ABSTRACT.
Although rare in Portugal, snakebite envenoming entails severe morbidity and mortality. We present the case of a 65-year-old woman bitten on her leg in a northern coastal region in Portugal, on a walk during the COVID-19 pandemic lockdown. Despite first looking for help at the nearest pharmacy, she developed anaphylactoid shock and was promptly driven to a tertiary hospital, where antivenom was administered in a timely manner under close monitoring. Prophylactic antibiotics were started and maintained based on elevated inflammatory markers and signs of wound inflammation. She evolved favorably, with rapid weaning of vasopressors and resolution of end-organ dysfunction. This case highlights the importance of prompt recognition and describes crucial steps in envenomation management in a country where snakebite is infrequent, but potentially fatal.
INTRODUCTION
Snakebite envenoming is a public health issue in tropical and subtropical climates in developing countries. In 2019, the WHO launched a global strategy on snakebite envenoming aimed at halving snakebite deaths and disabilities by 2030. However, just months later, restrictions imposed as a result of the ongoing COVID-19 pandemic have paused all projects. Unfortunately, against the backdrop of COVID-19, snakebites are still causing deaths and disabilities, and doctors should be aware of their clinical presentation and proper management.
Although less relevant from an epidemiological standpoint in Europe, snakebite is still an important cause of morbimortality. It is associated frequently with unpredictable complications such as tissue damage, coagulopathy and hemorrhage, kidney injury, paralysis, myocardial damage, and arrythmias.1,2 European vipers are the main culprits, with differences in geographic species distribution.2
There are two viper species mainly responsible for snakebite envenoming in Portugal: Vipera latastei and Vipera seoanei.3 Vipera latastei is a member of the Vipera genus, which includes other European vipers of major medical relevance, with which it shares morphological characteristics. It is responsible for 4% (n = 125) of snakebites, as noted in a systematic review of Vipera snakebites in Europe.4 Despite V. latastei being a species of major medical importance, its venom composition is not yet known.5
In Portugal, the National Poison Center (Centro de Informação Antivenenos), a part of the National Emergency Institute [Instituto Nacional de Emergência Médica (INEM I.P.)], provides support in the pre-hospital emergency setting. Seventy-five cases of viper bite have been recorded from 2017 through 2020, out of a total of 351 cases of snakebites (unpublished data, courtesy of INEM I.P.). There are no data available on clinical manifestations, severity, or mortality. The absence of reliable and systematic data collection, encompassing pre-hospital and in-hospital care, represents an obstacle to clinical awareness, optimized and standardized treatment, and hinders the prevention of such occurrence. This report illustrates an infrequent complication of snakebite envenoming: anaphylactoid shock with angioedema and its management.
CASE REPORT
A 65-year-old woman was admitted to our hospital center in May 2020. She had no history of allergies and had her latest tetanus immunization in 2007.
During a walk along a coastal pathway in the north of Portugal, near Póvoa de Varzim, the patient was bitten by a snake on the anteromedial portion of the left ankle, leading to sudden intense pain and profuse sweating. She was not able to identify the snake, and quickly developed incoercible vomiting, diarrhea, and periorbital and labial edema. At medical evaluation at the nearest hospital, she was obtunded with no focal deficits, afebrile, hemodynamically stable with low oxygen saturation with no signs of respiratory distress or relevant stetho-acoustic findings. Four fang marks were found on her left ankle, no larger than 5 mm each, with ecchymosis and local edema. Standard wound cleaning management was performed, and intravenous fluids and antiemetics were administered. Despite such measures, 2 hours after the snakebite, the patient maintained incoercible vomiting and worsening edema, and developed shock. Intravenous clemastine and hydrocortisone, followed by intramuscular epinephrine, showed no improvement, motivating vasopressor support with norepinephrine.
Initial blood tests showed metabolic and respiratory acidosis (pH, 7.29) with an elevated anion gap (17 mEq/L) and hyperlactatemia (2.1 mmol/L), leukocytosis (15,990 leukocytes/µL), neutrophilia (9,010 neutrophils/µL), and moderate eosinophilia (1,100 eosinophils/µL) with negative C-reactive protein; acute kidney injury [creatinine, 1.03 mg/dL (previously, 0.56 mg/dL in January 2020); and urea, 44.4 mg/dL], with mild hypokalemia (3.3 mmol/L) and microhematuria in urinalysis. No cytopenia, abnormal hepatic, muscle enzyme, or elevated bilirubin levels were found. D-dimers were elevated [2,650 ng/mL (< 500)], with normal prothrombin time, activated partial thromboplastin time, and international normalized ratio.
The patient was transferred to our hospital center (a tertiary reference center) and was admitted to the intensive care unit for further treatment with antivenom. On admission, the airway was patent, without respiratory distress signs under supplemental oxygen through nasal cannula. The patient was under norepinephrine support, with sinus tachycardia and adequate urine output. Neurologically, the patient was obtunded, with mydriatic pupils and no focal deficits. Progression of edema, affecting the lower third of her left leg, with larger ecchymosis but no signs of compartment syndrome, were noted.
Blood work 5 hours after being bitten revealed worsening leukocytosis (27,290 leukocytes/µL) and neutrophilia (23,930 neutrophils/µL), resolution of eosinophilia, and high procalcitonin levels (4.48 ng/mL; 0–0.050 ng/mL). There was not any other significant change regarding the initial results.
Antivenom (ViperFav®, Sanofi-Pasteur MSD, France) was administered approximately 6 hours after the snakebite and under close monitoring. The patient was started on prophylactic ceftriaxone and clindamycin, which were further maintained based on elevated inflammatory markers and wound inflammatory signs. Tetanus toxoid vaccine was administered in a timely manner.
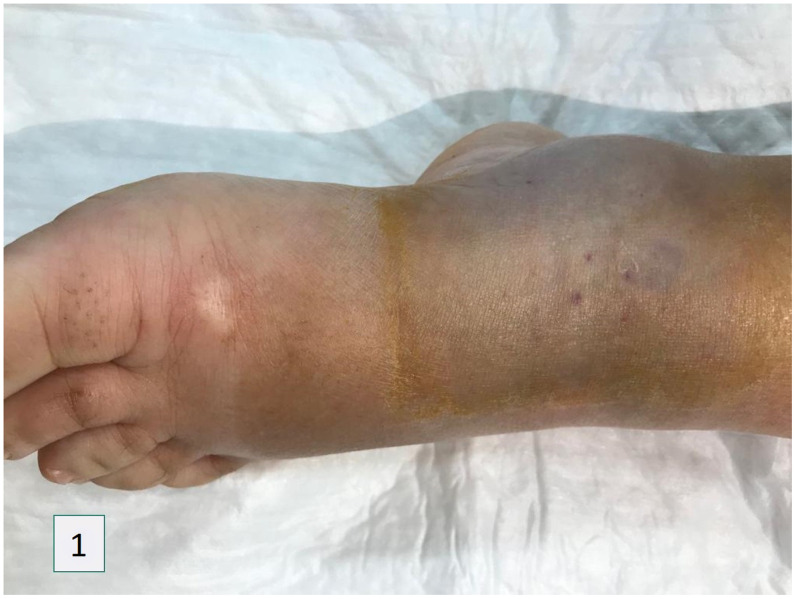
The patient evolved favorably in the ensuing hours, with rapid weaning of vasopressor support and improvement in clinical condition, and resolution of end-organ dysfunctions. In the following 24 hours, local hematoma developed, with no signs of deep vein thrombosis (Figure 1). Beyond this point, there was progressive clinical improvement in the extension of inflammatory markers. The patient was discharged on day 4, under oral antibiotic therapy, with ecchymosis and hematoma reabsorption and resolution of cutaneous inflammatory signs.
Figure 1.
Edema and hematoma 12 hours post-anti-venom and 18 hours post-bite. This figure appears in color at www.ajtmh.org.
DISCUSSION
We present a case of anaphylactoid shock in the context of a grade 3 severe systemic envenomation (clinical grading system proposed by Boels et al.6): systemic manifestations (gastrointestinal and anaphylactoid shock) and regional edema, plus laboratorial data compatible with viper bite envenoming—namely, V. latastei.
Hypotension is a frequent symptom associated with European viper bite envenoming, affecting up to 55.2% of patients, and shock may be present in 29.1% of patients.4 The hypotensive effects of the Vipera venom seems to be the result of the action of venom components, such as vascular endothelial growth factor-like heparin-binding dimeric hypotensive factor.7
Although uncommon, anaphylactoid reactions can occur in up to 5.3% of European viper bite cases,4 possibly mediated by kallikreins and bradykinins.8 Classic anaphylactic reactions (IgE mediated) can occur, but mostly in patients with a history of previous venom exposure.8 Anaphylactoid reactions are also described in the specific context of V. latastei.9 Hydrocortisone, clemastine, and intramuscular epinephrine were administered based on worsening labial and periorbital edema, respiratory distress, and hypotension. Despite this, the patient maintained distributive shock, which is associated with worse outcome rates.10
The prompt administration of antivenom is indicated in grades 2 and 3 of envenoming and is considered the gold standard of treatment.6,10 In Portugal, the antivenom available in reference centers is ViperFav®. It consists of purified equine immunoglobulin fragments F(ab')2 anti- venom for European vipers (V. aspis, V. berus, and V. ammodytes), not specifically for V. latastei, but interspecies cross-reactivity seems to guarantee effectiveness in V. latastei envenoming.11,12 Favorable clinical response to Viperfav® in V. latastei bites has been reported previously in Portugal.13 It is administered intravenously, without weight adjustment and under medical monitorization.14
Although it can still be beneficial up until 48 hours after the injury,6 when administered during the first 10 hours after the snakebite, it is associated with rapid resolution of systemic symptoms and critical laboratory markers, and a reduction in complication rates, functional disability, and duration of hospitalization.6 A single dose is enough, with no significant improvement with additional administration.7,11,15,16 In this case, the patient received antivenom in a timely manner, under medical supervision, and without any of the reported side effects that can occur in 1.5%—mainly, immediate or delayed hypersensitivity reactions—even though the patient presented with anaphylactoid shock.
The patient had notable leukocytosis, which is commonly associated with systemic snakebite envenoming.6 The risk of snakebite-associated infection is variable in different regions, but it is low overall, and empirical antibiotic therapy is not indicated routinely.6,16 The risk is greater in South America, Southeast Asia, and Africa, ranging from 10% to 53%.17,18 In Europe, its frequency is much lower, occurring in less than 2% of cases.6,10,16 Prophylactic antibiotics are often prescribed, despite not being associated with functional improvement or reduced hospitalization.6
Some authors advocate the use of prophylactic antibiotics limited to patients with severe local signs of envenoming, and empirical antibiotics limited to those who have local or systemic signs of infection, regardless of the severity of envenoming.18 When started, empirical antibiotics should be tailored to the potential bacteria that are present in the snake mouth flora, which includes anaerobes but also Gram-negative rods,17,18 as was done in our patient. In addition, because of an increase in inflammatory markers, sustained local edema, and hematoma, empirical antibiotics were maintained for 8 days, with progressive clinical and analytical improvement. Concerning tetanus prophylaxis, because the patient had more than three doses lifelong, only the tetanus toxoid vaccine was administered.
Although mild and self-limited, the patient developed acute kidney injury associated with the envenoming. Kidney toxicity can result from a variety of mechanisms, including ischemic damage secondary to hemodynamic instability, direct damage to the glomerular membrane by venom metalloproteinases, thrombotic microangiopathy, direct tubular cytotoxicity, and tubular obstruction by myoglobin in the setting of significant rhabdomyolysis.19
Thrombotic phenomena can occur, probably as a result of venom metalloproteinase-induced endothelial dysfunction.1 Ecchymoses and hematomas are reported frequently.6,16 In our patient, there was no significant coagulopathy, and hematoma may have resulted from a local cytotoxic venom effect on the skin, soft tissues, and microvasculature.
There was favorable response to antivenom, with resolution of shock and additional organ dysfunctions, and there were none of the side effects reported in other studies.6,11,15 Timely clinical response and antivenom administration were important to prevent progression of organ dysfunction.
CONCLUSION
Snakebite envenoming is a rare occurrence in Portugal. We believe that sharing this case of anaphylactoid shock after envenoming highlights the need for proper training for early recognition and timely administration of antivenom therapy alongside end-organ support therapies, changing the course of such rare but possibly fatal occurrences. Furthermore, it shows the need for reliable and systemic data collection regarding this issue, as snakebite envenoming is largely a preventable and treatable disease.
REFERENCES
- 1.Gutiérrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, Warrell DA, 2017. Snakebite envenoming. Nat Rev Dis Primers 3: 17063. [DOI] [PubMed] [Google Scholar]
- 2.Chippaux JP, 2012. Epidemiology of snakebites in Europe: a systematic review of the literature. Toxicon 59: 86–99. [DOI] [PubMed] [Google Scholar]
- 3.Loureiro A, Ferrand de Almeida N, Carretero MA, Paulo OS, 2008. Atlas dos Anfíbios e Répteis de Portugal, 1st edition. Lisboa, Portugal: Instituto da Conservação da Natureza e da Biodiversidade.
- 4.Paolino G, Di Nicola M, Pontara A, Didona D, Moliterni E, Mercuri S, Grano M, Borgianni N, Kumar R, Pampena R, 2020. Vipera snakebite in Europe: a systematic review of a neglected disease. J Eur Acad Dermatol Venereol 34: 2247–2260. [DOI] [PubMed] [Google Scholar]
- 5.Di Nicola MR, et al. 2021. Vipers of major clinical relevance in Europe: taxonomy, venom composition, toxicology and clinical management of human bites. Toxicology 453: 152724. [DOI] [PubMed] [Google Scholar]
- 6.Boels D, Hamel JF, Deguigne MB, Harry P, 2012. European viper envenomings: assessment of ViperfavTM and other symptomatic treatments. Clin Toxicol 50: 189–196. [DOI] [PubMed] [Google Scholar]
- 7.Komori Y, Nikai T, Taniguchi K, Masuda K, Sugihara H, 1999. Vascular endothelial growth factor VEGF-like heparin-binding protein from the venom of Viperar aspis aspis (Aspic viper). Biochemistry 38: 11796–11803. [DOI] [PubMed] [Google Scholar]
- 8.Reimers AR, Weber M, Müller UR, 2000. Are anaphylactic reactions to snake bites immunoglobulin E-mediated? Clin Exp Allergy 30: 276–282. [DOI] [PubMed] [Google Scholar]
- 9.González D, 1982. Clinical aspects of bites by viper in Spain. Toxicon 20: 349–353. [DOI] [PubMed] [Google Scholar]
- 10.De Haro L, Glaizal M, Tichadou L, Blanc-Brisset I, Hayek-Lanthois M, 2009. Asp viper (Vipera aspis) envenomation: experience of the Marseille Poison Centre from 1996 to 2008. Toxins (Basel) 1: 100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamb T, de Haro L, Lonati D, Brvar M, Eddleston M, 2017. Antivenom for European Vipera species envenoming. Clin Toxicol 55: 557–568. [DOI] [PubMed] [Google Scholar]
- 12.Casewell N, Al-Abdulla I, Smith D, Coxon R, Landon J, 2014. Immunological cross-reactivity and neutralisation of European viper venoms with the monospecific Vipera berus antivenom ViperaTAb. Toxins (Basel) 6: 2471–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvalho C, Carvalho L, Neves F, 2012. Mordedura de vıbora, situação rara mas potencialmente grave. Acta Pediatr Port 43: 125–127. [Google Scholar]
- 14. Autoridade Nacional do Medicamento e Produtos de Saúde, I.P. (Infarmed) , 2015. Formulário Nacional do Medicamento. Available at: https://www.infarmed.pt/documents/15786/1965139/CFT_18_Vacinas+e+imunoglobulinas_FNM+22_04_2015.zip/bae5ff7d-bde9-4918-a57f-73ded060adc7. Accessed September 30, 2021.
- 15.Boels D. et al. 2020. Snake bites by European vipers in mainland France in 2017–2018: comparison of two antivenoms Viperfav® and Viperatab® . Clin Toxicol 58: 1050–1057. [DOI] [PubMed] [Google Scholar]
- 16.Jollivet V. et al. 2015. European viper envenomation recorded by French poison control centers: a clinical assessment and management study. Toxicon 108: 97–103. [DOI] [PubMed] [Google Scholar]
- 17.Wagener M, Naidoo M, Aldous C, 2017. Wound infection secondary to snakebite. South Afr Med J 107: 315–319. [DOI] [PubMed] [Google Scholar]
- 18.Resiere D. et al. 2020. Infectious complications following snakebite by Bothrops lanceolatus in Martinique: a case series. Am J Trop Med Hyg 102: 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sitprija V, Sitprija S, 2012. Renal effects and injury induced by animal toxins. Toxicon 60: 943–953. [DOI] [PubMed] [Google Scholar]