ABSTRACT.
People deprived of liberty (PDL) are at high risk of acquiring Mycobacterium tuberculosis infection (latent tuberculosis infection [LTBI]) and progressing to active tuberculosis (TB). We sought to determine the incidence rates and factors associated with LTBI and active TB in Colombian prisons. Using information of four cohort studies, we included 240 PDL with two-step tuberculin skin test (TST) negative and followed them to evaluate TST conversion, as well as, 2,134 PDL that were investigated to rule out active TB (1,305 among people with lower respiratory symptoms of any duration, and 829 among people without respiratory symptoms and screened for LTBI). Latent tuberculosis infection incidence rate was 2,402.88 cases per 100,000 person-months (95% CI 1,364.62–4,231.10) in PDL with short incarceration at baseline, and 419.66 cases per 100,000 person-months (95% CI 225.80–779.95) in individuals with long incarceration at baseline (who were enrolled for the follow after at least 1 year of incarceration). The TB incidence rate among PDL with lower respiratory symptoms was 146.53 cases/100,000 person-months, and among PDL without respiratory symptoms screened for LTBI the incidence rate was 19.49 cases/100,000 person-months. History of Bacillus Calmette-Guerin vaccination decreased the risk of acquiring LTBI among PDL who were recently incarcerated. Female sex, smoked drugs, and current cigarette smoking were associated with an increased risk of developing active TB. This study shows that PDL have high risk for LTBI and active TB. It is important to perform LTBI testing at admission to prison, as well as regular follow-up to control TB in prisons.
BACKGROUND
Tuberculosis (TB) remains a significant public health problem, mainly in low- and middle-income countries, where more than 80% of the global TB burden resides.1 Despite global efforts to achieve the End TB Strategy goals for decreasing TB incidence,2 the goal of 20% reduction between 2015 and 2020 has not been met, with a worldwide cumulative reduction of 9% from 2015 to 2019, and the TB incidence slowly increasing in the Region of the Americas.1
People deprived of liberty (PDL) are considered one of the high-risk populations for the acquisition of latent tuberculosis infection (LTBI) and to progress to active TB. The overall pooled incidence rate of LTBI in PDL is 15.0 per 100,000 person-years,3 and the worldwide prevalence of active TB among PDL is 10 times higher than in the general population.3 South America has the highest TB incidence rate ratio (26.9) between PDL and the general population compared with other WHO regions (4.1–15.6).
The demographics of PDL (intravenous drug use, poverty, homelessness, etc.) coupled with overcrowded environmental conditions, poor ventilation, late case detection, and inadequate isolation and treatment of patients, increase the risk of active TB and LTBI,4–6 and it may have a substantial effect in the general population.5 Furthermore, the interaction between the community and the institutional amplifiers (visitors, guards, and administrative staff), results in generalized endemic TB.7
Identifying the period in which individuals are at the greatest risk of infection or developing active TB in PDL after entry to prison (i.e., identifying susceptible individuals at the time of their initial exposure to high burden settings within the prison environment) would allow to implement preventive strategies such as screening and treatment of people with LTBI, and diagnostic algorithms of all forms of TB to improve TB case finding.4,8–10
The objectives of this study were to estimate the incidence of LTBI and active TB as well as the factors associated with tuberculin skin test (TST) conversion and the development of active TB in Colombian prisons.
METHODOLOGY
We used four cohorts of PDL incarcerated in prisons of medium- and maximum-security level. The cohorts assembled between April 2010–December 2012 and November 2012–December 2013 were used to perform the analysis of active TB. The cohorts assembled between November 2012–April 2015 and September 2016–December 2018 were designed primarily for the analysis of LTBI. Full details about the protocol and procedures of the cohorts April 2010–December 2012, November 2012–December 2013, and November 2012–April 2015 are available elsewhere.9,11,12 Supplemental Table 1 summarizes pertinent descriptive details about prisons’ capacity, people incarcerated, eligible population, number of people screened, enrolled, analyzed, and the inclusion and exclusion criteria, as well as the definitions of the main outcomes and key procedures for each cohort included in this paper.
Latent tuberculosis infection.
In general, the methods for inclusion, follow-up, and diagnosis were the same for both LTBI cohorts. Differences are reported for each item, as applicable.
Inclusion criteria.
Individuals who had a negative two-step TST at baseline, who agreed to participate in the study and signed a written consent form.
Exclusion criteria.
1) Administration of live vaccines (measles, mumps, and rubella; varicella; or the live attenuated influenza vaccine) in the last 4 weeks before TST administration, 2) severe adverse reactions with a previous TST administration, and 3) previous active TB. For the cohort that started in September 2016, an additional exclusion criterion was included: people who were no longer incarcerated in prison (released or moved into house detention) because of the difficulties associated with follow-up.
Follow-up.
For the cohort recruited between November 2012 and December 2013, we administered the TST at 2 years of follow-up (February to April 2015) because of budget constraints. For the cohort recruited between September 2016 and December 2018, we administered the TST every 6 months for up 2 years.
Diagnosis of LTBI.
Tuberculin skin test with tuberculin PPD RT-23. 2 TU/0.1 mL (Statens Serum Institut®, Copenhagen, Denmark), was inoculated according to the CDC guidelines.13 Reading was performed within 48–72 hours of administration and an experienced nurse recorded the induration measurements in millimeters (mm). A second TST was administered to evaluate the booster effect. A positive result was defined as an induration of ≥ 10 mm diameter. Follow-up started after the two-step TST were negative. Subsequent TST administrations were used to identify new LTBI. New LTBI was defined as a PDL that converted the TST as follows: A TST test with ≥ 10 mm diameter with a difference of at least ≥ 6 mm between the second and subsequent administrations. All procedures were done according to Arroyave et al.12
Active tuberculosis.
We estimated the incidence rate of active TB in people with lower respiratory symptoms of any duration (cohort April 2010–December 2012),11 and in people without respiratory symptoms but screened for LTBI (cohort November 2012–December 2013).11,12
Lower respiratory symptoms were defined as the presence of cough of any duration and/or expectoration, and abnormal breath sounds on lung auscultation. People deprived of liberty who met this criterion were further investigated to rule out active TB. In addition, we searched the TB surveillance system whether participants included in the initial studies were diagnosed with TB between 2013 and December 2019.
Diagnosis.
All sputum samples were processed using the conventional sodium hydroxide-N-acetyl-L-cysteine method, with standard decontamination, and concentration methods. A smear was prepared for auramine-rhodamine staining to visualize acid-fast bacilli (AFB). The sputum sample from each PDL was inoculated into: 1) Lowenstein–Jensen (LJ) medium; 2) a mycobacterial growth indicator tube (MGIT) incubated in a MGIT 960 BACTEC instrument (BD Diagnostics, Sparks, MD); and 3) thin-layer agar (TLA) for the detection of resistance to rifampicin and isoniazid. Mycobacterium tuberculosis (MTB) was identified by standard biochemical tests.
Variables.
Outcomes.
We considered two outcomes: 1) new LTBI, and 2) microbiologically confirmed pulmonary TB as previously defined.
Predictor variables.
Sociodemographic and behavioral characteristics that have previously been reported as risk factors for active TB and LTBI were considered.9,12,14–19 The following information was collected for all individuals: age; history and time of prior incarceration; use of drugs (inhaled, injected, or smoked) or alcohol; comorbidities (chronic obstructive pulmonary disease, diabetes, chronic kidney disease, HIV, and any other immunosuppressive disease); previous contact with a TB case (outside and/or inside the prison); history of prior TB, including date of last episode, and outcome; weight and height; and city and neighborhood where the person lived before incarceration. To determine whether the PDL had previously been vaccinated with Bacillus Calmette–Guerin (BCG), they were visually inspected by an experienced nurse for the presence of a BCG scar.
Analysis.
All the analysis was performed using STATA v.14 (Stata Corp, College Station, TX). Descriptive statistics (median [IQR] and n [%]) were used to report the variables of interest.
We estimated the LTBI prevalence on admission to prison for the cohort recruited between 2016 and 2018.
Latent tuberculosis infection incidence rate and corresponding 95% CI were calculated in the 240 people from both cohorts (2012–2015 and 2016–2018) using the stptime command of STATA (the CI are calculated using the quadratic approximation to the Poisson log likelihood for the log-rate parameter).
Active TB incidence rate and corresponding 95% CI were calculated for each cohort (2010–2012 and 2012–2013).
To explore the factors associated with new LTBI, the cohorts studied for LTBI were divided into two subgroups: “PDL with short incarceration,” for those who were enrolled in follow-up upon incarceration or within the first 3 months of incarceration, and “PDL with long incarceration” for people who started their follow-up after 1 year or more of incarceration.
Chi-squared, Fishers Exact, or Mann–Whitney U tests were used for bivariate analysis to evaluate factors associated with new LTBI and active TB. For multivariable analyses, Poisson regression was used to estimate the risk ratios of variables associated with LTBI outcomes (LTBI with short or long incarceration), and with active TB. Variables included in the final model were selected based on biological plausibility and risk or protective factors previously identified in literature, no matter their statistical significance in the bivariate analysis. Standard errors in bivariate and multivariable models were adjusted by cluster (courtyards for LTBI models, and prisons for TB models). Courtyards were chosen for the LTBI models because evaluated prisons are in the same state (Antioquia) and prior studies identified clustering of cases and variation of TB prevalence between courtyards. We also adjusted by the variable prisons for the active TB model as the included prisons are in different states (Antioquia and Santander). A two-tailed P value < 0.05 was considered significant.
Finally, a Kaplan–Meier curve was plotted to compare the time from admission to prison to active TB diagnosis among individuals with lower respiratory symptoms, and without symptoms whom underwent screening for LTBI. Time zero was the time of entering prison. The outcome was active TB. Participants were censored at the end of the study period or at the time of release from prison when this occurred earlier.
Ethics statements.
All studies were approved by the Ethics Committees at each of the participating academic institutions (Facultad Nacional de Salud Pública, Universidad de Antioquia, and Universidad Pontificia Bolivariana), as well as by the Instituto Nacional Penitenciario y Carcelario (INPEC), and the director of each prison. Written consent forms were explained and signed by each PDL in the presence of two additional PDL witnesses. All PDL were treated with anti-TB treatment as soon as smear and/or culture results were received. New converters were reported to the prison health authority in all prisons, and PDL from prison number one were offered LTBI treatment. As LTBI treatment in PDL is not recommended as “should be performed” according to the international and Colombian guidelines, the healthcare personnel from prison number two opted not to offer LTBI treatment.
RESULTS
The Supplemental Table 1 summarizes the information regarding to prisons and population.
Prevalence of latent tuberculosis infection.
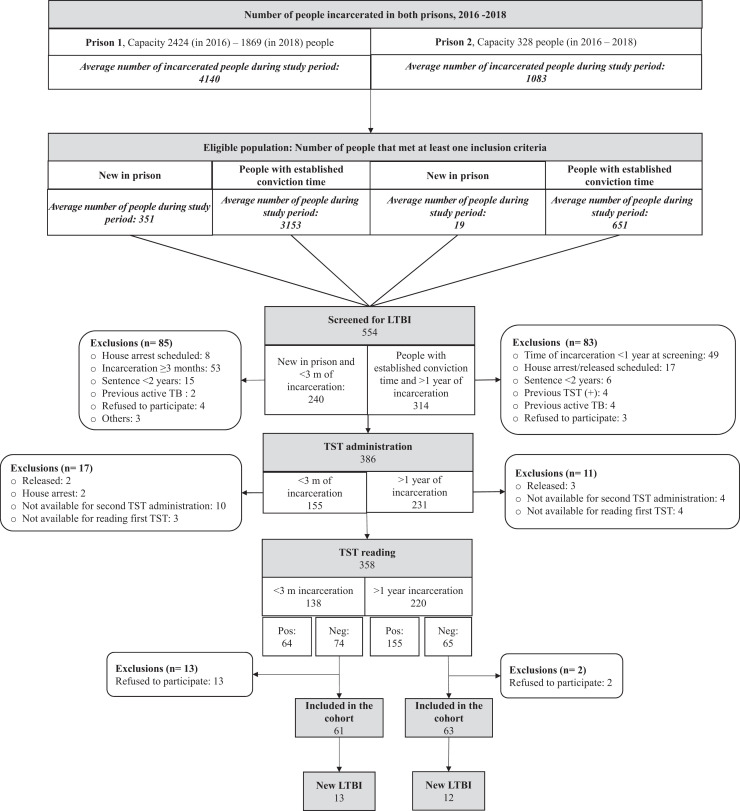
In the cohort 2016–2018, LTBI prevalence upon admission to prison (< 3 months of incarceration) was 46.38% (64/138) (95% CI 38.06–54.70), and in those who had spent more than 1 year of incarceration it was 70.45% (155/220) (95% CI 64.42–76.48) (Figure 1).
Figure 1.
Flow chart of persons screened for LTBI and included in the cohort between 2016 and 2018. TST: Tuberculin skin test. LTBI: Latent tuberculosis infection.
Incidence of latent tuberculosis infection.
Among the 240 two-step TST negative PDL (116 PDL from the cohort November 2012–April 2015, and 124 PDL from the cohort September 2016–December 2018), the cumulative incidence of LTBI was 27.08% and the median duration of follow-up was 33.90 months (IQR 14.90–52.55). The LTBI incidence rate was 2,402.88 cases per 100,000 person-months (95% CI 1,364.62–4,231.10) in PDL with short incarceration at baseline, and 419.66 cases per 100,000 person-months (95% CI 225.80–779.95) in individuals with long incarceration at baseline. Supplemental Table 2 reports the person-time, number of outcomes, incidence density, and respective 95% CI, every 6 months for each cohort.
The median age of the 240 people was 32 years (25.50–44.50). Most of the new infections occurred in prison one (89.23%). Individuals with LTBI and short incarceration had a low prevalence of comorbidities, and diabetes; neutropenia and cancer were absent among this group. People with LTBI and long incarceration had more comorbidities, however, those comorbidities have not been described as risk factors for LTBI (e.g., gastritis, high blood pressure). Table 1 shows general characteristics at baseline of new infected and noninfected people by the time of incarceration.
Table 1.
Baseline characteristics of people deprived of liberty evaluated for latent TB infection, 2012–2018, Colombia
| Variables | People deprived of liberty with short incarceration at baseline | People deprived of liberty with long incarceration at baseline | ||
|---|---|---|---|---|
| New LTBI N = 12 (%) |
Noninfected N = 49 (%) |
New LTBI N = 53 (%) |
Noninfected N = 126 (%) |
|
| Age, years, median [IQR] | 32 [25–36.50] | 31 [23–39] | 32 [25–56] | 33 [28–46] |
| Incarceration to first contact by researchers, months, median [IQR] | 0.20 [0.05–0.80] | 1.30 [0.6–20.20] | 10.80 [6.00–22.50] | 23.90 [7.80–44.40] |
| Incarceration to conversion time (or last follow-up), months, median [IQR] | 12.10 [8.00–12.7] | 6.6 [2.60–13.00] | 36.4 [30.70–47.50] | 43.90 [32.80–67.90] |
| Comorbidities | 2 (16.67) | 7 (14.29) | 17 (32.08) | 49 (38.89) |
| COPD | 1 (8.33) | 4 (8.16) | 7 (13.21) | 8 (6.35) |
| HIV | 1 (8.33) | 0 | 0 | 1 (0.79) |
| Diabetes | 0 | 0 | 2 (3.77) | 3 (2.38) |
| Other | 0 | 3 (6.12) | 8 (15.09) | 37 (29.36) |
| Current smoke drug use | 4 (33.33) | 14 (28.57) | 14 (26.42) | 36 (28.57) |
| Current inhaled drug use | 1 (8.33) | 7 (14.29) | 8 (15.09) | 20 (15.87) |
| Current cigarette smoking | 6 (50.00) | 25 (51.02) | 25 (47.17) | 49 (38.89) |
| Current alcohol consumption | 2 (16.67) | 14 (28.57) | 7 (13.21) | 18 (14.29) |
| BCG scar | 9 (75.00) | 46 (93.88) | 29 (59.18) | 95 (77.87) |
| Contact with a TB case | 0 | 4 (8.16) | 13 (24.53) | 41 (32.54) |
| Prison | ||||
| Prison 1 | 6 (50.00) | 14 (28.57) | 52 (98.11) | 85 (67.46) |
| Prison 3 | 6 (50.00) | 35 (71.43) | 1 (1.89) | 41 (32.54) |
| BMI < 18.5 kg/m2 | 0 | 1 (1.67) | 2 (3.77) | 3 (2.42) |
| Active TB during follow-up* | 1 (8.33) | 0 | 10 (18.52) | 0 |
BCG = Bacillus Calmette–Guerin vaccine; BMI = body mass index; COPD = chronic obstructive pulmonary disease; IQR = interquartile range; LTBI: latent tuberculosis infection; TB = tuberculosis; TST = tuberculin skin test.
Two persons who dropped out of the study were subsequently diagnosed with active TB, four and 16 months later. During our follow-up their TST remained negative. We do not know whether these persons were new converters or TST negative between their dropped out and the TB diagnosis.
Factors associated with new LTBI among people with short incarceration.
After adjusting by cluster effect, BCG vaccination was associated with a decreased risk of acquiring new LTBI in people with short incarceration (adjusted risk ratio [aRR] 0.30; 95% CI 0.11–0.79). Chronic obstructive pulmonary disease, age, inhaled or smoked drugs, and smoking were not associated with new LTBI in this group (Table 2). Contact with a TB case was not included in the multivariable model because only four individuals reported this exposure. There were no people with diabetes in this group; therefore, this variable was not included in the multivariable model.
Table 2.
Factors associated with new latent tuberculosis infection in people deprived of liberty with short incarceration
| Variable | New LTBI in people deprived of liberty short incarceration | |||
|---|---|---|---|---|
| Crude RR+ [95% CI] | P value | aRR*† [95% CI] | P value | |
| BCG scar | 0.33 [0.09–1.21] | 0.094 | 0.30 [0.11–0.79] | 0.014 |
| COPD | 1.02 [0.13–7.89] | 0.986 | 0.81 [0.16–4.04] | 0.794 |
| Age | 1.00 [0.94–1.05] | 0.959 | 1.00 [0.97–1.04] | 0.822 |
| Inhaled drugs [Ref Never] | 1.00 | 1.00 | ||
| Past | 1.21 [0.35–4.13] | 0.761 | 0.91 [0.16–5.15] | 0.917 |
| Current | 0.64 [0.08–5.22] | 0.679 | 0.73 [0.74–7.11] | 0.783 |
| Smoked drugs [Ref Never] | 1.00 | 1.00 | ||
| Past | 1.10 [0.22–5.45] | 0.907 | 1.24 [0.14–10.78] | 0.843 |
| Current | 1.22 [0.34–4.33] | 0.756 | 1.05 [0.16–6.90] | 0.952 |
| Cigarette smoking [Ref Never] | 1.00 | 1.00 | ||
| Past | 1.3 [0.15–11.12] | 0.811 | 1.52 [0.36–6.40] | 0.564 |
| Current | 1.01 [0.31–3.30] | 0.992 | 1.11 [0.28–4.33] | 0.872 |
aRR = adjusted risk ratio; BCG = Bacillus Calmette–Guerin vaccine; COPD = chronic obstructive pulmonary disease; LTBI = latent tuberculosis infection. *aRR are based on Poisson regression.
The standard errors of CI are adjusted for cluster effect (courtyard) in the bivariable and multivariable models.
Factors associated with new LTBI among people with long incarceration.
In the multivariable analysis, after 5 years of incarceration the risk of new LTBI decreased by 70%. Similarly, BCG vaccination decreased the risk of new LTBI by 41%, whereas the presence of COPD increased the risk of new LTBI among people with long incarceration (Table 3). Diabetes, age, smoking, inhaled drugs, and contact with a TB case were not associated with new LTBI. The HIV variable was not included in the multivariate model because there was only one non-LTBI participant living with HIV in this group, and none in the group with new LTBI and long incarceration.
Table 3.
Factors associated with new latent tuberculosis infection in PDL with long incarceration
| Variable | New LTBI in PDL with long incarceration | |||
|---|---|---|---|---|
| Crude RR+ [95% CI] | P value | aRR*† [95% CI] | P value | |
| Incarcerated > 60 months | 0.32 [0.12–0.82] | 0.001 | 0.30 [0.12–0.77] | 0.011 |
| BCG scar | 0.55 [0.36–0.84] | 0.013 | 0.59 [0.39–0.91] | 0.015 |
| COPD | 1.66 [0.93–2.96] | 0.131 | 2.04 [1.11–3.77] | 0.022 |
| Diabetes | 1.36 [0.46–4.09] | 0.606 | 2.14 [0.23–19.87] | 0.503 |
| Age | 1.00 [0.99–1.02] | – | 1.00 [0.98–1.02] | 0.952 |
| Inhaled drugs use [Ref Never] | 1.00 | 1.00 | ||
| Past | 0.93 [0.54–1.60] | 0.786 | 0.77 [0.53–1.12] | 0.166 |
| Current | 0.94 [0.49–1.80] | 0.846 | 0.78 [0.51–1.19] | 0.253 |
| Cigarette smoking [Ref Never] | 1.00 | 1.00 | ||
| Past | 1.07 [0.57–2.02] | 0.836 | 1.18 [0.74–1.89] | 0.485 |
| Current | 1.31 [0.76–2.24] | 0.332 | 1.46 [0.72–2.98] | 0.297 |
| Contact with a TB case | 0.75 [0.44–1.29] | 0.286 | 0.75 [0.42–1.35] | 0.341 |
aRR = adjusted risk ratio; BCG = Bacillus Calmette–Guerin vaccine; COPD = chronic obstructive pulmonary disease; LTBI = latent tuberculosis infection; PDL = people deprived of liberty.
aRR are based on Poisson regression.
The standard errors of CI are adjusted for cluster effect (courtyard) in the bivariable and multivariable models.
Active TB in PDL.
Among 2,134 PDL studied for TB (1,305 PDL from the cohort April 2010–December 2012, and 829 from the cohort November 2012–December 2013), 76 (3.56%) had a microbiological diagnosis of pulmonary TB; 12 of them were prevalent cases. The incidence among people with respiratory symptoms was 146.53 cases per 100,000 person-months (95% CI 114.24–187.94), and among people without respiratory symptoms screened for LTBI was 19.49 cases per 100,000 person-months (7.32–51.93). Supplemental Table 2 shows the person-time, active TB cases, incidence density, and their 95% CI, every 6 months for each cohort April 2010–December 2012 and November 2012–December 2013.
Table 4 shows the baseline characteristics, in general, people were young with a median of 31 [IQR 25–42] years old, and prison one had the highest number of TB cases (71.05%). Individuals with active TB had spent a median of 15.20 months [IQR 6.10–31.90] in the prison at the time of diagnosis.
Table 4.
Baseline characteristics of people deprived of liberty with and without active TB, 2010–2018, Colombia
| Variable | Active TB N = 76 (%) | Without TB N = 2,058 (%) |
|---|---|---|
| Age, years, median [IQR] | 30 [25–37] | 31 [25–42] |
| Time incarcerated, months, median [IQR] | 15.20 [6.10–31.90] | 13.30 [6.00–30.60] |
| Sex | ||
| Male | 67 (88.16) | 1,953 (94.89) |
| At least one comorbidity | 21 (27.63) | 627 (30.46) |
| COPD | 8 (10.53) | 149 (7.24) |
| Diabetes | 1 (1.31) | 34 (1.65) |
| HIV | 2 (2.63) | 13 (0.63) |
| Smoke drugs use | ||
| Never | 17 (22.37) | 738 (35.86) |
| Past | 22 (28.95) | 439 (21.33) |
| Currently | 37 (48.68) | 880 (42.76) |
| Inhaled drugs use | ||
| Never | 36 (47.37) | 1,070 (52.00) |
| Past | 26 (34.21) | 601 (29.20) |
| Currently | 14 (18.42) | 387 (19.29) |
| Cigarettes consume | ||
| Never | 19 (25.00) | 630 (30.61) |
| Past | 12 (15.79) | 437 (21.23) |
| Currently | 45 (59.21) | 991 (48.15) |
| Liquor consume | ||
| Never | 18 (23.68) | 297 (14.43) |
| Past | 46 (60.52) | 1,008 (48.98) |
| Currently | 12 (15.79) | 753 (36.59) |
| BCG scar | 63 (82.89) | 1,577 (76.62) |
| Contact with a TB case | ||
| No contact | 46 (60.52) | 1,473 (71.57) |
| External | 11 (14.47) | 248 (12.05) |
| Internal | 15 (19.73) | 220 (10.69) |
| Both | 0 (0.00) | 5 (0.24) |
| Unknown | 4 (5.26) | 112 (5.49) |
| Prison 1 | 54 (71.05) | 1,446 (70.26) |
| Prison 2 | 8 (10.53) | 129 (6.26) |
| Prison 3 | 11 (14.47) | 450 (21.87) |
| Prison 4 | 3 (3.95) | 33 (1.60) |
| BMI < 18.5 kg/m2 | 20/72 (27.78) | 108/1,193 (9.05) |
BCG = Bacillus Calmette–Guerin vaccine; BMI = body mass index; COPD = chronic obstructive pulmonary disease; IQR = interquartile range; TB = active tuberculosis.
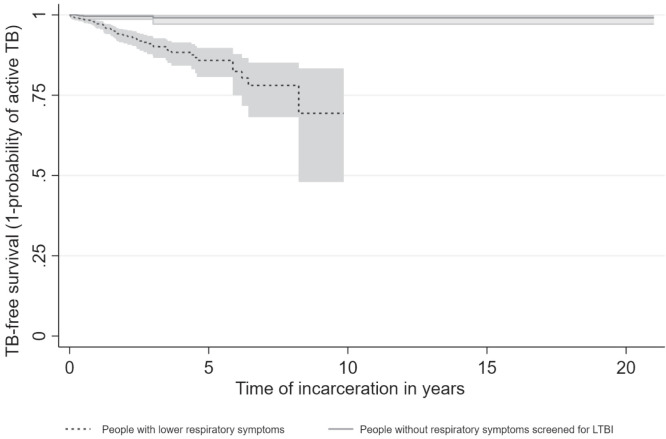
The majority of TB cases were in people who had been incarcerated between 1 and 7 years (Figure 2). Reported respiratory symptoms were associated with greater likelihood of receiving diagnosis of active TB. Conversely, individuals without respiratory symptoms remained free of active TB (Figure 2).
Figure 2.
Tuberculosis (TB) cases during incarceration (time in years). Time zero is the time of prison entry. Twelve TB cases were diagnosed within 3 months of incarceration (prevalent cases).
Factors associated with active TB.
Female sex, smoked drugs, and current cigarette smoking were associated with higher risk of developing active TB (Table 5). The factors HIV, diabetes, inhaled drugs, time of incarceration more than 1 year, BCG scar, and age were not associated with active TB.
Table 5.
Factors associated with active tuberculosis in prisons in Colombia
| Variable | Active TB | |||
|---|---|---|---|---|
| Crude RR+ [95% CI] | P value | aRR*† [95% CI] | P value | |
| Age ≤ 32 years | 1.45 [0.91–2.30] | 0.114 | 1.49 [0.78–2.82] | 0.225 |
| Incarceration for > 1 year | 1.22 [0.74–2.00] | 0.443 | 1.30 [0.92–1.82] | 0.135 |
| Male sex | 0.42 [0.22–0.82] | 0.010 | 0.38 [0.21–0.70] | 0.002 |
| HIV | 3.80 [1.03–14.14] | 0.040 | 1.95 [0.46–8.35] | 0.367 |
| Inhaled drugs use [Ref Never] | 1.00 | 1.00 | ||
| Past | 1.27 [0.78–2.09] | 0.338 | 0.96 [0.85–1.09] | 0.553 |
| Current | 1.07 [0.58–1.97] | 0.821 | 0.98 [0.86–1.13] | 0.814 |
| Smoked drugs use [Ref Never] | 1.00 | 1.00 | ||
| Past | 2.11 [1.14–3.95] | 0.018 | 2.29 [1.30–4.01] | 0.004 |
| Current | 1.79 [1.02–3.16] | 0.043 | 1.56 [1.13–2.15] | 0.006 |
| Cigarette smoking [Ref Never] | 1.00 | 1.00 | ||
| Past | 0.91 [0.45–1.86] | 0.802 | 0.91 [0.64–1.29] | 0.593 |
| Current | 1.48 [0.88–2.51] | 0.142 | 1.23 [1.10–1.38] | < 0.001 |
| BCG scar | 1.41 [0.77–2.59] | 0.265 | 1.36 [0.89–2.06] | 0.151 |
| Contact with a TB case | 1.61 [1.03–2.53] | 0.036 | 1.31 [0.74–2.33] | 0.354 |
aRR = adjusted risk ratio; BCG = Bacillus Calmette–Guerin vaccine; TB = tuberculosis.
aRR are based on based on Poisson regression.
The standard errors or CI are adjusted for cluster effect (prisons) in the bivariable and multivariable models. The multivariable model was adjusted by BCG vaccination. Body mass index was not included because there were a high number of participants (40%) with missing data.
DISCUSSION
In this study, we estimated the incidence and factors associated with LTBI and active TB in Colombian prisons. We observed that individuals who were recently incarcerated had high risk of acquiring LTBI. It suggests that identifying a group, which has increased risk of TB acquisition, provides an opportunity for intervention, through screening of PDL for LTBI upon prison entry, combined with repeated screening for PDL who are negative every year, and offering LTBI treatment to new converters. As previously reported by our group,12 the number of PDL needed to screen to detect one new LTBI case is low (one new LTBI in every 3.4 people that have a negative TST at baseline).
Prisons have several factors that influence TB transmission, that is, overcrowding conditions that cause closer proximity and longer duration of contact between an active TB case and susceptible individuals, delay in the diagnosis and TB treatment initiation, limited air circulation, high humidity, minimal ultraviolet (UV) light exposure, and host factors.6,7,20 Urrego et al.,20 reported that the mean estimated risk of TB transmission, in three prisons in Brazil, after exposure for 180 days to an infectious cellmate under the existing condition (combined mean ventilation of 19.4 L/s/person) was 65.6–81.1% depending on the prison, and the risk between cells ranges from 14.2% to 99.9%, variability due to different ventilation conditions. On the other hand, new infections in people with short incarceration may occur more frequently in individuals who are more susceptible as a result of innate dysfunction that may be inherited or acquired.
In our cohort of TST negative PDL, the cumulative percentage of new LTBI was similar to reports from LMICs like Brazil, where the cumulative incidence was 25.66% at 1 year of incarceration in 12 prisons,21 and 29% at 6 months in a female penitentiary.22 These values were higher than other studies that reported 7.6% of incident cases at 6 months in Iran,23 0.23% between 2004 and 2009 in United States,24 and 0.43% during 2 years in Japan.25
The prevalence of LTBI was high in PDL with long incarceration (70.45%) compared with the prevalence upon entering prison (46.38%). The prevalence at entry was similar to that reported by del Corral et al. (42.70%), which studied general population in Medellin, Colombia,26 highlighting the importance of infection transmission inside prisons. Prevalence among individuals who have spent more than 1 year of incarceration was similar to previous results from the same prisons reported by Rueda et al.,9 and to the results reported by Guerra et al. in Guaduas, another Colombian prison, (67.60%).27 Other researchers have also reported high LTBI prevalence in PDL in Iran (62.58%),23 Brazil (73%),28 and Malaysia (87.60–88.81%).29,30
Presence of a BCG scar was the only protective factor against LTBI acquisition among those with short incarceration in the multivariable model. The presence of a BCG scar has been reported as a protective factor of conversion in QuantiFERON-TB Gold In-Tube (QFT-GIF) (adjusted odds ratio [aOR] 0.53 95% CI 0.28–1.00) among physicians in China,31 in adult contacts of TB in a United Kingdom cohort (aOR 0.70; 0.56–0.87),32 and in HIV negative inmates from Taiwan, where QFT-IT results inversely correlated with the percentage of BCG scar, and the authors suggested that BCG vaccine seems to have a protective effect against LTBI.33 We acknowledge that BCG vaccination can boost TST results. However, the boosting effect lasts few years and is not likely to play a role in adults, as BCG vaccination in Colombia is administered at birth. Our findings are in agreement with other studies showing long-term protection provided by BCG vaccination in a retrospective population-based cohort study in Norway,34 and in Colombian general population.35
An important consideration is that most people incarcerated in these prisons are young individuals, who have other risk factors such as poor nutrition, and drug and alcohol abuse, which predisposes them to development of active TB. Additionally, from a broader perspective, infected young PDL become part of the LTBI reservoir and may later in life reactivate, contributing to TB transmission during their lifetime.36 Prior incarceration has been shown to increase the risk of TB (OR 12.42 [3.57–43.22]).37 This observation suggests that decreasing new infections among young PDL presents an opportunity to decrease the overall burden of TB.
Our study shows that 25% of all TB diagnoses occurred within 11 months of incarceration, and 75% of all TB cases had 42.2 months or less of incarceration, with an incidence rate of 31/1,000 person-years. This finding draws attention to the increased risk of progressing to active TB in people in prison. A systematic review published by Campbell et al., reported an incidence rate of TB in PDL with a positive TST of 45/1,000 person years, compared with silicosis (36.9/1,000 person-years), people living with HIV (PLHIV) (27.1/1,000 person-years) and previous contact with a known TB case (9.4–23.4/1,000 person-years). This finding demonstrates that PDL are one of the groups at highest risk of TB. In addition, Campbell et al. evaluated the incidence rate ratio (IRR) for TB after having a positive LTBI test (TST, interferon-gamma release assays, or both) in populations at risk according to the WHO. People deprived of liberty had an IRR (TST positive ≥ 10 mm/TST negative) of 31.0 [95% CI 4.1–233.9], which was higher than the IRR of PLHIV (11.1 [6.2–19.9]), those who had close and casual TB contacts (6.9 [3.3–14.4]), those who were recent immigrants (4.0 [2.1–7.9]), people with silicosis (1.7 [0.5–5.5]), and people who were on hemodialysis (2.6 [1.4–4.8]).38 The authors suggest that diagnostic tests for LTBI are useful in discriminating between people at higher and lower risk for TB.38 We agree that people in prisons must be screened for LTBI ideally performed at the time of entry to prison. However, in the prisons we studied, nearly half of the population had a positive TST at the time of incarceration, with an additional 25% becoming positive during the first 2 years of incarceration. The option of treating nearly half of newly incarcerated individuals is currently not feasible and not pursued in many LMICs.
Previous studies that looked at the risk factors associated with pulmonary TB that were conducted in PDL in the Democratic Republic of Congo,39 Cameroon,40 Brazil,41 Thailand,42 and Tajikistan16 showed that HIV infection,16,42 diabetes, malnutrition (BMI < 18.50 kg/m2),39,40 and the use of psychoactive substances,16,41 increased the risk of active TB. The last one was found in our study (smoked drugs) as associated with an increased risk of active TB. In addition, previously,39,42 duration of incarceration equal to or greater than 12 months was a significant risk factor for developing active TB. Incarceration may be associated with rapid changes in nutrition and weight, increased stress, and consequently alter the immune status of PDL,43 increasing the risk for active TB.
An additional important observation was the ability of screening for respiratory symptoms to identify individuals who are more likely to have active TB diagnosed. Conversely, among individuals without respiratory symptoms on repeated questioning, diagnosis of active TB was rare (4 over a 10-year period).
Additional factors reported in the literature that protect against or predispose individuals to LTBI and active TB, such as overcrowding,39 sex,44 and race,41 were not included in our models because of the fact that these variables were constant in our population. All PDL lived in overcrowded conditions (the mean living area per person was 1.28 ± 3.05 m2),11 100% of the people included in the LTBI studies were male, and in Colombia most people have a mixed genetic background.45
People deprived of liberty are frequently in contact with internal (other PDL, guards) and external (family, visitors) TB cases. Previous contact with a known TB case occurred in 18.4% and 22.9% of individuals with LTBI and active TB, respectively. Although previous contact was not significant in the model, we consider it an important albeit difficult to evaluate given the prisońs conditions. Being exposed to a TB case can occur frequently and go unnoticed in the context of crowded environment such as prisons. Continued contact with the community through prison staff, the frequent reentry and short stay and transfer, and the high prevalence of TB, make prisons a reservoir of TB, that may result in the amplification of infection and disease in the community.46 Undoubtedly, the management of TB within prisons would benefit TB control in the community.
Recently, Drain et al.47 proposed two intermediate states between LTBI and TB, “incipient TB infection” and “subclinical TB disease.” Both states are associated with prior MTB exposure, the person has viable MTB pathogen and MTB has metabolic activity—“incipient TB infection,” and “subclinical TB disease” has radiographic abnormalities or microbiological evidence of active MTB without clinical symptoms. In our studies, we could not evaluate all LTBI with chest X-ray or with microbiological cultures because of limited resources, which means that there could be people with subclinical TB disease, therefore, the TB incidence might be higher than we are reporting in this study.
Our results show that PDL, particularly those who had been recently incarcerated, are at increased risk for LTBI and for developing active TB and that a relatively simple, active case finding with respiratory symptoms screening may help to identify individuals who are at risk for developing active TB. We therefore propose, based on a previous study12 and on the high LTBI incidence during the first years of incarceration, that international and Colombian guidelines include “LTBI testing should be performed” instead of “should be considered” in PDL at the time of incarceration. In addition, we propose that follow-up screening be conducted each year in individuals who had a negative baseline LTBI screening result. When a new LTBI case is detected, LTBI treatment should be offered to decrease the incidence of active TB and new infections. In addition, follow-ups should be implemented for individuals who had an initial positive TST, especially in the presence of risk factors, to ensure a rapid diagnosis of active TB in the presence of any respiratory symptoms of any duration.
Supplemental tables
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1. World Health Organization , 2020. Global Tuberculosis Report 2020. Geneva, Switzerland: WHO. Available at: https://www.who.int/publications/i/item/9789240013131. Accessed March 3, 2021. [Google Scholar]
- 2. World Health Organization , 2014. The End TB Strategy. Geneva, Switzerland: WHO. Available at: http://www.who.int/tb/strategy/en/. Accessed March 9, 2020. [Google Scholar]
- 3.Cords O, Martinez L, Warren JL, O’Marr JM, Walter KS, Cohen T, Zheng J, Ko AI, Croda J, Andrews JR, 2021. Incidence and prevalence of tuberculosis in incarcerated populations: a systematic review and meta-analysis. Lancet Public Health 6: e300–e308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dara M. et al. 2015. Tuberculosis control in prisons: current situation and research gaps. Int J Infect Dis 32: 111–117. [DOI] [PubMed] [Google Scholar]
- 5.Baussano I, Williams BG, Nunn P, Beggiato M, Fedeli U, Scano F, 2010. Tuberculosis incidence in prisons: a systematic review. PLOS Med 7: e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melchers NVSV, van Elsland SL, Lange JMA, Borgdorff MW, van den Hombergh J, 2013. State of affairs of tuberculosis in prison facilities: a systematic review of screening practices and recommendations for best TB control. PLOS ONE 8: e53644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathema B, Andrews JR, Cohen T, Borgdorff MW, Behr M, Glynn JR, Rustomjee R, Silk BJ, Wood R, 2017. Drivers of tuberculosis transmission. J Infect Dis 216: S644–S653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dara M. et al. 2015. Tuberculosis control in prisons: current situation and research gaps. Int J Infect Dis 32: 111–117. [DOI] [PubMed] [Google Scholar]
- 9.Rueda ZV, Arroyave L, Marin D, López L, Keynan Y, Giraldo MR, Pulido H, Arbeláez MP, 2014. High prevalence and risk factors associated with latent tuberculous infection in two Colombian prisons. Int J Tuberc Lung Dis 18: 1166–1171. [DOI] [PubMed] [Google Scholar]
- 10. WHO-Europe , 2018. Good Practices in the Prevention and Care of Tuberculosis and Drug-Resistant Tuberculosis in Correctional Facilities. Available at: http://www.euro.who.int/__data/assets/pdf_file/0003/360543/TB-prisons-9789289052917-eng.PDF?ua=1. Accessed December 2, 2021.
- 11.Rueda ZV, López L, Vélez LA, Marín D, Giraldo MR, Pulido H, Orozco LC, Montes F, Arbeláez MP, 2013. High incidence of tuberculosis, low sensitivity of current diagnostic scheme and prolonged culture positivity in four Colombian prisons. A cohort study. PLoS One 8: e80592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arroyave L, Keynan Y, López L, Marin D, Arbeláez MP, Rueda ZV, 2017. Negative latent tuberculosis at time of incarceration: identifying a very high-risk group for infection. Epidemiol Infect 145: 2491–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. CDC , 2021. TB | Hojas Informativas-Pruebas de Tuberculosis. Available at: http://www.cdc.gov/tb/esp/publications/factsheets/testing/skintesting_es.htm. Accessed July 21, 2021.
- 14.Gizachew Beza M, Hunegnaw E, Tiruneh M, 2017. Prevalence and associated factors of tuberculosis in prisons settings of east Gojjam Zone, northwest Ethiopia. Int J Bacteriol 2017: 3826980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Séri B. et al. 2017. Prevalence of pulmonary tuberculosis among prison inmates: a cross-sectional survey at the correctional and detention facility of Abidjan, Côte d’Ivoire. PLoS One 12: e0181995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winetsky DE, Almukhamedov O, Pulatov D, Vezhnina N, Dooronbekova A, Zhussupov B, 2014. Prevalence, risk factors and social context of active pulmonary tuberculosis among prison inmates in Tajikistan. PLoS One 9: e86046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuge TG, Ayanto SY, 2016. Prevalence of smear positive pulmonary tuberculosis and associated risk factors among prisoners in Hadiya Zone prison, southern Ethiopia. BMC Res Notes 9: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navarro PD, Almeida IN, Kritski AL, Ceccato MD, Maciel MM, Carvalho WD, Miranda SS, 2016. Prevalence of latent Mycobacterium tuberculosis infection in prisoners. J Bras Pneumol 42: 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguilera XP. et al. 2016. Tuberculosis in prisoners and their contacts in Chile: estimating incidence and latent infection. Int J Tuberc Lung Dis 20: 63–70. [DOI] [PubMed] [Google Scholar]
- 20.Urrego J, Ko AI, da Silva Santos Carbone A, Paião DSG, Sgarbi RVE, Yeckel CW, Andrews JR, Croda J, 2015. The impact of ventilation and early diagnosis on tuberculosis transmission in Brazilian prisons. Am J Trop Med Hyg 93: 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paião DSLE, da Silva FMBL, Martins VSSS, Pompílio MA, Urrego J, Ko AI, Andrews JR, Croda J, 2016. Impact of mass-screening on tuberculosis incidence in a prospective cohort of Brazilian prisoners. BMC Infect Dis 16: 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira MM. et al. 1996. Tuberculosis and HIV infection among female inmates in São Paulo, Brazil: a prospective cohort study. J Acquir Immune Defic Syndr Hum Retrovirol 13: 177–183. [DOI] [PubMed] [Google Scholar]
- 23.Mamani M, Mahmudian H, Majzoobi MM, Poorolajal J, 2016. Prevalence and incidence rates of latent tuberculous infection in a large prison in Iran. Int J Tuberc Lung Dis 20: 1072–1077. [DOI] [PubMed] [Google Scholar]
- 24.Weant TE, Turner AN, Murphy-Weiss M, Murray DM, Wang S-H, 2012. Can social history variables predict prison inmates’ risk for latent tuberculosis infection? Tuberc Res Treat 2012: 132406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawatsu L, Uchimura K, Ohkado A, 2018. A situational analysis of latent tuberculosis infection among incarcerated population in Japan. PLoS One 13: e0203815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.del Corral H. et al. 2009. IFNgamma response to Mycobacterium tuberculosis, risk of infection and disease in household contacts of tuberculosis patients in Colombia. PLoS One 4: e8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guerra J, Mogollón D, González D, Sanchez R, Rueda ZV, Parra-López CA, Murcia MI, 2019. Active and latent tuberculosis among inmates in La Esperanza prison in Guaduas, Colombia. PLoS One 14: e0209895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nogueira PA, de Melo Abrahão RMC, Galesi VMN, 2012. Tuberculosis and latent tuberculosis in prison inmates. Rev Saude Publica 46: 119–127. [DOI] [PubMed] [Google Scholar]
- 29.Al-Darraji HAA, Kamarulzaman A, Altice FL, 2014. Latent tuberculosis infection in a Malaysian prison: implications for a comprehensive integrated control program in prisons. BMC Public Health 14: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margolis B, Al-Darraji HAA, Wickersham JA, Kamarulzaman A, Altice FL, 2013. Prevalence of tuberculosis symptoms and latent tuberculous infection among prisoners in northeastern Malaysia. Int J Tuberc Lung Dis 17: 1538–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He G. et al. 2015. The prevalence and incidence of latent tuberculosis infection and its associated factors among village doctors in China. PLoS One 10: e0124097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katelaris AL, Jackson C, Southern J, Gupta RK, Drobniewski F, Lalvani A, Lipman M, Mangtani P, Abubakar I, 2020. Effectiveness of BCG vaccination against Mycobacterium tuberculosis infection in adults: a cross-sectional analysis of a UK-based cohort. J Infect Dis 221: 146–155. [DOI] [PubMed] [Google Scholar]
- 33.Chan P-C, Yang C-H, Chang L-Y, Wang K-F, Kuo Y-C, Lin C-J, Lee S-W, Hsueh P-R, Fang C-T, Huang L-M, 2013. Lower prevalence of tuberculosis infection in BCG vaccinees: a cross-sectional study in adult prison inmates. Thorax 68: 263–268. [DOI] [PubMed] [Google Scholar]
- 34.Nguipdop-Djomo P, Heldal E, Rodrigues LC, Abubakar I, Mangtani P, 2016. Duration of BCG protection against tuberculosis and change in effectiveness with time since vaccination in Norway: a retrospective population-based cohort study. Lancet Infect Dis 16: 219–226. [DOI] [PubMed] [Google Scholar]
- 35.Marín D, Marín N, del Corral H, López L, Ramirez-Agudelo ME, Rojas CA, Arbeláez MP, García LF, Rojas M, 2017. PPD-induced monocyte mitochondrial damage is associated with a protective effect to develop tuberculosis in BCG vaccinated individuals: a cohort study. PLoS One 12: e0171930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy A. et al. 2014. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ 349: g4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tekkel M, Rahu M, Loit HM, Baburin A, 2002. Risk factors for pulmonary tuberculosis in Estonia. Int J Tuberc Lung Dis 6: 887–894. [PubMed]
- 38.Campbell JR, Winters N, Menzies D, 2020. Absolute risk of tuberculosis among untreated populations with a positive tuberculin skin test or interferon-gamma release assay result: systematic review and meta-analysis. BMJ 368: m549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalonji GM, De Connick G, Okenge Ngongo L, Kazumba Nsaka D, Kabengele T, Tshimungu Kandolo F, Ilunga-Ilunga F, Adelin A, Giet D, 2016. Prevalence of tuberculosis and associated risk factors in the central prison of Mbuji-Mayi, Democratic Republic of Congo. Trop Med Health 44: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noeske J, Ndi N, Mbondi S, 2011. Contrôle de la tuberculose dans les prisons face aux conditions de confi nement: une cause perdue? L’expérience du Cameroun. Int J Tuberc Lung Dis 15: 223–227. [PubMed] [Google Scholar]
- 41.Maceda EB, Gonçalves CCM, Andrews JR, Ko AI, Yeckel CW, Croda J, 2018. Serum vitamin D levels and risk of prevalent tuberculosis, incident tuberculosis and tuberculin skin test conversion among prisoners. Sci Rep 8: 997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morasert T, Worapas W, Kaewmahit R, Uphala W, 2018. Prevalence and risk factors associated with tuberculosis disease in Suratthani Central Prison, Thailand. Int J Tuberc Lung Dis 22: 1203–1209. [DOI] [PubMed] [Google Scholar]
- 43.Massoglia M, Pridemore WA, 2015. Incarceration and health. Annu Rev Sociol 41: 291–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitaker JA, Mirtskhulava V, Kipiani M, Harris DA, Tabagari N, Kempker RR, Blumberg HM, 2013. Prevalence and incidence of latent tuberculosis infection in Georgian healthcare workers. PLoS One 8: e58202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ossa H. et al. 2016. Outlining the ancestry landscape of Colombian admixed populations. PLoS One 11: e0164414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bick JA, 2007. Infection control in jails and prisons. Clin Infect Dis 45: 1047–1055. [DOI] [PubMed] [Google Scholar]
- 47.Drain PK, Bajema KL, Dowdy D, Dheda K, Naidoo K, Schumacher SG, Ma S, Meermeier E, Lewinsohn DM, Sherman DR, 2018. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev 31: e00021–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.