ABSTRACT.
Tuberculosis (TB) remains a global problem and a diagnostic challenge, especially in pediatrics. The aim of this study was to describe the clinical, microbiological, radiological, and histopathological data of TB in children. A 7-year retrospective and descriptive cohort study that included 127 patients under 18 years of age with diagnosis of active TB was conducted from 2011 to 2018 in a pediatric hospital. Tuberculosis was microbiologically confirmed using Ziehl–Neelsen (ZN) staining, culture or polymerase chain reaction (PCR) in a total of 94 (74%) cases. Thirty-three cases were defined as probable TB based on tuberculin skin test result and epidemiological evaluation. The TB forms found were lymph node (39.3%), bone (15.7%), lung (13.6%), and meningeal TB (8.6%). The most common symptoms were fever (48.8%) and adenopathy (45.6%). History of contact was established in 34.6%. Positive ZN staining (sensitivity 30%) and culture (sensitivity 37%) were found in 29% and 37.7% of subjects, respectively. About 64.5% depicted abnormal chest X-ray. Xpert MTB/RIF® (PCR) was positive in 9.4% and biopsy was compatible in 52.7% of these samples. It is fundamental to have laboratory and epidemiological evaluation that support the diagnosis of the disease in children and thus, define its management; since, in most cases, early microbiologic confirmation is lacking.
INTRODUCTION
Tuberculosis (TB) is a global health problem with a higher mortality rate than that reported for HIV. According to the latest report of the WHO, a total of 10 million TB cases consisting of 5.8 million men, 3.2 million women and 1.0 million children were registered in 2017.1 The report also showed that 90% of these cases were adults (aged ≥ 15 years) and that 75% of all the cases occurred in developing countries.1 International travel and massive movement of people from different geographical areas, especially from low-income countries to developed countries and vice versa, have led to the modification of TB epidemiology by exposing healthy individuals including non-vaccinated children to sick people.2,3
Tuberculosis diagnosis in pediatric age is a challenge because of its insidious onset and lack of constitutional signs and symptoms in up to 72%.4 Diagnosis of TB, based on international guidelines and epidemiological studies, considers clinical data that are supported by purified protein derivative (PPD), epidemiological contact, radiological and microbiological studies, record of vaccination with Bacillus of Calmette-Guérin (BCG) and specifically, histopathological studies, as dictated by the Centers for Disease Control and Prevention and the Pan American Health Organization regulated by the WHO.
The present study is a 7-year retrospective analysis of pediatric TB patients at a tertiary hospital, with the objective of describing the epidemiological, clinical, microbiological, radiological, and histopathological data of the disease in this population group.
METHODS
Retrospectively, we reviewed clinical records of all the patients under 18 years of age who were diagnosed with TB at the Clinic of Tuberculosis, Department of Pediatric Infectious Diseases in the period from January 2011 to April 2018. The hospital center has a unique outpatient program in Pediatric Tuberculosis, which permits the centralization of all patients with diagnosis of TB in the clinic for direct management by experts in the disease. All data were collected from the clinical files of the patients and include clinical and sociodemographical data, imaging studies, epidemiological contact, microbiological studies, the PPDs performed, and history of BCG vaccination (Table 2). In addition, information on the treatment and the final outcome at the end of the same were collected. Diagnostic data extracted included application of 2 international units (IU) of PDD, read at 72 hours (positive ≥ 15 mm); Ziehl–Neelsen (ZN) staining and/or culture for Mycobacterium tuberculosis (MTB) in Lowenstein–Jensen medium and/or liquid medium; Xpert MTB/RIF® polymerase chain reaction (PCR) test; and data of caseous necrosis with chronic granulomatous inflammation in the histopathological report. In all cases, assessment by the Immunology Service was requested to rule out the presence of primary immunodeficiency (PID).
Table 2.
Demographic data and clinical characteristics of patients with tuberculosis included in the study
| Characteristics | Number of cases | Percentage (%) |
|---|---|---|
| Age | ||
| ≤ 5 years | 60 | 47.2 |
| 6–10 years | 32 | 25.1 |
| 11–13 years | 14 | 11 |
| 14–16 years | 21 | 16.5 |
| Application of BCG | 88 | 69.2 |
| Primary Immunodeficiency | ||
| CGD | 37 | 29.1 |
| Axis defect | 16 | 12.5 |
| Severe combined | 10 | 7.7 |
| immunodeficiency | ||
| Variable common | 7 | 5.5 |
| immunodeficiency | 1 | 0.7 |
| Cyclic neutropenia | 1 | 0.7 |
| NEMO defect | 1 | 0.7 |
| Hemato-oncology | 2 | 1.6 |
| Use of immunosuppressants | 4 | 3.1 |
N = 127 patients. BCG = Bacillus of Calmette-Guérin; NEMO = cyclic neutropenia and nuclear factor-kappa B essential modulator; CGD = chronic granulomatous disease 18.
Statistical analysis.
All the data obtained were statistically analyzed with SPSS system, version 21 (IBM). Descriptive statistics and χ2 were used to report the population characteristics. The categorical variables were reported in percentages, whereas for the continuous variables, the most appropriate were measures of central tendency and dispersion (mean, mode, median, SD, and interquartile range). P < 0.05 was considered statistically significant.
Ethical considerations.
In the realization of this study, we counted on the authorization and support of the ethics committee and prevention of conflicts of interest (CEPCI) of the National Institute of Pediatrics (NIP). Ethics approbation and consent to participate was authorized by the Academic College of the National Institute of Pediatrics.
Definition of the cases.
The cases were admitted under two case-definitions: confirmed case and probable case. Confirmed case was defined as the presence of one or two of the following: positive isolation of Mycobacterium spp., positive ZN, compatible histopathological data and positive Xpert MTB/RIF®. Probable case was defined as the presence of two or more positive data such as patient with consistent clinical data (cough, fever, and weight loss), compatible pulmonary or extrapulmonary magnetic resonance imaging (MRI) and/or radiography plus the symptoms, history of contact with a case of active TB, PPD ≥ 0 mm and favorable evolution with the onset of empirical anti-TB treatment.
RESULTS
Sociodemographic characteristics.
A total of 127 cases of active TB were diagnosed. 94 (74%) were defined as confirmed cases and 33 (25%) as probable cases. Fifty-eight percent (74/127) of the cases were male and 42% (53/127) were female. Only 96 patients received BCG vaccine.
Of the total cases that received the BCG vaccine (75.5%), the application was done at birth in 98%, one patient at 1 month and another at 6 months. About 29.1% (37/127) of the patients had a baseline condition related to PID and 1.6% (2/127) to hematoncology.
The average age at diagnosis was 6.5 years. In particular, 47.2% (60/127) of the cases had less than 5 years old. The age of presentation according to type was miliary TB, 9 years; skin TB, 8 years; abdominal Tb, 7 years; lung TB, 7 years; lymph node TB, 5 years, meningeal TB, 6 years; and musculoskeletal TB, 4 years. No relationship was found between TB type and sex or age of the patients (P > 0.05).
Location of TB.
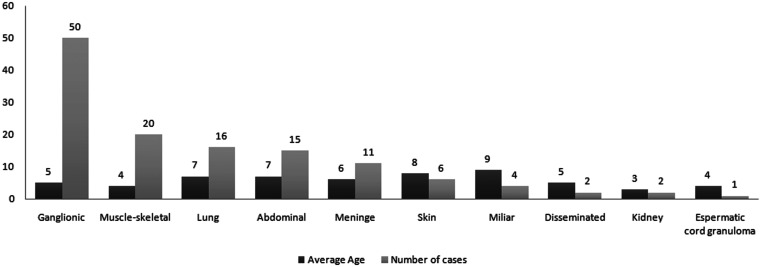
The extrapulmonary forms of TB were the most frequent (87.5% versus 12.5%). The most common extrapulmonary manifestations were lymph node (50 cases, 39%) followed by musculoskeletal form (20 cases, 15.7%), the rest is shown in Figure 1.
Figure 1.
Extrapulmonary forms of tuberculosis and ages (mean) of patients. The most common were ganglionic (50 cases, 39%) followed by muscle-skeletal form (20 cases, 15.7%).
According to the areas of bone affectation, vertebral TB was the most frequent (33% of the cases) followed by elbow and hip joints in 19% and long bones in 14%. Sporadic cases were found in the talus, maxilla, rib cage, and iliac bones. With regard to the location of atypical Mycobacteria, all cases were extrapulmonary forms, with lymph node involvement in 83% of the cases (15/18) in comparison to 39% in MTB complex. One case of renal TB and another of disseminated TB due to M. gordonae and M. avium, respectively, were documented.
Clinical presentation.
Table 1 summarizes the principal clinical and microbiological characteristics of all the patients with active TB. Depending on TB type, the most frequent signs and symptoms registered were fever (62, 48.8%) and adenopathy (58, 45.6%). Other clinical characteristic findings, based on the anatomic site affected in our analyzed cases, were fever in 100% of the cases of abdominal TB, 83% in skin TB, and 81% in lung TB. Other symptoms reported with lesser frequency were pain in 11%, edema in 6.8%, discharge of secretion in the affected area in 6.8% and dyspnea in 5.1%. Regarding cases of M. avium complex infection, adenopathy was documented in all cases.
Contact history.
Of all the cases, contact was not established in 34.6% (44/127) in questions regarding TB contacts obtained from the family. Of the history of positive contact, the most common contacts were with siblings in 28%, grandparents in 24%, father in 20%, and both the mother and uncles in 12%. Statistically, there was no relationship between the age and type of TB with history of positive contact. Regarding PPD, 42 patients underwent the test and it was negative in 22 of the cases (nine cases with skin TB, five with musculoskeletal TB, four with lymph node TB, two with miliary TB, and two with lung TB). PPD sensitivity was 66%. No study of Quantiferon was performed in any of the patients.
Microbiology.
The diagnosis of active TB was microbiologically confirmed using ZN staining and culture or PCR in a total of 94 (74%) cases. The positive results confirmed in this study were: culture, 53 cases (sensitivity 37%) out of which five also had positive PCR; Ziehl-Nelseen staining, 37 cases (sensitivity 30%); and PCR, 37 (29%) cases without report of resistance to rifampicin (Table 1). Only one child had multidrug-resistant TB. With regard to the isolates, the most prevalent was Mycobacterium bovis (MBV) in 54% (69/127) of the cases (including two cases of infection with vaccine strain Tokio 172) followed by MTB in 14.3% (18/127), atypical Mycobacteria (M. gordonae, M. avium complex, and M. noncrhomogenicum) in 14.3% (18/127) and MTB complex in 19% (24/127).
Table 1.
Clinic signs and microbiological results assayed in patients of all the patients with active tuberculosis
| Characteristic | Number of cases | Percentage (%) |
|---|---|---|
| Clinic signs | ||
| Fever | 62 | 48.8 |
| Adenopathy | 58 | 45.6 |
| Cough | 31 | 24.4 |
| Weight loss | 22 | 17.3 |
| Weakness and fatigue | 22/20 | 17.3/15.7 |
| Positive microbiological findings | ||
| Xpert/RIF PCR | 12 | 9.4 |
| MTB Culture | 48 | 37.7 |
| ZN staining | 37 | 29 |
| Data supporting TB diagnosis | ||
| PPD | 20 | 47.6 |
| History of contact | 44 | 34.6 |
| Radiological findings | 82 | 64.5 |
| Outcome | ||
| Alive | 114 | 89.7 |
| Died | 3 | 2.3 |
| Lost to follow-up | 10 | 7.8 |
N = 127 patients. MTB = Mycobacterium tuberculosis; PCR = polymerase chain reaction; PPD = purified protein derivative; ZN = Ziehl–Neelsen.
Imaging studies.
Compatible radiological findings were 42.5% by chest radiography and 40.7% by chest/brain tomography. Images with lymph node growth, extrinsic bronchial obstruction, atelectasis, lobe medium syndrome or hyper reactivity bronchial are suggestive of TB. Regarding musculoskeletal TB, the most common findings were osteolytic lesions in all the cases; abscess in 70% (89/127) of the cases and the triad of juxta-articular osteoporosis, peripheral bone erosion and decreased joint space in 17% (22/127) of the patients. In the cases with vertebral TB, the most frequent findings were osteolytic lesion, xiphosis, and spinal cord compression in all the cases followed by vertebral abscess (Pott’s disease) in 70%. For the cases with abdominal TB, the findings most frequent were intra-abdominal adenopathy (67 cases, 53%), abdominal mass (55 cases, 40%), ascites (42 cases, 33.3%), calcifications (34 cases, 26.6%), and thickening of the intestinal wall (34 cases, 26.6%).
Treatment.
Treatment was successful in 97.4% of the cases. Three cases (2.3%), out of which two had confirmed PID, died of TB as a direct cause. The first-line anti-TB drugs were used in 73.5% of the cases. Other drugs used, either concomitantly or to replace the primary medication because of its lack, side effect, drug interaction, or drug resistance of type of Mycobacterium (especially MBV); were given as follows: clarithromycin (5.9%), ciprofloxacin (4.4%), levofloxacin (4.4%), streptomycin (2.9%), and amikacin (1.7%).
With regard to atypical Mycobacteria, the first-line anti-TB drugs plus clarithromycin was used in M. avium cases. Cases of M. noncrhomogenicum and M. gordonae were treated with a scheme of first-line anti-TB drugs plus ciprofloxacin. Antimycobacterial drugs were withdrawn after 9 months, with no deaths documented. Adverse effects (paresthesia, cholestatic syndrome, and optic neuritis) were reported in 4.2% of the cases. The median duration for the intensive phase was 3 (2–5) months and 4 (3–4) months for maintenance phase. The median of the total duration treatment was 9 (6–14) months. Ten (7.8%) patients abandoned the treatment despite medical opinion.
In addition to medical treatment, surgical procedures were performed. Ten cases with bone TB were subjected to curettage, cryotherapy, and/or bone substitute placement. All the cases of vertebral TB required the stabilization with debridement and drainage of abscess. In the cases of abdominal TB, laparotomy was performed in 12 (80%), intestinal resection in eight (53.3%), and ileostomy in five (33.3%).
Immunodeficiency.
Among the most common PIDs found were 1) chronic granulomatous disease in 16 cases, 2) interleukin axis defect in 12, with gamma interferon accounting for 10 cases, and 3) severe combined immunodeficiency in seven cases. In an isolated way, a case related to variable common immunodeficiency, cyclic neutropenia, and nuclear factor-kappa B essential modulator (NEMO) defect was reported. In two (1.6%) cases of the total population, the immunodeficiency was secondary to hematological processes. In this study, lymphocytic leukemia was more frequently found.
The prevalence of confirmed immunodeficiency (0–83%) was variable depending on the type of TB. Skin and miliary TB were the most associated with immunodeficiency with 50% and 83%, respectively. Seventy-eight percent of the chronic granulomatous diseases were confirmed in patients diagnosed with lymph node TB and 80% of severe combined immunodeficiency in the skin TB cases. In the present study, none of the cases presented concomitant infection by HIV.
DISCUSSION
TB remains a global problem and a diagnostic challenge, especially in pediatrics. In this study, a population with confirmed and probable diagnosis of TB attended at pediatric hospital, during a period of 7 years with a total of 127 cases were considered. About 47.2% of the cases were in children under 5 years of age, which corresponds to that reported in the world literature because of the high risk of disease progression at younger ages.5,6 In the present study, it was found that half of the patients had fever and one of every four cases (25%) had cough, findings that were similar to that reported by Cruz and Starke.7 Other constitutional symptoms such as loss of weight and weakness and fatigue were found in 18.6% and 16.9%, respectively. This finding coincides with the findings of other authors.8,9 History of contact was positive in 34.6% of the cases. Our results emphasize that the absence of constitutional symptoms or history of negative contact does not rule out TB in differential diagnosis, especially when we take into consideration that the characteristic clinical picture of TB is not very frequent in pediatric age.
Microbiological confirmation (culture, Ziehl–Neelsen staining, or PCR) was possible in 82.4% of the cases. Culture had sensitivity of 40%; however, in the patients with lung TB, sputum cultures were positive in 6 of the 16 cases (37.5%). Ziehl–Neelsen staining had a sensitivity of 30%. The diagnosis was histopathological confirmed in 52.7% of the total cases, but in patients with oseo-articular TB, the histopathological confirmation was in 100% of the cases. Xpert/MTB RIF was performed in 33 cases with 12 positive results (sensitivity of 53%). This study was not available in 94 cases. Among the isolates identified, the presence of two cases of osteitis by the strain MBV of the BCG vaccine substrain Tokyo 172, previously not reported, draws attention. Our country has a national immunization program with BCG applied at birth in 99% of the children.10 In the present study, there was only one case of multidrug resistance (MDR) in a patient with abdominal TB by wild MBV, which represented < 1% of the cases. Multi-drug resistance in pediatric age have a global annual incidence of 35 thousand cases therefore, our study is within the average reported worldwide.11,12 Quantiferon was not performed in our study; however, some studies have reported indeterminate results, which vary between 0% and 17%, and in children under 4 years, the indeterminate results have been as high as 30%.13–15 Therefore, the value of quantiferon versus PPD in children under 4 years of age should be an area of active investigation.
All the patients in our cases underwent studies in search of PID, chronic granulomatous diseases, interleukin 12/interferon, gamma axis defect, and severe combined immunodeficiency or acquired immunodeficiency. Twenty-nine percent of the cases presented PID, mainly chronic granulomatous diseases (CGD), found in 43.2%. Of the total CGD cases, 78% of them were lymph node TB, a result that is similar to what was reported in the literature.16 The findings of PID were more common in miliary and skin TB with 50% and 83%, respectively. Tuberculosis treatment protocols in our institution are based on the WHO recommendation, which consist of 2 months intensive phase and 4 months of maintenance phase for pulmonary forms, and up to 12 months for extrapulmonary forms. However, TB management in immunocompromised children has not been well established. In this study, the total treatment was 6.3 ± 7.5 months for intensive phase and 10.1 ± 6.3 months for maintenance phase, contrary to the international guidelines that mark as standard treatment a daily administration in the intensive phase of 60 doses with four drugs and a time–time administration per week for maintenance phase of 45 doses with two drugs. This variation might be associated with a large sample of immunocompromised children (39%).
One limitation of the study is a prospective designed to control the variables between groups. Differences between MBV, MTB complexes and nontuberculous mycobacteria might be addressed with a larger sample size.
CONCLUSIONS
Tuberculosis in pediatrics presents a diagnostic challenge since it does not present the characteristic data reported in the literature in adult population. A high clinical suspicion should be maintained when there are epidemiological data and socioeconomic factors that favor its presentation. This is important to avoid late diagnosis since this condition leads to an increase in the morbidity and mortality of the patients. In all cases with diagnosis of TB, efforts in search of immunodeficiencies should be made, because this increases the difficulty of its management and prognosis.
ACKNOWLEDGMENTS
We thank Cyril Ndidi Nwoye Nnamezie, an expert translator whose native language is English, for his help in preparing this manuscript. Our thanks also go to the Instituto Nacional de Pediatria for facilitating the publication of this article.
REFERENCES
- 1. World Health Organization , 2018. Global Tuberculosis Control: WHO Report 2018. Geneva, Switzerland: WHO. Publication WHO/HTM/TB/2018. [Google Scholar]
- 2.Tuberculosis Collaborators GBD, 2015. The global burden of tuberculosis: results from the global burden of disease study. Lancet 18: 261–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guía práctica para la Atención de la Tuberculosis en niños, niñas adolescentes Secretaria de Salud de México. 2011. Practical guide for tuberculosis care in children, adolescent girls. Secretary of Health of Mexico.
- 4.Marais BJ, Gie RP, Hesseling AC, Schaaf HS, Lombard C, Enarson DA, Beyers N, 2006. A refined symptom based approach to diagnose pulmonary tuberculosis in children. Pediatrics 118: 1350–1359. [DOI] [PubMed] [Google Scholar]
- 5.Marais BJ, Hesseling AC, Gie RP, Schaaf HS, Beyers N, 2006. The burden of childhood tuberculosis and the accuracy of community-based surveillance data. Int J Tuberc Lung Dis 10: 259–263. [PubMed] [Google Scholar]
- 6.Swaminathan S, Rekha B, 2010. Pediatric tuberculosis: global overview and challenges. Clin Infect Dis 50 (Suppl 3): S184–S194. [DOI] [PubMed] [Google Scholar]
- 7.Cruz AT, Starke JR, 2010. Pediatric tuberculosis. Pediatr Rev 31: 13–25. [DOI] [PubMed] [Google Scholar]
- 8.Buonsenso D, Lancella L, 2012. A twenty-year retrospective study of pediatric tuberculosis in two tertiary hospitals in Rome. Pediatr Infect Dis J 31: 1022–1025. [DOI] [PubMed] [Google Scholar]
- 9.Perez Velez CM, Marais BJ, 2012. Tuberculosis in children. N Engl J Med 367: 348–361. [DOI] [PubMed] [Google Scholar]
- 10. Secretaría de Salud , 2017. Manual de Vacunación. Health Ministry. Vaccination manual.
- 11.Jenkins HE, Tolman AW, Yuen CM, Parr JB, Keshavjee S, Pérez-Vélez CM, Pagano M, Becerra MC, Cohen T, 2014. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet 383: 1572–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkins HE, Yuen CM, 2018. The burden of multidrug-resistant tuberculosis in children. Int J Tuberc Lung Dis 22: 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debord C, Lauzanne AD, Gourgouillon N, Guérin-El Khourouj V, Pédron B, Gaudelus J, Faye A, Sterkers G, 2011. Interferon-gamma release assay performance for diagnosing tuberculosis disease in 0- to 5-year-old children. Pediatr Infect Dis J 30: 995–997. [DOI] [PubMed] [Google Scholar]
- 14.Diel R. et al. 2011. Interferon-γ release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur Respir J 37: 88–99. [DOI] [PubMed] [Google Scholar]
- 15.Haustein T, Ridout DA, Hartley JC, Thaker U, Shingadia D, Klein NJ, Novelli V, Dixon GLJ, 2009. The likelihood of an indeterminate test result from a whole-blood interferon-gamma release assay for the diagnosis of Mycobacterium tuberculosis infection in children correlates with age and immune status. Pediatr Infect Dis J 28: 669–673. [DOI] [PubMed] [Google Scholar]
- 16.Conti F. et al. 2016. Mycobacterial disease in patients with chronic granulomatous disease: a retrospective analysis of 71 cases. J Allergy Clin Immunol 138: 241–248. [DOI] [PubMed] [Google Scholar]