Abstract
Objective:
Gene therapy is a promising approach in the treatment of cardiovascular diseases. Preclinical and clinical studies have demonstrated that adeno-associated viral (AAV) vectors are the most attractive vehicles for gene transfer. However, pre-existing immunity, delayed gene expression, and post-infection immune response limit the success of this technology. The aim of this study was to investigate efficacy of the first synthetic AAV lineage clone, Anc80L65 for cardiac gene therapy.
Methods:
Combining two different reporter approaches by fluorescence with green fluorescent protein (GFP), and by bioluminescence (Firefly luciferase) we compared transduction efficiency of Anc80L65 and AAV9 in neonatal rat cardiomyocytes ex vivo and rat hearts in vivo after intramyocardial and intracoronary administration.
Results:
In cardiomyocytes Anc80L65 provided a GFP expression of 28.9% (36.4 ± 3.34 cells/field) at 24 hours and close to 100% on day seven. In contrast, AAV9.GFP provided minimal GFP expression of 5.64% at 24 hours, and 11.8% on day seven. After intramyocardial injection, vector expression peaked on day seven with Anc80L65, however with AAV9 the peak expression was during week six. Administration of Anc80L65 demonstrated significantly more efficient expression of reporter gene than after AAV9 at 6 weeks (6.81±0.64 log10 gc/100 ng DNA versus 6.49±0.28 log10 gc/100 ng DNA, p<.05). These results were consistent with the amount of genome copy per cell observed in the heart.
Conclusion:
In conclusion, Anc80L65 vector allows fast and robust gene transduction compare to AAV9 vector in cardiac gene therapy. Anc80L65 did not adversely affect cardiac function, caused no inflammatory response, or toxicity.
Keywords: adeno-associated vector, gene therapy, cardiac function, inflammatory response
Graphical Abstract
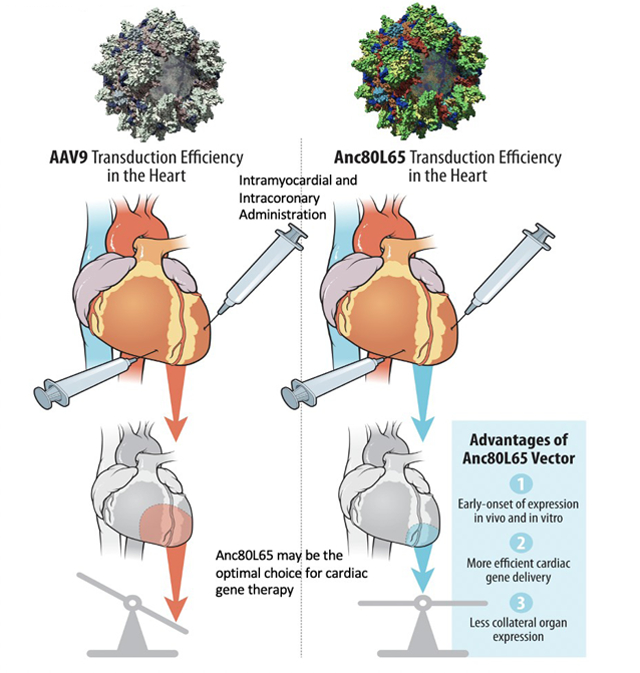
Central Picture: Advantages of Synthetic AAV Vector, Anc80L65 after intramyocardial administration.
Central Message:
Intramyocardial administration of synthetic Anc80L65 vector provides early onset and efficient expression in cardiomyocytes compared to AAV9 vector, and does not induce inflammatory response.
Introduction
Complications of cardiovascular diseases, specifically of acute myocardial infarction (MI) remain a leading cause of morbidity and mortality around the world1. Ischemic dysfunction of cardiomyocytes from an acute coronary event can impair functions of both ventricles and can cause extensive ischemic injury, leading to the development of heart failure in 20% to 30% of patients2,3. The results of the meta-analysis of complete (CR) versus incomplete revascularization (IR) in patients with coronary artery disease shows that CR is associated with a 30% reduction in long-term mortality4,5. However, as was demonstrated in the SYNTAX trial the rates of IR is pretty high (43.3% for PCI and 36.8% for CABG). The most common reasons for not achieving CR were the presence of chronic total occlusion, diffuse disease or small-vessel diameter. Moreover, attempts to achieve CR “at all costs” might expose patients to procedural complications6. The development of additional or concomitant therapeutic options for these patients remains a necessary field of inquiry. A growing body of animal studies has documented that gene therapy can reduce myocardial damage, with potential recovery of ventricular function7,8. Over the past decade, gene therapy technology has significantly advanced, and viral gene delivery tools have progressed to FDA approved clinical investigation for humans. Gene therapy is now considered an attractive strategy for combating molecular abnormalities underlying pathogenesis of myocardial ischemia9. Several viral vectors and non-viral gene delivery systems were tested for various cardiac applications10. AAV vectors gained significant attention due to important beneficial properties for therapeutic application in humans. AAV-mediated gene delivery allows high transduction efficiency and prolonged transgene expression in a broad range of tissue and organs, including the heart, while being less pathogenic11. Successful clinical application of the AAV vector therapeutic platform has been achieved for a variety of diseases, including cardiac among others12,13. AAV vectors are single-stranded DNA with a good safety profile14. Putatively, AAV9 vector is the most efficient serotype for cardiac gene delivery15,16.
However, AAV9-mediated gene expression does not plateau until four to six weeks post gene transfer15,16,17. This slow attenuation of gene expression significantly compromises its’ therapeutic impact, especially in regards to acute coronary events. Known AAV vehicles posses lower DNA packaging capacity, limiting size, and may fail to transfect the desired tissue because of pre-existing immunity18,19. However, a new synthetic clone, Anc80L65 created using a reverse genetics approach and ancestral AAV sequence reconstruction, can overcome the challenges of existed AAV vectors20.. Recently, it was demonstrated that Anc80L65 is an efficient gene transfer tool for central nervous system21 and retina22. The purpose of this study was to investigate the cardiac therapeutic potential of Anc80L65 vector, comparing its properties to AAV9 vector.
Methods
Animals studies and in vivo gene transfer: intramyocardial and intracoronary delivery
All animal procedures were performed under protocols approved by the Icahn School of Medicine at Mount Sinai Institutional Care and Use Committee as previously described23 (Video 1). 170 Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA) were used. Details are available in Appendix E1.
Neonatal Rat Cardiac Cells Isolation and In Vitro Transfection with AAV9 and Anc80L65 viral vector
Neonatal rat ventricular cardiomyocytes (CMs) were isolated from three days old rats by multiple rounds of digestion with 0.1% collagenase II (Invitrogen, Carlsbad, CA) in phosphate-buffered saline. Details are available in Appendix E1.
Animal imaging: detection of Luciferase activity
To visualize tissues expressing Luciferase (Luc) in vivo, gene construct Anc80L65.Luc and AAV9.Luc was injected intramyocardial or intracoronary. At day one, three, seven and six weeks after gene transfer the d-luciferin substrate (Biotium, Hayward, CA) was injected intraperitoneally, at a dose of 150 μg/g of body weight. Details are available in Appendix E1.
Viral Virus Production
A single stranded adeno-associated viral vector (AAV), serotype 9, encoding GFP under the control of the human cytomegalovirus promoter was constructed by the University of Pennsylvania Vector Core (http://www.med.upenn.edu/gtp/vectorcore/). Anc80L65 viral stocks were prepared and tittered at the Gene Tranfer Vector Core at Mass General Brigham (https://www.vdb-lab.org/vector-core/). Details are available in Appendix E1.
GFP signal analysis
The rat hearts were harvested and perfused using saline solution and 4% paraformaldehyde. Details are available in Appendix E1.
Real-Time qPCR Analyses and determination of Anc80L65 and AAV9 vectors genome copy number
Total RNA was isolated using the RNeasy mini kit (Qiagen, Hilden, Germany) and reverse transcribed using superscript reverse transcriptase, according to the manufacturer’s instructions. Details are available in Appendix E1.
Inflammatory response and cytokines expression analysis
Concentrations of pro- and anti-inflammatory cytokines in sera were determined using a 23-bioplex assay (Bio-Rad, Philadelphia, PA). Details are available in Appendix E1.
Evaluation of cardiac function by echocardiography
All animals underwent transthoracic echocardiography. Details are available in Appendix E1.
Assessment of toxicity with blood tests and blood gas analysis
Blood samples were collected from all rats under anesthesia. Details are available in Appendix E1.
Statistical Analysis
Continuous variables were tested for normality using skewness and kurtosis, applied to each data set, before further statistical testing. Outcomes are presented as mean ± standard deviation SD or median (interquartile range) based on normality. For cardiac mechanics and molecular biology assay data, one-way analyses of variance (ANOVAs) were used for time-independent factors that satisfied the normality assumptions in all groups, and post-hoc tests were used to assess differences between specific groups. Statistical comparisons for the box-and-whisker dot plots were made based on a one-way ANOVA comparing the median/50% percentile with Tukey multiple comparison tests. Prism GraphPad 5.0 was used for all data handling.
RESULTS
In vitro gene transfer
Neonatal rat cardiomyocytes were grown in tissue culture and infected with the 1×1010 gc Anc80L65.GFP or AAV9.GFP (Fig 1a, 1b). There was a time-dependent increase in Anc80L65-mediated transgene expression in cultured neonatal cardiomyocytes. At 3 days we found 73.6 ± 1.93 cells/field (p<.001) expressed GFP following transfection of Anc80L65.GFP (Fig 1a; 1bD). This number was increased, at 7 days to 193.6 ± 2.84 cells/field (p<.001) as shown by fluorescent microscopy (Fig 1a, 1bF). In contrast, AAV9.GFP-infected cells demonstrated only 7.8 ± 0.33 cells/field with positive staining for GFP at 3 days (Fig 1a; 1bC), and a displayed minimal transduction at seven days compared to Anc80L65 (18.4±0.41 cells/field) (p<.001) (Fig 1a; 1bE).
Figure 1. Fluorescence imaging of GFP in neonatal rats cardiomyocytes at 1 day, 3 days, and 7 days,. (n=30).
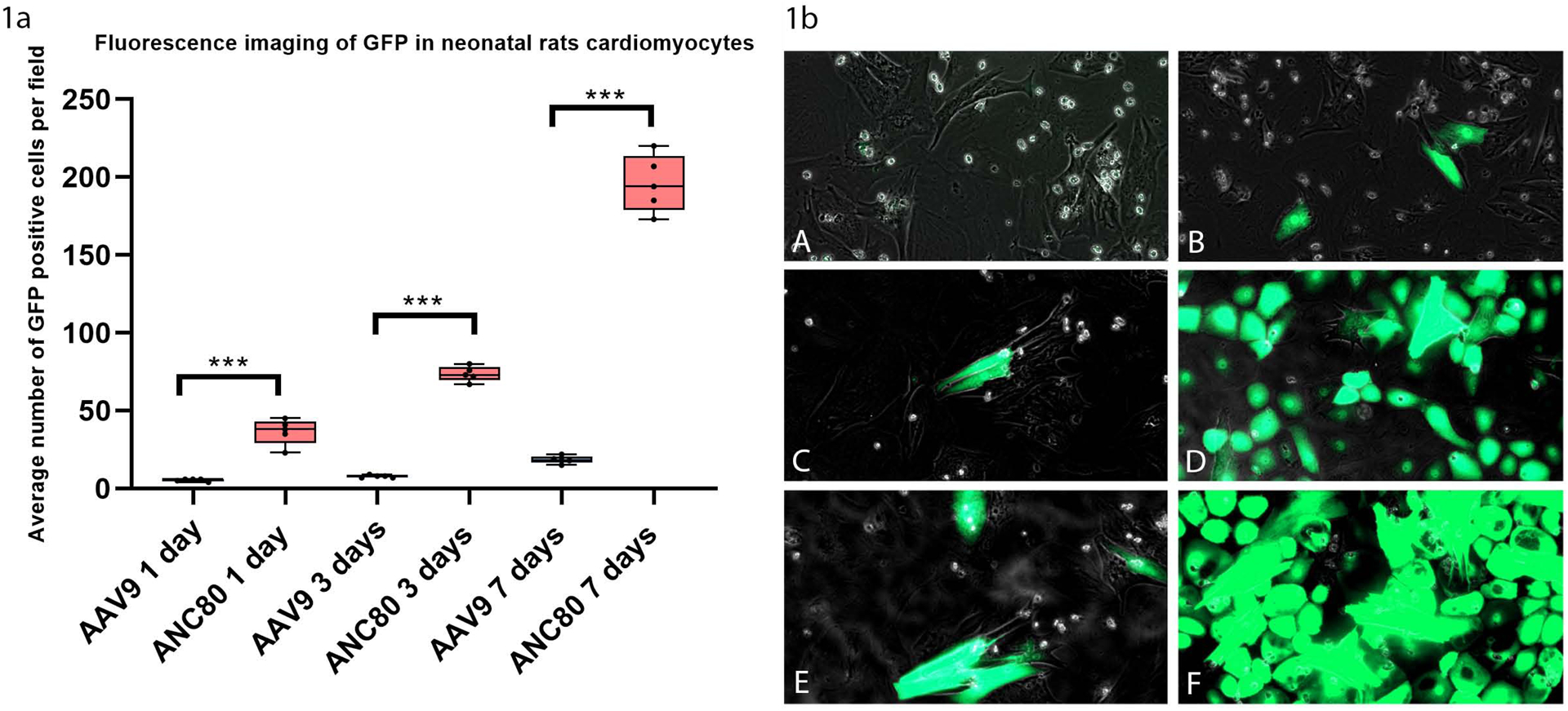
There was a time-dependent increase in Anc80L65-mediated transgene expression in cultured cardiomyocytes. Stained sections were imaged using a Zeiss Scanner Axio Scan. Positive-staining cells were counted, at a magnification of x100, as a percentage of total cells over six 0.5-mm2 fields and averaged.
ns P > 0.05
* P ≤ 0.05
** P ≤ 0.01
*** P ≤ 0.001
1a. Average number of GFP positive cells per field in neonatal rats cardiomyocytes at 1 day, 3 days, and 7 days,
1b. Fluorescence microscopy of neonatal rats cardiomyocytes:
1bA, AAV9.GFP 1 day expression. 1bB, Anc80L65.GFP 1 day expression. 1bC, AAV9.GFP 3 days expression. 1bD, Anc80L65.GFP 3 days expression. 1bE, AAV9.GFP 7 days expression. 1bF, Anc80L65.GFP 7 days expression.
Anc80L65 mediates faster onset and higher gene expression in cardiomyocytes in vivo
To investigate in vivo cardiac transduction efficiency, we injected of Anc80L65.GFP and AAV9.GFP into the left ventricular wall of the rat through an anterior thoracotomy in separate experiments. Intramyocardial delivery of the Anc80L65 vector resulted in much quicker GFP expression compared to AAV9 injection as shown by the increase in average cells displaying GFP positive cells/field between the 1 day mark and 6 weeks. (Fig 2a, 2b). Myocardial imaging demonstrated that onset of transgene expression occurred at 24 hours following administration of Anc80L65 vector (Fig 2aD). The amount of expression in the heart increased gradually over the first three days (Fig 2aE) (55.5 ± 0.34 cells/field, p<.001). Then the level peaked at six weeks post injection (Fig 2aF). During the course of the above experiments, the expression of GFP in myocardium with Anc80L65 was much higher compared with AAV9. Myocardium injected with AAV9 started to show sustained and visible levels of GFP expression only after six weeks (Fig 2aC) with maximum value of 79.6±2.68 cells/ field. However, these values were significantly less than after administration of Anc80L65 at six weeks (Fig 2aF), 109.5±4.07 cells/field (p<.01). Immunostaing with cardiomyocyte specific marker α-actinin and DAPI confirmed the data and demonstrated significantly high expression specifically in cardiomyocytes per field after Anc80L65 administration (p<.001) at 6 weeks (Fig 2b, 2bA–D).
Figure 2.
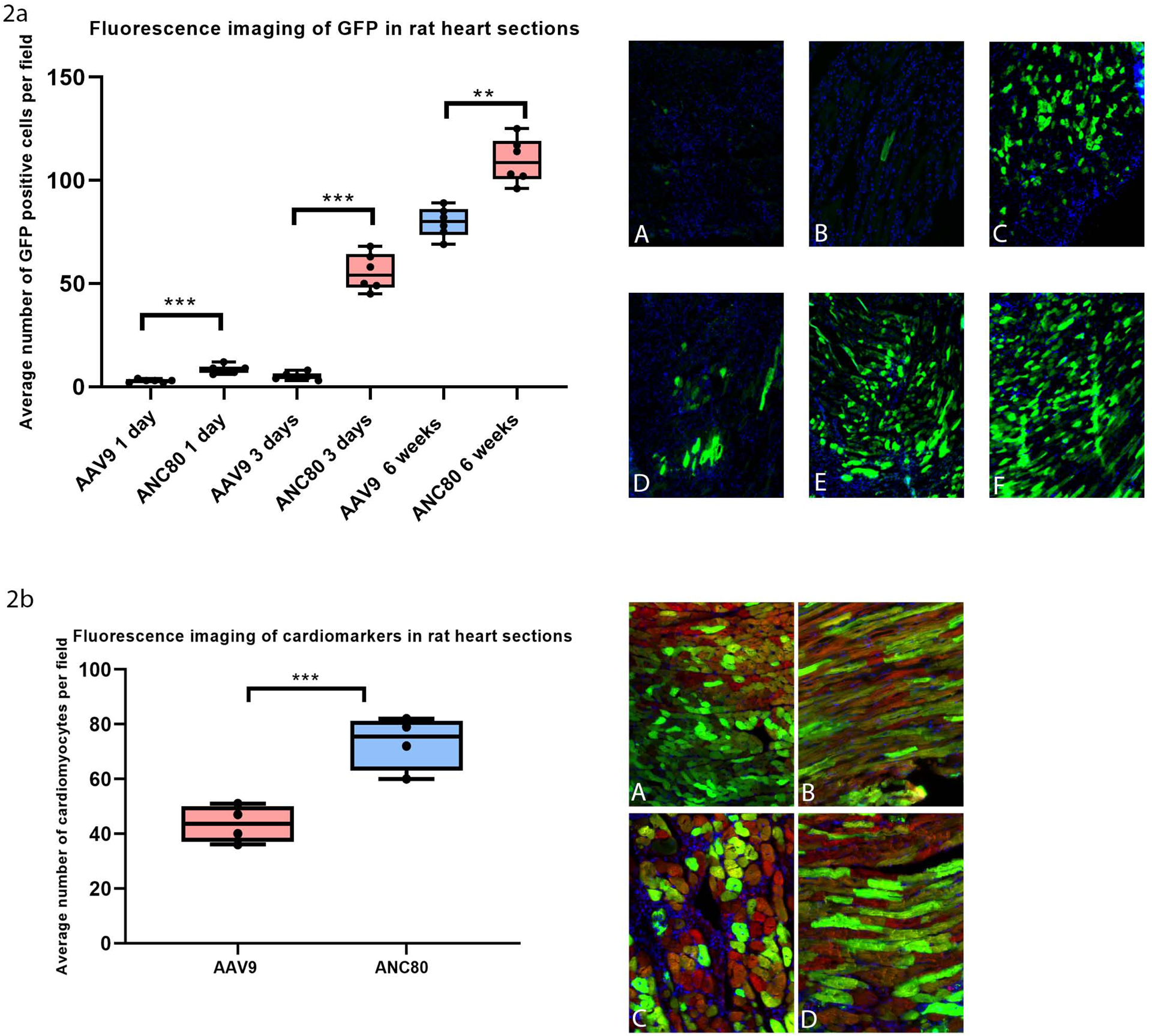
Fluorescence imaging of GFP and Immunostaing with cardiomyocyte specific marker α-actinin and DAPI demonstrated high expression in cardiomyocytes per field after Anc80L65 administration in rat heart sections after intramyocardial delivery AAV9.GFP and ANC80.GFP (n=30).
ns P > 0.05
* P ≤ 0.05
** P ≤ 0.01
*** P ≤ 0.001
2a. Average number of GFP positive cells per field: 2aA, AAV9.GFP 1 day expression. 2aB, AAV9.GFP 3 days expression. 2aC, AAV9.GFP 6 weeks expression. 2aD, Anc80L65.GFP 1 day expression. 2aE, Anc80L65.GFP 3 days expression. 2aF, Anc80L65.GFP 6 weeks expression.
2b. Fluorescence imaging of cardiomarkers in rat heart sections at 6 weeks: 2bA, AAV9.GFP coronal cutting. 2bB, AAV9.GFP sagittal cutting, 2bC, Anc80L65.GFP coronal cutting. 2bD, Anc80L65.GFP sagittal cutting. For nuclear labeling cells were immunostained with Red- actinin, blue-DAPI, Green-GFP.
Intramyocardial administration of Anc80L65 vectors is more efficient than intracoronary injection
We compared GFP transgene expression quantitatively with fluorescent microscopy on heart cross-sections at the midventricular level after intramyocardial and intracoronary injection. At six weeks strong GFP expression was exhibited after intramyocardial injection of Anc80L65 vector (46.14±4.67%) (Fig 3a, 3bB), while weaker expression was seen in AAV9-intramyocardial injected heart (19.89±6.87%) (p<.05) (Fig 3bA). Interestingly, GFP imaging revealed that Anc80L65 effectively transduced muscles not only in the left ventricle, but also in the right ventricle. AAV9.GFP had a much weaker GFP profile. We next investigated intracoronary delivery (IC) as an alternative approach, because we thought that intramyocardial transfer results preferentially in local transduction. Interestingly, Anc80L65.GFP rendered the more efficient and persistent systemic transduction in cardiac muscles after IM injection compared to IC injection (Fig 3bB, 3bC) (46.14±4.67% versus 12.14±3.32%, p<.001).
Figure 3. Mean area of total GFP fluorescence per cross-section after intramyocardial and intracoronary administration of Anc80L65 and AAV9 at 6 weeks (n=16).
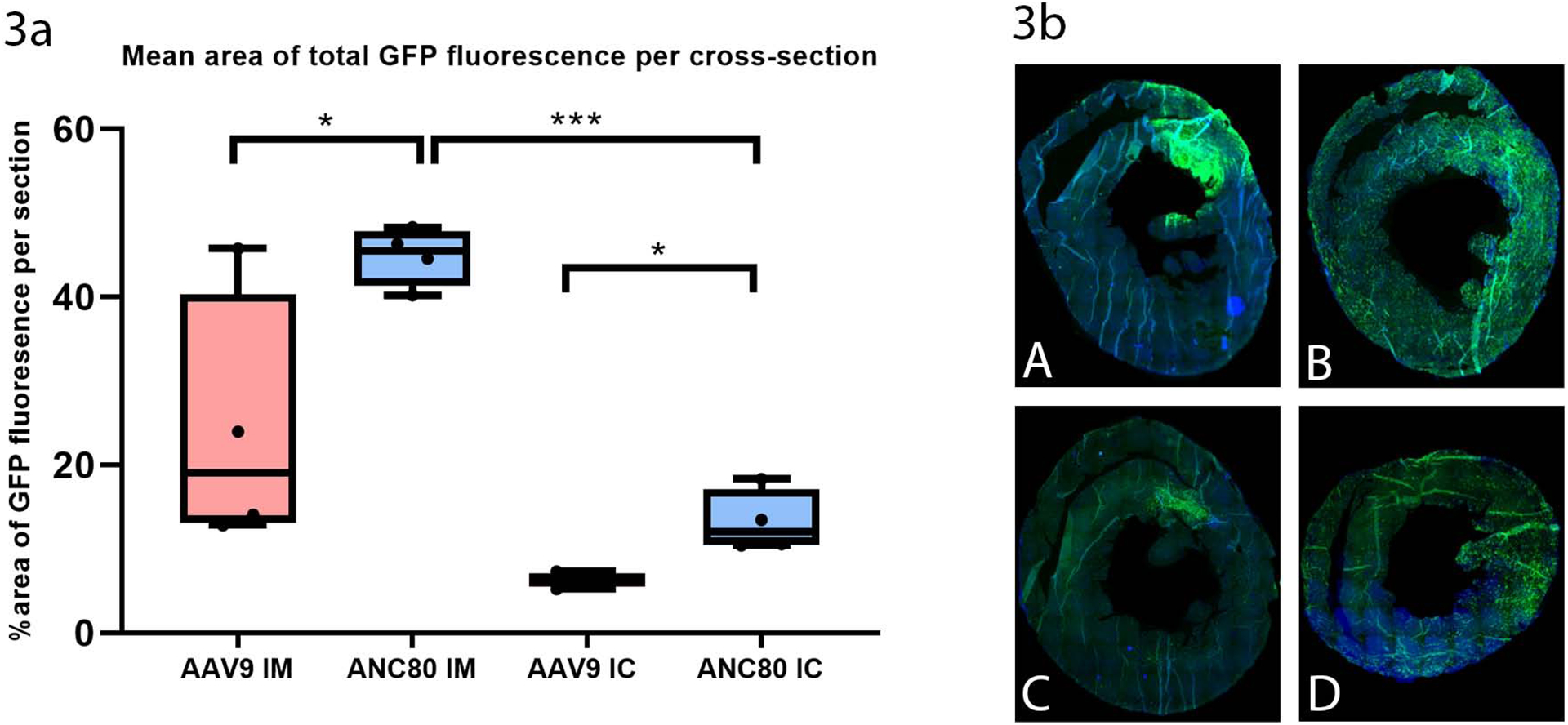
GFP transgene expression was compared on heart cross-sections after intramyocardial and intracoronary injection. Strong GFP expression was exhibited after injection of Anc80L65 vector
ns P > 0.05
* P ≤ 0.05
** P ≤ 0.01
*** P ≤ 0.001
3a. Percentage of area of GFP fluorescence per section
3b. Fluorescence imaging of GFP: 3bA, AAV9.GFP intramyocardial delivery. 3bB, Anc80L65.GFP intramyocardial delivery. 3bC, AAV9.GFP intracoronary delivery. 3bD, Anc80L65.GFP intracoronary delivery.
In vivo levels and kinetics of Luciferase (Luc) expression
Additional studies were performed to evaluate a time-course assay of Anc80L65.Luc and AAV9.Luc expression in the rats at different time points post injection (Fig 4a, 4b). In order to quantify the light released, representing luciferase activity, from the heart of each animal, we calculated the number of total flux in photons/sec/cm2/sr (tf) emitted from the thorax at each time point, after vector delivery, using Xenogen IVIS100 imaging software. In the Anc80L65.Luc group, the level of expression was maximized and stabilized (8.42 ± 0.05 log10 tf, p<.001) on day seven and stayed steady until six weeks (Fig 4b). Within the AAV9.Luc group, injected animals showed a mean peak of expression only at six weeks post injection (6.31 ± 0.03 log10 tf, p<.001). Thus, Anc80L65.Luc induced a rapid and robust expression detectable as soon as 24 hours post-injection and the expression levels of Anc80L65.Luc were much higher than the AAV9.Luc at all the time points studied.
Figure 4. Bioluminescence imaging of firefly Luciferase protein (n=40).
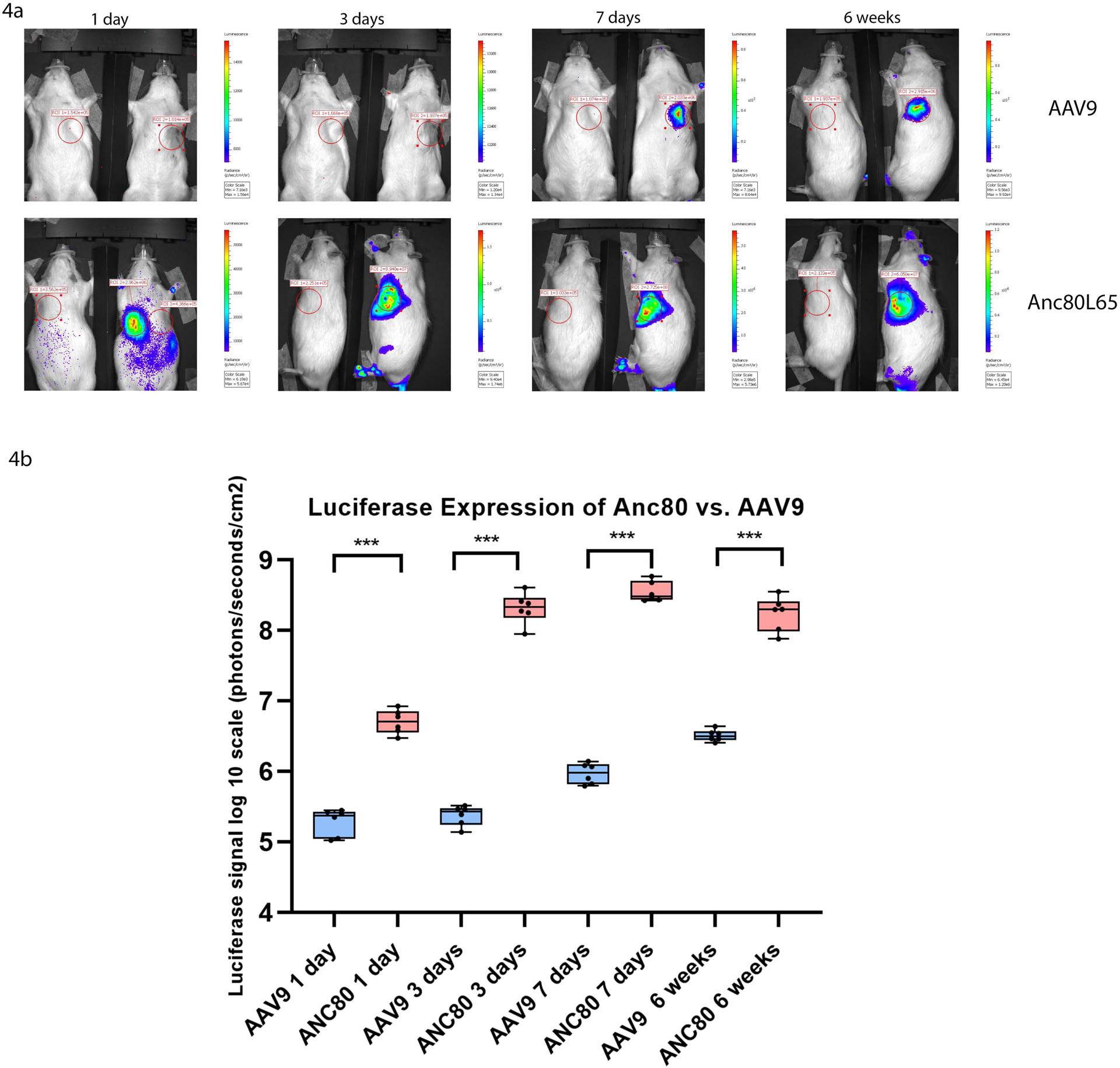
In order to quantify the luciferase activity, from the heart of each animal, we calculated the number of total flux in photons/sec/cm2/sr (tf) after vector delivery, using Xenogen IVIS100 imaging software. In the Anc80L65.Luc group, the level of expression was maximized and stabilized compared to AAV9 group.
ns P > 0.05
* P ≤ 0.05
** P ≤ 0.01
*** P ≤ 0.001
4a. Bioluminescence imaging at 1 day, 3 days, 7 days, and 6 weeks after Anc80L65.Luc and AAV9.Luc administration. The Bioluminescence imaging of the transfected cells represents the number of photons emitted/second/cm2 as a color image where the maximum is red and the minimum is dark blue.
4b. Luciferase expression of Anc80L65.Luc versus AAV9.Luc at different time points.
Biodistribution and quantification of Anc80L65 and AAV9 vectors
We next examined the viral genome copy number in several tissues against total genomic DNA in those tissues. Intramyocardial delivery of Anc80L65 resulted in robust infection as assessed by qPCR data and demonstrated increased expression in the heart (6.79±0.64 log10 gc per 100 ng of DNA) compared to AAV9 (6.54±0.28 log10 gc per 100 ng of DNA, p<.05) (Fig 5a). These data are consistent with genome copy per cell in the LV wall, which showed a significance difference between Anc80L65 and AAV9 at six weeks (64.5±4.81 versus 47.4±3.01 p<.05) (Fig 5b). Whereas, collateral liver expression was much lower in Anc80L65 compared to AAV9 (5.88±0.11 log10 gc per 100 ng of DNA versus 6.81±0.06 log10 gc per 100 ng of DNA, p<.001). These results demonstrated the ability of Anc80L65 to achieve higher cardiac transfection compared to transfection in kidneys, lungs and liver.
Figure 5.
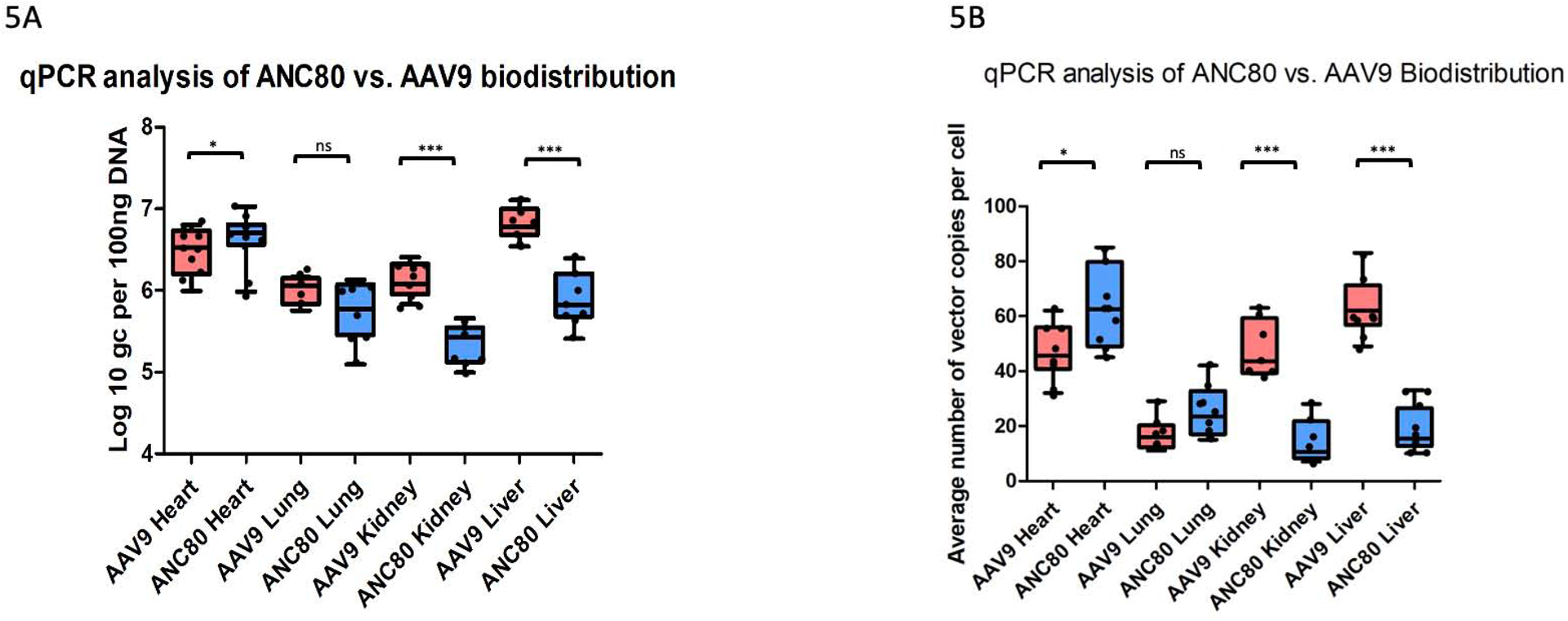
Intramyocardial delivery of Anc80L65 resulted in increased number of viral genome copies and number of vector copies per cell in the heart compared to AAV9. Whereas, collateral organs expression was much lower in Anc80L65 compared to AAV9 (n=30). The level of GAPDH DNA was used to calculate normalized transfection. Data were reported as viral genome copies (gc) per 100 ng of DNA.
ns P > 0.05
* P ≤ 0.05
** P ≤ 0.01
*** P ≤ 0.001
5a. qPCR analysis of Anc80L65 versus AAV9 vector distribution in different organs.
5b. Average number of vector genome copies Anc80L65 versus AAV9 per cell in different organs.
Evaluation the effect of viral transduction on heart function
Since there are could be potential cardiac adverse effects from viral exposure, transthoracic echocardiography was performed to evaluate whether heart function is affected after viral injection. As shown in Table E1, injection of AAV9 vector caused increased heart rate and reduction of stroke volume index at 7 days. At 6 weeks this effect decreased although tachycardia still remained. We did not see any differences in heart function after Anc80L65 administration.
Safety, inflammation and immunology
Rats were phlebotomized pre-injection and post-injection with Anc80L65 or AAV9 vectors. Blood was analyzed for cell blood counts (CBC), chemistry (Chem), and blood gases. CBC and Chem values for Anc80L65 were within normal range and did not demonstrate any signs of toxicity related to the viral transfer (Table E2). However, in AAV9 group white blood cells, lymphocytes (LYM%), and neutrophils increased remarkably at 7 days (p<.05) and a significantly higher LYM% was found at 6 weeks as well. Serum from the different time points were further evaluated for distributions of the main cytokines as a measure of inflammatory, and immune response to the vector antigens. Overall, among 23 cytokine analytes investigated, no significant differences were observed for Anc80L65. In contrast, significant changes of tumor necrosis factor α, interferon γ, interleukins 1β, 6, 1α, and 10 were observed in AAV9 group (Fig. 6a, 6b), (Table E3).
Figure 6. Changes in pro-inflammatory and anti-inflammatory cytokines expression.
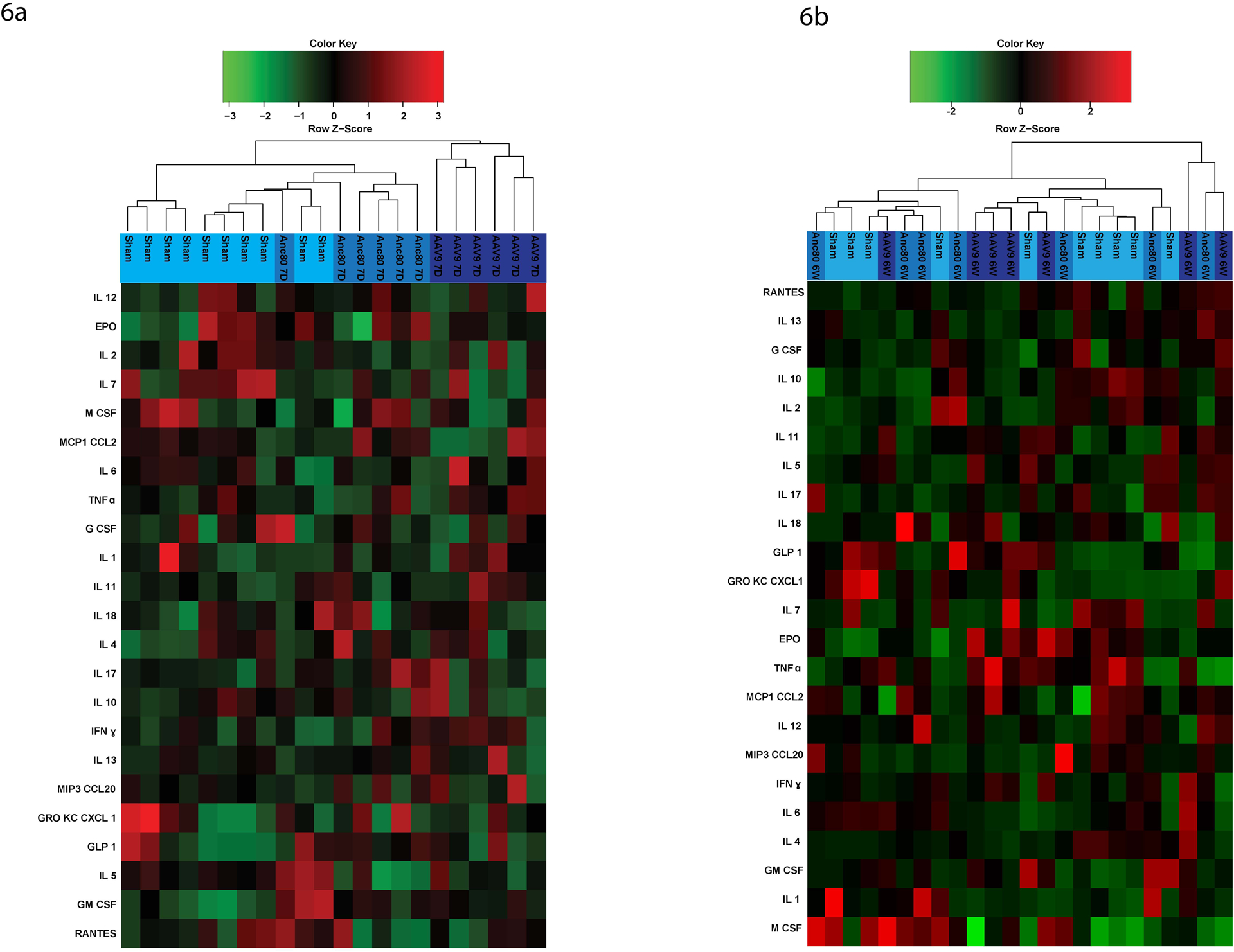
Among 23 cytokines investigated, no significant differences were observed for Anc80L65. In contrast, changes of tumor necrosis factor α, interferon γ, interleukins 1β, 6, 1α, and 10 were observed in AAV9 group (n=24).
ns P > 0.05
* P ≤ 0.05
** P ≤ 0.01
*** P ≤ 0.001
The left dendrogram shows the clustering of cytokines. The colors on the right side represent expression.. IL, interleukin; EPO, erythropoietin; M CSF, macrophage colony-stimulating factor; MCP1 CCL2, monocyte chemoattractant protein; TNFα, tumor necrosis factor; G CSF, granulocyte colony-stimulating factor; IFNγ, interferon gamma; MIP3 CCL20, macrophage inflammatory protein; GRO KC CXCL 1, chemokine ligand; GLP 1, glucagon-like peptide; GM CSF, granulocyte-macrophage colony-stimulating factor.
6a. Cytokines expression of Anc80L65 versus AAV9 viral vector at 7 days.
6b. Cytokines expression of Anc80L65 versus AAV9 viral vector at 6 weeks.
Discussion
Targeted genetic manipulation of cardiac function may offer a novel therapeutic strategy for patients with ischemic heart disease, serving as destination therapy or as concomitant intervention for patients who undergo cardiac catheterization or surgical procedures for coronary revascularization24,25. The field of gene therapy has been biased toward the development of organ-tropic vectors to achieve these conditions; these mechanisms theoretically address the problem of achieving cardiac cell target specificity while reducing the risks of collateral expression to other organs. However, regardless of tropism, the risk of host immune reactions to the vector capsid is inherently a function of the delivery strategy, since the upper dose limit is frequently challenged when the desired outcome is not obtained. These problems underscore the need for a novel, highly efficient, global, cardiac organ-specific vector for cardiac gene delivery that is also clinically translatable.
In this report, we have demonstrated that a novel synthetic AAV vector, Anc80L65 is an efficient tool for cardiomyocyte gene delivery with an early onset and stable gene expression in both in vitro and in vivo systems, with significant advantages over AAV9 vector. We believe that better transduction capabilities of Anc80L65 based upon the unique characteristics of the capsid of the vector, which preserves the full cloning capacity. This is the first study, to our knowledge, which compares the time course expression of synthetic vector, Anc80L65 and traditional vector, AAV9 in the same experimental heart model.
From the clinical and scientific standpoints, knowledge of transduction efficiency and kinetics of viral vectors in the myocardium is essential26,27. To compare both vectors, we used two different reporter gene approaches, marking by fluorescence and bioluminescence, after intramyocardial and intracoronary route of delivery. At day one and day three after gene delivery, cardiac GFP expression and luciferase bioluminescence was detectable only for Anc80L65. Of note, AAV9 bioluminescence was depicted at day seven after transgene delivery, but it was localized and revealed low-level expression. Moreover, in vivo imaging and qPCR showed that levels of cardiac expression of GFP and viral DNA were higher in Anc80L65 group compared to AAV9. This held true for all time points analyzed. The strong cardiac tropism from Anc80L65 was also confirmed by the highest levels of luciferase activity, subsequent fluorescent microscopy on cardiac tissue cross-sections, and genome copy number found at six weeks post gene delivery. Interestingly, we found a significant increase in GFP expression in right ventricle even though we made an injection only in the lateral wall of left ventricle. This suggests that Anc80L65 provides the ability to obtain a more homogenous expression profile than AAV9. The quantification of viral genome copy number in other tissues such as liver, lung, and kidney, did not reveal a significant Anc80L65 localization in these tissues. This confirms cardiac tropism after intramyocardial injection and is associated with a significantly lower transduction level in the collateral organs compared to AAV9. Taken together these results suggest that Anc80L65 is the vector with the earliest onset of expression and the highest myocardial transduction while causing much less collateral expression compared to AAV9. In addition, intramyocardial delivery of Anc80L65 vector resulted in preferential transduction to cardiomyocytes compared to intracoronary injection. Based on the results, we demonstrated that Anc80L65 is non-immunogenic in rat model. With the amount of Anc80L65 vector particles used in the present study, we observed much lower levels of lymphocyte and neutrophil activation compared to AAV9. Distributions of serum levels of the main inflammatory cytokines did not differ significantly from the baseline. Analysis of transthoracic echocardiographic results demonstrated that global cardiac function had no major differences between the Anc80L65 group and the baseline level.
Limitations.
In the present study, we did not investigate AAV-mediated transgene expression in different cell types besides cardiomyocytes and did not assess CNS expression. We did not include group of animals with combined intramyocardial and intracoronary administration. The study shows valuable information with a single vector dose and non-immunogenic effect of Anc80L65 in rat model. Evaluation of Anc80L65 cardiac tropism in a model with myocardial infarction or ischemia/reperfusion and determining a dose-response relationship warrants further investigation.
Conclusions.
The therapy that offers rapid and efficient expression of gene products in patients with acute and chronic CAD can potentially provide a promising approach to minimizing the damage to the myocardium and preventing negative postinfarction remodeling28. In the present study, we have shown the robust gene expression in cardiac muscle after administration of Anc80L65 vector at different time points, with no functional impairment to the heart and no activation of immune response. Further studies are necessary to evaluate the long-term functional consequences of Anc80L65 administration and potential benefit of repeated Anc80L65 administration in large animal models.
Supplementary Material
Video 1. Intramyocardial and intracoronary administration of AAV-mediated vectors in a rat.
Perspective:
Anc80L65 may be the optimal choice for cardiac gene delivery, because of its efficacy in heart transduction, lowered collateral expression, unique trafficking properties, and its suitability for clinically relevant gene transfer.
Acknowledgements
We would like to acknowledge Anthony S. Fargnoli and Sarah M. Gubara for technical assistance.
Conflict of Interest Statement
Dr Katz and Dr Eliyahu reports grant from the National Heart, Lung, and Blood Institute, and Icahn School of Medicine at Mount Sinai. Dr Eliyahu and Dr Vincek have a pending patent application relevant to the synthetic AAV vector (ID 3710.047P). Dr Vandenberghe (LHV) holds equity in Affinia Therapeutics and Akouos and serves on the Board of Directors of Affinia Therapeutics, Addgene and Odylia. LHV is inventor of AncAAV technology licensed to Affinia, Akouos and/or other biopharmaceutical companies from which they may receive royalties. LHV is an SAB member to Akouos, consultant to Affinia and Novartis and receives research support from Novartis. LHV’s interests were reviewed and are managed by Mass Eye and Ear and Mass General Brigham in accordance with their conflict-of-interest policies. All other authors have nothing to disclose with regard to commercial support.
Abbreviations and Acronyms
- AAV9
adeno-associated virus, serotype 9
- Anc80L65
synthetic adeno-associated virus lineage clone
- RT-qPCR
real-time quantitative polymerase chain reaction
- GFP
green fluorescent protein-reporter that exhibits fluorescence
- Luciferase (Luc)
protein-reporter that produce bioluminescence
- CR
complete revascularization
- IR
incomplete revascularization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 22nd Meeting of American Society of Gene & Cell Therapy, Washington, D.C. May, 2019
All animal procedures were performed under protocols approved by the Icahn School of Medicine at Mount Sinai Institutional Care and Use Committee.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019; 139: e56–528. [DOI] [PubMed] [Google Scholar]
- 2.Torabi A, Cleland JG, Rigby AS, Sherwi N. Development and course of heart failure after a myocardial infarction in younger and older people. J Geriatr Cardiol. 2014; 11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torabi A, Cleland JG, Khan NK, Loh PH, Clark AL, Alamgir F, et al. The timing of development and subsequent clinical course of heart failure after a myocardial infarction. Eur Heart J. 2008; 29: 859–70. [DOI] [PubMed] [Google Scholar]
- 4.Garcia S, Sandoval Y, Roukoz H, Adabag S, Canoniero M, Yannopoulos D, et al. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease. J Am Coll Cardiol. 2013; 62 (16): 1421–31. [DOI] [PubMed] [Google Scholar]
- 5.Leviner DB, Torregrosssa G, Puskas JD. Incomplete revascularization: what the surgeon needs to know. Ann Cardiothorac Surg. 2018; 7: 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Syntax Investigators et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009; 360 (10): 961–72. [DOI] [PubMed] [Google Scholar]
- 7.Katz MG, Gubara SM, Hadas Y, Weber T, Kumar A, Eliyahu E, et al. Effects of genetic transfection on calcium cycling pathways mediated by double-stranded adeno-associated virus in postinfarction remodeling. J Thorac Cardiovasc Surg 2020; 159:1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathison M, Singh VP, Chiuchiolo MJ, Sanagasetti D, Mao Y, Patel VP, et al. In situ reprogramming to transdifferentiate fibroblasts into cardiomyocytes using adenoviral vectors: Implications for clinical myocardial regeneration J Thorac Cardiovasc Surg 2017; 153:329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz MG, Fargnoli AS, Williams RD, Bridges CR. Cell and gene therapies for cardiovascular disease, in Templeton NS (Ed.), Gene and Cell Therapy, CRC Press, New York: 2015, pp.861–901. [Google Scholar]
- 10.Katz MG, Fargnoli AS, Williams RD, Bridges CR. Gene therapy delivery systems for enhancing viral and nonviral vectors for cardiac diseases: current concepts and future applications. Hum Gene Ther. 2013; 24:914–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan D Systemic delivery of adeno-associated viral vectors. Curr Opin Virol.2016; 21:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rangarajan S, Walsh L, Lester W, Perry D, Madan B, Laffan M, et al. AAV5-factor VIII gene transfer in severe hemophilia A. N. Engl. J. Med 2017; 377:2519–2530. [DOI] [PubMed] [Google Scholar]
- 13.Keeler AM, Flotte TR Recombinant adeno-associated virus gene therapy in light of luxturna (and zolgensma and glybera): where are we, and how did we get here? Annu. Rev. Virol 2019; 6:601–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004; 78:6381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE, Zolotukhin I, Tarantal AF, Byrne BJ. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res. 2006; 99:e3–9. [DOI] [PubMed] [Google Scholar]
- 16.Inagaki K, Fuess S, Storm TA, Gibson GA, Mctiernan CF, Kay MA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006; 14:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bish LT, Morine K, Sleeper MM, Sanmiguel J, Wu D, Gao G, et al. Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat. Hum Gene Ther. 2008; 19:1359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orlowski A, Katz MG, Gubara SM, Fargnoli AS, Fish KM, Weber T. Successful transduction with AAV Vectors after selective depletion of anti-AAV antibodies by immunoadsorption. Mol Ther Methods Clin Dev. 2020; 16:192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asokan A, Samulski RJ. An emerging adeno-associated viral vector pipeline for cardiac gene therapy. Hum Gene Ther. 2013; 24: 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zinn E, Pacouret S, Khaychuk V, et al. In silico reconstruction of the viral evolutionary lineage yields a potent gene therapy vector. Cell Rep 2015; 12: 1056–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hudry E, Andres-Mateos E, Lerner EP, Volak A, Cohen O, Hyman BT, et al. Efficient gene transfer to the central nervous system by single-stranded Anc80L65. Mol Ther Methods Clin Dev. 2018; 10:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carvalho LS, Xiao R, Wassmer SJ, Langsdorf A, Zinn E, Pacouret S, et al. Synthetic adeno-associated viral vector efficiently targets mouse and nonhuman primate retina in vivo. Hum Gene Ther. 201; 29:771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz MG, Fargnoli AS, Gubara SM, Chepurko E, Bridges CR, Hajjar RJ. Surgical and physiological challenges in the development of left and right heart failure in rat models. Heart Fail Rev. 2019; 24:759–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sellke FW. Gene therapy in cardiac surgery: is there a role? J Thorac Cardiovasc Surg. 2003; 125:994–997. [DOI] [PubMed] [Google Scholar]
- 25.Katz MG, Fargnoli AS, Kendle AP, Hajjar RJ, Bridges CR. Gene therapy in cardiac surgery: clinical trials, challenges, and perspectives. Ann Thorac Surg. 2016; 101:2407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scimia MC, Gumpert AM, Koch WJ. Cardiovascular gene therapy for myocardial infarction. Expert Opin Biol Ther. 2014; 14:183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lähteenvuo J, Ylä-Herttuala S. Advances and challenges in cardiovascular gene therapy. Hum Gene Ther. 2017; 28:1024–1032. [DOI] [PubMed] [Google Scholar]
- 28.Ylä-Herttuala S, Baker AH. Cardiovascular gene therapy: past, present, and future. Mol Ther. 2017; 25:1095–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Intramyocardial and intracoronary administration of AAV-mediated vectors in a rat.


