Abstract
Introduction: The aim of this study was to perform a clinicopathologic analysis of PHLPP1 expression in gastric cancer patients and analyze AKT activity with chemotherapy drug treatment in cancer subtypes. Materials and Methods: Surgically resected gastric cancer tissue specimens were obtained from 309 patients who underwent gastrectomy, and PHLPP1 expression was validated by tissue microarray analysis with immunohistochemistry. We assessed whether PHLPP1 selectively dephosphorylates Ser473 of AKT in an in-vitro study. Results: We found that the PHLPP1 overexpression (OE) group showed significantly greater proportions of differentiated subtype samples and early T stage samples, lower lymph node metastasis, and lower TNM stage than the PHLPP1 underexpression (UE) group. The overall survival of the PHLPP1-OE group was significantly higher (53.39 ± 0.96 months) than that of the PHLPP1-UE group (47.82 ± 2.57 months) (P = .01). In vitro analysis, we found that the PHLPP1-OE group showed a significant decrease in relative AKT S-473 levels in both cell lines (MKN-74 and KATO-III). We found that treatment with chemotherapy drugs decreased the activity of Ser473 in the MKN-74 cell line with PHLPP1 OE, but it did not affect the activity of Ser473 in KATO-III cells. Conclusion: We found that patients who overexpressed PHLPP1 showed low recurrence and good prognosis. PHLPP1 was found to work by lowering the activity of AKT Ser473 in gastric cancer. Additionally, we found a clue regarding the mechanism of chemotherapeutic drug resistance in a cell line of signet ring cell origin and will uncover this mechanism in the future.
Keywords: stomach neoplasm, NGS, immunohistochemistry, biomarker, prognosis
Introduction
According to the 2020 GLOBOCAN report, gastric cancer is the world's fifth most common cancer and fourth ranking in cancer mortality. 1 Known as an important cause of stomach cancer, Helicobacter pylori infection is known to exist in approximately 50% of the world's population. 2 The higher the infection rate is, the higher the incidence of stomach cancer. Dr Corea established the hypothesis that intestinal type gastric cancer is caused by H pylori. 3 However, in the case of diffuse types, no obvious cause or hypothesis has been established. Some studies have reported that the diffuse type shows poorer survival and higher recurrence than the intestinal type.4–6 The signet ring cell (SRC) type is a subtype that has poor prognosis as long as it is included in the diffuse type. SRC gastric accounts for approximately 3.4% to 50% of gastric cancer cases.5,7,8 SRCs have a small nucleus on one side and a cytoplasm that is full of mucus and rarely form lines or coronary structures. The prognosis of SRC gastric cancer is different in early and advanced stages. In the early stage, the early detection and treatment of SRC are better than those of cancers of other tissue types, but in the case of progressive gastric cancer, cancer cells tend to invade, and the lymph node metastasis rate is generally high, resulting in a poor prognosis compared to that of cancers of other tissue types.8–10
Previously, we compared the differentiated and SRC subtypes of gastric adenocarcinoma by next-generation sequencing (NGS), and we found 30 biomarker candidates. 11 Among these, PHLPP1 was identified, and it is assessed in this study. Pleckstrin homology domain leucine-rich repeat protein phosphatases (PHLPPs) include a pair of protein phosphatases (PHLPP1 and PHLPP2) involved in the regulation of Akt serine–threonine kinase and protein kinase C (PKC) signaling. The N-terminus of Akt includes PH domains that PIP2 and PIP3 can bind and threonine 308 (T308), which is important for active loop activation of catalytic domains.12,13 The C-terminus contains serine 473 (S473), which is important for full activation of the hydrophobic domain.14–16 It has been reported that PHLPPs inhibit cancer cell proliferation and may act as tumor suppressors. 17
Moreover, some studies have revealed that decreased or lost expression of PHLPPs is present in many human tumors, such as prostate cancer, colon cancer, lung cancer, chronic lymphocytic leukemia, pancreas cancer, and glioblastoma.14,15,17–20 Human colorectal cancer (CRC) patient microarray data revealed that the expression of PHLPPs is positively correlated with that of CDH1. The researchers found that silencing PHLPP expression increased the invasiveness of CRC cells by inducing epithelial–mesenchymal transition in vitro. 21 In melanoma, PHLPP1 downregulates Akt activation and inhibits the proliferation and survival of melanoma cells. 22 Overexpressed PHLPP1 induces the activation of Akt, which inhibits the proliferation and survival of melanoma cells in vitro. In melanoma, PHLPP1 was reported to be controlled by Akt S473, but this relationship has not been identified in stomach cancer. Additionally, retardation of melanoma tumor growth has also been reported in a xenograft mouse model.
The aim of this study was to perform a clinicopathologic analysis of PHLPP1 expression and analyze Akt activity with chemotherapy drug treatment in gastric cancer subtypes.
Materials and Methods
The reporting of this study conforms to STROBE guidelines. 23
Ethics Statement
This study was designed and conducted in accordance with the principles of the Helsinki Declaration (as revised in 2013). The study was approved by the Institutional Review Board of Gyeongsang National University Hospital (GNUHIRB 2009-54). Prior to the experiment, informed consent was obtained from all patients.
RNA Extraction and NGS
This study is a retrospective cohort comparative study of intestinal-type gastric cancer (IGC, well and moderately differentiated cell types) and diffuse-type gastric cancer (DGC, SRC type). We collected 8 total surgical samples: 5 IGC samples and 3 diffuse-type gastric cancer DGC samples. We compared expression profiles between clinically comparable samples using transcriptome resequencing data. We performed variant analysis and network analysis via the Ingenuity Pathway Analysis system (Qiagen). 11
Validation of PHLPP1 Expression by Tissue Microarray Analysis with Immunohistochemistry
Surgically resected gastric cancer tissue specimens were obtained from 309 patients who underwent gastrectomy at the Gyeongsang National University Hospital between January 1, 2004, and December 31, 2007. The patients selected by consecutively who agree to informed consent. The following inclusion criteria were used in this study: histologically proven primary gastric adenocarcinoma; subtotal and total gastrectomy as the surgical method. Medical charts and pathological reports were reviewed. Immunohistochemistry staining and quantitation of immunostained cells were performed as described previously. 11 We assessed the mortality via an examination of the medical chart after a recent visit, and we called patients who had not visited the hospital for 6 months. We censored patients who did not visit for more than 6 months and did not receive the telephone call. For survival analysis, we consulted the National Statistical Office of the Republic of Korea.
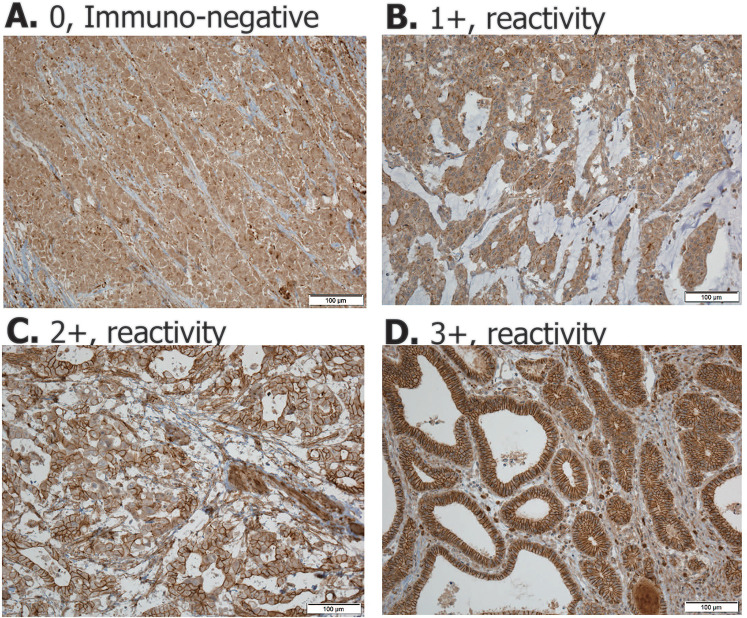
We conducted the study by deleting patient information from all the information in the research process. When reading the immunohistochemistry staining, the reading was conducted without patient information. Immunohistochemistry staining of PHLPP1 was performed using polyclonal anti-rabbit PHLPP1 antibody (2 µg/mL; ab71972, Cambridge). Signal intensity was scored as the percentage of PHLPP1-positive cells in the following manner: score 0 (<0%), score 1 (1%-25%), score 2 (25%-49%), and score 3 (50%-74%; Figure 1).
Figure 1.
Immunohistochemistry staining of PHLPP1 was performed using a polyclonal anti-rabbit PHLPP1 antibody. Signal intensity was scored as the percentage of PHLPP1-positive cells in the following manner: score 0 (<0%), score 1 (1%-25%), score 2 (25%-49%), and score 3.
Cell Lines and Transfection
Human gastric cell lines (MKN-74 and KATO-III) were purchased from the Cell Line Bank. Cells were maintained in RPMI 1640 media (Gibco, Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco, Thermo Fisher Scientific, Inc.) and penicillin (100 U/mL, Thermo Fisher Scientific, Inc.) and cultured at 37 °C in a humidified air atmosphere containing 5% CO2. A full-length human PHLPP1 expression vector was purchased from Addgene (pcDNA3 HA-PHLPP1 full length #37100). The overexpression (OE) plasmid transfection was performed using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. The chemotherapy drugs 5-FU (10 μM) (Sigma, MO), cisplatin (250 nM) (Dong-A ST), and oxaliplatin (1 μM) (Eloxatin, Sanofi-Avents, NJ) were administered for 3 days.
Western Blotting
Twenty micrograms of protein from each extract was loaded onto 8% to 12% SDS-polyacrylamide gel electrophoresis gels and transferred to PVDF membranes (iBlot, 2PVDF Regular Stacks, Invitrogen, Thermo Fisher Scientific, Inc.) Membranes were blocked in 5% w/v bovine serum albumin (BSA), 1X TBS and 0.1% Tween® 20 at room temperature for 1 h. The membrane was incubated with diluted primary antibody (phospho-Akt [Ser473] #4060, phospho-Akt [Thr308] [D25E6] #13038, or Akt #9272 HA-Tag [C29F4] #3724; 1:1000; Cell Signaling Technology) in TBS/Tween 20 (0.1% v/v) containing 5% BSA at 4 °C overnight. After washing, the membranes were incubated with a secondary antibody (anti-mouse IgG or anti-rabbit IgG; 1:2000; Thermo Fisher Scientific, Inc.) conjugated with horseradish peroxidase, followed by detection with enhanced chemiluminescence reagents (Bio-RAD Laboratories, Inc.).
Chemotherapy Drug Treatment
We measured and compared the concentrations of total AKT, threonine 308, and serine 473 in the control and PHLPP1-overexpressing MKN-74 and KATO-III cells. We checked the AKT change in the treatment of anticancer drugs in MKN-74 and KATO-III cells. 5-FU, cisplatin, and oxaliplatin were administered to the control and OE cell lines, and the effects were observed after a day.
Statistical Analysis
All statistical analyses were performed using SPSS Statistics version 24 (IBM SPSS, Inc.). Data represent the mean ± SD. The statistical analyses of the mean values were performed via T tests, and the statistical analyses of the frequency were performed with χ2 tests. The Kaplan-Meier method was used for survival analysis. In all analyses, P values less than .05 were considered statistically significant.
Results
The PHLPP1-OE Group Showed Better Prognosis Than the PHLPP1-UE Group
We selected PHLPP1 out of 30 cancer driver genes and analyzed the manifestations and clinical prognosis with gastric cancer tissue samples. We scored PHLPP1 expression in gastric cancer samples using immunohistochemistry. The PHLPP1 score distribution was as follows: score 0, N = 17 (5.5%); score 1, N = 33 (10.7%); score 2, N = 83 (26.9%); and score 3, N = 176 (57%) (Figure 1). We divided patients scoring 2 + or 3 + into the PHLPP1 OE group and those scoring 0 or 1 + into the PHLPP1-underexpression (UE) group to analyze clinicopathological factors.
In the WHO classification, the PHLPP1-OE group had the largest proportion of moderately differentiated (MD) samples (40%), the PHLPP1-UE group had the largest proportion of poorly differentiated (PD) samples (63%), and the proportion of SRC samples was 14.3% (P < .01). In the T stage subgroup, there were obviously more cases of advanced gastric cancer (AGC) in PHLPP1-UE (74%) than in PHLPP1-OE (48.3%) (P < .01). In the N stage subgroup analysis, the lymph node metastasis group had a significantly higher proportion of PHLPP1-UE samples (64%) than the PHLPP1-OE group (36.7%) (P < .01). In the TNM classification subgroup, the stage III-IV group had a significantly higher proportion of PHLPP1-UE samples (58%), while the proportion of PHLPP1-OE samples was 22.8%. The PHLPP1-UE group also had a much higher rate of recurrence (44%) than the PHLPP1-OE group (21.6%) (P < .01) (Table 1).
Table 1.
Comparison of the clinicopathological features of the PHLPP1-OE and PHLPP1-UE groups according to immunohistochemistry analysis of tissues from 309 gastric cancer patients.
| Level of PHLPP1 expression | P | ||
|---|---|---|---|
| Under expression (0-1 + ) | Overexpression (2 + -3 + ) | ||
| WHO classification | <.001 | ||
| WD/MD/PD/Mucinous/SRC | 2/6/31/3/7 | 60/102/68/5/20 | |
| Lauren classification | <.001 | ||
| Intestinal | 6 (12%) | 162 (62.5%) | |
| Diffuse | 27 (54%) | 33 (12.7%) | |
| Mixed | 1 (2%) | 10 (3.9%) | |
| Tumor invasion | .001 | ||
| EGC (T1) | 13 (26%) | 134 (51.7%) | |
| AGC (T2∼4) | 37 (74%) | 125 (48.3%) | |
| LN metastasis | <.001 | ||
| Absent | 18 (36%) | 164 (63.3%) | |
| Metastasis (≥ 1) | 32 (64%) | 95 (36.7%) | |
| TNM stage | <.001 | ||
| I | 15 (30%) | 150 (57.9%) | |
| II | 6 (12%) | 50 (19.3%) | |
| III-IV | 29 (58%) | 59 (22.8%) | |
| Cancer related death | 18/50 (28.1%) | 46/259 (17.8%) | .007 |
| Recurrence | 22/50 (44%) | 56/259 (21.6%) | .002 |
Abbreviations: WHO, World Health Organization; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; SRC, signet ring cell carcinoma; LN, lymph node; PPase, inorganic pyrophosphatase; EGC, early gastric cancer; AGC, advanced gastric cancer.
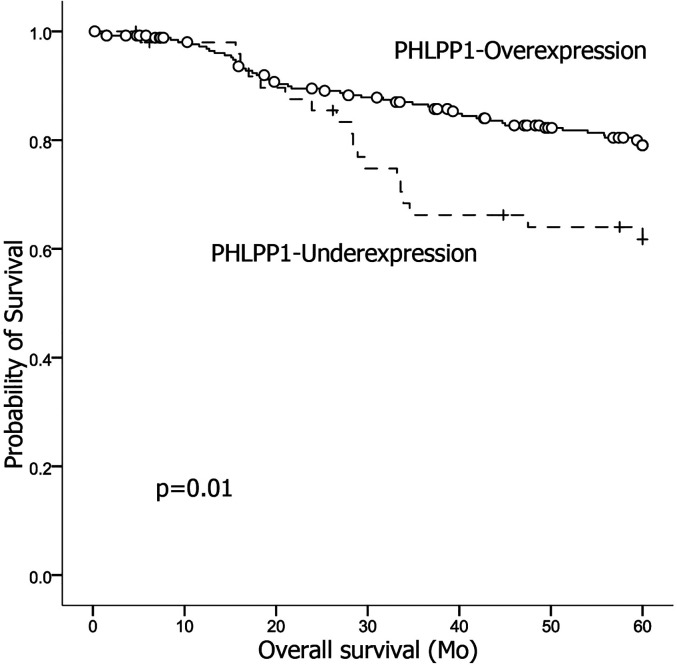
We found the PHLPP1-OE group showed significantly higher proportions of differentiated samples and early T stage samples, lower lymph node metastasis, and lower TNM stage than the PHPP1-UE group. The overall survival of the PHLPP1-OE group was significantly higher (53.39 ± 0.96 months, 95% CI, 51.51-55.28 months) than that of the PHLPP1-UE group (47.82 ± 2.57 months, 95% CI 42.78-52.85) (P = .01) (Figure 2).
Figure 2.
The overall survival of the PHLPP1-OE group was significantly higher than that of the PHLPP1-UE group (P = .01).
PHLPP1-OE Reduces the Activity of AKT S-473, a Moderate Cancer-Activating Signal
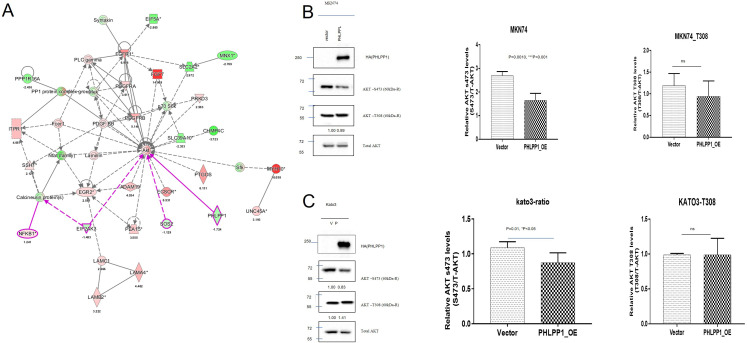
We conducted a comparative study of SRC and WD samples using NGS in a previous study. Network analysis of cancer driver genes extracted through variant analysis was performed. As a result, PHLPP1 was confirmed to be expressed through the AKT pathway (Figure 3A).
Figure 3.
Network and expression analyses of PHLPP1 and Akt. (A) Network analysis of PHLPP1 and Akt. (B) We measured the levels of total AKT, T308, and S473 in MKN-74 gastric cancer cells with PHLPP1 overexpression. (C) We measured the levels of total AKT, T308, and S473 in KATO-III gastric cancer cells with PHLPP1 overexpression.
We measured total AKT, T308, and S473 after overexpressing PHLPP1 in KATO-III cells (DGC origin) and MKN-74 cells (IGC origin). We found that the PHLPP1-OE group showed a significant decrease in relative Akt S473 levels in both cell lines (MKN-74 and KATO-III), but there were no differences in relative Akt T308 levels in either cell line (Figure 3B and C).
PHLPP1-OE Does not Reduce AKT S-473 After Treatment With Chemotherapeutic Drugs in KATO-III Cells
To determine the relationship between PHLPP1 and chemotherapy drug resistance, we assessed the change in S473 levels after treating PHLPP1-OE gastric cancer cell lines with chemotherapy drugs.
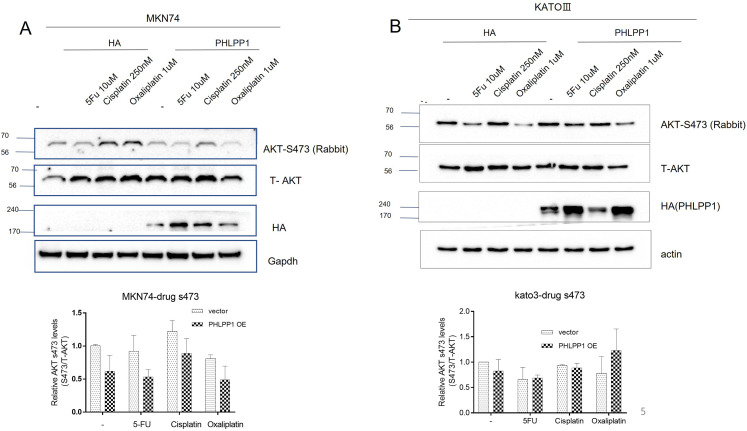
We found that the level of S473 in PHLPP1-OE MKN-74 cells (differentiated cancer origin) was decreased after treatment with chemotherapy drugs (5-FU, cisplatin, and oxaliplatin) (Figure 4A). However, the level of S473 was not affected in the PHLPP1-OE KATO-III cell line (SRC cancer origin) compared with that in the control groups (Figure 4B).
Figure 4.
PHLPP1 was overexpressed in stomach cancer cells after treatment with various chemotherapeutic drugs. (A) The overall level of AKT S473 in MKN-74 cells was decreased compared with that in the control groups. (B) The level of AKT-S473 in KATO-III cells was not affected by drugs compared with that in the control groups.
Discussion
The aim of this study was to perform a clinicopathologic analysis of PHLPP1 and analyze the relationship of Akt expression with chemoresistance in gastric cancer subtypes. We found that patients who overexpressed PHLPP1 showed lower recurrence and better prognosis than those who underexpressed PHLPP1. Additionally, the PHLPP1-OE group showed a decreased level of AKT s473 in differentiated cells (MKN-74), but the level of AKT s473 was not reduced in SRC-origin cells (KATO-III) after treatment with chemotherapy drugs.
Previously, we compared 2 groups of extremes by comparing DGC samples, which have the poorest prognosis (SRC type), with IGC samples, which have the best prognosis. We found that there was a large difference in adhesion, vascular development, and cell-to-cell junction components between the 2 subtypes. We performed whole transcriptome analysis by NGS, and we found 30 biomarker candidates by variant analysis. 11 Among these biomarker candidates, PHLPP1 was addressed in this study.
PHLPP1 belongs to a novel family of serine–threonine protein phosphatases that play an important role in maintaining the balance in cell signaling. 24 Two isoforms of PHLPPs, namely, PHLPP1 and PHLPP2, have been reported in this phosphatase family. PHLPP1 has also been reported as an important regulator of AKT serine–threonine kinases (AKT1, AKT2, and AKT3) and conventional/novel PKC isoforms. PHLPPs dephosphorylate S473 (the hydrophobic motif) in AKT, thus partially inactivating the kinase. Threonine 308, within the activation loop, is phosphorylated by PDK1, allowing full AKT activation. Among PHLPP1 targets is the S473 regulatory region, and the effect of its phosphorylation on AKT function is controversial. The Yanlin Yu group reported that PHLPP1 inhibits the activity of Akt2 but not AKT1, thereby inhibiting metastasis of melanoma. 25 Sigeki Miyamoto reported that PHLPP1 inhibits the activity of AKT (S473) and cell survival in the heart. 26
PHLPPs may act as tumor suppressors in several types of cancer due to their ability to block growth factor-induced signaling in cancer cells. 21 In stomach cancer, 2 studies from China have reported that gastric cancer with low or lack of expression of PHLPP1 has poor prognosis.27,28 Studies have shown that there are high proportions of advanced TNM stage and poor survival samples in PHLPP1-UE groups of gastric cancer patients, which is similar to the results of our studies. We additionally found that recurrence was also higher in the PHLPP1-UE group than in the PHLPP1-OE group. Previously, we found that the PHLPP1 variant gene was present in 2/3 of SRC samples via NGS, but there were no gene variations in differentiated cancer samples (0/5). This result gives insight into cancer development because PHLPP1 is a tumor suppressor. To understand PHLPPs in gastric cancer, we performed a study and found that Akt was also a target of PHLPPs, as it is in other cancers.
We confirmed the reduction in Akt S473 in PHLPP1-OE MKN-74 cells treated with chemotherapy drugs. However, Akt S473 activity was reduced only in the control group, and Akt S473 activity was the same or increased activity after treatment with 5-FU, cisplatin, and oxaliplatin in the KATO-III groups. It has been clinically reported that SRC patients have a worse prognosis than non-SRC AGC patients. 8 One cause of this may be the development of chemotherapy drug resistance in SRC patients. Understanding chemotherapy drug-resistance mechanism is important for the development of chemotherapy. In the future, we will conduct a miRNA/gene regulation study that affects the development and progression of gastric cancer to investigate carcinogenesis of diffuse type gastric cancer.29–31
This study has limitations. (1) We did not perform power calculation to determine the number of patients before conducting the study. (2) We could not conduct knock-in or knock-out functional studies in vitro. Additionally, (3) the number of SRC patients was small, so it was not possible to determine if they had more chemoresistance than the non-SRC group. However, network analysis of PHLPP1 revealed the relationship between PHLPP1 and Akt in gastric cancer and confirmed the increase in Akt expression caused by PHLPP1.
Conclusion
We found that patients who overexpressed PHLPP1 showed lower recurrence and a better prognosis than those who underexpressed PHLPP1. Additionally, overexpressed PHLPP1 showed targetability and the potential to activate Akt.
Glossary
Abbreviations
- PHLPPs
Pleckstrin homology domain leucine-rich repeat protein phosphatases; OE, overexpression; UE, underexpression; SRC, signet ring cell; NGS, next-generation sequencing; C, PKC, protein kinase; T308, threonine 308; S473, serine 473; IGC, intestinal-type gastric cancer; DGC, diffuse-type gastric cancer; MD, moderately differentiated; WHO, World Health Organization; PD, poorly differentiated; LN, lymph node; AGC, advanced gastric cancer
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval: This study was designed and carried out according to the principles of the Declaration of Helsinki (1989). Written consent was obtained from all participants before inclusion in the trial. The study was approved by the Institutional Review Board of Gyeongsang National University Hospital (GNUHIRB 2009-54).
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by biomedical research institute fund (GNUHBRIF-2016-0006) from the Gyeongsang National University Hospital, and a National Research Foundation of Korea (NRF) grant funded by the Republic of Korean government (2020R1F1A1074077).
ORCID iDs: Sang-Ho Jeong https://orcid.org/0000-0001-9061-6236
Chi-Young Jeong https://orcid.org/0000-0001-9061-6236
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. . 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420-429. doi: 10.1053/j.gastro.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 3.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13(1):2-9. doi: 10.1111/j.1751-2980.2011.00550.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koemans WJ, Luijten J, van der Kaaij RT, et al. The metastatic pattern of intestinal and diffuse type gastric carcinoma - A Dutch national cohort study. Cancer Epidemiol. 2020;69:101846. doi: 10.1016/j.canep.2020.101846 [DOI] [PubMed] [Google Scholar]
- 5.Sarriugarte Lasarte A, Garcia Alberdi E, Martinez Indart L, et al. From Lauren's Diffuse gastric cancer to WHO's Poorly cohesive carcinoma. Clinicopathological and prognostic characteristics. Rev Esp Enferm Dig. 2021;113(5):324-331. doi: 10.17235/reed.2020.7184/2020 [DOI] [PubMed] [Google Scholar]
- 6.Chen YC, Fang WL, Wang RF, et al. Clinicopathological variation of Lauren classification in gastric cancer. Pathol Oncol Res. 2016;22(1):197-202. doi: 10.1007/s12253-015-9996-6 [DOI] [PubMed] [Google Scholar]
- 7.Nie RC, Yuan SQ, Li YF, et al. Clinicopathological characteristics and prognostic value of signet ring cells in gastric carcinoma: a meta-analysis. J Cancer. 2017;8(17):3396-3404. doi: 10.7150/jca.21017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao S, Lv L, Zheng K, Tian Y, Zheng JC, Jiang CG. Prognosis and biological behavior of gastric signet-ring cell carcinoma better or worse: a meta-analysis. Front Oncol. 2021;11:603070. doi: 10.3389/fonc.2021.603070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg. 2009;250(6):878-887. doi: 10.1097/SLA.0b013e3181b21c7b [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro MM, Sarmento JA, Sobrinho Simoes MA, Bastos J. Prognostic significance of Lauren and Ming classifications and other pathologic parameters in gastric carcinoma. Cancer. 1981;47(4):780-784. doi:. [DOI] [PubMed] [Google Scholar]
- 11.Jeong SH, Park M, Park SY, et al. Transcriptome analysis and the prognostic role of NUDC in diffuse and intestinal gastric cancer. Technol Cancer Res Treat. 2021;20:15330338211019501. doi: 10.1177/15330338211019501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andjelkovic M, Alessi DR, Meier R, et al. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272(50):31515-31524. doi: 10.1074/jbc.272.50.31515 [DOI] [PubMed] [Google Scholar]
- 13.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275(5300):665-668. doi: 10.1126/science.275.5300.665 [DOI] [PubMed] [Google Scholar]
- 14.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Molecular Cell. 2005;18(1):13-24. doi: 10.1016/j.molcel.2005.03.008 [DOI] [PubMed] [Google Scholar]
- 15.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP And a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Molecular Cell. 2007;25(6):917-931. doi: 10.1016/j.molcel.2007.02.017 [DOI] [PubMed] [Google Scholar]
- 16.Gao T, Brognard J, Newton AC. The phosphatase PHLPP controls the cellular levels of protein kinase C. J Biol Chem. 2008;283(10):6300-6311. doi: 10.1074/jbc.M707319200 [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Weiss HL, Rychahou P, Jackson LN, Evers BM, Gao T. Loss of PHLPP expression in colon cancer: role in proliferation and tumorigenesis. Oncogene. 2009;28(7):994-1004. doi: 10.1038/onc.2008.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M, Pratt CP, Zeeman ME, et al. Identification of PHLPP1 as a tumor suppressor reveals the role of feedback activation in PTEN-mutant prostate cancer progression. Cancer Cell. 2011;20(2):173-186. doi: 10.1016/j.ccr.2011.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molina JR, Agarwal NK, Morales FC, et al. PTEN, NHERF1 and PHLPP form a tumor suppressor network that is disabled in glioblastoma. Oncogene. 2012;31(10):1264-1274. doi: 10.1038/onc.2011.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nitsche C, Edderkaoui M, Moore RM, et al. The phosphatase PHLPP1 regulates Akt2, promotes pancreatic cancer cell death, and inhibits tumor formation. Gastroenterology. 2012;142(2):377-387 e1-5. doi: 10.1053/j.gastro.2011.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Stevens PD, Liu J, et al. PHLPP Is a negative regulator of RAF1, which reduces colorectal cancer cell motility and prevents tumor progression in mice. Gastroenterology. 2014;146(5):1301-1312.e1-10. doi: 10.1053/j.gastro.2014.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong L, Jin L, Tseng HY, et al. Oncogenic suppression of PHLPP1 in human melanoma. Oncogene. 2014;33(39):4756-4766. doi: 10.1038/onc.2013.420 [DOI] [PubMed] [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Internal Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 24.Brognard J, Newton AC. PHLiPPing the switch on Akt and protein kinase C signaling. Trends Endocrinol Metab. 2008;19(6):223-230. doi: 10.1016/j.tem.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Y, Dai M, Lu A, Yu E, Merlino G. PHLPP1 Mediates melanoma metastasis suppression through repressing AKT2 activation. Oncogene. 2018;37(17):2225-2236. doi: 10.1038/s41388-017-0061-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyamoto S, Purcell NH, Smith JM, et al. PHLPP-1 negatively regulates Akt activity and survival in the heart. Circ Res. 2010;107(4):476-484. doi: 10.1161/CIRCRESAHA.109.215020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Shu H, Wang Z, et al. Loss expression of PHLPP1 correlates with lymph node metastasis and exhibits a poor prognosis in patients with gastric cancer. J Surg Oncol. 2013;108(7):427-432. doi: 10.1002/jso.23419 [DOI] [PubMed] [Google Scholar]
- 28.Hou Y, Deng J, Zhang L, et al. Lower expression of PH domain leucine-rich repeat protein phosphatase 1 (PHLPP1) association with poor prognosis of gastric cancer. Int J Clin Exp Med. 2015;8(11):20481-9. [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira J, Santos M, Delabio R, et al. Analysis of gene expression of miRNA-106b-5p and TRAIL in the apoptosis pathway in gastric cancer. Genes (Basel). 2020;11(4), 393. doi: 10.3390/genes11040393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu C, Xie J, Liu Y, et al. MicroRNA expression profiling and target gene analysis in gastric cancer. Medicine (Baltimore). 2020;99(37):e21963. doi: 10.1097/md.0000000000021963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu X, Liu Y, Xu J, et al. miR-608 rs4919510 polymorphism may affect susceptibility to colorectal cancer by upregulating MRPL43 expression. DNA Cell Biol. 2020;39(11):2017-2027. doi: 10.1089/dna.2020.5689 [DOI] [PubMed] [Google Scholar]