ABSTRACT.
Melioidosis is an infection caused by the bacterium Burkholderia pseudomallei. The most common presentation is bacteremia occurring in 38–73% of all patients, and the mortality rate ranges from 9% to 42%. Although there is abundant data representing risk factors for infection and patient outcomes, there is limited information regarding laboratory investigations associated with bacteremia and mortality. We assessed a range of baseline and diagnostic investigations and their association with patient outcomes in a retrospective cohort study in Townsville, Australia. 124 patients’ medical and laboratory records were reviewed between January 1, 1997 and December 31, 2020. Twenty-seven patients died and 87 patients were bacteremic. The presence of lymphopenia (< 1.5 × 109 cells/L) was the highest risk for bacteremia (relative risk [RR] 2.2; 95% CI: 1.3–3.7, P < 0.001). Factors associated with mortality included lymphopenia, (RR: 1.4; 95% CI: 1.2–1.6, P = 0.004); uremia (RR: 1.7; 95% CI: 1.1–2.5, P = 0.03); and an elevated international normalized ratio (RR: 1.5; 95% CI: 1.2–2.0, P = 0.006). Median incubation to positive blood culture result was 28 hours with 15/82 (18%) positive in ≤ 24 hours. For serological testing during admission only 53/121 (44%) were indirect hemagglutination assay positive, 67/120 (56%) enzyme immunoassay IgG positive, and 23/89 (26%) IgM positive. Simple baseline investigations at time of presentation may be used to stratify patients at high risk for both bacteremia and mortality. This information can be used as a decision aid for early intensive management.
INTRODUCTION
Melioidosis is an infection caused by the bacterium Burkholderia pseudomallei, an organism that is endemic to many tropical and subtropical regions including but not limited to south east Asia and northern Australia.1 The most common laboratory feature of infection is bacteremia occurring in 38–73% of all patients. The overall mortality rate of infection has improved over time, however, the upper range has been reported at 42%.1
Risk factors and clinical manifestations of infection have been well documented in relation to clinical outcomes. Comparatively, there is significantly less information in the literature regarding the laboratory investigations performed on these patients at the time of or during admission and their association with clinical outcome. From an hematological perspective these include a neutrophil and lymphocyte count, where neutrophilia and lymphopenia have been associated with increased mortality.2 Biochemical analysis with features suggestive of end organ dysfunction such as uremia or hyperbilirubinaemia have also demonstrated an association with both melioidosis compared with other infections, and also poor clinical outcomes.3–5 Additionally, a scoring system with the aim of predicting mortality was developed using five laboratory and two clinical variables.3 While a score of > 3 had a sensitivity of 81% and a specificity of 67%, it has not been validated on an external dataset.
In terms of diagnostic testing, time from incubation to positive blood culture is associated with the patient’s burden of infection and therefore mortality.6 To date only one study has examined the blood culture incubation time and clinical outcome.6 Finally, serological investigation for the diagnosis of melioidosis was examined in the form of a retrospective cohort review.7 This study analyzed the indirect hemagglutination assay (IHA) and the association with both risk factors and bacteremia.7 Unfortunately, it did not compare these results in relation to mortality, nor were other serological investigations such as the enzyme immunoassay (EIA) assessed.
We assess multiple baseline and diagnostic laboratory-based investigations on presentation or during admission to determine the factors associated with patient outcomes. Additionally, this data was used to assess the utility of the previously proposed scoring system.
METHODS
Townsville University Hospital (TUH) is a 742-bed tertiary referral center in Far North Queensland serving a local population of approximately 195,000 inhabitants.8 All patients admitted to and treated at TUH, 18 years of age or older, with culture-confirmed melioidosis identified between January 1, 1997 and December 31, 2020 were included. Retrospective clinical details, including Aboriginal and Torres Strait Islander (ATSI) status, were obtained from the hospital medical records and laboratory data were extracted from the state-wide electronic database. The follow-up period ranged from 1 day to 4 years, with all documented deaths occurring within 120 days of diagnosis. Disease focus was identified by radiological features and/or site-specific positive cultures where available. Alcohol excess was defined as > 14 units of alcohol per week.9 Chronic kidney disease (CKD) was defined as per the National Kidney Foundation-Kidney Disease Outcomes Quality Initiative guidelines.10 Chronic lung disease was present if diagnosis or treatment of lung disease included in the medical record. Chronic liver disease was present if diagnosis documented in medical record. Immunosuppression was present if the patient was receiving treatment with an immunosuppressing agent including corticosteroids, immunomodulatory therapy including monoclonal antibodies, or chemotherapy. Malignancy included either solid or hematological disease. Septic shock was defined as organ dysfunction requiring vasopressor support and lactate > 2 mmol/L.11 Biochemical investigations were defined as such, lymphopenia (total lymphocyte count < 1.5 × 109 cells/L); neutrophilia (neutrophil count > 8.1 × 109 cells/L); thrombocytosis (platelet count > 400 × 109/L); hyperbilirubinaemia (total bilirubin > 20 µmol/L); uremia (urea > 8.0 mmol/L); low serum bicarbonate < 22 mmol/L; and abnormal international normalized ratio (INR) (INR > 1.1). Serology on presentation was defined as within 5 days of admission. An indirect hemagglutination assay (IHA) for detection of total antibody was performed, and interpretation included titers of ≤ 1:5 considered to be negative; titers of 1:10–1:20 considered borderline; and titers ≥ 1:40 positive.12 An enzyme immunoassay (EIA) specific for detection of IgG and IgM was performed, with results considered positive, borderline, or negative depending on EIA units as defined by Ashdown et al.12 Blood culture time to positivity was defined as the incubation time in hours required for detection by the BacT/ALERT system (bioMérieux, Marcy l’Etoile, France).
Statistical analysis.
Data were analyzed using STATA version 16 statistical software package (StataCorp, College Station, TX). Categorical variables were analyzed using χ2 or Fisher’s exact test. Numerical variables were analyzed using Student’s t test or Mann–Whitney U test.
Ethics.
This study received ethical approval from the Royal Brisbane and Women’s Hospital Ethics Committee LNR/2020/QRBW/65573, with site-specific authority obtained from the Townsville Hospital and Health Service and approval under the Queensland Public Health Act.
RESULTS
In total, 134 patients with culture-confirmed melioidosis were admitted to TUH over the study period. Of these patients only 124 (93%) had both medical records available for analysis, and a combination of baseline hematological, biochemical, or diagnostic investigations available at the time of presentation. Mortality data was missing for one patient. In total, 27/123 (22%) patients died.
There are a number of risk factors associated with infection including, but not limited to, age, ATSI status, immunosuppression, and chronic lung disease.1 Table 1 identifies the risk factors that were associated with abnormal hematological and biochemical markers on presentation. On Admission, 24% of those aged 18–49 years were uremic compared with 44% in the 50–69, and 55% in the over 70 group. The ATSI cohort presented more frequently with neutrophilia (P = 0.02), as did patients with chronic lung disease (P = 0.001). Unsurprisingly, CKD was associated with uremia, which was associated with mortality (P = 0.001). Patients receiving immunosuppressing agents were less likely to be neutrophilic (P = 0.02), but there was no difference in lymphopenia (P = 0.92). Finally, patients without risk factors were less likely to be lymphopenic, 46% versus 80% (P = 0.006) or neutrophilic, 23% versus 70% (P = 0.001).
Table 1.
Hematological and biochemical investigations and their association with clinical risk factors
| N (%) | ||||||
|---|---|---|---|---|---|---|
| Variable | Lymphopenia | Neutrophilia | Uremia | Low bicarbonate | Elevated INR | |
| Age | 18–49 | 25/34 (74) | 26/34 (76) | 8/34 (24) | 17/37 (46) | 18/23 (78) |
| 50–69 | 42/59 (71) | 33/59 (56) | 26/59 (44) | 22/60 (37) | 24/37 (65) | |
| 70–99 | 26/29 (90) | 21/29 (72) | 16/29 (55) | 8/31 (26) | 10/17 (59) | |
| P value | 0.15 | 0.09 | 0.03 * | 0.23 | 0.3 | |
| Sex | Male | 61/77 (79) | 52/77 (65) | 30/77 (39) | 28/82 (34) | 35/49 (71) |
| Female | 32/45 (71) | 28/48 (62) | 20/45 (44) | 19/46 (41) | 17/28 (61) | |
| P value | 0.31 | 0.55 | 0.55 | 0.42 | 0.33 | |
| ATSI | Yes | 26/33 (79) | 27/33 (81) | 12/33 (36) | 15/34 (44) | 16/20 (80) |
| No | 62/80 (78) | 48/80 (60) | 3/80 (46) | 31/81 (38) | 33/54 (61) | |
| P value | 0.88 | 0.02 * | 0.33 | 0.56 | 0.13 | |
| Diabetes mellitus | Yes | 46/58 (79) | 41/58 (71) | 27/58 (47) | 24/59 (41) | 24/37 (65) |
| No | 47/64 (73) | 39/64 (61) | 23/64 (36) | 23/65 (35) | 28/40 (70) | |
| P value | 0.45 | 0.25 | 0.23 | 0.54 | 0.63 | |
| Alcohol excess | Yes | 51/64 (80) | 44/64 (69) | 24/64 (38) | 25/46 (54) | 32/42 (76) |
| No | 39/53 (74) | 33/53 (62) | 24/53 (45) | 21/53 (40) | 19/34 (56) | |
| P value | 0.43 | 0.46 | 0.39 | 0.85 | 0.06 | |
| Chronic kidney disease | Yes | 7/7 (100) | 5/7 (71) | 7/7 (100) | 4/7 (57) | 2/5 (40) |
| No | 78/107 (73) | 68/107 (64) | 38/107 (36) | 40/107 (37) | 48/70 (69) | |
| P value | 0.12 | 1.00 | 0.001 * | 0.29 | 0.33 | |
| Lung disease | Yes | 29/35 (83) | 31/35 (89) | 16/35 (46) | 17/37 (46) | 13/21 (62) |
| No | 64/87 (74) | 49/87 (56) | 34/87 (39) | 30/87 (34) | 39/56 (70) | |
| P value | 0.27 | 0.001 * | 0.50 | 0.23 | 0.52 | |
| Immunosuppression | Yes | 9/12 (75) | 4/12 (33) | 4/12 (33) | 6/12 (50) | 5/8 (63) |
| No | 84/110 (76) | 76/110 (69) | 46/110 (42) | 41/110 (37) | 47/69 (68) | |
| P value | 0.92 | 0.02 * | 0.57 | 0.39 | 0.75 | |
| Malignancy | Yes | 13/16 (81) | 9/16 (56) | 8/16 (50) | 3/16 (19) | 9/12 (75) |
| No | 80/106 (75) | 71/106 (67) | 42/106 (40) | 44/106 (42) | 43/65 (66) | |
| P value | 0.76 | 0.40 | 0.43 | 0.08 | 0.74 | |
| No risk factor† | Yes | 6/13 (46) | 3/13 (23) | 4/13 (31) | 3/13 (23) | 6/10 (60) |
| No | 86/107 (80) | 75/107 (70) | 45/107 (42) | 42/112 (38) | 44/65 (68) | |
| P value | 0.006 * | 0.001 * | 0.43 | 0.30 | 0.63 | |
ATSI = Aboriginal and Torres Strait Islander; INR = international normalized ratio.
Denotes statistical significance.
Excludes age and sex.
Of the seven categorical hematological and biochemical variables analyzed, four had a significant association with mortality (Table 2). Lymphopenia on presentation was associated with increased mortality (relative risk [RR] 1.4; 95% CI: 1.2–1.6, P = 0.004). Additional factors associated with a higher mortality include uremia (RR: 1.7; 95% CI: 1.1–2.5, P = 0.03); low bicarbonate (RR: 2.2; 95% CI: 1.4–3.3, P < 0.001); and an elevated INR (RR: 1.5; 95% CI: 1.2–2.0, P = 0.006).
Table 2.
Categorical hematological and biochemical investigation on presentation and association with bacteremia and mortality
| Variable | N (%) | Relative risk | P value | Death | Relative risk | P value | ||
|---|---|---|---|---|---|---|---|---|
| Total | Bacteremia | (95% CI) | N (%) | (95% CI) | ||||
| Lymphopenia | Yes | 90/119 (75) | 73/92 (80) | 2.2 (1.3–3.7) | < 0.001 | 26/90 (29) | 1.4 (1.2–1.6) | 0.004 |
| No | 29/119 (25) | 10/28 (36) | 1/29 (3) | |||||
| Neutrophilia | Yes | 77/119 (65) | 60/79 (76) | 1.3 (1.0–1.8) | 0.02 | 20/77 (26) | 1.2 (0.9–1.6) | 0.25 |
| No | 42/119 (35) | 23/41 (56) | 7/42 (17) | |||||
| Thrombocytosis | Yes | 13/119 (11) | 6/13 (46) | 0.6 (0.4–1.1) | 0.06 | 3/13 (23) | 1.0 (0.3–3.4) | 0.97 |
| No | 106/119 (89) | 77/107 (72) | 24/106 (23) | |||||
| Uremia | Yes | 49/119 (41) | 40/49 (82) | 1.3 (1.1–1.7) | 0.01 | 16/49 (33) | 1.7 (1.1–2.5) | 0.03 |
| No | 70/119 (59) | 43/71 (61) | 11/70 (16) | |||||
| Hyperbilirubinaemia | Yes | 37/119 (31) | 28/37 (76) | 1.3 (0.7–2.3) | 0.28 | 10/37 (27) | 1.3 (0.–2.3) | 0.45 |
| No | 82/119 (69) | 54/82 (66) | 17/82 (21) | |||||
| Low Bicarbonate | Yes | 46/119 (39) | 38/46 (83) | 2.0 (1.0–3.9) | 0.02 | 18/46 (39) | 2.2 (1.5–3.3) | 0.001 |
| No | 73/119 (61) | 49/78 (63) | 9/73 (12) | |||||
| Yes | 52/77 (68) | 40/51 (78) | 1.4 (1.0–2.0) | 0.04 | 20/52 (38) | 1.5 (1.2–2.0) | 0.006 | |
| No | 25/77 (32) | 14/25 (56) | 2/25 (8) | |||||
Although categorical variables are somewhat easier to interpret in a clinical setting, using the reported value of each test performed is relevant when assessing these associations with either bacteremia or mortality. For example, bacteremic patients had a median lymphocyte count of 0.75 × 109 cells/L (95% CI: 0.4–1.1) as compared with nonbacteremic patients 1.5 × 109 cells/L (95% CI: 1–1.9, P < 0.001), Table 3. This form of analysis is also useful when reviewing platelet counts as there is a clear difference in median platelet count and bacteremia, although neither are in the thrombocytosis or thrombocytopenic range, 207 versus 285 × 109/L, P < 0.001. Similarly, a higher neutrophil count may assist in distinguishing patients with neutrophilia and mortality risk, 15.2 versus 9.5 × 109/L, P = 0.05.
Table 3.
Hematological and biochemical results on admission and association with bacteremia and mortality
| Variable median (IQR) | Bacteremia | Dead | ||||
|---|---|---|---|---|---|---|
| Yes (N = 87) | No (N = 37) | P value | Yes (N = 27) | No (N = 92) | P value | |
| Lymphocytes, × 109 cells/L | 0.75 (0.4–1.1) | 1.5 (1–1.9) | < 0.001 | 0.5 (0.2–0.8) | 1.1 (0.7–1.6) | < 0.001 |
| Neutrophils, × 109 cells/L | 12.1 (8.0–18.1) | 8.7 (5.1–12.4) | 0.06 | 15.2 (8.1–19.0) | 9.5 (6.3–14.6) | 0.05 |
| Platelets, × 109/L | 207 (148–297) | 285 (228–394) | < 0.001 | 190 (131–320) | 237 (169–324) | 0.3 |
| Bilirubin, µmol/L | 17 (11–25) | 13 (10–20) | 0.15 | 19 (12–30) | 14 (10–24) | 0.2 |
| Urea, mmol/L | 8.0 (5.6–12.6) | 5.8 (4.0–7.9) | < 0.001 | 10 (7.2–14.6) | 6.4 (4.1–10.3) | 0.002 |
| Bicarbonate, mmol/L | 22 (18–23) | 25 (22–28) | < 0.001 | 18 (13–23) | 23 (21–26) | 0.002 |
| INR | 1.3 (1.1–1.6) | 1.1 (1.0–1.3) | 0.005 | 1.4 (1.3–1.8) | 1.2 (1.1–1.4) | < 0.001 |
INR = international normalized ratio; IQR = interquartile range.
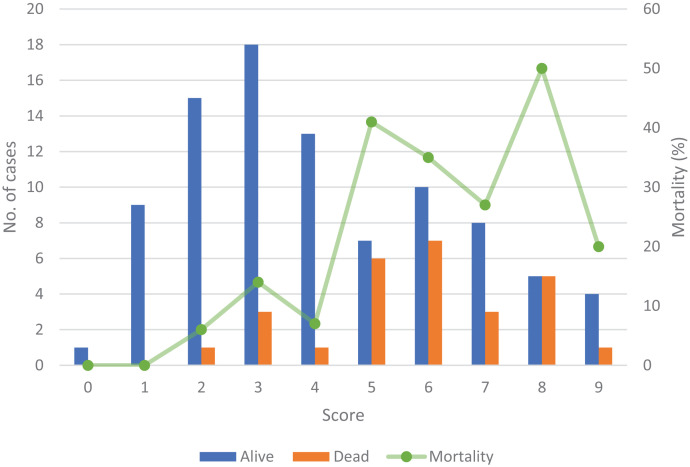
In assessing the predictive mortality score, 72/119 (61%) of the patients in our cohort achieved a score of > 3. Of these patients, 23/72 (32%) died as compared with only 4/47 (9%) in those patients with a score ≤ 3, (RR: 1.6; 95% CI: 1.2–2.0, P = 0.004), Figure 1. Of the 27 patients who died 23/27 (i.e., 85% sensitivity) presented with a score > 3 on admission. The specificity was 47%, with a positive predictive value of 32% and negative predictive value of 91%.
Figure 1.
Scoring system results derived from Cheng et al. applied to the Townsville patient cohort. This figure appears in color at www.ajtmh.org.
In our cohort, 87/124 (70%) patients who had a blood culture performed were bacteremic. Of these, 82/87 (94%) had a documented time from incubation to positive result with a median of 28 hours (IQR: 25–35) and 15/82 (18%) cultures were positive in less than or 24 hours. The hematological and biochemical factors associated with bacteremia are listed in Table 2. Of these, the highest risk for bacteremia was lymphopenia (RR: 2.2; 95% CI: 1.3–3.7, P < 0.001). When reviewing the hours of incubation required for the automated instrument to register a positive blood culture, the time to positivity was not affected by age, sex, ATSI status, nor any risk factors for infection assessed in this study. With regard to categorical hematological variables there was a decreased time to positivity in patients with lymphopenia of 30 hours versus 36 hours in nonlymphopenic patients. Although this delay was not statistically significant, P = 0.08. Neutrophilia was associated with greater time to positive blood culture 32 versus 27 hours, P = 0.03, Table 4.
Table 4.
Time to positive blood culture and associated factors
| Variable | Hours, median (IQR) | P value | |
|---|---|---|---|
| Pneumonia | Yes | 27.7 (25.0–32.3) | 0.05 |
| No | 31.5 (26.5–38.2) | ||
| ICU admission | Yes | 25.0 (23.0–28.0) | 0.001 |
| No | 31.0 (26.3–38.0) | ||
| Septic shock | Yes | 24.7 (22.5–27.9) | 0.002 |
| No | 30.0 (26.3–35.0) | ||
| Ventilated | Yes | 24.7 (22.5–27.5) | 0.002 |
| No | 30.0 (26.3–35.0) | ||
| Dialysis | Yes | 24.2 (21.0–28.0) | 0.03 |
| No | 30.0 (26.0–35.0) | ||
| Neutrophilia | Yes | 29.7 (26.0–35.0) | 0.03 |
| No | 26.7 (23.0–31.0) | ||
| Dead | Yes | 26.1 (24.0–31.0) | 0.06 |
| No | 28.3 (26.0–35.0) | ||
ICU = intensive care unit; IQR = interquartile range.
For serological analysis, an IHA was performed in 121/124 (98%), an IgG EIA in 120/124 (97%), and an IgM in 89/124 (72%) patients. Only 53/121 (44%) patients were IHA positive, 67/120 (56%) EIA IgG positive, and 23/89 (26%) IgM positive. Combining IHA and EIA IgG an additional 21 patients who were IHA negative were positive by EIA IgG. Therefore, 74/121 (61%) of patients were positive by either test. The addition of an EIA IgM was of limited benefit with 2/121 (2%) additional positive results when either the IHA or EIA IgG was negative. Evaluating only positive and negative serological results in relation to risk factors for infection, patients with diabetes were more likely to present with a positive EIA IgG, 37/52 (71%), P = 0.02 (Table 5). Septic arthritis (100%) and pneumonia (53%) were both more likely to induce a positive IgG, P = 0.02. Although the septic arthritis EIA results are identical via IHA, pneumonia (37%) was not associated with a positive IHA result, P = 0.04. Skin and soft tissue infection was most likely to present with a positive IgM (58%), P = 0.04. All other variables were more likely to be associated with negative EIA serological results. Additionally, when comparing all three interpretive categories and mortality, a negative result was strongly associated with mortality for both a negative IgM (RR: 1.5; 95% CI: 1.2–1.9) and IgG (RR: 1.4; 95% CI: 1.1–1.9, P < 0.001) (Table 6).
Table 5.
Variables associated with serological results
| Variable | EIA IgM | P value | EIA IgG | P value | IHA | P value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | |||||
| Risk factor | ||||||||||
| Diabetes | Yes | 12/37 (32) | 25/37 (68) | 0.78 | 37/52 (71) | 15/52 (29) | 0.02 | 26/48 (54) | 22/48 (46) | 0.4 |
| No | 10/34 (29) | 24/34 (71) | 28/57 (49) | 29/57 (51) | 25/53 (47) | 28/53 (53) | ||||
| Lung diseases | Yes | 2/23 (9) | 21/23 (91) | 0.006 | 15/33 (45) | 18/33 (55) | 0.05 | 9/29 (31) | 20/29 (69) | 0.02 |
| No | 20/48 (42) | 28/48 (58) | 49/75 (65) | 26/75 (35) | 41/70 (59) | 29/70 (41) | ||||
| Disease focus | ||||||||||
| Bacteremia | Yes | 13/50 (26) | 37/50 (74) | 0.07 | 41/75 (55) | 34/75 (45) | 0.07 | 28/71 (39) | 43/71 (61) | 0.001 |
| No | 10/21 (48) | 11/21 (52) | 24/33 (73) | 9/33 (27) | 23/29 (79) | 6/29 (21) | ||||
| Pneumonia | Yes | 9/51 (18) | 42/51 (82) | < 0.001 | 40/76 (53) | 36/76 (47) | 0.02 | 29/67 (43) | 38/67 (57) | 0.04 |
| No | 13/19 (68) | 6/19 (32) | 24/31 (77) | 7/31 (23) | 21/31 (68) | 10/31 (32) | ||||
| Skin and soft tissue | Yes | 7/12 (58) | 5/12 (42) | 0.04 | 12/15(80) | 3/15 (20) | 0.09 | 10/14 (71) | 4/10 (29) | 0.09 |
| No | 15/57 (26) | 42/57 (74) | – | 51/91 (56) | 40/91 (44) | – | 40/84 (48) | 44/84 (52) | – | |
| Septic arthritis | Yes | 3/4 (75) | 1/4 (25) | 0.09 | 8/8 (100) | 0 | 0.02 | 8/8 (100) | 0 | 0.001 |
| No | 19/65 (29) | 46/65 (71) | 54/97 (56) | 43/97 (44) | 41/89 (46) | 47/89 (54) | ||||
| Severity | ||||||||||
| ICU admission | Yes | 2/20 (10) | 18/20 (90) | 0.01 | 10/27 (37) | 17/27 (63) | 0.005 | 8/26 (31) | 18/26 (69) | 0.06 |
| No | 20/45 (44) | 25/45 (56) | 51/75 (68) | 24/75 (32) | 42/71 (59) | 29/71 (41) | ||||
| Septic shock | Yes | 2/16 (12) | 14/16 (88) | 0.02 | 9/23 (39) | 14/23 (61) | 0.02 | 7/22 (32) | 15/22 (68) | 0.09 |
| No | 20/48 (42) | 28/48 (58) | 51/77 (66) | 26/77 (34) | 43/74 (58) | 31/74 (42) | ||||
| Ventilator | Yes | 2/17 (12) | 15/17 (88) | 0.03 | 8/23 (35) | 15/23 (65) | 0.005 | 7/22 (32) | 15/22 (68) | 0.09 |
| No | 20/47 (43) | 27/477 (57) | 52/77 (68) | 25/77 (32) | 43/74 (58) | 31/74 (42) | ||||
| Biochemistry | ||||||||||
| Lymphopenia | Yes | 15/54 (28) | 39/54 (72) | 0.29 | 47/85 (55) | 38/85 (45) | 0.11 | 33/75 (44) | 42/75 (56) | 0.02 |
| No | 7/12 (41) | 10/17 (59) | 17/23 (74) | 6/23 (26) | 17/24 (71) | 7/24 (29) | ||||
| Neutrophilia | Yes | 11/49 (22) | 38/49 (78) | 0.02 | 40/72 (56) | 32/72 (44) | 0.27 | 28/65 (43) | 37/65 (57) | 0.05 |
| No | 11/22 (50 | 11/22 (50) | 24/36 (67) | 12/36 (33) | 22/34 (65) | 12/34 (35) | ||||
| Low bicarbonate | Yes | 4/35 (11) | 31/35 (89) | 0.01 | 17/45 (38) | 28/45 (62) | 0.002 | 10/46 (22) | 36/46 (78) | < 0.001 |
| No | 19/54 (35) | 35/54 (65) | 50/75 (67) | 25/75 (33) | 43/75 (57) | 32/75 (43) | ||||
EIA = enzyme immunoassay; ICU = intensive care unit; IHA = indirect hemagglutination assay.
Table 6.
Serology and association with mortality
| Test | N (%) | Relative risk | P value | ||
|---|---|---|---|---|---|
| Total | Deaths | RR (95% CI) | |||
| EIA IgM | Positive | 22/83 (26) | 2/22 (9) | Reference | < 0.001 |
| Equivocal | 14/83 (17) | 0/14 (0) | NA | ||
| Negative | 47/83 (57) | 18/47 (38) | 1.5 (1.2–1.9) | ||
| EIA IgG | Positive | 62/114 (54) | 8/62 (13) | Reference | < 0.001 |
| Equivocal | 9/114 (8) | 1/9 (11) | N/A | ||
| Negative | 43/114 (38) | 17/43 (40) | 1.4 (1.1–1.9) | ||
| IHA | Positive | 50/114 (44) | 3/50 (6) | Reference | < 0.001 |
| Borderline | 17/114 (15) | 7/17 (41) | N/A | ||
| Negative | 47/114 (41) | 15/47 (32) | 1.4 (1.2–1.7) | ||
EIA = enzyme immunoassay; IHA = indirect hemagglutination assay; NA = not applicable.
DISCUSSION
The objective of this study was to analyze and summarize multiple laboratory-based investigations from melioidosis patients at the time of presentation, with the aim of assessing their association with poor patient outcomes. This information may then aid clinicians in early recognition of patients at risk for premature death.
There were a number of risk factors with significant associations to abnormal hematological or biochemical investigations. Although age was associated with an increased risk of uremia, ATSI status with neutrophilia, and having no risk factor was associated with both a normal lymphocyte and neutrophil count, none of these factors were associated with increased mortality. However, the presence of lymphopenia on admission irrespective of risk factor was unequivocally associated with both bacteremia and poor patient outcomes. This is concordant with previous studies that demonstrated that a component of the B. pseudomallei cell wall, lipopolysaccharide, may have an endotoxin effect resulting in lymphopenia.13 Furthermore, patients with melioidosis are able to make B. pseudomallei–specific lymphocytes, with lower concentrations found in those patients that succumbed to infection.2 When stratifying for neutrophilia there appeared to be no difference in mortality between patients with a normal or elevated neutrophil count. However, this was not true for mean neutrophil count. Similar to previous data, patients in our cohort with a higher neutrophil count were more likely to die.2 This potentially excessive neutrophil activation represents a hematopoietic stem cell shift from lymphoid to myeloid lineage, and B. pseudomallei–infected neutrophils are able to inhibit T-cell proliferation and interferon-gamma production, therefore, limiting host immune response.14 Similar to neutrophil count the total platelet count was more relevant than an abnormal level. In keeping with recently published research a higher platelet count is associated with a decreased risk of mortality.15 It is important to note the average reported platelet count in that study was 216 × 109/L in those patients who died and 256 × 109/L in those who survived. Similar to this study, that data may be of limited value to a clinician. It may be easier to use thrombocytopenia (< 150 × 109/L) as a marker of severity; however, this appears to be a rare event in our cohort.16
Chronic kidney disease is both a risk factor for infection and increased mortality. While history of CKD on admission was not associated with increased mortality, the presence of uremia was associated with an almost 2-fold increased risk of death, which is similar to that reported by Domthong et al.5 Elevated bilirubin levels have been reported as a factor associated with mortality in two studies and biologically could be viewed as a marker of hepatic dysfunction; however, these results were not reproduced in our dataset.3,5 The proposed scoring system by Cheng et al. was a useful tool in this cohort, demonstrating 32% mortality in those patients with a score > 3 compared with 9% with a score ≤ 3. Unfortunately, low specificity and positive predictive value suggest that this score requires optimization, potentially with additional risk factors or biochemical markers.
In our cohort, 87 patients were bacteremic on presentation. The median incubation time to a positive result of 28 hours, with 82% occurring > 24 hours, suggests that a majority of these patients had a low concentration of organism in blood at the time of collection.17 This is in contrast to a mean incubation time of 24 hours with 62% of positive results occurring within 24 hours in Thailand.6 This discrepancy in incubation time between regions may be one factor underlying the greater burden of mortality in Thailand, perhaps suggesting patients in that region present later in the disease process or have a greater bacterial load at the time of presentation. The variable demonstrating greatest association with bacteremia was lymphopenia, with those patients possessing a greater than 2-fold risk compared with those with a normal or elevated lymphocyte count. This information would be of significant clinical utility as it may enable stratification of patients by likelihood of bacteremia 21–38 hours earlier.18 Because of the association with increased mortality in bacteremic patients, in epidemiologically relevant regions a patient presenting with lymphopenia on admission may prompt early intensive management.
Serology remains a poor method for diagnosis of an acute melioidosis infection, with only 61% of patients positive for either IHA or EIA IgG. The use of the EIA IgM appears to be of limited clinical utility in this setting, which is discordant with results from two IgM assays assessed in Thailand with reported sensitivities of 75% and 88%, although only 16 melioidosis patients were included in this analysis.19 One reason for this low positive rate may be in relation to the number of patients presenting with pneumonia 84/121 (69%), possibly suggesting acuity of infection and therefore decreased time for antibody formation before presentation. Additionally, 76% of our cohort presented with lymphopenia that may also contribute to these serological results.
There are a number of limitations in this study. As the data were collected retrospectively not all risk factor variables were verifiable. The follow-up period for patients was not consistent and therefore deaths occurring after 120 days may have been missed. However, this is unlikely to account for a significant proportion of mortality. The serological testing was not uniform as not all samples were taken on the same day of admission, and therefore may affect the number of positive results. Multivariable regression analysis was not performed because of the limited bacteremia-free and morality rates. Finally, this is a small cohort study, and ideally should be repeated with a larger dataset and compared across multiple regions for greater generalizability.
CONCLUSION
Melioidosis is associated with a high mortality rate. This study demonstrates that a number of routine laboratory investigations including lymphocyte count, serum urea, serum bicarbonate, and INR may be used to stratify patients at high risk for bacteremia and mortality. Ultimately, this may lead to early recognition of illness severity and improve patient outcomes.
REFERENCES
- 1.Gassiep I, Armstrong M, Norton R, 2020. Human melioidosis. Clin Microbiol Rev 33: e00006–e00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenjaroen K. et al. , 2015. T-cell responses are associated with survival in acute melioidosis patients. PLoS Negl Trop Dis 9: e0004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng AC, Jacups SP, Anstey NM, Currie BJ, 2003. A proposed scoring system for predicting mortality in melioidosis. Trans R Soc Trop Med Hyg 97: 577–581. [DOI] [PubMed] [Google Scholar]
- 4.Chaowagul W, White NJ, Dance DAB, Wattanagoon Y, Naigowit P, Davis TME, Looareesuwan S, Pitakwatchara N, 1989. Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J Infect Dis 159: 890–899. [DOI] [PubMed] [Google Scholar]
- 5.Domthong P, Chaisuksant S, Sawanyawisuth K, 2016. What clinical factors are associated with mortality in septicemic melioidosis? A report from an endemic area. J Infect Dev Ctries 10: 404–409. [DOI] [PubMed] [Google Scholar]
- 6.Tiangpitayakorn C, Songsivilai S, Piyasangthong N, Dharakul T, 1997. Speed of detection of Burkholderia pseudomallei in blood cultures and its correlation with the clinical outcome. Am J Trop Med Hyg 57: 96–99. [DOI] [PubMed] [Google Scholar]
- 7.Harris PN, Ketheesan N, Owens L, Norton RE, 2009. Clinical features that affect indirect-hemagglutination-assay responses to Burkholderia pseudomallei. Clin Vaccine Immunol 16: 924–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Australian Bureau of Statistics , 2016. 2016 Census. Available at: https://www.abs.gov.au/census. Accessed March 9, 2021.
- 9. Australian Research Council and Universities Australia , 2020. Australian Guidelines to Reduce Health Risks from Drinking Alcohol. Commonwealth of Australia, Canberra: National Health and Medical Research Council. [Google Scholar]
- 10.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI, 2014. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63: 713–735. [DOI] [PubMed] [Google Scholar]
- 11.Singer M. et al. , 2016. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashdown LR, Johnson RW, Koehler JM, Cooney CA, 1989. Enzyme-linked immunosorbent assay for the diagnosis of clinical and subclinical melioidosis. J Infect Dis 160: 253–260. [DOI] [PubMed] [Google Scholar]
- 13.Ramsay S, Ketheesan N, Norton R, Watson AM, LaBrooy J, 2002. Peripheral blood lymphocyte subsets in acute human melioidosis. Eur J Clin Microbiol Infect Dis 21: 566–568. [DOI] [PubMed] [Google Scholar]
- 14.Buddhisa S, Rinchai D, Ato M, Bancroft GJ, Lertmemongkolchai G, 2015. Programmed death ligand 1 on Burkholderia pseudomallei–infected human polymorphonuclear neutrophils impairs T cell functions. J Immunol 194: 4413–4421. [DOI] [PubMed] [Google Scholar]
- 15.Kirby P, Smith S, Ward L, Hanson J, Currie BJ, 2019. Clinical utility of platelet count as a prognostic marker for melioidosis. Am J Trop Med Hyg 100: 1085–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birnie E. et al. , 2019. Thrombocytopenia impairs host defense against Burkholderia pseudomallei (Melioidosis). J Infect Dis 219: 648–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh AL, Smith MD, Wuthiekanun V, Suputtamongkol Y, Chaowagul W, Dance DA, Angus B, White NJ, 1995. Prognostic significance of quantitative bacteremia in septicemic melioidosis. Clin Infect Dis 21: 1498–1500. [DOI] [PubMed] [Google Scholar]
- 18.Russell CD, Parajuli A, Gale HJ, Bulteel NS, Schuetz P, de Jager CPC, Loonen AJM, Merekoulias GI, Baillie JK, 2019. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: a systematic review and meta-analysis. J Infect 78: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunakorn M, Boonma P, Khupulsup K, Petchclai B, 1990. Enzyme-linked immunosorbent assay for immunoglobulin M specific antibody for the diagnosis of melioidosis. J Clin Microbiol 28: 1249–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]