0.264 |
| Eerthella | g | 0.00 [0.00; 0.02] | 0.00 [0.00; 0.02] | 0.814 |
| Enterococcus | g | 0.01 [0.01; 0.03] | 0.01 [0.00; 0.02] | 0.209 |
| Escherichia.Shilla | g | 0.01 [0.00; 0.06] | 0.01 [0.00; 0.05] | 0.71 |
| Eubacterium | g | 0.01 [0.01; 0.02] | 0.01 [0.01; 0.03] | 0.699 |
| Faecalibacterium | g | 13.1 [9.81; 16.0] | 11.9 [7.84; 15.7] | 0.535 |
| Flavonifractor | g | 0.03 [0.02; 0.04] | 0.02 [0.02; 0.10] | 0.61 |
| Gemmir | g | 1.11 [0.57; 1.78] | 1.15 [0.64; 2.51] | 0.572 |
| Gordonibacter | g | 0.01 [0.00; 0.03] | 0.01 [0.00; 0.02] | 0.571 |
| Haemophilus | g | 0.01 [0.00; 0.04] | 0.02 [0.00; 0.06] | 0.374 |
| Holdemania | g | 0.01 [0.01; 0.01] | 0.01 [0.01; 0.02] | 0.581 |
| Lachnospira | g | 0.23 [0.02; 0.72] | 0.24 [0.03; 1.12] | 0.836 |
| Lactobacillus | g | 0.01 [0.00; 0.09] | 0.01 [0.01; 0.03] | 0.689 |
| Odoribacter | g | 0.07 [0.02; 0.17] | 0.06 [0.02; 0.16] | 0.783 |
| Oscillibacter | g | 0.20 [0.09; 0.29] | 0.17 [0.08; 0.39] | 0.858 |
| Parabacteroides | g | 0.59 [0.28; 0.84] | 0.41 [0.25; 0.83] | 0.491 |
| Parasutterella | g | 0.01 [0.00; 0.07] | 0.01 [0.00; 0.02] | 0.562 |
| Phascolarctobacterium | g | 0.01 [0.00; 0.55] | 0.22 [0.00; 1.36] | 0.214 |
| Prevotella | g | 0.07 [0.00; 4.29] | 0.01 [0.00; 1.71] | 0.276 |
| Roseburia | g | 7.48 [6.28; 13.2] | 10.4 [5.73; 12.3] | 0.639 |
| Ruminococcus | g | 4.59 [3.23; 6.60] | 5.35 [1.66; 7.42] | 0.526 |
| Ruminococcus2 | g | 1.16 [0.93; 1.81] | 1.23 [0.72; 1.87] | 0.783 |
| Streptococcus | g | 0.34 [0.12; 0.68] | 0.37 [0.14; 0.65] | 0.699 |
| Sutterella | g | 0.01 [0.00; 0.15] | 0.02 [0.00; 0.11] | 0.78 |
| Turicibacter | g | 0.04 [0.01; 0.14] | 0.08 [0.01; 0.20] | 0.416 |
| Veillonella | g | 0.04 [0.01; 0.12] | 0.06 [0.01; 0.19] | 0.62 |
Relative abundance data (Median [1st Quartile; 3rd Quartile] at phylum (p), family (f) and genus (g) level, as tested with a Mann-Whitney U test. The p-values are correct for multiple testing at a defined false discovery. Significant effects (p < 0.05) are given in bold. Vaginal birth = VB; C-section = CS.
4. Discussion
Mode of delivery at birth is a vital factor regulating the initial composition and subsequent assembly pattern of the gut microbiota in infants (Brestoff and Artis, 2013; Makino et al., 2013). Alterations in the normal colonisation of the gut microbial profile can lead to long-term negative consequences for health, including increased risk for immune and metabolic disorders (Brestoff and Artis, 2013). At the same time, C-section rates are dramatically increasing across the developing and developed world. The long-term consequences that this may have on health, including brain health, remain poorly understood. Here, to our knowledge for the first time, we report that the known negative outcomes of C-section can include lasting changes in stress sensitivity, demonstrated by increased psychological distress and anxiety in healthy young males born by C-section.
We demonstrate that, by adulthood, any differences in early life gut microbiota composition which could have occurred due to differing birth modes (Dominguez-Bello et al., 2010; Fouhy et al., 2019; Shao et al., 2019; Stewart et al., 2018) were not robustly evident in adulthood, with modest changes in relative abundance. At the phylum level, there was an increase in Verrucomicrobia in vaginally born participants, similarly at the family level, Verrucomicrobiaceae was also increased in the vaginally born group while at the genus level, Akkermansia was significantly increased compared to participants born by C-section. This supports the majority of previous work describing progression towards normalisation of gut microbiota profile in C-section babies during early life (Bokulich et al., 2016; Chu et al., 2017; Hill et al., 2017; Jakobsson et al., 2014; Palmer et al., 2007), and differs from other work showing long-lasting microbial effects of C-section in adulthood (Goedert et al., 2014). Nonetheless, altered microbiome composition at critical periods during early life, at a time during which the central nervous system is in a state of rapid development, has been negatively implicated in a number of behavioural changes in both animals (Cowan et al., 2020; O'Mahony et al., 2017) and humans (Carlson et al., 2018; Christian et al., 2015). It is interesting to note that while the alterations in microbial composition mostly stabilise in this young adult cohort, the negative psychological effects of C-section endure. It is tempting to postulate that interventions during the early critical periods could reverse these potential C-section-induced changes in adulthood.
The data from these healthy human volunteers, although preliminary, implies that mode of delivery at birth has an enduring effect on host immune system and behavioural response to stress. Indeed, while the microbiota of both groups is indistinguishable in adulthood, the higher levels of IL-1β and IL-10 in C-section participants compared with vaginally-delivered participants supports a dysregulation of immune-brain signalling in regulating behaviour (Sternberg, 2006). Some early gut bacteria colonizers (i.e. Bacteroides; Bifidobacteria and Lactobacillus species) promote regulatory T cells Foxp3(+) regulatory T cells (Tregs) and induce IL-10 production (Johansson et al., 2012; Round and Mazmanian, 2010). Indeed C-section has been associated with a lower production of T lymphocytes (Schlinzig et al., 2017). These early bacterial colonizers are important for the activation of regulatory B cells that can drive IL-10 and IL1-β production (Rosser et al., 2014). The association between the mode of delivery and the production of IL-10 and IL1- β has been previously described in early life in humans (Malamitsi-Puchner et al., 2005). Future work should examine if mode-of-delivery can influence specific aspects of cellular immune function in adulthood.
Measures of positive affect in the cohort born via C-section were significantly lower throughout the acute stress procedure and these individuals reported greater psychological stress in response to the TSST. Perhaps surprisingly, there is a dissociation between self-reported stress measures and cortisol output in the TSST. However, there are a large number of studies which have not reported any relationship between self-reported stress and cortisol output [See (Campbell and Ehlert, 2012) for review]. To probe this dysregulation in stress sensitivity we took advantage of a naturalistic stressor, University examination stress, and found that the anti-inflammatory cytokine IL-10 was significantly elevated during the Exam-Stress period in C-section participants and levels of TNF-α were increased. Individuals born by C-section also reported significantly greater levels of trait anxiety and perceived stress during the Exam Stress period but not during the Non-Stress period. Given the importance of mode of delivery in microbiota composition and subsequent immune and HPA axis priming, it is tempting to speculate that it may be causally related to the changes observed (Zijlmans et al., 2015). However, the nature of the current study design does not allow us to investigate factors that are responsible for such changes in this cohort as they occurred >20 years prior to testing. Throughout life the composition of the gut microbiome is further influenced by numerous factors including diet (David et al., 2014; Sandhu et al., 2017), exercise (Clarke et al., 2014; O'Sullivan et al., 2015), medication usage (Falony et al., 2016; Zhernakova et al., 2016), and geography (De Filippo et al., 2010; Vujkovic-Cvijin et al., 2020). Although the participants did not significantly differ with regard to these factors during the study, they still are potential confounds to any causal relationship. Furthermore, the participants did not differ significantly with regard to self-reported early life or recent life stressors (data not shown). Future cohort studies, which have temporal microbiota analysis coupled with different mode of delivery stratifications, across the lifespan, are now warranted to investigate mechanisms. Such studies should take into account the rationale underpinning the C-section (e.g. complicated pregnancy) and could also include prenatal maternal stress measures which are known to affect microbiome composition and offspring stress responses (Jasarevic et al., 2015a, 2015b). Moreover, the role of sex, birth-order and season of testing should be explored in future studies. Until recently there has been limited epidemiological data examining behavioural and psychiatric outcomes in individuals born by C-section. Indeed, where associations have been made in autism and psychosis they disappear when familial confounding is taken into place (Curran et al., 2015; O'Neill et al., 2016). However, associations between mode of delivery and attention deficit disorder (Curran et al., 2016) and school performance in Swedish adolescents (Curran et al., 2017), and numeracy skills in childhood (Polidano et al., 2017) have been described, however, direct measures of stress and cognitive performance in adulthood have not been investigated to date. Thus, our human data are to our knowledge the first attempt at stratification of stress response by mode of delivery. Longitudinal studies are required to determine if the alterations in stress responses seen in C-section delivered adults result in vulnerability to stress-related disorders such as depression.
Together these findings raise significant concerns regarding the increased use of C-section deliveries because of likely consequential stress effects. We postulate that the gut microbiome has a role in mediating such effects. However, it is clear that C-section may modify other physiological changes, such as stress and immune priming, during the birthing process that may also contribute to the phenotype (Golubeva et al., 2015; Jasarevic et al., 2015b, 2018; Lagercrantz and Slotkin, 1986; Zijlmans et al., 2015). Finally, since C-section deliveries when medically indicated are unavoidable lifesaving interventions, our data point to the importance of developing strategies to counteract the long-term negative consequences of C-section (Tribe et al., 2018) on stress responsivity later in life.
5. Strengths and limitations
The strengths of this study include the interdisciplinary analyses undertaken on the sample set, including psychological, cognitive, gut microbial, endocrine, and immune markers. This allowed for a thorough investigation of the potential enduring effects of C-section on multiple aspects of stress and anxiety in the young adults. Furthermore, use of a naturalistic stressor allowed within-subject analysis of stress outcomes during both times of high and low stress. Limitations of the study include the use of a male cohort only, in addition to the high percentage of Caucasian participants, and a relatively young population, therefore limiting the generalisability of our findings. Future studies should focus on females and the role that mode-of-delivery plays in the stress response. An assessment of sleep quality along with a more rigorous profiling of peripheral immune status would have also strengthened our conclusions. Furthermore, reasons for the C-section procedure were not determined. Future studies should include female participants, as well as a wider age-range as well as a more racially and ethnically diverse population.
6. Conclusion
Major differences in gut microbial profile found between infants born by C-section versus those vaginally delivered are not found to the same extent in a young adult population. However, an enduring impact of birth by C-section on psychological measures in young adulthood was observed. This has significant implications for stress-related disorders and warrants consideration of early intervention strategies to prevent the impact of c-section delivery on the developing gut microbiota from manifesting as psychological vulnerability to stress exposures.
Financial support
This work was supported in part by a Centre grant from Science Foundation Ireland (SFI) to the APC Microbiome Ireland under Grant Number SFI/12/RC/2273_P2; the European Community's Seventh Framework Programme Grant MyNewGut under Grant Agreement No. FP7/2007-2013. LHM was funded by Science Without Borders, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), under the Grant Agreement Number No. 11601-13-2.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
CRediT authorship contribution statement
Timothy G. Dinan: Conceptualization, Methodology, Writing – review & editing, Supervision. Paul J. Kennedy: Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Livia H. Morais: Formal analysis, Writing – original draft, Writing – review & editing. Amy Murphy: Formal analysis. Caitriona M. Long-Smith: Writing – original draft, Writing – review & editing. Gerard M. Moloney: Writing – original draft, Writing – review & editing. Thomaz F.S. Bastiaanssen: Formal analysis. Andrew P. Allen: Formal analysis. Aoife Collery: Formal analysis. David Mullins: Formal analysis. Anne-Marie Cusack: Methodology, Formal analysis. Kirsten Berding: Formal analysis. Paul W. O'Toole: Conceptualization, Methodology, Writing – review & editing. Gerard Clarke: Conceptualization, Methodology, Writing – review & editing, Supervision. Catherine Stanton: Conceptualization, Methodology, Writing – review & editing, Supervision. John F. Cryan: Conceptualization, Methodology, Writing – review & editing, Supervision.
Declaration of competing interest
APC Microbiome Ireland has conducted studies in collaboration with several companies, including GSK, Pfizer, Cremo, Wyeth, Mead Johnson, Nutricia, 4D Pharma, and DuPont. T. G. Dinan has been an invited speaker at meetings organized by Servier, Lundbeck, Janssen, and AstraZeneca and has received research funding from Mead Johnson, Cremo, Nutricia, and 4D Pharma. J. F. Cryan has been an invited speaker at meetings organized by Mead Johnson, Yakult, and Alkermes, and has received research funding from Mead Johnson, Cremo, Nutricia, and IFF. GC has received honoraria from Janssen, Probi and Apsen as an invited speaker, is in receipt of research funding from Pharmavite and Fonterra and is a paid consultant for Yakult and Zentiva.
Acknowledgements
The authors would like to thank all the volunteers who participated in the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100425.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Allen A.P., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G. Biological and psychological markers of stress in humans: focus on the Trier Social Stress Test. Neurosci. Biobehav. Rev. 2014;38:94–124. doi: 10.1016/j.neubiorev.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Azad M.B., Konya T., Persaud R.R., Guttman D.S., Chari R.S., Field C.J., Sears M.R., Mandhane P.J., Turvey S.E., Subbarao P., Becker A.B., Scott J.A., Kozyrskyj A.L., Investigators C.S. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. 2016;123:983–993. doi: 10.1111/1471-0528.13601. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K.J.S.A., TX: Psychological Corporation . vol. 1. 1996. p. 82. (Manual for the Beck Depression Inventory-II). [Google Scholar]
- Bernstein D.P., Stein J.A., Newcomb M.D., Walker E., Pogge D., Ahluvalia T., Stokes J., Handelsman L., Medrano M., Desmond D., Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Betran A.P., Ye J., Moller A.B., Zhang J., Gulmezoglu A.M., Torloni M.R. The increasing trend in caesarean section rates: global, regional and national estimates: 1990-2014. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasucci G., Rubini M., Riboni S., Morelli L., Bessi E., Retetangos C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum. Dev. 2010;86(Suppl. 1):13–15. doi: 10.1016/j.earlhumdev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Bokulich N.A., Chung J., Battaglia T., Henderson N., Jay M., Li H., A D.L., Wu F., Perez-Perez G.I., Chen Y., Schweizer W., Zheng X., Contreras M., Dominguez-Bello M.G., Blaser M.J. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016;8:343ra82. doi: 10.1126/scitranslmed.aad7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff J.R., Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat. Immunol. 2013;14:676–684. doi: 10.1038/ni.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J., Ehlert U. Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology. 2012;37:1111–1134. doi: 10.1016/j.psyneuen.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Carlson A.L., Xia K., Azcarate-Peril M.A., Goldman B.D., Ahn M., Styner M.A., Thompson A.L., Geng X., Gilmore J.H., Knickmeyer R.C. Infant gut microbiome associated with cognitive development. Biol. Psychiatr. 2018;83:148–159. doi: 10.1016/j.biopsych.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra J.J. Influences of microbiota on intestinal immune system development. Am. J. Clin. Nutr. 1999;69:1046S–1051S. doi: 10.1093/ajcn/69.5.1046s. [DOI] [PubMed] [Google Scholar]
- Cho C.E., Norman M. Cesarean section and development of the immune system in the offspring. Am. J. Obstet. Gynecol. 2013;208:249–254. doi: 10.1016/j.ajog.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Christian L.M., Galley J.D., Hade E.M., Schoppe-Sullivan S., Kamp Dush C., Bailey M.T. Gut microbiome composition is associated with temperament during early childhood. Brain Behav. Immun. 2015;45:118–127. doi: 10.1016/j.bbi.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.M., Ma J., Prince A.L., Antony K.M., Seferovic M.D., Aagaard K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017;23:314–326. doi: 10.1038/nm.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S.F., Murphy E.F., O'Sullivan O., Lucey A.J., Humphreys M., Hogan A., Hayes P., O'Reilly M., Jeffery I.B., Wood-Martin R., Kerins D.M., Quigley E., Ross R.P., O'Toole P.W., Molloy M.G., Falvey E., Shanahan F., Cotter P.D. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–1920. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Cowan C.S.M., Dinan T.G., Cryan J.F. Annual Research Review: critical windows - the microbiota-gut-brain axis in neurocognitive development. JCPP (J. Child Psychol. Psychiatry) 2020;61:353–371. doi: 10.1111/jcpp.13156. [DOI] [PubMed] [Google Scholar]
- Curran E.A., Dalman C., Kearney P.M., Kenny L.C., Cryan J.F., Dinan T.G., Khashan A.S. Association between obstetric mode of delivery and autism spectrum disorder: a population-based sibling design study. JAMA Psychiatr. 2015;72:935–942. doi: 10.1001/jamapsychiatry.2015.0846. [DOI] [PubMed] [Google Scholar]
- Curran E.A., Kenny L.C., Dalman C., Kearney P.M., Cryan J.F., Dinan T.G., Khashan A.S. Birth by caesarean section and school performance in Swedish adolescents- a population-based study. BMC Pregnancy Childbirth. 2017;17:121. doi: 10.1186/s12884-017-1304-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran E.A., Khashan A.S., Dalman C., Kenny L.C., Cryan J.F., Dinan T.G., Kearney P.M. Obstetric mode of delivery and attention-deficit/hyperactivity disorder: a sibling-matched study. Int. J. Epidemiol. 2016;45:532–542. doi: 10.1093/ije/dyw001. [DOI] [PubMed] [Google Scholar]
- Darabi B., Rahmati S., HafeziAhmadi M.R., Badfar G., Azami M. The association between caesarean section and childhood asthma: an updated systematic review and meta-analysis. Allergy Asthma Clin. Immunol. 2019;15:62. doi: 10.1186/s13223-019-0367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., Ling A.V., Devlin A.S., Varma Y., Fischbach M.A., Biddinger S.B., Dutton R.J., Turnbaugh P.J. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J.B., Massart S., Collini S., Pieraccini G., Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan T.G., Cryan J.F., Stanton C. Gut microbes and brain development have black box connectivity. Biol. Psychiatr. 2018;83:97–99. doi: 10.1016/j.biopsych.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Dominguez-Bello M.G., Costello E.K., Contreras M., Magris M., Hidalgo G., Fierer N., Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falony G., Joossens M., Vieira-Silva S., Wang J., Darzi Y., Faust K., Kurilshikov A., Bonder M.J., Valles-Colomer M., Vandeputte D., Tito R.Y., Chaffron S., Rymenans L., Verspecht C., De Sutter L., Lima-Mendez G., D'Hoe K., Jonckheere K., Homola D., Garcia R., Tigchelaar E.F., Eeckhaudt L., Fu J., Henckaerts L., Zhernakova A., Wijmenga C., Raes J. Population-level analysis of gut microbiome variation. Science. 2016;352:560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fouhy F., Watkins C., Hill C.J., O'Shea C.A., Nagle B., Dempsey E.M., O'Toole P.W., Ross R.P., Ryan C.A., Stanton C. Perinatal factors affect the gut microbiota up to four years after birth. Nat. Commun. 2019;10:1517. doi: 10.1038/s41467-019-09252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert J.J., Hua X., Yu G., Shi J. Diversity and composition of the adult fecal microbiome associated with history of cesarean birth or appendectomy: analysis of the American Gut Project. EBioMedicine. 2014;1:167–172. doi: 10.1016/j.ebiom.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubeva A.V., Crampton S., Desbonnet L., Edge D., O'Sullivan O., Lomasney K.W., Zhdanov A.V., Crispie F., Moloney R.D., Borre Y.E., Cotter P.D., Hyland N.P., O'Halloran K.D., Dinan T.G., O'Keeffe G.W., Cryan J.F. Prenatal stress-induced alterations in major physiological systems correlate with gut microbiota composition in adulthood. Psychoneuroendocrinology. 2015;60:58–74. doi: 10.1016/j.psyneuen.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Harrington J., Fitzgerald A.P., Layte R., Lutomski J., Molcho M., Perry I.J. Sociodemographic, health and lifestyle predictors of poor diets. Publ. Health Nutr. 2011;14:2166–2175. doi: 10.1017/S136898001100098X. [DOI] [PubMed] [Google Scholar]
- Hill C.J., Lynch D.B., Murphy K., Ulaszewska M., Jeffery I.B., O'Shea C.A., Watkins C., Dempsey E., Mattivi F., Tuohy K., Ross R.P., Ryan C.A., PW O.T., Stanton C. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. doi: 10.1186/s40168-016-0213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland C.S. O.o. Census of Ireland. 2016. Census of Ireland, chapter 5, diversity.https://www.cso.ie/en/census/ 2016. [Google Scholar]
- Jakobsson H.E., Abrahamsson T.R., Jenmalm M.C., Harris K., Quince C., Jernberg C., Bjorksten B., Engstrand L., Andersson A.F. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63:559–566. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- Jasarevic E., Howard C.D., Morrison K., Misic A., Weinkopff T., Scott P., Hunter C., Beiting D., Bale T.L. The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus. Nat. Neurosci. 2018;21:1061–1071. doi: 10.1038/s41593-018-0182-5. [DOI] [PubMed] [Google Scholar]
- Jasarevic E., Howerton C.L., Howard C.D., Bale T.L. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology. 2015;156:3265–3276. doi: 10.1210/en.2015-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasarevic E., Rodgers A.B., Bale T.L. A novel role for maternal stress and microbial transmission in early life programming and neurodevelopment. Neurobiol. Stress. 2015;1:81–88. doi: 10.1016/j.ynstr.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.A., Saghafian-Hedengren S., Haileselassie Y., Roos S., Troye-Blomberg M., Nilsson C., Sverremark-Ekstrom E. Early-life gut bacteria associate with IL-4-, IL-10- and IFN-gamma production at two years of age. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keag O.E., Norman J.E., Stock S.J. Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: systematic review and meta-analysis. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy Paul J., Cryan John F., Dinan Timothy G., Clarke G. Irritable bowel syndrome: a microbiome-gut-brain axis disorder? World J. Gastroenterol. 2014;20(39):14105–14125. doi: 10.3748/wjg.v20.i39.14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagercrantz H. The good stress of being born. Acta Paediatr. 2016;105:1413–1416. doi: 10.1111/apa.13615. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H., Slotkin T.A. The "stress" of being born. Sci. Am. 1986;254:100–107. doi: 10.1038/scientificamerican0486-100. [DOI] [PubMed] [Google Scholar]
- Li H.T., Zhou Y.B., Liu J.M. The impact of cesarean section on offspring overweight and obesity: a systematic review and meta-analysis. Int. J. Obes. 2013;37:893–899. doi: 10.1038/ijo.2012.195. [DOI] [PubMed] [Google Scholar]
- Makino H., Kushiro A., Ishikawa E., Kubota H., Gawad A., Sakai T., Oishi K., Martin R., Ben-Amor K., Knol J., Tanaka R. Mother-to-infant transmission of intestinal bifidobacterial strains has an impact on the early development of vaginally delivered infant's microbiota. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamitsi-Puchner A., Protonotariou E., Boutsikou T., Makrakis E., Sarandakou A., Creatsas G. The influence of the mode of delivery on circulating cytokine concentrations in the perinatal period. Early Hum. Dev. 2005;81:387–392. doi: 10.1016/j.earlhumdev.2004.10.017. [DOI] [PubMed] [Google Scholar]
- McCann A., Ryan F.J., Stockdale S.R., Dalmasso M., Blake T., Ryan C.A., Stanton C., Mills S., Ross P.R., Hill C. Viromes of one year old infants reveal the impact of birth mode on microbiome diversity. PeerJ. 2018;6 doi: 10.7717/peerj.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsala J., Lundqvist A., Virta L.J., Kaila M., Gissler M., Virtanen S.M. Prenatal and post-natal exposure to antibiotics and risk of asthma in childhood. Clin. Exp. Allergy. 2015;45:137–145. doi: 10.1111/cea.12356. [DOI] [PubMed] [Google Scholar]
- Morais L.H., Golubeva A.V., Moloney G.M., Moya-Pérez A., Ventura-Silva A.P., Arboleya S., Bastiaanssen T.F.S., O'Sullivan O., Rea K., Borre Y., Scott K.A., Patterson E., Cherry P., Stilling R., Hoban A.E., El Aidy S., Sequeira A.M., Beers S., Moloney R.D., Renes I.B., Wang S., Knol J., Ross R.P., O'Toole P.W., Cotter P.D., Stanton C., Dinan T.G., Cryan J.F. Enduring behavioral effects induced by birth by caesarean section in the mouse. Curr. Biol. 2020;30:3761–3774. doi: 10.1016/j.cub.2020.07.044. e6. [DOI] [PubMed] [Google Scholar]
- Mueller N.T., Whyatt R., Hoepner L., Oberfield S., Dominguez-Bello M.G., Widen E.M., Hassoun A., Perera F., Rundle A. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int. J. Obes. 2015;39:665–670. doi: 10.1038/ijo.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng P.C. The fetal and neonatal hypothalamic-pituitary-adrenal axis. Arch. Dis. Child. Fetal Neonatal Ed. 2000;82:F250–F254. doi: 10.1136/fn.82.3.F250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony S.M., Clarke G., Dinan T.G., Cryan J.F. Early-life adversity and brain development: is the microbiome a missing piece of the puzzle? Neuroscience. 2017;342:37–54. doi: 10.1016/j.neuroscience.2015.09.068. [DOI] [PubMed] [Google Scholar]
- O'Neill S.M., Curran E.A., Dalman C., Kenny L.C., Kearney P.M., Clarke G., Cryan J.F., Dinan T.G., Khashan A.S. Birth by caesarean section and the risk of adult psychosis: a population-based cohort study. Schizophr. Bull. 2016;42:633–641. doi: 10.1093/schbul/sbv152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan O., Cronin O., Clarke S.F., Murphy E.F., Molloy M.G., Shanahan F., Cotter P.D. Exercise and the microbiota. Gut Microb. 2015;6:131–136. doi: 10.1080/19490976.2015.1011875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C., Bik E.M., DiGiulio D.B., Relman D.A., Brown P.O. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penders J., Thijs C., Vink C., Stelma F.F., Snijders B., Kummeling I., van den Brandt P.A., Stobberingh E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- Polidano C., Zhu A., Bornstein J.C. The relation between cesarean birth and child cognitive development. Sci. Rep. 2017;7:11483. doi: 10.1038/s41598-017-10831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser E.C., Oleinika K., Tonon S., Doyle R., Bosma A., Carter N.A., Harris K.A., Jones S.A., Klein N., Mauri C. Regulatory B cells are induced by gut microbiota-driven interleukin-1beta and interleukin-6 production. Nat. Med. 2014;20:1334–1339. doi: 10.1038/nm.3680. [DOI] [PubMed] [Google Scholar]
- Round J.L., Mazmanian S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. U. S. A. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu K.V., Sherwin E., Schellekens H., Stanton C., Dinan T.G., Cryan J.F. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl. Res. 2017;179:223–244. doi: 10.1016/j.trsl.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Schlinzig T., Johansson S., Stephansson O., Hammarstrom L., Zetterstrom R.H., von Dobeln U., Cnattingius S., Norman M. Surge of immune cell formation at birth differs by mode of delivery and infant characteristics-A population-based cohort study. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Forster S.C., Tsaliki E., Vervier K., Strang A., Simpson N., Kumar N., Stares M.D., Rodger A., Brocklehurst P., Field N., Lawley T.D. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. 2019;574:117–121. doi: 10.1038/s41586-019-1560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D. 1983. State-trait Anxiety Inventory for Adults. [Google Scholar]
- Sternberg E.M. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C.J., Ajami N.J., O'Brien J.L., Hutchinson D.S., Smith D.P., Wong M.C., Ross M.C., Lloyd R.E., Doddapaneni H., Metcalf G.A., Muzny D., Gibbs R.A., Vatanen T., Huttenhower C., Xavier R.J., Rewers M., Hagopian W., Toppari J., Ziegler A.G., She J.X., Akolkar B., Lernmark A., Hyoty H., Vehik K., Krischer J.P., Petrosino J.F. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562:583–588. doi: 10.1038/s41586-018-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribe R.M., Taylor P.D., Kelly N.M., Rees D., Sandall J., Kennedy H.P. Parturition and the perinatal period: can mode of delivery impact on the future health of the neonate? J. Physiol. 2018;596:5709–5722. doi: 10.1113/JP275429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatanen T., Plichta D.R., Somani J., Munch P.C., Arthur T.D., Hall A.B., Rudolf S., Oakeley E.J., Ke X., Young R.A., Haiser H.J., Kolde R., Yassour M., Luopajarvi K., Siljander H., Virtanen S.M., Ilonen J., Uibo R., Tillmann V., Mokurov S., Dorshakova N., Porter J.A., McHardy A.C., Lahdesmaki H., Vlamakis H., Huttenhower C., Knip M., Xavier R.J. Genomic variation and strain-specific functional adaptation in the human gut microbiome during early life. Nat. Microbiol. 2019;4:470–479. doi: 10.1038/s41564-018-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujkovic-Cvijin I., Sklar J., Jiang L., Natarajan L., Knight R., Belkaid Y. Host variables confound gut microbiota studies of human disease. Nature. 2020;587:448–454. doi: 10.1038/s41586-020-2881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- World Health Organization Human Reproduction Programme, A WHO Statement on caesarean section rates. Reprod. Health Matters. 2015;23:149–150. doi: 10.1016/j.rhm.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Yuan C., Gaskins A.J., Blaine A.I., Zhang C., Gillman M.W., Missmer S.A., Field A.E., Chavarro J.E. Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatr. 2016;170 doi: 10.1001/jamapediatrics.2016.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhernakova A., Kurilshikov A., Bonder M.J., Tigchelaar E.F., Schirmer M., Vatanen T., Mujagic Z., Vila A.V., Falony G., Vieira-Silva S., Wang J., Imhann F., Brandsma E., Jankipersadsing S.A., Joossens M., Cenit M.C., Deelen P., Swertz M.A., LifeLines cohort s., Weersma R.K., Feskens E.J., Netea M.G., Gevers D., Jonkers D., Franke L., Aulchenko Y.S., Huttenhower C., Raes J., Hofker M.H., Xavier R.J., Wijmenga C., Fu J. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352:565–569. doi: 10.1126/science.aad3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans M.A., Korpela K., Riksen-Walraven J.M., de Vos W.M., de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology. 2015;53:233–245. doi: 10.1016/j.psyneuen.2015.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.