Abstract
Background
The disease-modifying therapies (DMTs) largely used in multiple sclerosis (MS) may result in higher infectious risk.
Objective
We aimed to investigate the infectious risk in DMT-treated MS patients.
Methods
MS patients were evaluated for infectious risk before starting, switching or during DMT.
Results
In this three-year observational cohort study 174 MS patients were enrolled. Among them, 18 patients were anti-HBc + and 19 patients were QuantiFERON®-TB Gold In-Tube (QFT) + . No patients with anti-HBc + showed a detectable HBV-DNA and all started DMT. Among QTB + patients, 17 latent TB infections (LTBIs) and 2 active TB infections (TBIs) were identified. After one month of LTBI prophylaxis or TB treatment, respectively, all patients started DMTs.
Overall, 149 started DMTs. During DMTs, one ocrelizumab-treated patient with anti-HBc + developed HBV reactivation and six patients (3 on natalizumab, 2 on ocrelizumab and 1 on IFN-β) showed reactivation of HSV-1, with detectable plasma DNA. Finally, 1 cladribine-treated patient experienced VZV reactivation. All the reactivations of latent infections have been successfully treated.
Conclusion
Screening of infectious diseases in DMT candidate MS patients helps to mitigate the infectious risk. During DMTs, a regular assessment of infectious risk allows to avoid discontinuing MS therapy and guarantees a higher degree of safety.
Keywords: Multiple sclerosis, infectious risk, disease-modifying therapies
Introduction
Disease-modifying therapies (DMTs) are able to modify the course of multiple sclerosis (MS) by suppressing or modulating the immune system and by exerting an anti-inflammatory function during the MS “flare-up” phases. Although highly effective in the MS treatment, DMTs carry an array of adverse effects,1,2 inducing patients to delay or discontinue MS treatment. Accordingly, the induction of varying degrees of immunosuppression in DMT-treated MS patients, especially significant with last-generation drugs, has been associated to an increased infectious risk, secondary to newly acquired or latent pathogens.3–6 Although susceptibility to infections is determined by the interaction of multiple host and pathogen-related factors, and cannot be merely circumscribed to a specific DMT, it seems advisable to consider patient's infective history and pre-existing infections when selecting a therapeutic plan, and if needed, take potential precautionary measures.7–9
The first-generation DMTs such as IFN-β or glatiramer acetate are not thought to be associated with a significantly increased risk of infection, whereas more effective new generation DMTs have demonstrated a higher risk profile 10 However, most of these observations are derived from clinical trials of DMT-treated MS patients, which may underestimate or not correctly determine the overall associated risk, due to their restricted inclusion criteria and short-term follow-up. Real-life long-term experience is needed to confirm data from clinical trials.
The most frequent infections in DMT-treated MS patients are caused by herpes viruses such as herpes simplex virus-1 and 2 (HSV-1 and 2), varicella zoster virus (VZV), as well as hepatitis B virus (HBV), and Mycobacterium tuberculosis (MTB) (both primary tuberculosis infection [TBI] and reactivation of latent tuberculosis infection [LTBI])11–15 LTBI is defined as a state of persistent immune response to stimulation by MTB antigens without evidence of clinically manifested active TB. This condition identifies the individuals who have being in contact with MTB and have developed an immune response, but it does not necessarily imply the persistence of living pathogens in the human body. 16 The vast majority of infected people have no signs or symptoms of TB and will never develop the disease. Therefore, TB diagnosis remains a challenge. A direct measurement tool for MTB infection in humans is currently unavailable. Most infected persons have no signs or symptoms of TB, nevertheless they are at risk for eventually developing active TB disease.
Although less frequent, immunocompromised patients may also develop other opportunistic infections such as cryptococcosis, Pneumocystis jirovecii pneumonia11,15 and progressive multifocal leukoencephalopathy (PML), especially during or after natalizumab-based therapy.17,18
Hence, individualized surveillance should be warranted to patients with a history of pre-existing infections as well as patients that have previously experienced therapy-related infectious complications. However, the published experience for such cases is considerably limited.
The aim of the study was to collect real-world data on the infectious rates and risk in MS patients before starting or switching or during DMTs. We thus propose an infectious assessment method that may reduce the infectious risk in DMT-treated MS patients.
Methods
Standard protocol approvals and patient consents
The study was approved by Ethics Committee of Policlinico Umberto I, Sapienza University of Rome (protocol numbers 130/13 and 353/20). All patients gave written informed consent for participation in the study.
Study design and participants
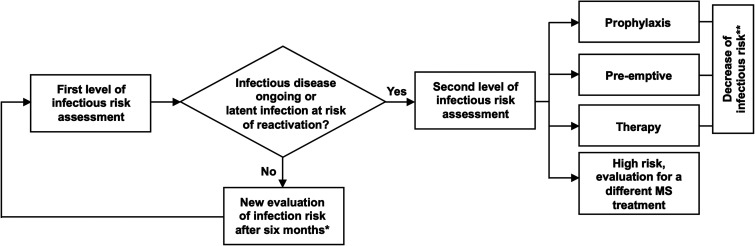
From February 1, 2018, to April 1, 2021, at the Neuroinfectious Unit of Policlinico Umberto I, Sapienza University of Rome, MS patients were evaluated for infectious risk before starting or switching or during ongoing DMTs. The infectious risk assessment was stratified into two separate levels (Figure 1).
Figure 1.
Flow chart of infectious risk assessment
* to evaluate new infections acquired during DMT or the reactivation of latent infection. The evaluation was performed after six months for injective DMTs and after twelve months for oral DMTs.
** serial assessment based on the infectious risk and first-level of infectious risk assessment after six months.
The first-level evaluation included: human immunodeficiency virus (HIV; Ag/Ab Combo), hepatitis B virus (HBV; hepatitis B surface antigen [HBsAg], hepatitis B core antibody [anti-HBc], hepatitis B surface antibody [anti-HBs]), hepatitis C virus (HCV; anti-HCV), hepatitis A virus (HAV; IgG and IgM), cytomegalovirus (CMV; IgG and IgM), Epstein-Barr virus (EBV; viral capsid antigen [VCA] IgG, VCA IgM, EBNA IgG), herpes simplex-1/2 (HSV-1/2; IgG and IgM), varicella zoster virus (VZV; IgG and IgM), rubella virus (IgG and IgM), measles virus (IgG and IgM), JC virus (Stratify® JCV Assay), and Toxoplasma gondii (IgG and IgM). Mycobacterium tuberculosis (MTB) was investigated by QuantiFERON®-TB Gold In-Tube (QFT) assay.
The second-level assessment was performed only for those patients who resulted positive in the first-level survey. As a rule, any MS patient that showed IgM positivity for CMV, EBV, Toxoplasma gondii, HSV-1/2, measles virus, and rubella virus at the first-level assessment was further investigated by RT-PCR to exclude plasma viral or parasitic replication. The diagnosis of HBV infection was based on medical history, physical examination, and exclusion of acute hepatitis B. For each anti-HBc + patient, acute hepatitis B was ruled out via further serological evaluations of HBeAg, anti-HBe, AST, ALT, total and direct bilirubin, and molecular quantification of HBV-DNA. Moreover, evaluation of hepatitis D virus (HDV; IgG and IgM) was performed to identify possible coinfections. After exclusion of acute hepatitis B, patients with HBsAb<10 IU were monitored monthly, through assessment of liver enzymes, HBsAg and HBV-DNA, due to potential risk for HBV flares or reactivation. On the contrary, patients with HBsAb>10 IU were monitored every three months.
Finally, in QFT + patients, active TBI was excluded by medical history and chest X-ray. All patients that presented with abnormal chest X-ray, such as pulmonary infiltrates, have been subjected to further microbiological tests. MTB presence was investigated on sputum smear microscopy, sputum culture, and MTB RT-PCR was also performed. Bronchoalveolar lavage (BAL) via fiberoptic bronchoscopy (FBS) was performed in patients with elevated clinical suspicion of pulmonary TB who either could not expectorate or had negative sputum smear and MTB culture. 19 Fluorescence microscopy, Ziehl-Neelsen (ZN) staining technique, and MTB culture was performed on the BAL fluid. All patients with latent tuberculosis infection (LTBI) underwent TB prophylaxis (isoniazid plus rifampicin) for 3 months. If the patients showed no further medical complications, DMTs were started after one month of prophylactic regimen. On the other hand, active TBI patients underwent TB therapy with first-line anti-TB agents: isoniazid, rifampin, ethambutol, and pyrazinamide. Regimen for treating TB included a primary intensive phase of 2 months with isoniazid, rifampin, ethambutol, and pyrazinamide, followed by a continuation phase of 4 months with isoniazid, rifampin (total 6 months for treatment). Likewise, DMTs were introduced after one month of uncomplicated TB treatment. Liver function tests (AST, ALT, total and direct bilirubin, lipase, amylase, creatinine, urea, alkaline phosphatase, and gamma-GT) were monitored every 15 days in all patients undergoing LTBI prophylaxis or TB treatment.
In the context of a collaboration between the MS Centre and the Neuroinfectious Unit, all the patients were evaluated every six months to identify new or reactivation of latent infections. In case of increased infectious risk or active infectious diseases needing a specific treatment, an official report was sent to the MS Centre to inform the reference neurologist and discuss about the therapeutic approach. Vaccination was suggested for those patients who didn't need to start immediately DMT.
Data sharing
Requests for access to the data reported in this Article will be considered by the corresponding author.
Results
Demographics and clinical characteristics among all patients
At the Neuroinfectious Unit, Policlinico Umberto I, Sapienza University of Rome, 174 MS patients (101 females/73 males) with a median age (interquartile range, IQR) of 49 (40–57), median years of disease of 10 (4–19) and median Expanded Disability Status Scale (EDSS) of 3.5 (1.5–6.0) were enrolled. Overall, 37.9% (66/174) of the MS patients were naïve to MS treatment, while 62.1% (108/174) were non-naïve. Among non-naïve MS patients, 84.3% (91/108) of the patients were referred before DMT switch, and the most common previous treatments were IFN-β (23.1%), fingolimod (18.5%), dimethyl fumarate (16.7%) and natalizumab (14.8%). For 15.7% (17/108) of the patients the infectious evaluation was performed during ongoing DMT-treatment (non-switchers).
Demographical and clinical features are reported in Table 1.
Table 1.
Demographic and yyyyclinical features of study population.
| all MS patients | all non-naïve MS patients | ||||
|---|---|---|---|---|---|
| all MS patients (n = 174) | naïve (n = 66) |
non-naïve (n = 108) | switcher (n = 91) | non-switcher (n = 17) | |
| Female/Male | 101/73 | 34/32 | 67/41 | 55/36 | 12/5 |
| Age, median (IQR) | 49 (40–57) | 47 (33–58) | 49 (41–57) | 49 (41–57) | 53 (44–59) |
| Years of disease, median (IQR) | 10 (4–19) | 2 (2–6) | 14 (8–21) | 14 (8–21) | 14 (9–22) |
| EDSS score, median (IQR) | 3.5 (1.5–6.0) | 3.0 (1.0–5.3) | 3.5 (2.0–6.0) | 3.5 (2.0–6.0) | 3.0 (2.5–6.0) |
| Previous treatment | |||||
| Alemtuzumab | 1 | - | 1 | 1 | - |
| Azathioprine | 2 | - | 2 | 2 | - |
| Cladribine | 1 | - | 1 | 1 | - |
| Daclizumab | 3 | - | 3 | 3 | - |
| Dimethyl fumarate | 18 | - | 18 | 16 | 2 |
| Fingolimod | 20 | - | 20 | 14 | 6 |
| Glatiramer acetate | 12 | - | 12 | 12 | - |
| IFN-β | 25 | - | 25 | 18 | 7 |
| Mycophenolate | 1 | - | 1 | 1 | - |
| Natalizumab | 16 | - | 16 | 14 | 2 |
| Rituximab | 1 | - | 1 | 1 | - |
| Teriflunomide | 8 | - | 8 | 8 | - |
| Current treatment | |||||
| Alemtuzumab | 2 | - | 2 | 2 | - |
| Cladribine | 20 | 3 | 17 | 17 | - |
| Dimethyl fumarate | 10 | 8 | 2 | 1 | 1 |
| Fingolimod | 9 | 2 | 7 | 4 | 3 |
| Glatiramer acetate | 3 | - | 3 | 1 | 2 |
| IFN-β | 6 | 2 | 4 | 2 | 2 |
| Natalizumab | 15 | 7 | 8 | 4 | 4 |
| Ocrelizumab | 71 | 26 | 45 | 41 | 4 |
| Teriflunomide | 13 | 5 | 8 | 7 | 1 |
| None | 25 | 13 | 12 | 12 | - |
| Years of current treatment, median (IQR) | 2.1 (1.4–3.1) | 2.1 (1.3–2.5) | 2.1 (1.7–3.1) | 1.9 (1.4–2.8) | 5.0 (2.9–8.3) |
MS: multiple sclerosis; IQR: interquartile range; EDSS: Expanded disability status scale; IFN-β: interferon-beta.
First-level of infectious risk assessment
Through the first-level infectious risk assessment, we determined that 23.6% of the MS patients had a potential infectious risk, therefore they required a second-level evaluation.
No patient had active HBV infection (HBsAg + , anti-HBc + ), 12.8% of the MS patients were HBsAg-, anti-HBs + and anti-HBc + , whereas 58.9% had no immunization to HBV (Table 2, Figure 2). 3.7% of the patients showed positivity for anti-HCV (Table 2, Figure 2).
Table 2.
Screening for infectious disease in study population.
| all MS patients | all non-naïve MS patients | ||||
|---|---|---|---|---|---|
| all MS patients (n = 174) | naïve (n = 66) |
non-naïve (n = 108) |
switcher (n = 91) |
non-switcher (n = 17) |
|
| HAV | 58/174 | 22/66 | 35/108 | 33/91 | 2/17 |
| IgG + IgM- | 15/57 | 6/22 | 9/35 | 8/33 | 1/1 |
| IgG-IgM- | 43/57 | 16/22 | 26/35 | 25/33 | 1/1 |
| HBV | 142/174 | 51/66 | 90/108 | 80/91 | 10/17 |
| HBsAg- HBsAb + anti-HBc- | 41/141 | 19/50 | 21/90 | 19/80 | 2/10 |
| HBsAg- HBsAb + anti-HBc + | 18/141 | 5/50 | 13/90 | 10/80 | 3/10 |
| HBsAg- HBsAb- anti-HBc- | 83/141 | 27/50 | 56/90 | 51/80 | 5/10 |
| HCV-Ab | 108/174 | 44/66 | 64/108 | 59/91 | 5/17 |
| positive | 4/108 | 1/43 | 3/64 | 3/59 | 0/5 |
| negative | 104/108 | 42/43 | 61/64 | 56/59 | 5/5 |
| HIV Ab/Ag | 172/174 | 66/66 | 108/108 | 91/91 | 17/17 |
| positive | 0/172 | 0/66 | 0/108 | 0/91 | 0/15 |
| negative | 172/172 | 66/66 | 106/108 | 91/91 | 17/17 |
| Measle | 100/174 | 36/66 | 64/108 | 57/91 | 7/17 |
| IgG + IgM- | 94/100 | 34/35 | 60/64 | 54/57 | 6/7 |
| IgG-IgM- | 6/100 | 2/35 | 4/35 | 3/57 | 1/7 |
| Rubella | 126/174 | 44/66 | 81/108 | 74/91 | 7/17 |
| IgG + IgM- | 108/125 | 36/43 | 71/81 | 65/74 | 6/7 |
| IgG-IgM- | 18/125 | 8/43 | 10/71 | 9/74 | 1/7 |
| Toxoplasma | 128/174 | 47/66 | 80/108 | 73/91 | 7/17 |
| IgG + IgM- | 30/127 | 8/46 | 22/80 | 19/73 | 3/7 |
| IgG-IgM- | 98/127 | 39/46 | 58/80 | 54/73 | 4/7 |
| CMV | 130/174 | 48/66 | 81/108 | 74/91 | 7/17 |
| IgG + IgM- | 87/129 | 31/47 | 55/81 | 50/74 | 5/7 |
| IgG-IgM- | 43/129 | 17/47 | 26/81 | 24/74 | 2/7 |
| HSV-1 | 113/174 | 43/66 | 70/108 | 61/91 | 9/17 |
| IgG + IgM- | 84/112 | 32/42 | 52/70 | 48/61 | 4/9 |
| IgG + IgM + | 2/112 | 0/42 | 2/70 | 0/61 | 2/9 |
| IgG-IgM- | 27/112 | 11/42 | 16/70 | 15/61 | 1/9 |
| HSV-2 | 112/174 | 42/66 | 70/108 | 61/91 | 9/17 |
| IgG + IgM- | 14/112 | 3/41 | 11/70 | 11/61 | 0/9 |
| IgG-IgM- | 98/112 | 39/41 | 59/70 | 50/61 | 9/9 |
| VZV | 116/174 | 43/66 | 73/108 | 64/91 | 9/17 |
| IgG + IgM- | 114/116 | 43/42 | 71/73 | 62/64 | 9/9 |
| IgG-IgM- | 2/116 | 0/42 | 2/73 | 2/64 | 0/9 |
| EBV | 133/174 | 49/66 | 83/108 | 75/91 | 8/17 |
| VCA IgG + EBNA IgG + VCA IgM- | 131/132 | 48/48 | 81/83 | 73/75 | 8/8 |
| VCA IgG + EBNA IgG- VCA IgM- | 2/132 | 0/48 | 2/83 | 2/75 | 0/8 |
| VCA IgG- EBNA IgG- VCA IgM- | 0/132 | 0/48 | 0/83 | 0/75 | 0/8 |
| JCV Stratify® assay | 33/174 | 12/66 | 21/108 | 16/94 | 5/17 |
| positive | 5/33 | 0/12 | 5/21 | 4/5 | 1/4 |
| negative | 28/33 | 12/12 | 16/21 | 12/16 | 4/5 |
| QuantiFERON® TB-gold | 127/174 | 47/66 | 80/108 | 69/91 | 11/17 |
| positive | 19/127 | 4/47 | 15/80 | 11/71 | 4/11 |
| indeterminate | 3/127 | 2/47 | 1/80 | 1/71 | 0/11 |
| negative | 105/127 | 13/47 | 64/80 | 57/71 | 7/15 |
MS: multiple sclerosis; HAV: hepatitis A virus; HBV: hepatitis B virus, HCV: hepatitis C virus; HIV: human immunodeficiency virus; Ab: antibody; Ag: antigen; CMV: cytomegalovirus; HSV-1: Herpes Simplex-1; HSV-2: Herpes Simplex-2; VZV: varicella zoster virus; EBV: Epstein-Barr virus; JCV: John Cunningham virus.
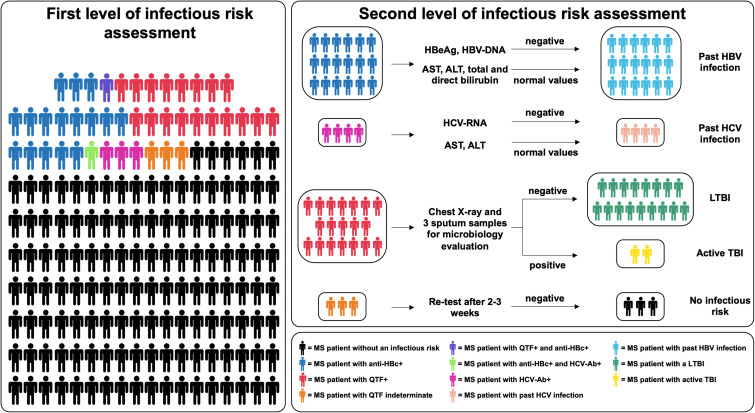
Figure 2.
First and second level of infection risk assessment
MS: multiple sclerosis, QFT: QuantiFERON® TB gold in tube, LTBI: latent tuberculosis infection, TBI: Tuberculosis infection, aspartate aminotransferase (AST), alanine aminotransferase (ALT).
Serological evaluations showed that 6% of MS patients had no measles immunity and 1.7% of MS patients presented findings indicating a lack of immunization against VZV (Table 2). Concerning the EBV serological evaluation, no EBV-naïve patients were observed. Indeed, 98.5% of the MS patients showed a past EBV infection, while 1.5% resulted to be VCA IgG + , VCA IgM- and EBNA IgG- (Table 2).
Stratify® JCV assay was performed in 33 patients, of which 15.2% had a positive result. Finally, 15.0% of QFT-tested MS patients were positive, whereas 2.4% showed indeterminate values (Table 2, Figure 2).
Overall, we observed only one QTF + and anti-HBc + patient, and another one with positivity for both anti-HBc and anti-HCV (Figure 2). No MS patients tested positive for HIV Ab/Ag (Table 2).
Second-level of infectious risk assessment
As reported in Figure 2, all MS patients who resulted positive at the infectious risk first-level assessment, were further evaluated via a tailored second-level survey. To exclude active HBV infection, the second-level assessment for the eighteen anti-HBc + patients consisted of the following investigations: HBeAg, anti-HBe, AST, ALT, total and direct bilirubin, and HBV-DNA. All these MS patients resulted negative for HBeAg and HBV-DNA, but positive for anti-HBe. AST, ALT, and total and direct bilirubin levels were in normal range. These patients were considered having latent HBV infection (Figure 2). After the infectious risk second-level evaluation, twelve patients started ocrelizumab, two natalizumab, one glatiramer acetate, one teriflunomide, and one IFN-β. To date, among them only one patient has yet to start MS therapy.
All four MS patients with anti-HCV positivity were further investigated for plasma HCV-RNA as well as AST and ALT levels. All patients resulted negative for HCV-RNA and showed normal AST and ALT levels (Figure 2). Among them, two patients started ocrelizumab, one natalizumab, and one patient has yet to start MS treatment.
All nineteen QFT + MS patients were further investigated by chest X-ray. Two patients showed an active TBI and in those cases, microbiology evaluation was performed on three sputum samples per patient. Treatment with isoniazid, rifampin, ethambutol, and pyrazinamide for two months plus 4 months with isoniazid and rifampin (total of 6 months for treatment) was administered. Both patients initiated DMT after one month of uncomplicated TB treatment. Specifically, one patient started ocrelizumab and the other patient natalizumab. For the other 17 QFT + patients, the second-level assessment indicated LTBI and prophylaxis with isoniazid plus rifampicin for three months was prescribed. Among them, fourteen MS patients were initiated on DMT after one month of LTBI prophylaxis. Specifically, two patients started natalizumab, five ocrelizumab, three teriflunomide, two cladribine, and two glatiramer acetate. To date, three patients have yet to start MS therapy.
For the three patients with an indeterminate QFT value, a new QFT evaluation was performed after two-three weeks and in all cases, it yielded negative results.
Reactivation of latent infection during DMT-treatment
To date, out of the evaluated 174 MS patients, 151 MS patients started DMTs with a median duration (IQR) of current treatment of 2.1 years (1.4–3.1). Overall, the prevalent current treatments were ocrelizumab (47.0%, 71/151), cladribine (13.2%, 20/151), natalizumab (9.9%, 15/151), and teriflunomide (8.6%, 13/151).
In our cohort, during DMT-treatment we observed one HBV reactivation in an ocrelizumab-treated patient (Table 3). As already described in a previous report, 20 patient was asymptomatic and liver enzymes remained within the normal range. Treatment for HBV reactivation (entecavir 0.5 mg once daily) was started and 4 weeks after HBV-DNA was undetectable. Thus, the patient was able to receive the scheduled dose of ocrelizumab. In another anti-HBc + patient, a transient HBV-DNA detection was observed one month after the third ocrelizumab infusion (Table 3). Patient was asymptomatic and liver enzymes remained within the normal range.
Table 3.
Reactivations of latent infection during DMTs.
| Number of reactivations | DMT | Time of reactivation from starting DMT | |
|---|---|---|---|
| HBV | 2 | ocrelizumab | HBV reactivation after the first infusion (13 weeks) 20 |
| ocrelizumab | A transient HBV-DNA detection after the third infusion (13 months) | ||
| HSV-1 | 6 | IFN-β | HSV-1 reactivation after three months |
| natalizumab | Recurrent HSV-1 reactivation at 72 infusions | ||
| ocrelizumab | HSV-1 reactivation after the first infusion (3 months) | ||
| ocrelizumab | HSV-1 reactivation after the first infusion (2 months) | ||
| natalizumab | Recurrent HSV-1 reactivation at 56 infusions | ||
| natalizumab | Recurrent HSV-1 reactivation at 42 infusions | ||
| JCV | 2 | fingolimod (switching from natalizumab) |
PML onset after 40 days 18 |
| dimethyl fumarate | PML onset after 21 months 18 | ||
| VZV | 1 | cladribine | VZV reactivation at 4 months |
DMT: disease-modifying therapy; HBV: hepatitis B virus, HSV-1: Herpes Simplex-1; JCV: John Cunningham virus; PML: progressive multifocal leukoencephalopathy; VZV: Varicella Zoster virus.
Six MS DMT-treated patients showed HSV-1 reactivation with detectable plasma DNA (Table 3). Specifically, one patient was under IFN-β treatment, two patients were under ocrelizumab, while three patients under natalizumab. Prophylaxis was started (acyclovir or valacyclovir) in all six cases.
As described in a previous report, 18 in two patients we observed the development of progressive multifocal leukoencephalopathy (PML). One patient was on fingolimod for 40 days after switching from natalizumab (last infusion of natalizumab less than six months earlier, Stratify JCV index = 3.61) while the other one was under dimethyl fumarate. At hospital admission patients were leukopenic (0.83 × 109/L and 0.88 × 10 9 /L, respectively). Both patients had to stop DMTs. For both patients three months after hospitalization, JCV-DNA was undetectable in CSF and a brain MRI showed the reduction in the size of the lesions, with complete regression of PML related symptoms. Finally, in one cladribine-treated patient we observed VZV reactivation and prophylaxis was started (Table 3).
De novo infection during DMT-treatment
After one year of cladribine treatment, one patient, who initially was QFT- before starting DMT, resulted QFT + . The infection risk second-level assessment was performed for excluding active TBI and prophylaxis was started. Patient received the scheduled dose of cladribine.
Another MS patient who had received fingolimod for two years showed primary CMV infection with seroconversion. PCR quantification of CMV-DNA in blood, urine and stool resulted positive. CMV treatment was not required.
Discussion
In this three-year observational cohort study, we collected real-world data on the infectious risk before starting or switching or during DMT in MS patients. We worked out a two-level infectious risk assessment. At the first-level evaluation, we were able to identify MS patients with a potential infectious risk and for whom a second-level assessment was necessary.
The first main result was that screening for HBV and TB allowed to identify patients with past HBV infection and LTBI or TBI, respectively. HBV reactivation can be devastating for patients treated with ocrelizumab and alemtuzumab, whereas MS oral agents do not likely pose a substantial risk related to HBV. HBV reactivation monitoring, through HBV-DNA periodic assessment, can prevent DMT discontinuation and could spare patients from receiving HBV treatment in avoidable cases. This approach has been applied to our patient who experienced a transient reactivation with detection of HBV-DNA, but without any clinical sign or liver enzyme changes. Discontinuation of DMT was deemed unnecessary and to date, the patient has been successfully managed with ocrelizumab. Regarding patients with LTBI and TBI, although screening for TB should be prioritized for patients receiving alemtuzumab, other DMTs such as fingolimod, natalizumab, rituximab, dimethyl fumarate, ocrelizumab, and teriflunomide induce varying degrees of immunosuppression. Taking into account this concept, DMTs can bring about a risk of opportunistic infections, including progression of a primary TB infection or reactivation of LTBI due to their impact on cellular immunity. 21 Furthermore, the prevalence of TBI justifies the need to identify at risk groups among MS patients to be selectively screened for LTBI, as to avoid development of active TB, resulting from either primary infection or reactivation. 21 Currently, there is limited information regarding the clinical parameters to address MS patients according to their probability of having LTBI. The regimen for treating TB disease includes a first intensive phase of two months with isoniazid, rifampin, ethambutol, and pyrazinamide, followed by a continuation phase of four months with isoniazid plus rifampin (total of 6 months for treatment). DMT-treatment can be started after the first month of TB treatment. Three-month prophylaxis with two molecules (isoniazid plus rifampicin) is effective, safe, and has higher completion rates compared to longer 6 to 9-month isoniazid-based monotherapy. Moreover, shorter, rifamycin-based treatment regimens generally have a lower risk of hepatotoxicity than 6 to 9-month isoniazid-based monotherapy. Nevertheless, given the inherent risk of hepatotoxicity associated with TB treatment and LTBI prophylaxis, it is necessary to periodically perform clinical and liver function (transaminase level) monitoring, especially if the selected MS treatment may also unavoidably carry a higher risk of hepatotoxicity.
The second main result of our study can be ascribed to the fact that in our cohort we identified unimmunized patients for VZV, HBV, measles, and rubella. Vaccination is an important tool in preventing infections, though vaccine timing, adverse effects and relative contraindications can be challenging. 22 Vaccines are generally considered safe for MS patients. Contraindications concern MS patients with a medical history of allergic reactions to one of the vaccine components and MS immunosuppressed patients in the case of live-attenuated vaccines. Indeed, the consensus being that they are contraindicated for MS patients who receive immunosuppressing or immunomodulating treatment unless the risk of infection is high. 23 Vaccination should be covered during treatment discussion with patients; vaccines should be preferably administered prior to DMT initiation, alternatively they may be momentarily delayed while patients are experiencing a MS relapse. 24 Preventive vaccination can be offered to eligible patients, in particular against HBV (especially common in the 45–65 age range) and against VZV (recombinant zoster vaccine Shingrix® is indicated for prevention of zoster reactivation in individuals ≥50 years).12,25,26
In our cohort, almost all MS patients were EBV VCA IgG and EBNA IgG positive and VCA IgM negative, indicating a previous infection. However, for a small percentage of MS patients we observed the lack of EBNA IgG despite the presence of VCA IgG. This is in line with a previous report, 27 which indicates that some patients with either chronic infections or immunosuppression may be negative for EBNA IgG or have only low level titres, despite the presence of VCA IgG detectable titres.
The third main result of our study was that the infectious risk evaluation during DMT allowed us to observe several herpes virus reactivations and one HBV reactivation without specific clinical signs. Particularly, in three natalizumab-treated patients and one IFN-β-treated patient, HSV-1 recurrent reactivations were observed. In other two ocrelizumab-treated patients, only one episode of reactivation was observed. Moreover, one cladribine-treated patient developed a VZV reactivation after four months from starting treatment and prophylaxis was administered. Concerning HBV reactivation, treatment with a potent antiviral agent seemed to be an effective and safe option for HBsAg negative, anti-HBc positive and HBV-DNA negative patients who show HBV-DNA detection during ocrelizumab. Indeed, in our patient HBV-DNA was no longer detectable soon after the initiation of an HBV-specific antiviral treatment. To date, the patient has been continuously managed with an ocrelizumab-based treatment.
PML is a particular concern when patients are managed with natalizumab. The risk of PML development in natalizumab-treated patients with JCV seropositivity is significantly elevated. However, PML cases are reported also in JCV seronegative subjects. 18 The risk of PML associated to fingolimod is low, with only a few cases reported as of now. Likewise, with dimethyl fumarate only a few cases of PML have been reported in MS patients so far, 18 but the drug has been recently introduced for general use, and PML has also been described in association to psoriasis treatment with fumarates. 28 In our cohort, we observed PML onset in two patients: one had been treated with fingolimod after switching from natalizumab, and the other one was managed with dimethyl fumarate. 18
Finally, during DMT-treatment we observed a CMV de novo infection in a fingolimod-treated patient. The patient did not require any therapy. Indeed, though fingolimod may cause an unexplained, selective reactivation of herpes virus infection, the immune responses against CMV and EBV remain unaffected. 29 In another patient, a conversion of QFT from negative to positive was observed and after excluding active TBI, prophylaxis was started. This episode underlines the need to annually repeat QFT also in those patients that have been started on an MS drug after initial negative screening. This annual monitoring allows the attending physician to promptly identify QFT conversions. In case of positive conversion, anti-TB treatment or prophylaxis must be started according to recommendations, to reduce the risk of progression to active TB. Presently, LTBI prophylaxis significantly reduces the risk of developing active disease and is justified in virtually all cases.
As the old aphorism goes, prevention is indeed better than cure. In this regard, risk mitigation strategies can play a significant role. Consequently, to ensure the effectiveness of such approach, we first need to recognize the presence of specific infectious risk in MS patient. A comprehensive infectious screening of each patient appears to be useful not only as a short-term strategy but may also become a guiding factor when a subsequent therapeutic option is considered. Moreover, this approach may aid in the investigation of the possible long-term additive effects of several DMTs in the single individual.
In conclusion, a thorough and individualized evaluation of each patient's infectious risk is necessary to determine which interventions should be performed to limit infectious complications in MS patients undergoing DMT. Each infectious risk identified in MS patients candidate for DMTs was discussed with the neurologist in charge of MS patient care. This combined approach allowed the identification of the best strategy to guarantee the optimal treatment with the lowest infectious risk for each patient. Moreover, this approach allowed the management of some latent infection and avoided the exclusion of some patients from starting DMTs, as well as prevented premature DMT discontinuation. Immunosuppressed MS patients are at risk of reactivation of latent pathogens, worsening of asymptomatic chronic infections, and contracting de novo infections. In those cases, prevention is preferable to treatment, for reducing both infectious morbidity and mortality, as well as limiting MS therapy discontinuation. Therefore, preventive approaches should be tailored to each patient, according to personal and treatment-related risk factors.
Acknowledgements
We are grateful to all the staff in in Neuroinfectious Unit of Policlinico Umberto I, Sapienza University of Rome, Department of Human Neurosciences, Sapienza University of Rome, and Department of Molecular Medicine, Sapienza University of Rome, that collaborate in the management of MS patients.
Footnotes
Contributors: MAZ, PP, MI were responsible for data acquisition, analysis, and interpretation and drafting of the manuscript. MRC, CMM, AC, GA was responsible for study conception, design, supervision, funding, analysis, and interpretation of data, and drafting of the manuscript. VP, MT, SGC, CM, VB, LM, AT, AG were responsible for data acquisition, analysis, and interpretation. MAZ, PP, MT, FP, MF, MA, AC were responsible for enrollment of the patients. All authors had full access to all the data in the study, verified the data, and had final responsibility for the decision to submit for publication.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Maria Antonella Zingaropoli https://orcid.org/0000-0001-9479-8559
Contributor Information
Patrizia Pasculli, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Italy.
Marco Iannetta, Department of System Medicine, Tor Vergata University of Rome, Italy.
Valentina Perri, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Italy.
Viola Baione, Department of Human Neurosciences, Sapienza University of Rome, Italy.
Ambra Taglietti, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Italy.
Marta Altieri, Department of Human Neurosciences, Sapienza University of Rome, Italy.
Aurelia Gaeta, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Italy.
Guido Antonelli, Department of Molecular Medicine, Sapienza University of Rome, Italy.
Antonella Conte, Department of Human Neurosciences, Sapienza University of Rome, Italy; IRCCS Neuromed, Pozzilli, Isernia, Italy.
Maria Rosa Ciardi, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Italy.
References
- 1.Kurtzke JF. A reassessment of the distribution of multiple sclerosis. Acta Neurol Scand 1975; 51: 137–157. [DOI] [PubMed] [Google Scholar]
- 2.Kurtzke JF. Multiple sclerosis in time and space–geographic clues to cause. J Neurovirol 2000; 6 : S134–S140. [PubMed] [Google Scholar]
- 3.Brück W, Gold R, Lund BT, et al. Therapeutic decisions in multiple sclerosis: moving beyond efficacy. JAMA Neurol 2013; 70: 1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pourcher V. What are the infectious risks with disease-modifying drugs for multiple sclerosis and how to reduce them? A review of literature. Rev Neurol (Paris) 2020; 176: 235–243. [DOI] [PubMed] [Google Scholar]
- 5.Grebenciucova E, Pruitt A. Infections in patients receiving multiple sclerosis disease-modifying therapies. Curr Neurol Neurosci Rep 2017; 17: 88. [DOI] [PubMed] [Google Scholar]
- 6.Luna G, Alping P, Burman J, et al. Infection risks Among patients With multiple sclerosis treated With fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol 2020; 77: 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celius EG. Infections in patients with multiple sclerosis: implications for disease-modifying therapy. Acta Neurol Scand 2017; 136 : 34–36. [DOI] [PubMed] [Google Scholar]
- 8.Montgomery S, Hillert J, Bahmanyar S. Hospital admission due to infections in multiple sclerosis patients. Eur J Neurol 2013; 20: 1153–1160. [DOI] [PubMed] [Google Scholar]
- 9.Moiola L, Barcella V, Benatti S, et al. The risk of infection in patients with multiple sclerosis treated with disease-modifying therapies: a delphi consensus statement. Mult Scler 2021; 27: 331–346. [DOI] [PubMed] [Google Scholar]
- 10.Rommer PS, Zettl UK. Managing the side effects of multiple sclerosis therapy: pharmacotherapy options for patients. Expert Opin Pharmacother 2018; 19: 483–498. [DOI] [PubMed] [Google Scholar]
- 11.Epstein DJ, Dunn J, Deresinski S. Infectious complications of multiple sclerosis therapies: implications for screening, prophylaxis, and management. Open Forum Infect Dis 2018; 5: ofy174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy KR, Beavers KL, Hammond SP, et al. American Gastroenterological association institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015; 148: 215–219; quiz e16–17. [DOI] [PubMed] [Google Scholar]
- 13.Bittner S, Engel S, Lange C, et al. [Diagnostics and treatment of tuberculosis under immunotherapy for multiple sclerosis : current status and recommendations in Germany]. Nervenarzt 2019; 90: 1245–1253. [DOI] [PubMed] [Google Scholar]
- 14.Navas C, Torres-Duque CA, Munoz-Ceron J, et al. Diagnosis and treatment of latent tuberculosis in patients with multiple sclerosis, expert consensus. On behalf of the Colombian association of neurology, committee of multiple sclerosis. Mult Scler J Exp Transl Clin 2018; 4: 2055217317752202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persson R, Lee S, Ulcickas Yood M, et al. Infections in patients diagnosed with multiple sclerosis: a multi-database study. Mult Scler Relat Disord 2020; 41: 101982. [DOI] [PubMed] [Google Scholar]
- 16.Mack U, Migliori GB, Sester M, et al. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J 2009; 33: 956–973. [DOI] [PubMed] [Google Scholar]
- 17.Ho P-R, Koendgen H, Campbell N, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies. Lancet Neurol 2017; 16: 925–933. [DOI] [PubMed] [Google Scholar]
- 18.Ciardi MR, Zingaropoli MA, Iannetta M, et al. JCPyv NCCR analysis in PML patients with different risk factors: exploring common rearrangements as essential changes for neuropathogenesis. Virol J 2020; 17: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmad M, Ibrahim WH, Sarafandi SA, et al. Diagnostic value of bronchoalveolar lavage in the subset of patients with negative sputum/smear and mycobacterial culture and a suspicion of pulmonary tuberculosis. Int J Infect Dis 2019; 82: 96–101. [DOI] [PubMed] [Google Scholar]
- 20.Ciardi MR, Iannetta M, Zingaropoli MA, et al. Reactivation of hepatitis B virus With immune-Escape mutations after ocrelizumab treatment for multiple sclerosis. Open Forum Infect Dis 2019; 6: ofy356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fragoso YD, Adoni T, Anacleto A, et al. How do we manage and treat a patient with multiple sclerosis at risk of tuberculosis? Expert Rev Neurother 2014; 14: 1251–1260. [DOI] [PubMed] [Google Scholar]
- 22.Riva A, Barcella V, Benatti SV, et al. Vaccinations in patients with multiple sclerosis: a delphi consensus statement. Mult Scler 2021; 27: 347–359. [DOI] [PubMed] [Google Scholar]
- 23.Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology. Neurology 2019; 93: 584–594. [DOI] [PubMed] [Google Scholar]
- 24.Yong KP, Kim HJ. Disease modifying therapies and infection risks in multiple sclerosis-a decision-making conundrum. Ann Transl Med 2020; 8: 722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klotz L, Havla J, Schwab N, et al. Risks and risk management in modern multiple sclerosis immunotherapeutic treatment. Ther Adv Neurol Disord 2019; 12: 1756286419836571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Syed YY. Recombinant zoster vaccine (shingrix ®): a review in herpes zoster. Drugs Aging 2018; 35: 1031–1040. [DOI] [PubMed] [Google Scholar]
- 27.Miller G, Grogan E, Rowe D, et al. Selective lack of antibody to a component of EB nuclear antigen in patients with chronic active epstein-barr virus infection. J Infect Dis 1987; 156: 26–35. [DOI] [PubMed] [Google Scholar]
- 28.Berger JR. Classifying PML risk with disease modifying therapies. Mult Scler Relat Disord 2017; 12: 59–63. [DOI] [PubMed] [Google Scholar]
- 29.Pfender N, Jelcic I, Linnebank M, et al. Reactivation of herpesvirus under fingolimod: a case of severe herpes simplex encephalitis. Neurology 2015; 84: 2377–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]