Background and purpose
Previous studies have indicated that fibrinogen and low serum albumin levels are associated with poor outcomes of acute ischemic stroke. The role of the fibrinogen-to-albumin ratio (FAR) as a novel inflammatory and thrombotic biomarker in acute ischemic stroke is unclear. This study aims to investigate the relationship between the FAR and 3-month outcomes of acute pontine infarction. Methods: Patients with acute pontine infarction were consecutively included. All patients were followed up at 3 months after onset, and the 3-month outcome was evaluated using modified Rankin Scale (mRS) scores. A score of 0 to 2 was defined as a good outcome, and a score ≥ 3 was defined as a poor outcome. Receiver operating curve (ROC) analysis was used to calculate the optimal cutoff values for patients with acute pontine infarction. Then, a binary logistic regression model was used to evaluate the risk factors for a poor outcome after acute pontine infarction. Results: A total of 264 patients with acute pontine infarction were included. Eighty (30.3%) patients were included in the poor outcome group. The optimal cutoff value of the FAR for predicting the 3-month outcome of acute pontine infarction was 8.199. The FAR was independently associated with a poor outcome at 3 months in patients with acute pontine infarction (odds ratio [OR] = 1.293, 95% confidence interval [CI]: 1.150-1.453). Conclusions: We found that a high FAR predicted poor 3-month outcomes in patients with acute pontine infarction.
Keywords: pontine infarction, fibrinogen-to-albumin ratio, diffusion-weighted imaging, 3-month outcome, inflammation
Introduction
Cerebral infarction is the most common type of stroke and is characterized by high morbidity, mortality, and disability. Despite advances in treatment strategies and diagnostic methods in the past two decades, some patients still have severe neurological deficits.1,2 In cases of pontine infarction in particular, the fibrous conduction bundles are relatively concentrated; consequently, the clinical symptoms at disease onset are generally severe, and the outcome is usually poor. Thus, identifying a marker that can reliably predict the clinical outcome of pontine infarction is particularly important to determine the disease condition. Fibrinogen is an inflammation-related protein and is the main factor involved in blood viscosity and fibrin formation. Elevated blood fibrinogen levels indicate an increased risk of acute cerebral infarction and can predict short-term outcomes.3,4 Physiological levels of albumin inhibit vascular cell adhesion molecule-1 (VCAM-1) expression and increase oxygen free radical clearance, which reduce inflammatory responses and endothelial cell apoptosis, and therefore albumin is an anti-inflammatory and antioxidant factor. Reduced serum albumin levels can predict the short-term outcomes of acute cerebral infarction.5–7 Recent studies have indicated that the fibrinogen-to-albumin ratio (FAR) is a novel inflammation marker that is associated with the severity of coronary artery disease and with disease activity in ankylosing spondylitis.8,9 There are few studies of the association between FAR and the prognosis of acute cerebral infarction. Zheng et al. indicated that a FAR value ≥ 0.077 might be a predictor of poor 3-month clinical outcomes after acute lacunar cerebral infarction. 10 However, it is not clear whether an elevated FAR is associated with poor outcomes after pontine infarction. Therefore, the main purpose of this study was to elucidate the association between the FAR and the 3-month clinical outcomes of patients with acute pontine infarction.
Methods
Subjects
This is a prospective, observational study conducted during January 2012 to June 2019. Patients with acute pontine infarction admitted to the Affiliated Hefei Hospital of Anhui Medical University were continuously enrolled during the study period. The inclusion criteria for patients were as follows: older than 18 years old and diagnosed with acute pontine infarction by diffusion weighted imaging (DWI). All patients received antiplatelet drugs and statin therapy. Patients with the following conditions were excluded: (1) the time between onset and admission was longer than 7 days; (2) coexisting nonischemic lesions, such as subdural hematoma and intracranial tumor; (3) patients with severe dementia; (4) patients with tumors, liver disease, autoimmune disease, renal failure, or Parkinson's disease; and (5) prestroke disability with a modified Rankin Scale (mRS) score > 2. The study protocol (201727)was approved by the Research Ethics Committee of the Affiliated Hefei Hospital of Anhui Medical University, and written informed consent was obtained from all patients or their guardians before inclusion in the study.
Clinical Data
We collected demographic information, including age and sex; medical history records including hypertension, diabetes, atrial fibrillation, prior stroke or transient ischemic attack (TIA), and current smoking or drinking; blood pressure level on admission including systolic blood pressure (SBP) and diastolic blood pressure (DBP); and National Institutes of Health Stroke Scale (NIHSS) scores on admission. Hypertension was determined by prior use of antihypertensive drugs, SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg. Diabetes was determined by prior use of antidiabetic drugs, Fasting blood glucose (FBG) ≥ 7.0 mmol/L or 2-h postprandial blood glucose ≥ 11.1 mmol/L.
We considered the following as the potential causes of pontine infarction: 11 (1) Vertebrobasilar large-artery disease (VLAD): patients had a stenosis of at least 50% of the lumen diameter in the large artery (either vertebral or basilar artery (BA)). (2) Basilar artery branch disease (BABD): patients had hypertension or diabetes, with an infarct extending to the pontine surface and no large artery occlusive disease (LAOD) or potential source of cardioembolism. (3) Small artery disease (SAD): patients had hypertension or diabetes, the lesion (< 15 mm) did not reach the pontine surface, and patients had no other etiology (OE). (4) Cardiac embolism (CE): patients had potential cardiogenic embolism sources, including nonvalvular atrial movement, left ventricular wall segmental movement disorders, intracardiac thrombus or tumor, mitral stenosis, or other minor sources. (5) Other etiologies and unknown causes.
Laboratory Data
Fasting blood samples were collected on the morning of the second day after admission following an overnight fast. The fibrinogen concentration in plasma was measured using an automatic coagulation analyzer (Stago STAR Max, France). FBG, triglycerides (TG), low-density lipoprotein (LDL), albumin, and other biochemical parameters were assayed using an automatic biochemical analyzer (HITACHI Automatic Analyzer 7600-020, Japan)
Magnetic Resonance Imaging (MRI) Data Acquisition and Analysis
Within 3 days after admission, all patients underwent MRI with a 1.5 Tesla MRI scanner (Siemens Healthineers, Model: Avanto I class, Germany). The imaging parameters and evaluation method were described in a previously published study. 12 The DWI sequence was performed with the following parameters: repetition time/echo time, 3400/102 ms; slice thickness, 5 mm; dispersion mode: three-scan trace; b value, 0 to 1000. Three-dimensional time-of-fight (3-D TOF) magnetic resonance angiography (MRA) was acquired in the axial plane using the following parameters: repetition time 25 ms, echo time 7 ms, and section thickness 0.6 mm. The MRI and MRA results were analyzed, mainly including the infarct lesion size at the infarct site (the maximum diameter of the infarct lesion at the largest infarct level on DWI), and a BA with 50% stenosis was diagnosed as BA stenosis according to the North American symptomatic carotid endarterectomy test. 13 The radiologist was not aware of the fibrinogen, albumin, and mRS data.
Evaluation of 3-Month Outcomes
The clinical outcomes of all enrolled patients were determined by a qualified trained neurologist who was unaware of patient MRI data via telephone or by appointment in the outpatient department. The outcome was evaluated using mRS scores: an mRS ≤ 2 was defined as a good outcome, and an mRS ≥ 3 was defined as a poor outcome (severe disability or death).
Statistical Analyses
Data analysis was performed using SPSS 22.0 version for Windows (SPSS Inc., Chicago, IL). Continuous data were assessed for normality using the Kolmogorov-Smirnov test. Continuous variables with a normal distribution are expressed as the mean ± SD. Continuous variables without a normal distribution are expressed as the median (M) and interquartile range (IQR). Categorical variables are reported as absolute numbers and percentages (%). Differences in continuous variables between groups were assessed with Student's t test or the Mann-Whitney U test. Differences in categorical variable distributions between groups were assessed by the χ2 test or Fisher's exact test as appropriate. A receiver operating characteristic (ROC) curve was used to identify the FAR cutoff that best predicted good and poor outcomes. Binary logistic regression was used to verify factors independently associated with the 3-month outcome. Odds ratios (ORs) and 95% confidence intervals (CIs) were subsequently calculated. Spearman's correlation coefficient was used to determine the correlations of the FAR with NIHSS scores and mRS scores. All tests were two-tailed, and a value of P < 0.05 was considered statistically significant. The figures were drawn using PowerPoint and GraphPad Prism software (version 8.0).
Results
Clinical and Demographic Data
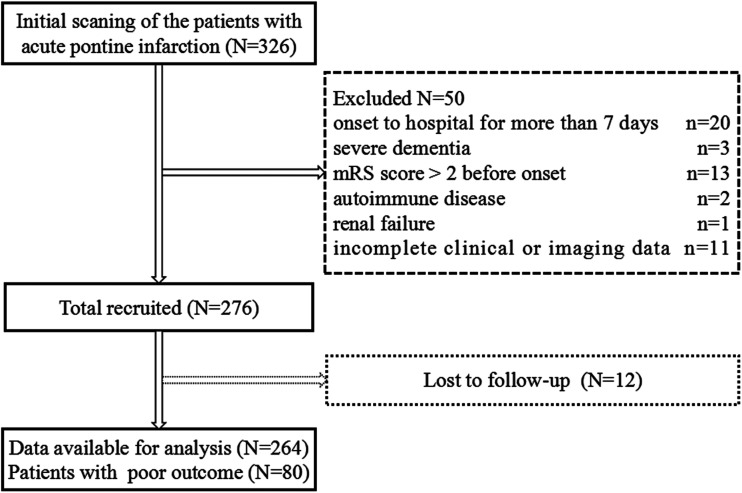
A total of 326 patients with acute pontine infarction were admitted to our hospital during the study period between January 2012 and June 2019. Among these patients, 50 were excluded according to the exclusion criteria. Twelve patients who were lost to follow-up during the 90-day follow-up period were not included in the analysis. Therefore, 264 patients were included in this study (Figure 1). Among the 264 patients, the mean age was (66.7 ± 11.0) years, 159 (60.2%) patients were male, and 105 (39.8%) were female. In terms of etiological classification, BABD accounted for the highest proportion of patients (108/264, 40.9%), followed by VLAD (60/264, 22.6%), SAD (50/264, 18.9%), and unknown etiology (36/264, 13.6%), and CE accounted for the lowest (10/264, 3.8%) proportion. The FAR was 8.11 ± 2.68. The clinical and demographic data are shown in Table 1. According to the mRS scores, 184 (69.7%) patients were in the good outcome group, and 80 (30.3%) were in the poor outcome group. Compared with the poor outcome group, the albumin level was significantly higher in the good outcome group (P = 0.037). The FBG, fibrinogen, and FAR values in the poor outcome group were significantly higher than those in the good outcome group (P < 0.05), while there was no statistically significant difference in other indicators. There was no statistically significant difference in the pontine infarction size and BA stenosis between the two groups.
Figure 1.
The diagram showed the selection of patients with acute pontine infarction.
Table 1.
Comparison of baseline characteristics in patients with good and poor 3-month outcomes.
| Variable | Good outcome (n = 184) | Poor outcome (n = 80) | OR (95% CI) | P value |
|---|---|---|---|---|
| Age (years) | 65.2 ± 11.0 | 70.1 ± 10.3 | 1.043 (1.017-1.071) | 0.001 |
| Male, n (%) | 113 (61.4) | 46 (57.5) | 0.850 (0.499-1.449) | 0.551 |
| Hypertension, n (%) | 147 (79.9) | 68 (85.0) | 1.426 (0.700-2.906) | 0.328 |
| Diabetes, n (%) | 79 (42.9) | 35 (43.8) | 1.034 (0.609-1.755) | 0.902 |
| Atrial fibrillation, n (%) | 4 (2.2) | 4 (5.0) | 2.368 (0.577-9.716) | 0.231 |
| Stroke history, n (%) | 37 (20.1) | 21 (26.3) | 1.414 (0.765-2.615) | 0.269 |
| Smoker, n (%) | 54 (29.3) | 19 (23.8) | 0.750 (0.410-1.373) | 0.351 |
| Alcohol user, n (%) | 32 (17.4) | 11 (13.8) | 0.757 (0.361-1.590) | 0.463 |
| Etiological subtype, n (%) | ||||
| LAOD | 40 (21.7) | 20 (25.0) | 1.500 (0.594-3.786) | 0.391 |
| CE | 7 (3.8) | 3 (3.8) | 1.286 (0.273-6.050) | 0.750 |
| BABD | 73 (39.7) | 35 (43.8) | 1.438 (0.612-3.383) | 0.405 |
| SAD | 37 (20.1) | 13 (16.2) | 1.054 (0.394-2.820) | 0.916 |
| OE | 27 (14.7) | 9 (11.2) | Ref | |
| SBP (mm Hg) | 152.6 ± 20.3 | 152.3 ± 21.2 | 0.999 (0.987-1.012) | 0.916 |
| DBP (mm Hg) | 88.3 ± 12.9 | 89.5 ± 10.1 | 1.009 (0.986-1.032) | 0.450 |
| FBG (mmol/l) | 6.40 ± 2.38 | 7.30 ± 3.47 | 1.117 (1.018-1.225) | 0.019 |
| TG (mmol/l) | 2.14 ± 1.45 | 1.99 ± 0.97 | 0.907 (0.732-1.125) | 0.375 |
| LDL (mmol/l) | 2.75 ± 0.78 | 2.98 ± 1.05 | 1.342 (0.992-1.816) | 0.056 |
| Fibrinogen (g/l) | 3.09 ± 0.77 | 3.77 ± 0.98 | 2.430 (1.735-3.403) | < 0.001 |
| Albumin (g/l) | 41.59 ± 3.27 | 40.57 ± 4.21 | 0.925 (0.859-0.995) | 0.037 |
| FAR | 7.52 ± 2.25 | 9.49 ± 3.07 | 1.334 (1.187-1.500) | < 0.001 |
| Infarct size (mm) | 13.44 ± 5.01 | 14.38 ± 5.28 | 1.037 (0.984-1.093) | 0.171 |
| BA stenosis, n (%) | 38 (20.7) | 20 (25.0) | 1.281 (0.689-2.379) | 0.434 |
Values are expressed as the mean ± standard deviation, n (%); OR, odds ratio; 95% CI, 95% confidence interval; LAOD, large artery occlusive disease; CE, cardiac embolism; BABD, basilar artery branch disease; SAD, small artery disease; OE, other etiology; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; TG, triglyceride; LDL, low-density lipoprotein cholesterol; FAR, fibrinogen-to-albumin ratio; BA, basilar artery.
The Relationship Between the FAR and 3-Month Outcomes in Patients with Acute Pontine Infarction
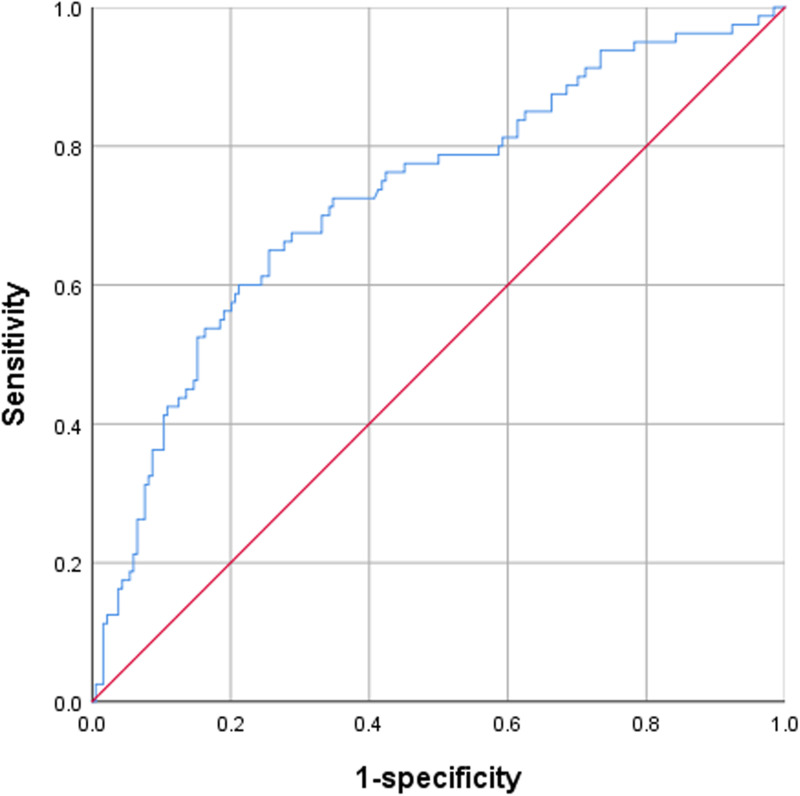
The cutoff points for the sensitivity and specificity of the FAR were estimated by performing ROC analysis. A FAR value of 8.199 was the optimal cutoff value to differentiate good outcomes from poor outcomes in patients with acute pontine infarction, and the area under the ROC curve was 0.728 (95% CI: 0.659-0.796). The FAR exhibited a sensitivity of 65.0% and a specificity of 74.5% for discriminating between good and poor outcomes (Figure 2).
Figure 2.
Receiver operating curve (ROC) showed predictive value of FAR for poor outcome (sensitivity = 0.650; 1-specificity = 0.255; FAR = 8.199; area under curve [AUC] = 0.728).
Comparison of Clinical Characteristics in the High and low FAR Groups
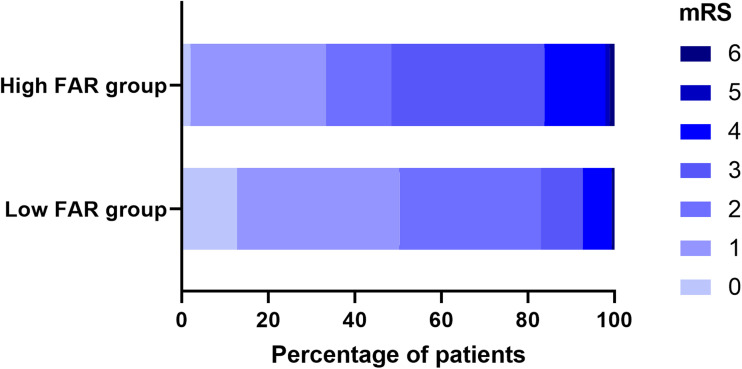
Using a FAR cutoff value of 8.199, we divided our patients into two groups: a low FAR group including 165 patients (FAR < 8.199) and a high FAR group including 99 patients (FAR ≥ 8.199). The characteristics of these two groups are summarized (Table 2) and (Figure 3). The NIHSS and mRS scores in the high FAR group were significantly higher than those in the low FAR group (all P < 0.05).
Table 2.
Demographic and clinical characteristics of patients with acute pontine infarction in the low ( < 8.199) and high (≥ 8.199) FAR groups.
| Variable | Low FAR (n = 165) | High FAR (n = 99) | P value |
|---|---|---|---|
| Age (years) | 65.35 ± 10.84 | 68.94 ± 10.84 | 0.010 |
| Male, n (%) | 96 (58.2) | 63 (63.6) | 0.381 |
| Hypertension, n (%) | 132 (80.0) | 83 (83.8) | 0.437 |
| Diabetes, n (%) | 64 (38.8) | 50 (50.5) | 0.063 |
| Atrial fibrillation, n (%) | 4 (2.4) | 4 (4.0) | 0.458 |
| Stroke history, n (%) | 35 (21.2) | 23 (23.2) | 0.701 |
| Smoker, n (%) | 46 (s27.9) | 27 (27.3) | 0.915 |
| Alcohol user, n (%) | 25 (15.2) | 18 (18.2) | 0.519 |
| NIHSS score | 5 (3-6) | 7 (4-9) | < 0.001 |
| Etiological subtype, n (%) | 0.804 | ||
| LAOD | 36 (21.8) | 24 (24.2) | |
| CE | 5 (3.0) | 5 (5.1) | |
| BABD | 67 (40.6) | 41 (41.4) | |
| SAD | 32 (19.4) | 18 (18.2) | |
| OE | 25 (15.2) | 11 (11.1) | |
| SBP (mm Hg) | 151.30 ± 20.35 | 154.47 ± 20.81 | 0.224 |
| DBP (mm Hg) | 88.36 ± 11.14 | 89.18 ± 11.88 | 0.574 |
| FBG (mmol/l) | 6.57 ± 2.54 | 6.84 ± 3.14 | 0.444 |
| TG (mmol/l) | 2.18 ± 1.47 | 1.96 ± 1.03 | 0.197 |
| LDL (mmol/l) | 2.80 ± 0.81 | 2.86 ± 0.97 | 0.584 |
| Infarct size (mm) | 13.35 ± 4.92 | 14.36 ± 5.37 | 0.121 |
| BA stenosis, n (%) | 35 (21.2) | 23 (23.2) | 0.701 |
| mRS score | 1 (1-2) | 3 (1-3) | < 0.001 |
Values are expressed as the mean ± standard deviation, n (%); OR, odds ratio; 95% CI, 95% confidence interval; LAOD, large artery occlusive disease; CE, cardiac embolism; BABD, basilar artery branch disease; SAD, small artery disease; OE, other etiology; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; TG, triglyceride; LDL, low-density lipoprotein cholesterol; FAR, fibrinogen-to-albumin ratio; BA, basilar artery.
Figure 3.
The mRS score distribution between the high FAR group and low FAR group.
Associations of the FAR with Baseline NIHSS and mRS Scores in Patients with Acute Pontine Infarction
Spearman correlation analysis showed that the FAR was positively correlated with baseline NIHSS scores (r = 0.352, P < 0.001) and mRS scores (r = 0.308, P < 0.001).
The FAR was Associated with Poor Outcomes at 3 Months After Acute Pontine Infarction
We used binary logistic regression models to analyze the associations between risk factors and poor outcomes at 3 months. Our results showed that age, FBG, LDL, fibrinogen, and the FAR were significantly associated with a poor outcome at 3 months after acute pontine infarction (all P < 0.05) (Table 3).
Table 3.
Binary logistic regression analysis for the prediction of poor outcomes.
| Model | Adjusted OR (95% CI) | P value |
|---|---|---|
| Model 1 (with FAR) | ||
| Age | 1.043 (1.012-1.074) | 0.005 |
| Male | 0.999 (0.548-1.819) | 0.996 |
| FBG | 1.157 (1.044-1.283) | 0.006 |
| LDL | 1.443 (1.037-2.008) | 0.030 |
| FAR | 1.293 (1.150-1.453) | < 0.001 |
| Model 2 (with fibrinogen) | ||
| Age | 1.044 (1.014-1.075) | 0.004 |
| Male | 0.996 (0.546-1.816) | 0.989 |
| FBG | 1.151 (1.036-1.278) | 0.009 |
| LDL | 1.401 (1.003-1.957) | 0.048 |
| Fibrinogen | 2.224 (1.577-3.136) | < 0.001 |
| Model 3 (with albumin) | ||
| Age | 1.050 (1.020-1.080) | 0.001 |
| Male | 1.038 (0.583-1.846) | 0.900 |
| FBG | 1.168 (1.057-1.290) | 0.002 |
| LDL | 1.492 (1.084-2.053) | 0.014 |
| albumin | 0.930 (0.859-1.007) | 0.074 |
OR, odds ratio; 95% CI, 95% confidence interval; FBG, fasting blood glucose; LDL, low-density lipoprotein cholesterol; FAR, fibrinogen-to-albumin ratio.
Discussion
As far of our knowledge this is the first report that shows the relationship between serum FAR levels and poor outcomes in patients with acute pontine infarction. In this study, 264 patients with acute pontine infarction were followed up for 3 months. The results showed significantly positive associations between the FAR and the NIHSS scores at admission and the mRS scores at 3 months. In addition, the FAR was significantly associated with poor 3-month outcomes of acute pontine infarction. Therefore, an elevated FAR might be a potential predictor of poor short-term outcomes of acute pontine infarction.
The association between the FAR and the severity and outcome of cerebral infarction is mainly related to fibrinogen and albumin levels. Fibrinogen is a coagulation factor that is a major determining factor of blood viscosity and a key factor in platelet activation. 14 It is also a component of acute phase proteins. Fibrinogen and its degradation product participate in the inflammatory response of atherosclerosis by binding to the interaction sites of lymphocytes and endothelial cells. 15 Elevated fibrinogen levels can also increase the binding rate of fibrinogen with platelets, causing platelet aggregation and increased blood flow resistance. This in turn slows blood flow and maintains the blood in a hypercoagulable state, thus promoting the formation of atherosclerotic plaques and thrombosis. 16 Furthermore, fibrinogen is an important component of the coagulation cascade reaction. Elevated fibrinogen levels may lead to the formation of fibrin clots through activation of the coagulation cascade reaction, thus accelerating the occurrence and development of arterial thrombosis and resulting in neurological function deterioration and a poor prognosis in patients with cerebral infarction. 17 Our study indicated that elevated fibrinogen was a risk factor for a poor 3-month clinical outcome in patients with acute pontine infarction.
Albumin is a major protein in serum. In addition to regulating the osmotic pressure of extracellular fluid, albumin inhibits platelet aggregation, has antioxidant functions, and is inversely associated with inflammation.18,19 Reduced albumin increases VCAM-1 activity in endothelial cells and attenuates anti-inflammatory functions, causing further vascular endothelial injury. Furthermore, reduced albumin levels can increase the free lysophosphatidylcholine concentration and stimulate the synthesis of lipid and coagulation factors, which increases blood viscosity, causes hyperlipidemia and hypercoagulability, and promotes atherosclerotic plaques and thrombosis. 20 Idicula et al. showed that albumin has neuroprotective functions in patients with cerebral infarction and can reduce neurotoxicity and clear free radicals. 21 In addition, high albumin levels can improve the functional outcome and reduce mortality in patients with cerebral infarction. Our study also indicated that when the albumin level was increased, the relative risk of poor 3-month clinical outcomes of pontine infarction was decreased (OR = 0.930), although the result was not statistically significant (P = 0.074).
By combining the aforementioned pathophysiological characteristics of fibrinogen and albumin, the FAR can provide more comprehensive information and better predict the prognosis of related diseases. Previous studies confirmed the association between this new indicator and many diseases, including acute coronary syndrome, cervical cancer, esophageal squamous cell carcinoma, and sleep apnea, and showed that the FAR has high clinical application value.22–25 Both serum fibrinogen and albumin levels were studied in chronic venous insufficiency and it was reported that disease severity and the FAR ratio has strongly correlated. 26 In these patients impaired the blood viscosity is claimed as one of the major factor in the pathogenesis of the disease. 27 Another point is albumin, another important factor that can be measured in the blood, which also regulates oncotic pressure and is responsible for blood viscosity. 28 In another biomarker study Cimsir et al. found that increased FAR levels seem to effective predictive factor for recurrent pregnancy loss. 29 The main foresight of all these studies concurred that increased FAR levels are associated with advanced disease or poor clinical outcomes.
Some studies also confirmed that the FAR was associated with the occurrence and outcomes of cerebral infarction. Acharya et al. indicated that a high FAR was closely associated with a greater risk of acute cerebral infarction during the first 24 h of venoarterial extracorporeal membrane oxygenation. 30 Recently, Zheng et al. specified that the FAR was associated with the severity and was a predictor of a poor 3-month outcome in patients with acute lacunar cerebral infarction. 10 Our study also showed that the FAR was associated with a poor 3-month clinical outcome of acute pontine infarction and further confirmed that the FAR was positively correlated with both the baseline NIHSS score and the mRS score. Patients with a high FAR had more severe neurological deficits and a worse outcome. Furthermore, although the infarct sizes were not significantly different between the high and low FAR groups, the infarct sizes were larger in the high FAR group, and more fiber bundles of the pons might be involved, especially the corticospinal tract in the basal pontine nuclei. Thus, corresponding baseline NIHSS scores were higher and the outcomes were worse in the high FAR group. These results more strongly support the predictive value of this indicator for the short-term outcome of acute pontine infarction.
This study, for the first time, investigated the association between the FAR and the short-term outcomes of acute pontine infarction. This study also had some limitations. First, this was a single-center observational study. The sample was limited, and there were regional restrictions; therefore, some selection bias may have occurred. Second, nutritional status was not assessed at admission, and this assessment might help to understand the prognostic roles of the FAR in patients with cerebral infarction. Furthermore, the mechanism underlying the association between the FAR and the short-term outcome of acute pontine infarction is still not completely clear, and multicenter and large-sample studies are needed for further verification.
Conclusion
In present study, we found higher fibrinogen, a higher FAR and lower albumin mainly in the group with poor outcomes. The FAR is a potential marker that can be used to predict a poor 3-month outcome of acute pontine infarction. However, further large-scale research is needed to demonstrate the prognostic value of these parameters in patients with acute pontine infarction.
Acknowledgments
We acknowledge the American Journal Experts (AJE) team for their help with language editing. This study was supported by grants from the Major Research Development Program of Anhui Province (1804h08020233)
Footnotes
Authors’ Note: MZ was involved in the design of the study, data collection, interpretation of the data, and manuscript writing and was a recipient of the obtained funding. SC participated in the study design, data collection, and statistical analysis and was a recipient of the obtained funding. JL, HX, and ZL participated in the data analysis, interpretation of the data, and manuscript revision. MX was the guarantor of this paper. The studies involving human participants were reviewed and approved by the Institutional Review Board of the Affiliated Hefei Hospital of Anhui Medical University.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Major Research Development Program of Anhui Province (grant number 1804h08020233).
ORCID iD: Mingfeng Zhai https://orcid.org/0000-0003-1934-2740
References
- 1.Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;394(10204):1145-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radu RA, Terecoasă EO, Băjenaru OA, et al. Etiologic classification of ischemic stroke: where do we stand? Clin Neurol Neurosurg. 2017;159:93-106. [DOI] [PubMed] [Google Scholar]
- 3.Li D, Xing C, Li Y, et al. Elevated plasma fibrinogen indicates short-term poor outcome in patients with acute ischemic stroke after intravenous thrombolysis. J Stroke Cerebrovasc Dis. 2020;29(8):104991. [DOI] [PubMed] [Google Scholar]
- 4.Shi J, Shi R, Qin W, et al. Dynamic changes in fibrinogen and prognosis of acute ischemic stroke patients treated with intravenous thrombolysis. Neurotox Res. 2020;38(3):775-784. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Shu Y, Zhang J, et al. Dynamics of nutritional status in dying patients with acute cerebral infarction in central China: a preliminary study. Neurol Res. 2011;33(5):503-507. [DOI] [PubMed] [Google Scholar]
- 6.Prajapati KD, Sharma SS, Roy N. Current perspectives on potential role of albumin in neuroprotection. Rev Neurosci. 2011;22(3):355-363. [DOI] [PubMed] [Google Scholar]
- 7.Hashem SS, Helmy SM, El-Fayomy NM, et al. Predictors of stroke outcome: the role of hemorheology, natural anticoagulants, and serum albumin. Egypt J Neurol Psychiatr Neurosurg. 2018;54(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang DP, Mao XF, Wu TT, et al. The fibrinogen-to-albumin ratio is associated with outcomes in patients With coronary artery disease Who underwent percutaneous coronary intervention. Clin Appl Thromb Hemost. 2020;26:1076029620933008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celebi S, Ozcan Celebi O, Berkalp B, et al. The association between the fibrinogen-to-albumin ratio and coronary artery disease severity in patients with stable coronary artery disease. Coron Artery Dis. 2020;31(6):512-517. [DOI] [PubMed] [Google Scholar]
- 10.Zheng L, Wang Z, Liu J, et al. Association between admission blood fibrinogen-to-albumin ratio and clinical outcomes after acute lacunar stroke. Biomark Med. 2021;15(2):87-96. [DOI] [PubMed] [Google Scholar]
- 11.Kumral E, Bayülkem G, Evyapan D. Clinical spectrum of pontine infarction. Clinical-MRI correlations. J Neurol. 2002;249(12):1659-1670. [DOI] [PubMed] [Google Scholar]
- 12.Cao SG, Ni X, Wu Q, et al. Basilar artery dolichosis is associated with a poor 90-day outcome in acute isolated pontine infarction. Sci Rep. 2020;10(1):6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnett HJM, Taylor DW, Haynes RB, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325(7):445-453. [DOI] [PubMed] [Google Scholar]
- 14.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3(8):1894-1904. [DOI] [PubMed] [Google Scholar]
- 15.Guo YH, Hernandez I, Isermann B, et al. Caveolin-1-dependent apoptosis induced by fibrin degradation products. Blood. 2009;113(18):4431-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tousoulis D, Papageorgiou N, Androulakis E, et al. Fibrinogen and cardiovascular disease: genetics and biomarkers. Blood Rev. 2011;25(6):239-245. [DOI] [PubMed] [Google Scholar]
- 17.Herrick S, Blanc-Brude O, Gray A, et al. Fibrinogen. Int J Biochem Cell Biol. 1999;31(7):741-746. [DOI] [PubMed] [Google Scholar]
- 18.Roche M, Rondeau P, Singh NR, et al. The antioxidant properties of serum albumin. FEBS Lett. 2008;582(13):1783-1787. [DOI] [PubMed] [Google Scholar]
- 19.Rael LT, Leonard J, Salottolo K, et al. Plasma oxidized albumin in acute ischemic stroke is associated with better outcomes. Front Neurol. 2019;10(709). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joles J A, Willekes-Koolschijn N, Koomans H A. Hypoalbuminemia causes high blood viscosity by increasing red cell lysophosphatidylcholine. Kidney Int. 1997;52(3):761-770. [DOI] [PubMed] [Google Scholar]
- 21.Idicula TT, Waje-Andreassen U, Brogger J, et al. Serum albumin in ischemic stroke patients: the higher the better. The bergen stroke study. Cerebrovasc Dis. 2009;28(1):13-17. [DOI] [PubMed] [Google Scholar]
- 22.Gao QF, Qiu JC, Huang XH, et al. The predictive and prognostic role of a novel ADS score in esophageal squamous cell carcinoma patients undergoing esophagectomy. Cancer Cell Int. 2018;18(153). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An Q, Liu W, Yang Y, et al. Preoperative fibrinogen-to-albumin ratio, a potential prognostic factor for patients with stage IB-IIA cervical cancer. BMC Cancer. 2020;20(1):691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hizli O, Cayir S, Coluk Y, et al. The novel indicators of moderate to severe sleep apnea: fibrinogen to albumin ratio versus CRP to albumin ratio. Eur Arch Otorhinolaryngol. 2021;278(3):851-855. [DOI] [PubMed] [Google Scholar]
- 25.Karahan O, Acet H, Ertaş F, et al. The relationship between fibrinogen to albumin ratio and severity of coronary artery disease in patients with STEMI. Am J Emerg Med. 2016;34(6):1037-1042. [DOI] [PubMed] [Google Scholar]
- 26.Karahan O, Yavuz C, Kankilic N, et al. Simple blood tests as predictive markers of disease severity and clinical condition in patients with venous insufficiency. Blood Coagul Fibrinolysis. 2016;27(6):684-690. [DOI] [PubMed] [Google Scholar]
- 27.Açıkgöz B, Kavala AA, Türkyılmaz S, et al. The evaluation of C-reactive protein values in patients with primary chronic venous insufficiency. Turk J Vasc Surg. 2019;28(1):15-18. [Google Scholar]
- 28.Sapmaz I, Manduz S, Sanri US, et al. Influence of albumin concentration in priming solution on blood viscosity under hypothermic conditions. Cardiovasc J Afr. 2009;20(3):168-169. [PMC free article] [PubMed] [Google Scholar]
- 29.Cimsir MT, Yildiz MS. Could fibrinogen to albumin ratio be a predictive marker for recurrent pregnancy loss. Int J Clin Pract. 2021;75(10):e14520. [DOI] [PubMed] [Google Scholar]
- 30.Acharya P, Jakobleff WA, Forest SJ, et al. Fibrinogen albumin ratio and ischemic stroke during venoarterial extracorporeal membrane oxygenation. ASAIO J. 2020;66(3):277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]