ABSTRACT.
With an increasing number of adolescents participating in international travel, little is known about travel-related behaviors and health risks in this age group. In the years 2015–2016, we conducted an anonymous, posttravel, questionnaire-based survey with the aim to compare self-reported practices and travel-related symptoms between adolescents (< 18 years old, N = 87) and adults (≥ 18 years old, N = 149) who came to our travel clinic before their humanitarian missions. They had the same pretravel health education, and traveled together to perform similar activities. In univariate analysis, compared with adults, we found that adolescents reported less prior international travel (P < 0.001), more often wore long-sleeved clothing for malaria prevention (P < 0.001) but less often for sun protection (P = 0.009), more often used insect repellents (P = 0.011), and less often had diarrhea (P = 0.024). All other practices and health outcomes were similar between the groups. Multivariate analyses using Bayesian network show strong associations between adults and prior travel experience, and not wearing long-sleeve clothing for malaria prevention. We also found strong associations between prior international travel and sustaining an injury, and having jet lag, as well as between taking malaria prophylaxis and not having diarrhea. Overall, most practices and health outcomes were similar between age groups. Adolescent age and lack of prior international travel experience did not have significant impacts on practices and health outcomes. Our findings highlight the need for more effective strategies to improve the behaviors and health outcomes in both adolescents and adults.
INTRODUCTION
Every year, thousands of people embark on humanitarian aid trips to international destinations, not only among adults, but also among adolescents, including high school students. Top destinations for such trips are in the African region, regions of the Americas, and South East Asia region. Many participants are volunteers who apply their efforts to help address ongoing problems in economically underdeveloped or developing countries, where there may be deficiencies in clean water and sanitation infrastructure, and access to healthcare. As a result, they are at increased risks of personal health and well-being, including various infectious diseases, noninfectious illnesses, and injuries.
Studies have shown that a large proportion of international travelers exhibit poor knowledge, attitudes, and practices (KAP) with regard to vaccines, malaria, traveler’s diarrhea, and noninfectious illnesses.1–3 A substantial portion of travelers to at-risk areas do not seek pretravel health education and advice, oftentimes forgoing recommended vaccines and malaria chemoprophylaxis before their trips.4–8 Most of these studies featured predominantly older adults, and studies of travelers of younger age groups are limited.9–15 In particular, research on adolescent travelers is extremely limited and may be biased by having different travel destinations and travel activities than comparison groups of older adults. Data from one study in youths showed higher risk-taking attitudes in males and older youths than females and younger youths, and travelers who did not seek pretravel health advices had higher risk-taking attitudes than those travelers who did.16
The aims of this study are to evaluate health-related practices and travel-related illnesses of individuals who received the same pretravel education in a classroom and through a travel guide book, and participated in short-term international service trips through a local humanitarian program, to investigate whether there are differences in practices and health outcomes between adolescents and adults. We leverage an ongoing pretravel education arrangement, where travelers of different age groups were offered the same pretravel education and healthcare, and traveled together to the same destinations.
MATERIALS AND METHODS
Study subjects.
All participants were adolescents and adults who received a 30-minute pretravel education classroom session provided by two travel clinic physicians before their short-term (duration of less than 1 month) international humanitarian service missions in the years 2015 and 2016. They attended the same pretravel education session, which was done together in the same classroom with general information about their destination, and presentation of detailed information regarding sanitation, food and water handling, food-borne and water-borne infections, insect bite precaution, malaria chemoprophylaxis, immunization, as well as heat-related illnesses, injury precaution, jet lag disorders, and animal exposure. The leaders of humanitarian service provided food and water during the trip but the participants were also able to get their own food and water from local stores.
Additionally, all participants were given travelers’ health literature in the form of guide book. We also offered participants a comprehensive individualized consultation based on individual’s health background and specific health risks, and gave them recommendations and advices for mitigating risks, including vaccine administration and medication prescriptions. The medications we offered for malaria chemoprophylaxis were doxycycline, mefloquine, and atovaquone/proguanil. The decision was based on destination countries, adverse reactions, drug–drug interactions, cost, and individual’s preference. The consultation did not include a physical examination. The participants traveled together to the same destination countries and performed similar service activities. Participants of all ages were accepted, and it is possible that more than one member of the family were on the same trip, and participated in the study, though because of the anonymous nature of the study, this was not ascertained. The study subjects were recruited after their return to the United States.
Study design and data collection.
This is a cross-sectional study conducted at the University of Utah Travel Clinic. The data were collected through an electronic, self-administered, anonymous questionnaire, which was sent to the participants’ e-mails 4 weeks after their return. The 4-week posttravel period was chosen given the need to report on completion of malaria chemoprophylactic regimens, which in some travelers included a 4-week posttravel course of doxycycline. Informed consent was obtained electronically through e-mails. Given the exploratory nature of this study, no sample size calculation was performed, and a convenience sample was enrolled over a period of 2 years. The study protocol was approved by the Institutional Review Board (IRB) at the University of Utah (IRB no. 82842).
The questionnaire used in this study consisted of three parts. The first part contained demographics, travel history, and countries of destination. The second part queried behaviors and practices the participants exhibited while being abroad. The last part was about symptoms of illnesses and infections after returning from the trip. The questionnaire was anonymous, thus we were unable to trace whether any of the participants of our study were family members. Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at the University of Utah.17,18
Analysis objectives.
The primary objective was to investigate whether travel-related practices and travel-related illnesses were different between adolescents (< 18 years old) and adults (≥ 18 years old) who received the same pretravel education in a classroom and a travel guide book, and participated in short-term international service trips through a local humanitarian program. The secondary objective was to use a Bayesian network to evaluate associations and strengths of the associations between the participants’ demographics and practices during the trip, and posttravel illnesses.
Statistical methods.
Statistical analyses were carried out using R program version 3.62. Categorical variables were described using percentage, whereas continuous variables were described by mean, median, and SD. Comparisons of the categorical variables were performed using the Fisher’s exact test or the χ2 test of independence, as appropriate. A P value of < 0.05 was considered significant.
Given the conditional nature of the data because of the variables that occur before, during, and after travel, we used a Bayesian network to examine multivariate associations that included the relationship between a travelers’ health outcomes given their health behaviors and adherence to appropriate traveler’s practices, and between these behaviors and practices given the travelers’ characteristics and demographic. The Bayesian network allowed us to evaluate these conditional relationships in a single network model. The structure of the Bayesian network was generated with a hill-climbing learning algorithm assessed using the Bayesian Information Criterion (BIC).19 We disallowed implausible and reverse directions of the associations between some factors to maintain interpretability of the model. Bootstrap resamplings were applied to the available data to assess the robustness of the associations. We calculated the strength of each connection using BIC as well as strength of the connections and directional strength of these associations through their presence in the chosen bootstrapped network structures.
RESULTS
Demographics and characteristics.
The electronic survey was distributed to 719 people and 328 participants (47%) responded to the survey. Ninety-two questionnaires were marked incomplete because of missing data and not included in our analysis. There were 236 questionnaires available for univariate analysis, and 228 questionnaires were included in Bayesian network analysis. The additional eight questionnaires were excluded because of missing predictors. Ages of the respondents were between 15 and 65 years with a mean (±SD) age of 24.8 (±12.7) years. Of all the respondents, 87 (37%) were younger than 18 years old. Their destinations were to seven countries, including Thailand, Cambodia, Kenya, Madagascar, Peru, Guatemala, and Nepal. All of the destinations were malaria-endemic countries; however, only 133 (56%) stayed in malaria-endemic areas. Among 236 respondents, 188 (80%) were on their first international trip and 48 (20%) had previously traveled abroad. The adult participants had more international travel experience (31.5% versus 1.1%, P < 0.001). 189 (80%) received pretravel healthcare before their trip (Table 1).
Table 1.
Demographics and characteristics of the participants
| Characteristics | Total | < 18 years | ≥ 18 years | P value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Age | 236 (100) | 87 (36.9) | 149 (63.1) | < 0.001 |
| History of traveling outside of the United States | 48 (20.3) | 1 (1.1) | 47 (31.5) | < 0.001 |
| Destination country | – | – | – | 0.37 |
| Thailand | 55 (23.4) | 25 (29.1) | 30 (20.1) | – |
| Cambodia | 44 (18.7) | 16 (18.6) | 28 (18.8) | – |
| Kenya | 30 (12.8) | 8 (9.3) | 22 (14.8) | – |
| Madagascar | 28 (11.9) | 13 (15.1) | 15 (10.1) | – |
| Peru | 31 (13.2) | 8 (9.3) | 23 (15.4) | – |
| Guatemala | 23 (9.8) | 9 (10.5) | 14 (9.4) | – |
| Nepal | 24 (10.2) | 7 (8.1) | 17 (11.4) | – |
| Stayed in rural area | 169 (71.9) | 61 (70.1) | 108 (73.0) | 0.64 |
| Stayed in malaria endemic area | 133 (56.4) | 48 (55.2) | 85 (57.0) | 0.24 |
| Received pretravel healthcare | 189 (80.1) | 73 (83.9) | 116 (77.9) | 0.26 |
Behaviors and practices during the trip.
Relating to malaria prevention, 182 (77.1%) respondents indicated that they used an insect repellent. A total of 102 (43.2%) covered their skin with long-sleeved clothing to prevent malaria. Adolescents were significantly more likely to report that they used an insect repellent for insect bite prevention (P = 0.01) and that they wore long-sleeved clothing (P < 0.001). About 176 (75%) took malaria chemoprophylaxis and less than half of the respondents used bed nets and closed windows during the night with no significant differences between age groups (Table 2).
Table 2.
Behaviors and practices of adolescents and adults on short-term international missions
| Behaviors and practices | Total | < 18 years | ≥ 18 years | P value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Malaria prevention measures | ||||
| Took malaria chemoprophylaxis | 176 (74.6) | 70 (80.5) | 106 (71.1) | 0.11 |
| Wore long-sleeved shirts | 102 (43.2) | 54 (62.2) | 48 (32.2) | < 0.001 |
| Used insect repellent | 182 (77.1) | 75 (86.2) | 107 (71.8) | 0.011 |
| Used bed net | 47 (19.9) | 18 (20.7) | 29 (19.5) | 0.82 |
| Closed windows | 67 (28.4) | 24 (27.6) | 43 (28.9) | 0.83 |
| Walked bare foot | 73 (31.1) | 33 (37.9) | 40 (27.0) | 0.08 |
| Sun protection measures | ||||
| Used sun screen | 182 (77.1) | 67 (77.0) | 115 (77.2) | 0.98 |
| Wore long-sleeved shirt | 102 (43.2) | 28 (32.2) | 74 (49.7) | 0.009 |
| Wore hat and sunglasses | 142 (60.2) | 50 (57.5) | 92 (61.7) | 0.52 |
| Had animal contact | 34 (14.5) | 14 (16.1) | 20 (13.5) | 0.59 |
The participants reported walking bare foot 31% and having animal contact during their trip 15%. To prevent sun-related illnesses, the majority (77%) of the participants used sun screen, 60% wore sunglasses and a hat, whereas only 43% covered their skin with clothing. Interestingly, while adolescents wore more likely to report wearing long-sleeved clothing to prevent insect bites more than adult travelers, the adult travelers were significantly (P = 0.009) more likely to report wearing long-sleeved clothing for sun protection.
Travel-related symptoms and illnesses.
With regards to reporting symptoms and illnesses, 44 (19%) of travelers sustained injuries during the trip and 44 (19%) reported that they had travel-related nonspecific symptoms after return. Other travel-related symptoms included jetlag (50%), diarrhea (32%), and fever (8%). These travel-related symptoms and illnesses were not associated with being an adolescent or an adult traveler except for traveler’s diarrhea, which was reported significantly more in adult travelers (P = 0.024) (Table 3).
Table 3.
Travel-related symptoms and illnesses of adolescents and adults
| Symptoms/illnesses | Total | < 18 years | ≥ 18 years | P value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Travel-related injury | 44 (18.6) | 12 (13.8) | 32 (21.5) | 0.14 |
| Travel-related non-specific symptoms | 44 (18.6) | 13 (14.9) | 31 (20.9) | 0.25 |
| Jetlag | 116 (49.8) | 38 (43.7) | 78 (53.4) | 0.15 |
| Diarrhea | 76 (32.3) | 20 (23.3) | 56 (37.6) | 0.024 |
| Fever | 18 (7.7) | 5 (5.7) | 13 (8.9) | 0.38 |
Bayesian network.
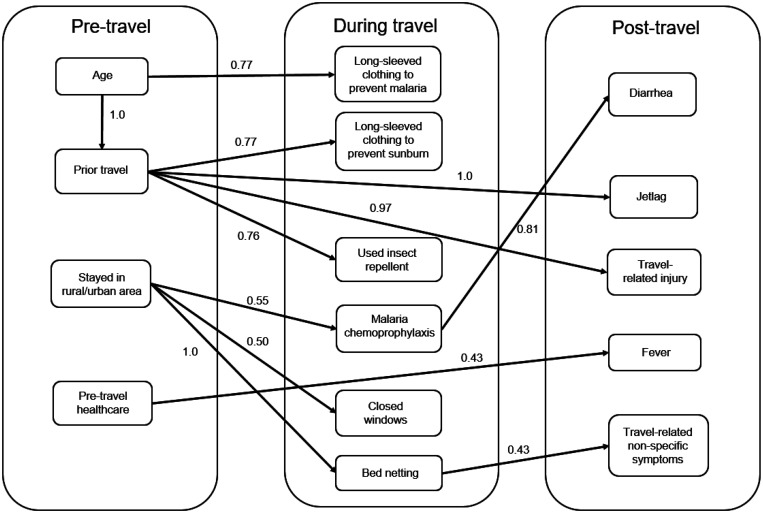
We further investigated multivariate associations and directional associations between the demographics, practices, and health outcomes by fitting a Bayesian network structure that includes the quantified strength of association between the connected variables using BIC (Table 4) and bootstrapped samples (Figure 1). A total of 228 questionnaires were available for analyses after excluding participants with at least one data variable missing. Using the full dataset to fit a Bayesian network, we obtained the following joint probability distribution P(x) as a product of conditional distributions of variables chosen by the algorithm:
Table 4.
Conditional probabilities from the Bayesian network fit
| Outcome | Conditioned on | Probability | Strength (BIC) | Strength (Bootstrap) | Direction (Bootstrap) |
|---|---|---|---|---|---|
| Age ≥ 18 | – | 0.62 | – | – | – |
| Stayed in rural area | – | 0.72 | – | – | – |
| Pretravel healthcare | – | 0.80 | – | – | – |
| Prior travel | Age < 18 | 0.01 | −15.41 | 1.00 | 1.00 |
| Age ≥ 18 | 0.29 | – | – | – | |
| Jetlag | No prior travel | 0.40 | −14.53 | 1.00 | 1.00 |
| Prior travel | 0.88 | – | – | – | |
| Travel injury | No prior travel | 0.11 | −10.06 | 0.97 | 1.00 |
| Prior travel | 0.48 | – | – | – | |
| Bednetting | Stayed in urban area | 0.03 | −7.65 | 1.00 | 1.00 |
| Stayed in rural area | 0.27 | – | – | – | |
| Long sleeves to prevent malaria | Age < 18 | 0.63 | −7.40 | 0.77 | 1.00 |
| Age ≥ 18 | 0.32 | – | – | – | |
| Diarrhea | No malaria chemoprophylaxis | 0.57 | −6.74 | 0.81 | 1.00 |
| Malaria chemoprophylaxis | 0.25 | – | – | – | |
| Long sleeves to prevent sunburn | No prior travel | 0.37 | −4.42 | 0.77 | 1.00 |
| Prior travel | 0.69 | – | – | – | |
| Used insect repellent | No prior travel | 0.83 | −3.45 | 0.76 | 1.00 |
| Prior travel | 0.57 | – | – | – | |
| Fever | No pretravel healthcare | 0.20 | −1.68 | 0.43 | 1.00 |
| Pretravel healthcare | 0.05 | – | – | – | |
| Malaria chemoprophylaxis | Stayed in urban area | 0.63 | −1.10 | 0.55 | 1.00 |
| Stayed in rural area | 0.80 | – | – | – | |
| Travel-related non-specific symptoms | No bed netting | 0.21 | −0.55 | 0.43 | 1.00 |
| Used bed net | 0.07 | – | – | – | |
| Closed windows | Stayed in urban area | 0.17 | −0.27 | 0.50 | 1.00 |
| Stayed in rural area | 0.33 | – | – | – |
BIC = Bayesian Information Criterion.
Figure 1.
Bayesian network showing associations between variables before, during, and after the trip. Numbers indicate the proportion of bootstrap samples that contain the same connections. The connections chosen by a Bayesian network fit on all available variables using Bayesian Information Criterion (BIC).
We divided all variables into three categories: prior to the trip, during the trip, and after the trip. All connections showed the same direction of associations in all 1,000 bootstrap samples (bootstrap direction proportion of 1.0) (Table 4). Those with age greater than 18 years were more likely to have prior travel experience (29% versus 1% with a strong association according to BIC) but less likely to wear long-sleeve clothing to prevent malaria (32% versus 63%). These outcomes are consistent with the univariate analysis in the Tables 1 and 2. Surprisingly, we found strong associations that those with prior travel experience were more likely to sustain an injury (48% versus 11%) and had jet lag (88% versus 40%). There was a moderately strong association between not prior travel experience and the report of using an insect repellent (83% versus 57%). We also found an association (in 81% of bootstrapped variables) between using malaria chemoprophylaxis and decreased the risk of traveler’s diarrhea with the probability of diarrhea in those not taking malaria chemoprophylaxis being 57% compared with 25% in those taking malaria chemoprophylaxis. The following variables included in the model fit had weak associations according to BIC and bootstrapping: not receiving pretravel healthcare and developing fever after the trip, not using a bed net and having a nonspecific illnesses after travel, and staying in a rural area and sleeping with closed windows or taking malaria chemoprophylaxis (Table 4).
DISCUSSION
There is a limited number of published studies focusing on travel-related health in adolescents, an age group whose risk-taking behavior may put them at an increased risk of travel-related illnesses.16 In this study, we leverage a program in which adolescents and adults go to the same destinations for humanitarian missions, and were offered the same pretravel education and healthcare. Using a posttravel web-based questionnaire, we ascertained differences in travel-related behaviors and outcomes between the age groups.
Our univariate analysis show that despite having less international travel experience, adolescents more often reported wearing long-sleeved clothing to prevent malaria and using insect repellents. In addition, adolescents reported less wearing long-sleeved clothing for sun protection and traveler’s diarrhea compared with adult travelers. We then used Bayesian network analysis, which models probability distributions and outputs directed acyclic graphs, to explore causal probabilities through multivariate associations. Through this, we confirmed the strong associations between older age and prior international travel and wearing long sleeves for malaria prevention. Among all travelers, we found strong associations between prior international travel and sustaining an injury, and experiencing jet lag. In addition, we found a moderately strong association between those not taking malaria chemoprophylaxis and having diarrhea, a finding consistent with prior studies.20,21 This finding suggests bacterial enteropathogen susceptibility to doxycycline in those countries. On the other hand, our analysis revealed no strong associations between receiving pretravel healthcare and the development of fever or any illnesses after the trip. Previous international travel experience, age, and pretravel healthcare did not have strong associations with the other practices or health outcomes of travelers in our study.
Misperception of risk places travelers at an increased risk of travel-related illnesses while abroad and after return.12,22–25 Previous reports, almost exclusively of adults, found a lack of pretravel education, inadequate pretravel medical advice, and nonadherence to advices and recommendations among travelers.4–8 In our study, receiving pretravel healthcare was not found to have strong associations with injuries, jet lag, or posttravel illnesses, though there was a weak association with fever after return. While adolescents, who have less travel experience, are thought to be higher risk, we found that they were more likely to report wearing long-sleeve clothing to prevent malaria, using an insect repellent, and having less diarrhea. Furthermore, in our Bayesian network analysis across all ages, not having prior international travel was associated with less injuries and jet lag. We postulate that having the same pretravel health education and traveling together to same destinations may mitigate risk-taking behaviors, and that those with a lack of prior international travel experience might have paid more attention to the education session. Additionally, adolescents may be more familiar with lectures, which is a formal method of conveying information and instructions to groups of learners, while adults’ attention span and attitude toward the classroom format might be different. The pretravel education in the form of classroom might not be appropriate for adult travelers who have more travel experience. For adult travelers, other education formats such as peer instructions, small discussion groups, and individually presented advice could be better options, and need to be explored in future studies. Additionally, travel clinic providers often spend less time advising experienced travelers. This could lead to inadequate knowledge, wrong practices, and increased risks of travel-related illnesses in adult travelers. However, our study did not monitor time spent for each participant’s individual consultation.
Our study adds to the very limited knowledge base regarding travel-related practices, health risks, and travel-related illnesses in adolescents, especially in our ability to compare different age groups who received the same pretravel education and participated in similar activities in the same locations during their travels. However, our findings had several limitations. Although an electronic questionnaire gives sense of anonymity and privacy, response was voluntary, and may be subject to participation biases. Furthermore, there was potential that family members who traveled together could influence one another’s practices and behaviors, but we were unable to track down whether any of the participants of our study were family members. Additionally, there could be differences in the extent to which participants interpreted questionnaire items. Also, since the questionnaires were administered 4 weeks after return, we do not know whether recall bias differentially affects the age groups, and future studies using diaries are warranted. Lastly, given our low response rate of 47%, our results may be subject to nonresponse bias.
CONCLUSIONS
We found that despite pretravel education, significant proportions of travelers exhibited poor practices and nonadherence to pretravel recommendations in both adolescent and adult travelers. Adolescent age, lack of prior international travel experience, and pretravel healthcare did not strongly associate with positive impacts on practices and health outcomes. Our findings highlight the need to emphasize pretravel education and improve the current strategies to guide travel behaviors and health outcomes in both adolescents and adults.
ACKNOWLEDGMENTS
We are grateful for the collaboration with Nora Sooklaris, who helped in the development of the questionnaire.
REFERENCES
- 1.Toovey S, Jamieson A, Holloway M, 2004. Travelers’ knowledge, attitudes and practices on the prevention of infectious diseases: results from a study at Johannesburg International Airport. J Travel Med 11: 16–22. [DOI] [PubMed] [Google Scholar]
- 2.Van Herck K. et al. , 2004. Knowledge, attitudes and practices in travel-related infectious diseases: the European airport survey. J Travel Med 11: 3–8. [DOI] [PubMed] [Google Scholar]
- 3.Kittitrakul C, Lawpoolsri S, Kusolsuk T, Olanwijitwong J, Tangkanakul W, Piyaphanee W, 2015. Traveler’s diarrhea in foreign travelers in Southeast Asia: a cross-sectional survey study in Bangkok, Thailand. Am J Trop Med Hyg 93: 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamer DH, Connor BA, 2004. Travel health knowledge, attitudes and practices among United States travelers. J Travel Med 11: 23–26. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Velez R, Bayas JM, 2007. Spanish travelers to high-risk areas in the tropics: airport survey of travel health knowledge, attitudes, and practices in vaccination and malaria prevention. J Travel Med 14: 297–305. [DOI] [PubMed] [Google Scholar]
- 6.Van Herck K, Zuckerman J, Castelli F, Van Damme P, Walker E, Steffen R, 2003. Travelers’ knowledge, attitudes, and practices on prevention of infectious diseases: results from a pilot study. J Travel Med 10: 75–78. [DOI] [PubMed] [Google Scholar]
- 7.Hill DR, 2000. Health problems in a large cohort of Americans traveling to developing countries. J Travel Med 7: 259–266. [DOI] [PubMed] [Google Scholar]
- 8.LaRocque RC, Rao SR, Tsibris A, Lawton T, Barry MA, Marano N, Brunette G, Yanni E, Ryan ET, 2010. Pre-travel health advice-seeking behavior among US international travelers departing from Boston Logan International Airport. J Travel Med 17: 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartjes LB, Baumann LC, Henriques JB, 2009. Travel health risk perceptions and prevention behaviors of US study abroad students. J Travel Med 16: 338–343. [DOI] [PubMed] [Google Scholar]
- 10.Kupper T, Rieke B, Neppach K, Morrison A, Martin J, 2014. Health hazards and medical treatment of volunteers aged 18–30 years working in international social projects of non-governmental organizations (NGO). Travel Med Infect Dis 12: 385–395. [DOI] [PubMed] [Google Scholar]
- 11.Vora N, Chang M, Pandya H, Hasham A, Lazarus C, 2010. A student-initiated and student-facilitated international health elective for preclinical medical students. Med Educ Online 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maltezou HC, Pavli A, 2018. Adolescents traveling to high-risk destinations: review and considerations for clinicians. Int J Travel Med Glob Health 6: 141–148. [Google Scholar]
- 13.Hagmann S, Neugebauer R, Schwartz E, Perret C, Castelli F, Barnett ED, Stauffer WM, 2010. Illness in children after international travel: analysis from the GeoSentinel Surveillance Network. Pediatrics 125: e1072–e1080. [DOI] [PubMed] [Google Scholar]
- 14.Herbinger KH, Drerup L, Alberer M, Nothdurft HD, von Sonnenburg F, Löscher T, 2012. Spectrum of imported infectious diseases among children and adolescents returning from the tropics and subtropics. J Travel Med 19: 150–157. [DOI] [PubMed] [Google Scholar]
- 15.van Rijn SF, Driessen G, Overbosch D, van Genderen PJJ, 2011. Travel‐related morbidity in children: a prospective observational study. J Travel Med 19: 144–149. [DOI] [PubMed] [Google Scholar]
- 16.Han P, Balaban V, Marano C, 2010. Travel characteristics and risk-taking attitudes in youths traveling to nonindustrialized countries. J Travel Med 17: 316–321. [DOI] [PubMed] [Google Scholar]
- 17.Harris PA. et al. , 2019. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, 2009. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scutari M, 2010. Learning Bayesian networks with the bnlearn R package. J Stat Softw 35: 22. [Google Scholar]
- 20.Lago K, Telu K, Tribble D, Ganesan A, Kunz A, Geist C, Fraser J, Mitra I, Lalani T, Yun HC, For The Infectious Disease Clinical Research Program TravMil Study Group , 2020. Doxycycline malaria prophylaxis impact on risk of travelers’ diarrhea among international travelers. Am J Trop Med Hyg 103: 1864–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sack RB, Santosham M, Froehlich JL, Medina C, Orskov F, Orskov I, 1984. Doxycycline prophylaxis of travelers’ diarrhea in Honduras, an area where resistance to doxycycline is common among enterotoxigenic Escherichia coli. Am J Trop Med Hyg 33: 460–466. [DOI] [PubMed] [Google Scholar]
- 22.Winer L, Alkan M, 2002. Incidence and precipitating factors of morbidity among Israeli travelers abroad. J Travel Med 9: 227–232. [DOI] [PubMed] [Google Scholar]
- 23.Angelo KM, Kozarsky PE, Ryan ET, Chen LH, Sotir MJ, 2017. What proportion of international travellers acquire a travel-related illness? A review of the literature. J Travel Med 24. Available at: https://doi.org/10.1093/jtm/tax046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vilkman K, Pakkanen SH, Laaveri T, Siikamaki H, Kantele A, 2016. Travelers’ health problems and behavior: prospective study with post-travel follow-up. BMC Infect Dis 16: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kain D, Findlater A, Lightfoot D, Maxim T, Kraemer MUG, Brady OJ, Watts A, Khan K, Bogoch II, 2019. Factors affecting pre-travel health seeking behaviour and adherence to pre-travel health advice: a systematic review. J Travel Med 26. Available at: https://doi.org/10.1093/jtm/taz059. [DOI] [PubMed] [Google Scholar]