ABSTRACT.
Aedes albopictus is a competent vector of numerous pathogens, representing a range of transmission cycles involving unique hosts. Despite the important status of this vector, variation in its feeding patterns is poorly understood. We examined the feeding patterns of Ae. albopictus utilizing resting collections in Long Island, NY, and contextualized blood meal sources with host availability measured by household interviews and camera traps. We identified 90 blood meals, including 29 humans, 22 cats, 16 horses, 12 opossums, 5 dogs, 2 goats, and 1 each of rabbit, rat, squirrel, and raccoon. This is only the third study of Ae. albopictus blood feeding biology that quantitatively assessed domestic host availability and is the first to do so with wild animals. Host feeding indices showed that cats and dogs were fed upon disproportionately often compared with humans. Forage ratios suggested a tendency to feed on cats and opossums and to avoid raccoons, squirrels, and birds. This feeding pattern was different from another published study from Baltimore, where Ae. albopictus fed more often on rats than humans. To understand whether these differences were because of host availability or mosquito population variation, we compared the fitness of New York and Baltimore Ae. albopictus after feeding on rat and human blood. In addition, we examined fitness within the New York population after feeding on human, rat, cat, horse, and opossum blood. Together, our results do not indicate major mosquito fitness differences by blood hosts, suggesting that fitness benefits do not drive Northeastern Ae. albopictus feeding patterns.
INTRODUCTION
Aedes albopictus is a widely invasive mosquito of human and veterinary health importance. This species is capable of transmitting over 20 pathogens in laboratory assays,1 and is a confirmed natural vector of dengue, Zika, and chikungunya viruses, and dog heartworm.1,2 Additionally, virus detection in field-collected mosquitoes has led Ae. albopictus to be a suspected vector of numerous additional pathogens, including eastern equine encephalitis and West Nile because of virus detection in field-collected mosquitoes, although to date, there is no direct evidence of transmission to humans.1 These pathogens encompass vastly different transmission cycles. Some are anthroponoses, transmitted from human to mosquito (e.g., Zika virus), whereas others are zoonoses, transmitted from nonhuman animals to mosquitoes (e.g., dog heartworm). Transmission of these zoonoses may occasionally result in human infection (e.g., West Nile virus). In light of the broad vector potential of Ae. albopictus and variation in feeding patterns in nature, it is critical to perform host feeding studies in locations relevant to human and animal health risk.
Variation in mosquito host feeding patterns can be influenced by a number of factors including innate host preference, environmental conditions, host availability, and the design of the studies themselves.3,4 These factors may explain the variability in host feeding patterns reported for Ae. albopictus in the literature, which range from generalist or mammalophagic to highly anthropophagic (=human feeding).5 For example, a high percentage of mosquitoes with human-derived blood meals were identified in tropical countries such as Thailand (100%) and Cameroon (99.4%).6,7 In Thailand, aspirator collections were conducted around human dwellings, however, in Cameroon, mosquitoes were collected at a leisure and equestrian center, both of which were surrounded by human dwellings. In some parts of the United States, human feeding frequency was much lower, such as at a tire dump in Missouri (6.5%), urban Baltimore, MD (13.6%), urban and rural sites in Hawaii (18.1%) and Virginia (7.3%), and suburban North Carolina (20%).8–12 Additional studies have reported moderate human feeding rates such as in urban and peripheral sites in Brazil, urban and suburban Japan, and suburban New Jersey.13–15 Of those populations that did not feed predominantly on humans, most fed on a diverse array of animals, with the exception of Baltimore, where a striking number of Ae. albopictus fed on rats (72.3%).8
One notable consistency among all published studies (with a sample size over 60) is a tendency for Ae. albopictus to feed primarily on mammals compared with birds and reptiles.6–22 About half of studies report feeding on birds at low rates (1.7% to 25.6% of all blood meals).9,11–14,16,18,19,23 A tendency to feed even sporadically on birds is particularly important because of their role as amplifying hosts of arboviruses such as West Nile and Eastern equine encephalitis.
Host availability is rarely considered in the design of mosquito blood meal collection studies conducted in the field despite its importance in driving mosquito blood feeding patterns and thus interpreting study results. In Italy, Ae. albopictus from urban and rural sites had replicable differences in feeding patterns, mirroring differences in host availability at these sites.16 Similar observations were made in Singapore and India.23,24 However, the authors only described the site qualitatively (e.g., rural versus urban) and did not quantify host availability. We are aware of only two published studies (in North Carolina and Brazil) that have quantitatively assessed the link between host availability and blood feeding for Ae. albopictus.12,14 Results from these two studies do not provide a clear picture of whether Ae. albopictus feeds disproportionately often on humans compared with other mammals, with results varying depending on measurement type (abundance versus time-weighted), stratification level (household versus hectare), and which nonhuman animals were included in the paired comparison.
In addition to host availability, host attraction may also influence blood feeding patterns.25 Only two published studies have explored host attraction in Ae. albopictus.26,27 The authors of both studies reported higher attraction to humans compared with numerous other hosts including dogs and chickens. Preferential attraction to hosts is determined genetically, and may evolve as a result of elevated mosquito fitness after ingesting a given species’ blood.25,28 This has been demonstrated for Ae. aegypti, which maximizes the reproductive fitness on human blood, its preferred host.29 Only two studies have addressed the impact of blood from different host species on Ae. albopictus egg production,30,31 but none have compared both survival and fecundity following blood meals from the most ecologically relevant hosts.
We sought to determine Ae. albopictus feeding patterns in select suburban and farm landscapes along its front of active northward expansion in New York (NY) State.32 Our aim was to investigate the feeding patterns in the context of host availability and consequences for mosquito fitness. Ultimately, we wanted to fill a gap in our understanding of Ae. albopictus feeding ecology along its northeast United States range limit and how it might relate to public health risk. To meet our objectives, we performed host censuses for use in calculating host feeding indices (HFIs) and forage ratios (FR). We then assessed whether fitness of NY Ae. albopictus varied by host blood source ingested under laboratory conditions through a series of life table studies. To explore potential population-level differences, we compared fitness of Ae. albopictus individuals from New York and Baltimore after ingesting human and rat blood meals.
METHODS
Field sites.
Eight sites were selected in Suffolk County on Long Island, NY (Supplemental Figure 1): four farms and four residential areas, each containing between nine and 17 collection properties. Aedes albopictus has been present in Suffolk County since 2004, although its distribution is not uniform or complete across the county (Moses Cucura, personal communication). Residential sites were selected based on Ae. albopictus presence reported by the Suffolk County Vector Control and Arthropod-Borne Disease Laboratory and larval distribution data.33 All residential sites were suburban, with variable human population density: Central Islip (1,853 people/sq. km), Bay Shore (1,853 people/sq. km), Babylon (1,660 people/sq. km), and Hauppauge (734 people/sq. km). All four farms were partially bordered by suburban residential and forested natural landscapes. One was a petting zoo and three were riding stables.
Mosquito collection.
Weekly collections were conducted at each site between June 20 and August 15, 2018 with large custom-designed aspirators (30.5 cm diameter, 114 cm height, 12 V PM DC 2350 RPM, 1/35 horsepower, 3.7 amp motor).6 Collections were conducted once per week at each site between 08:00 and 19:00 hours, with two sites visited per day (one in the morning, and one in the afternoon). Morning and afternoon visitation was typically rotated from week to week. Two teams of three researchers worked simultaneously at separate properties at residential sites and together at farm sites. Two aspirators were operated per team for the length of time necessary to sample the full property (most collection times were between 7 and 12 minutes; range from 2.5 to 17 minutes). Mosquitoes were immobilized in acetone-treated jars (3 minutes) and sorted in the field to remove non-mosquito by-catch. The samples were transported on ice to the laboratory for identification according to a taxonomic key.34 Aedes albopictus were considered engorged if blood was visible in the abdomen upon examination. Mosquitoes were stored at −20°C and transported to Cornell University on dry ice for blood meal identification.
Blood meal identification.
Abdomens were removed from mosquitoes using forceps and transferred to sterile microcentrifuge tubes. To avoid cross-contamination, forceps were dipped in ethanol35 and flame-sterilized36 between each sample. The DNA was extracted from abdomens using Qiagen Puregene Cell kit (Qiagen Sciences, Germantown, MD). To identify blood meals, we amplified templates from the vertebrate-specific cytochrome c oxidase subunit I (COI) “barcoding” gene. Primers designed by Reeves et al. (2018) were used to amplify a 395 base pair amplicon37 (Table 1).
Table 1.
Primer sequences designed by Reeves et al. (2018)
| Primer name | Sequence |
|---|---|
| VertCOI_7194_F | 5′- CGM ATR AAY AAY ATR AGC TTC TGA Y −3′ |
| Mod_RepCOI_R | 5′- TTC DGG RTG NCC RAA RAA TCA −3′ |
Other Reeves COI primers were not used because of the co-amplification of Ae. albopictus DNA. Co-amplification is a recurrent issue with identifying Ae. albopictus blood meals because of matching sequences between its own genome and primers designed for use in blood meal studies of other mosquito species.17 Notably, cytochrome b primers designed by Egizi et al. (2013) were used initially, but as a result of a low success rate in our hands, we switched to the Reeves primers.37 Three blood meals identified with the Egizi primers were not successfully amplified by the Reeves primers; results with both primer sets were combined for our data analysis.
Polymerase chain reaction (PCR) conditions were slightly modified from Reeves et al. (2018) to minimize co-amplification of Ae. albopictus DNA and maximize the amplification of the desired amplicon.37 Reactions were performed with total volume of 20 µL, consisting of 10 µL of 2.0X Apex Taq RED Master Mix (Genesee Scientific Corp., San Diego, CA), 0.75 µL of VertCOI_7194_F forward primer (10 µM), 0.75 µL of Mod_RepCOI_R reverse primer (10 µM), 6.5 µL sterile nuclease-free H2O, and 2 µL of extracted DNA. Most reactions were conducted with the following thermocycling conditions: 94°C for 3 minutes, followed by 40 cycles of 94°C for 40 seconds, 53.5°C for 30 seconds, and 72°C for 60 seconds, and a final extension step at 72°C for 7 minutes. The annealing temperature was modified from Reeves et al. (2018) to minimize amplification of Ae. albopictus DNA according to a temperature gradient test conducted on positive (human-fed) and negative (nonfed) mosquito controls. Conditions were further modified for a subset of reactions to optimize amplification: 94°C for 3 minutes, followed by 5 cycles of 94°C for 40 seconds, 45°C for 30 seconds, and 72°C for 60 seconds, and then 35 cycles of 94°C for 40 seconds, 48.5°C for 30 seconds, and 72°C for 60 seconds, and a final extension step at 72°C for 7 minutes. All reactions were conducted alongside a positive (human-fed mosquito) and negative (sterile nuclease-free water) control. PCR products (5 µL) were loaded onto a 1% agarose gel stained with gelRED, electrophoresed, and visualized with UV light (Mighty Bright, Hoefer Scientific Instruments, San Francisco, CA).
Samples with positive bands after gel electrophoresis were purified with FastAP and Exonuclease (ThermoFisher Scientific, Waltham, MA) and submitted for Sanger sequencing at the Cornell University Biotechnology Resources Center. Sequences were compared with the available database in National Center for Biotechnology Information (NCBI) Basic Local Alignment Search Tool (BLASTn) and were identified to a source if matches were ≥ 98% with a sequence of known origin (with the exception of an eastern gray squirrel (Sciurus carolinensis) sequence, which had a 95.5% match).
Host availability.
Household interviews.
To estimate domestic host availability, household interviews were conducted weekly at time of collection. Interviews were conducted by trained field collection staff with a set of uniform questions (see Supplemental Materials 1). Interviewers were typically rotated between houses to further reduce interviewer bias. Residents were asked about the number of people and pets living in their house and the amount of time each host type spent outside that day and the two days prior. This time frame was investigated because digestion may prevent blood meal identification at approximately 48 hours after feeding under field relevant temperatures.38 Interviews were conducted in English or Spanish depending on homeowner preference.
Camera traps.
To estimate wild host availability, two motion-triggered camera traps (Moultrie M-880, #MCG-12691, Calera, AL) were set at each site as soon as they were available, from July 16 to August 13, 2018 on selected properties in residential sites and different locations within farm sites. Cameras were operated according to the specifications described by Linske et al.,39 with the exclusion of scent lures: 30-second detection delay between images, high passive infrared sensitivity, single still-image photo, 1.0 m above ground, and slight downward angle to capture both small and large hosts. Camera data were used to estimate host abundance by host type by determining the number of animal encounters with the camera per trap day. If a given host type was photographed within 30 minutes of the last image of that animal, it was considered the same individual and was not counted separately. If multiple individuals were captured in one image within 30 minutes of last sighting, the count was equal to the maximum number captured together in an image.
Fitness by host blood source.
Mosquito rearing.
Aedes albopictus eggs were collected from five towns in New York (three on Long Island and two in the Hudson Valley region) for a previous study,40 including two of the residential sites studied here (see Supplemental Materials 2 for colony information). Each location was reared separately in colony for a few generations and then combined into one large NY colony, totaling six to 10 generations of laboratory rearing prior to use. Eggs from Baltimore, MD (between F3 and F6 depending on replicate) were reared synchronously with the NY colony to assess between population differences. For each replicate, eggs were vacuum hatched, provided with a pinch of pulverized fish food (crushed Cichlid Gold™ fish food pellets; Hikari, Himeji, Japan), and one day later, separated into trays of 200 larvae, with 1 L of distilled water, and 4 Cichlid Gold fish food pellets. Adult mosquitoes were maintained in an environmental chamber (28°C, 71.9% ± 9.5% relative humidity, 10 hour light, 10 hour dark, and 2 hour dusk/dawn). Cups of 200 pupae were placed into cages inside the chamber, and upon eclosion, 10% sucrose was provided for 2–4 days. Males were removed and sucrose was replaced with distilled water for 1 day before blood feeding.
Blood.
Human (Lampire Biologicals, Pipersville, PA), opossum (The Janet L. Swanson Wildlife Health Center, Ithaca, NY), rat (The Center for Animal Resources and Education, Cornell University), cat (The Center for Animal Resources and Education at Cornell University, Ithaca, NY), and horse (Lampire Biologicals, Pipersville, PA) blood treated with anticoagulant (sodium citrate) was stored at −20°C upon arrival. Blood was thawed in warm water immediately before use. Mosquito blood feeding was conducted with artificial feeders (water reservoir at 37°C and desalted sausage casings as membrane) as described previously.41
Within-population fitness impacts for NY Ae. albopictus.
To determine whether fitness advantages for different host blood sources reflected the feeding pattern and level of host usage of NY Ae. albopictus, we assessed fecundity and survival of females after feeding on human, cat, horse, opossum, and rat blood. These blood sources were chosen based on host species identified in our blood meal analysis. For the purpose of this study, the NY colony was considered one population, although it was established with Ae. albopictus collected from sites across southern New York.
Fecundity and survival.
Fully engorged mosquitoes (approximately 35 per blood source per replicate and three to four replicates per group) were gently transferred individually into 0.5 L paper cups with a dry oviposition vessel. Mosquitoes were maintained in individual cups in the environmental chamber as described above. One day after blood feeding, strained larval rearing water was added to oviposition vessels to encourage egg laying. No additional water or sugar was provided. Each mosquito was checked daily for presence of eggs (first day of egg lay) and mortality until all females had died. Total number of eggs laid per female was recorded at the end of experiment. Dead mosquitoes were frozen at −20°C and later dissected to determine the number of mature retained eggs, if any. We compared the total eggs produced (retained + laid eggs). In replicate two, mosquitoes with a large number of retained eggs were not counted and were therefore not included in the egg analyses but were included in survival analyses. For individuals where egg retention data was not available, number of eggs laid was used. The following blood types were tested: replicate one included human, rat, cat, and horse; replicates two and three included human, rat, cat, horse, and opossum; and replicate four included human, rat and opossum.
Between-population differences of NY and Baltimore Ae. albopictus.
Because of the striking differences in field-collected host blood meal sources between our study and a prior Baltimore study (where Ae. albopictus fed more often on rats and less often on humans than in NY8), we assessed whether there were also differences in fitness between Ae. albopictus from these two locations after feeding on rat and human blood.
Fecundity and survival.
The NY and Baltimore Ae. albopictus were fed rat and human blood and observed synchronously using the methods described above. The rat and human-fed NY individual mosquitoes from replicates one to three of the within-population fitness assessment described above were used to compare both between-population fitness of NY and Baltimore Ae. albopictus and within-population fitness of NY Ae. albopictus. The wing length of a subset of NY and Baltimore individuals was measured to control for body size differences between the two population cohorts.42,43
Data analysis.
Host availability.
Residential host feeding index.
Abundance and time-weighted HFI were calculated using blood meal identification data from residential areas and household interview data for humans, cats, and dogs. Feeding indices were calculated according to equations described by Kay et al. (1979) and modified by Richards et al. (2006) as follows12,44:
where Bx and By represent the average number of blood meals from host x and host y per household and Hx and Hy represent the average number of host x and host y residing per household. Averages were calculated with data from households positive for at least one blood meal. Data were aggregated across all four residential sites because household and site-specific calculations frequently resulted in undefined values because of zeroes in the denominators.
A time-weighted feeding index12 was calculated as follows:
where Ty and Tx represent the time spent outside by hosts y and x, respectively. When household interview data was missing on the date of blood meal collection (26 of 66 surveys), the average of all other interview responses from that household was used as an approximation.
An HFI or HFIT greater than 1 indicated that host x was fed upon more often than expected compared with host y given their abundance or time spent outside. An HFI or HFIT equal to 1 indicated that the hosts were fed upon in proportion to their availability and an HFI or HFIT less than 1 indicated that host y was fed upon more often than expected compared with host x. Note that while an HFI or HFIT greater or less than 1 may reflect Ae. albopictus preference, it does not conclusively demonstrate it, as we cannot rule out influences from other factors such as host defenses, timing of host availability, or host location in the yard.
Residential forage ratio.
Forage ratios are another method for determining host feeding frequency by host availability.6 In our study, these were calculated using blood meal identification data and camera trap images from residential sites. Forage ratios were calculated for each host type that was captured by camera traps as follows45:
In the case of this study, the proportion of all hosts represented by host x was approximated by the proportion of all camera trap images that were taken of host x.
An FR greater than one suggests that the host was fed upon more often than expected given its abundance and less than one suggests that the host was fed upon less often than expected. An FR equal to one indicates that the host was fed upon in proportion to its abundance in the population. As with HFIs, FR may reflect preference but do not prove it because the same sources of bias may impact these results.
Farm host availability.
At the farm sites, HFI and FR were not calculated because of small sample sizes and technical difficulties of defining host availability, making quantification of FR and feeding indices uninformative. Interviews of human and domestic animal availability were only conducted once at farms during the last week of collections. Farm owners could not accurately estimate human exposure because of unpredictable influx of people on site for riding lessons and farm work. Animal exposure could not be reliably measured because of inconsistent use of fenced paddocks and semi-enclosed barns. Camera traps were positioned to picture wild animals at the outskirts of the fenced paddocks and therefore did not often picture domestic farm animals. Interview and camera trap data are reported for each but are only qualitatively compared with blood meal data; no further calculations were conducted.
Life table studies-fitness by host blood source.
Within-population fitness impact.
The effect of host blood source on egg production (fecundity) for the NY colony was assessed with a linear model, including replicate and mosquito survival as covariates. The effect of host blood source on mosquito survival was also determined using a linear model, including replicate as a covariate. Estimated marginal means post hoc analyses were conducted using the emmeans package.46 Survival curves were created with the average proportion surviving across the replicates and compared for each host blood source. The basic reproductive rate (R0) was calculated for each blood type and replicate according to previously described equations.47 The effect of blood type on R0 was compared via a linear model.
Between-population differences.
Egg production and survival were compared between human/rat, NY/Baltimore groups using linear models, as described above. However, in this case, number of eggs produced by each individual was divided by average wing length of the cohort, reported as eggs per mm wing length (eggs/mm wl), to control for the effect of body size, which differed between Baltimore and NY colonies despite identical rearing methods.
Ethics approval.
Survey protocols were reviewed and considered human subjects research exempt by Cornell University’s Institutional Review Board (IRB). Blood was acquired from vendors or groups that already had appropriate permits and thus blood feeding was not regulated by the Institutional Animal Care and Use Committee (IACUC).
RESULTS
Blood meal identification.
A total of 3,241 Ae. albopictus were collected between June 20 and August 15 (1,575 female and 1,666 male), of which 182 (14% of aspirator-collected females) were blood-fed. Of the females designated blood fed, 149 blood meals (81.9%) were between half-digested and fully engorged. An additional six mosquitoes were captured by hand nets with nonfresh blood while host-seeking near collectors, indicating that the blood meal was not taken from collectors. Host identity was successfully assigned to 90 samples (49.5%), including 29 humans (Homo sapiens; 32.2%), 22 cats (Felis catus; 24.4%), 16 horses (Equus caballus; 17.8%), 12 opossums (Didelphis virginiana; 13.3%), 5 dogs (Canis lupus familiaris; 5.6%), 2 goats (Capra hircus; 2.2%), and 1 each of rabbit (Sylvilagus floridanus; 1.1%), rat (Rattus norvegicus; 1.1%), squirrel (Sciurus carolinensis; 1.1%), and racoon (Procyon lotor; 1.1%). One of these was captured by hand net (with a human blood meal). When categorized by residential (N = 66) or farm sites (N = 24), most of the blood fed female Ae. albopictus from residential sampling sites indicated blood meals from humans (27; 40.9%), followed by cats (21; 31.8%) and opossums (12; 18.2%). The majority of farm blood meals were from horses (16; 66.7%), followed by humans (2; 8.3%) and goats (2; 8.3%) (Supplemental Figure 2).
Host availability.
Residential host feeding index.
Household interview and blood meal data were used to calculate HFIs, indicative of relative tendency to feed on certain vertebrate hosts across the residential properties where blood fed Ae. albopictus with identified blood meals were collected (N = 28) (Supplemental Table 1). The mean number (±SE) of blood meals from a given host type was calculated per residential property sampled: the most human blood meals were collected per residential property (0.96 ± 0.21), followed by cats (0.75 ± 0.17), and dogs (0.18 ± 0.09). Similarly, there were the most human residents per residential property sampled (3.18 ± 0.36), followed by cats (0.39 ± 0.19), and dogs (0.29 ± 0.10) according to household interviews. However, cats were reported to spend the most time outside over the 2 days before collection per residential property sampled (278.74 ± 232.93 minutes), followed by humans (234.26 ± 49.83 minutes), and dogs (53.61 ± 22.05 minutes) (Supplemental Figure 3). The standard error in cat time was large because some properties had outdoor cats (24 hours/day), whereas others did not have cats or had indoor cats.
Mean numbers of blood meals and residents were used to calculate paired comparisons of feeding between humans, cats, and dogs through abundance and time-weighted HFIs (Supplemental Table 2). Human versus cat HFI and HFIT both demonstrated a tendency to feed on cats compared with humans (0.16 and 0.20). Likewise, human versus dog HFI and HFIT both suggest that Ae. albopictus fed disproportionately often on dogs compared with humans (0.49 and 0.14). However, cat versus dog HFI and HFIT produced opposite results: according to abundance measures, cats were fed upon disproportionately more often compared with dogs (3.05), but when time-weighted, dogs were fed upon disproportionately more often compared with cats (0.73). On average, cats spent much more time outside than dogs, causing the directionality change of the index. Furthermore, neither HFI metric demonstrates a particularly strong deviance from the expected feeding proportions, suggesting that Ae. albopictus may not have a strong preference between cats and dogs.
Residential forage ratio.
Forage ratios were calculated from camera trap data at the four residential sites for all animals for which camera trap images were taken or blood meals identified (Table 2). Cats and opossums were fed upon more often than expected given their relative abundance in the host population. Of all residential blood meals taken from free roaming animals (i.e., not humans and dogs, which are largely constrained by property fences in residential sites sampled), 65.7 ± 10.2% were derived from cats, but only 27.4 ± 10.9% of all images were taken of cats, resulting in a 3.56 ± 0.98 FR (above the FR = 1 threshold to infer preference). Opossum blood meals accounted for 31.8 ± 10.8% of all blood meals but no opossums were pictured, resulting in an undefined FR, but suggesting preference for opossums. Raccoons, the other nocturnal animal detected by camera traps, were pictured often (24.8 ± 16.4% of all animals pictured) but only represented 2.5 ± 2.5% of all blood meals, resulting in an FR below 1 (0.046 ± 0.046), suggesting avoidance. Squirrels and birds were also pictured often (21.6 ± 10.5% and 26.2 ± 11.2% of all animals pictured, respectively) but no blood meals were identified from these host types in the blood fed Ae. albopictus collected at residential sites, resulting in an FR of 0, suggesting avoidance.
Table 2.
Mean (±SE) percentage of blood meals, percentage of camera trap images, and FR for all animal types for which camera trap images were taken or blood meals identified at residential sites (N = 4) in Suffolk County, NY
| Mean (±SE) | |||
|---|---|---|---|
| % Blood meals | % Images | FR | |
| Cat | 65.7 (10.2) | 27.3 (10.9) | 3.6 (1.0) |
| Possum | 31.8 (10.8) | 0 (0) | ∞* |
| Raccoon | 2.5 (2.5) | 24.8 (16.4) | 0.05 (0.05) |
| Squirrel | 0 (0) | 21.6 (10.5) | 0 (0) |
| Bird | 0 (0) | 26.2 (11.2) | 0 (0) |
FR = forage ratio.
FR was infinite because division by zero is undefined.
Farm host availability.
Approximate numbers and time spent outside for humans and domestic animals were reported by the farm owners. At Farm A, approximately nine people spent time at the farm for a total of 52 hours per day. The farm also had 40 horses, spending a total of 70 hours/day outside. At Farm A, 3.6% of camera trap images were of cats, 67.9% of raccoons, 17.9% of foxes, 3.6% of deer, and 7.1% of squirrels. Blood meals collected at Farm A included six horses and one squirrel.
Farm B estimated that 30 people (180 hours), 100 horses (200 hours), two dogs (26 hours), and two goats (26 hours) were outside on the property per day. Of all camera trap images at Farm B, 37.1% were of cats, 44.3% of raccoons, 4.1% of opossums, 5.2% of deer, 5.2% of squirrels, and 4.1% of rabbits. The blood meals consisted of five horses, one human, and one rabbit.
Farm C estimated that seven people (11 hours), 46 horses (420 hours), two dogs (12 hours), 18 chickens (171 hours), four ducks (38 hours), and one goose (24 hours) spent time outside per day. The most images were taken of cats (48.8%), followed by birds (23.3%), raccoons (14.0%), squirrels (9.3%), and rabbits (4.7%). Blood meals included four horses and one cat.
Farm D estimated that three people (14 hours), eight horses (48 hours), two dogs (8 hours), 20 goats (260 hours), four sheep (52 hours), one alpaca (24 hours), one llama (24 hours), 20 rabbits (260 hours), nine ducks (117 hours), and 30 chickens (720 hours) spent time outside per day. The camera trap pictured raccoons (33.3%) and birds (66.7%). Blood meals collected included: two goats, one horse, one human, and one rat.
Despite the diversity of hosts available at the four farm sites, the predominant blood meal identified at three of these sites was horse. The fourth farm was an anomaly, with more blood meals collected from goats than horses, but it was also the only farm where more goats were available than horses. Once again, raccoons were pictured at all sites, but no blood meals were collected, further suggesting avoidance of this animal. Birds were pictured frequently at two sites, and no blood meals collected, also suggesting avoidance.
Fitness by host blood source.
Within-population fitness impacts for NY Ae. albopictus.
Table 3 presents the proportions of Ae. albopictus that laid and retained mature eggs and mean (±SE) number of eggs produced by blood source.
Table 3.
Egg production by blood meal source for NY Aedes albopictus
| Blood source | Proportion with laid eggs (%) | Proportion with retained eggs (%)* | Mean eggs produced (±SE)† |
|---|---|---|---|
| Human | 104/121 (86.0) | 23/121 (19.0) | 61.0 (2.9)‡ |
| Opossum | 64/86 (74.4) | 10/86 (11.6) | 58.7 (4.8)‡ |
| Rat | 100/122 (82.0) | 16/122 (13.1) | 53.5 (3.7)ठ|
| Horse | 70/97 (72.2) | 11/97 (11.3) | 48.5 (3.9)ठ|
| Cat | 57/89 (64.0) | 10/89 (11.2) | 40.3 (4.0)§ |
Includes mosquitoes with any number of retained eggs.
Groups that do not share a superscript letter are significantly different (P < 0.02).
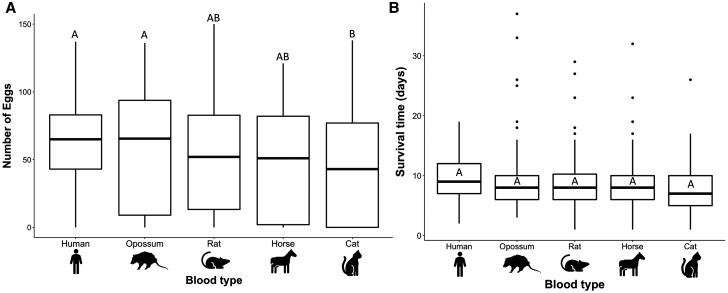
Females that ingested cat blood resulted in lower fecundity compared with those fed human and opossum blood (β = −17.3, SE = 5.3, P = 0.01 and β = −20.9, SE = 5.9, P = 0.004, respectively). There was no significant difference between any other blood groups (Figure 1A). There was also no significant effect of survival time on number of eggs produced (although only one blood meal was provided in this study, which may limit the impact of extended survival). On average, female Ae. albopictus began laying on day 3 post–blood meal, regardless of blood meal source, and survived for 7–9 days. Notably, there were significant differences between replicates (P < 0.0001).
Figure 1.
Box plots for NY Aedes albopictus female mosquitoes for (A) number of eggs produced and (B) survival time in days when fed cat (N = 89 females for egg production and N = 90 females for survival), horse (N = 97 egg production; N = 98 survival), human (N = 121 egg production; N = 123 survival), rat (N = 122 egg production; N = 124 survival), and opossum blood (N = 86 egg production; N = 92 survival). Groups that do not share a letter are significantly different.
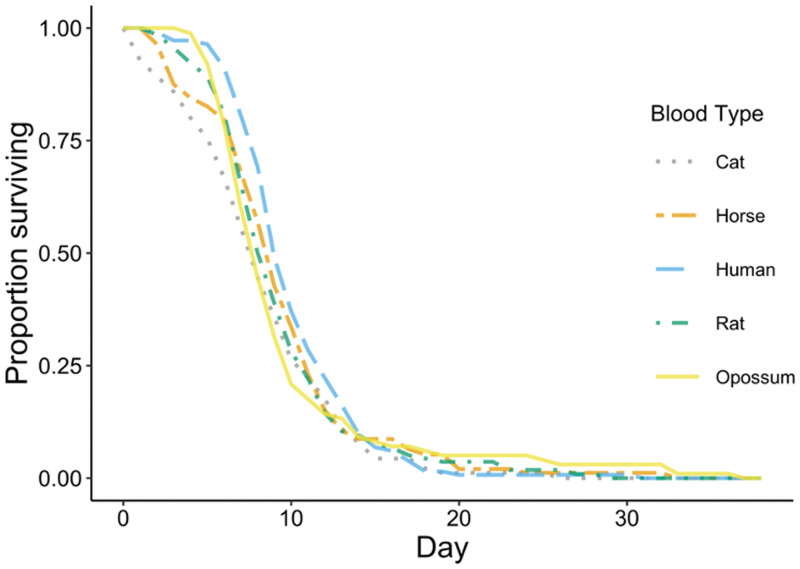
There were no significant differences in Ae. albopictus female survival time between any of the host blood groups (Figure 1B). Mosquitoes fed human blood survived 9.6 (±0.3) days, opossum-fed survived 9.5 (±0.6) days, rat-fed survived 8.7 (±0.4) days, horse-fed survived 8.6 (±0.48) days, and cat-fed survived 7.6 (±0.45) days. There were significant differences in survival by replicate (P < 0.0001). Daily survival curves averaged over replicates performed for each host type are presented in Figure 2.
Figure 2.
Survival (days) of NY Aedes albopictus by host blood type ingested, including cat (N = 90 females), horse (N = 98), human (N = 123), rat (N = 124), and opossum (N = 92) blood. This figure appears in color at www.ajtmh.org.
The mean (±SE) R0 across replicates was 29.7 (±4.1) for Ae. albopictus fed human blood, 27.1 (±8.9) for opossum blood, 27.0 (±4.1) for rat, 22.9 (±5.7) for horse, and 19.5 (±6.5) for cat. No significant differences in (R0) were found by host blood group.
Between-population differences of NY and Baltimore Ae. albopictus.
The proportions of Ae. albopictus that laid and retained mature eggs, mean (±SE) eggs, and mean (±SE) eggs/mm wing length is reported in Table 4. Wing lengths were measured for 26–33 females per colony per replicate.
Table 4.
Egg production for NY and Baltimore Aedes albopictus females fed human or rat blood
| Origin and blood source | Proportion with laid eggs (%) | Proportion with retained eggs (%) | Mean eggs produced (±SE) | Mean eggs/mm wl produced (±SE)* |
|---|---|---|---|---|
| NY human | 76/89 (85.4) | 17/89 (19.1) | 58.8 (3.6) | 20.7 (1.3)† |
| NY rat | 75/95 (78.9) | 13/95 (13.7) | 46.1 (4.0) | 16.2 (1.4)‡ |
| Baltimore human | 73/89 (82.0) | 12/89 (13.5) | 41.4 (3.2) | 14.7 (1.1)‡ |
| Baltimore rat | 70/95 (73.7) | 11/95 (11.6) | 38.2 (3.5) | 13.6 (1.3)‡ |
Groups that do not share a superscript letter are significantly different (P < 0.03).
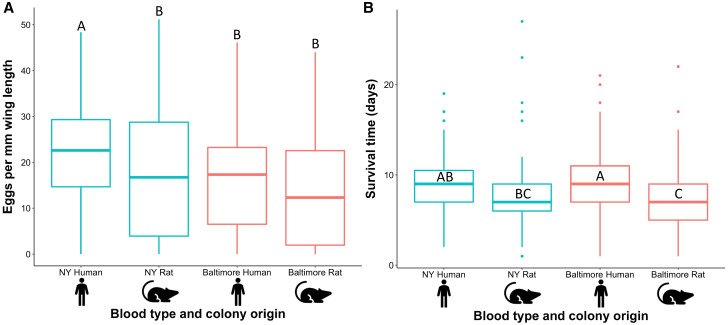
The only significant differences in number of eggs produced per mm wing length were between NY mosquitoes fed human blood and the three other host blood source groups (Figure 3A). Baltimore mosquitoes fed human (β = −6.0, SE = 1.8, P = 0.0008) and rat blood (β = −6.9, SE = 1.8, P = 0.0001) produced fewer eggs/mm wl than NY mosquitoes fed human blood. The NY mosquitoes fed human blood produced more eggs per mm wl than those fed rat blood (β = 3.8, SE = 1.8, P = 0.03). Baltimore mosquitoes fed rat blood produced marginally fewer eggs/mm wl than NY mosquitoes fed rat blood (β = −3.1, SE = 1.7, P = 0.07). There was no significant difference in eggs produced/mm wl between Baltimore mosquitoes fed human and rat blood (β = 1.0, SE = 1.8, P = 0.6) or Baltimore mosquitoes fed human blood and NY mosquitoes fed rat blood (β = −2.1, SE = 1.8, P = 0.2). There were significant differences in between replicates (P < 0.0001).
Figure 3.
Box plots for Baltimore and NY Aedes albopictus female mosquitoes of (A) number of eggs produced when fed rat and human blood; and (B) survival in days. Sample sizes for Baltimore human and rat and NY human and rat were, respectively: N = 89, 95, 89, and 95 for egg production and N = 94, 95, 91, and 97 for survival. Groups that do not share a common letter are significantly different. This figure appears in color at www.ajtmh.org.
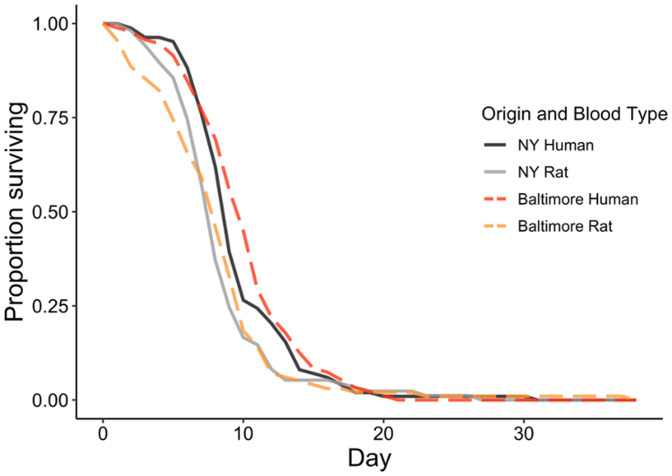
The mean (±SE) survival time of Baltimore Ae. albopictus was significantly higher when fed human blood (9.6 days ± 0.4) compared with rat blood (7.2 days ± 0.4) (β = 2.3, SE = 0.5, P = 0.0001). The same survival trend was observed for NY Ae. albopictus where mosquitoes fed human blood survived marginally longer than those fed rat blood (9.0 days ± 0.3 and 7.7 days ± 0.4 respectively: β = 1.3, SE = 0.5, P = 0.08) (Figure 3B). Baltimore mosquitoes fed human blood survived significantly longer compared with NY mosquitoes fed rat blood (β = 1.9, SE = 0.5, P = 0.002). Survival time was significantly lower for Baltimore mosquitoes fed rat blood compared with NY mosquitoes fed human blood (β = −1.7, SE = 0.5, P = 0.008). There was no significant difference in survival time between mosquitoes fed human blood from both sites (β = 0.6, SE = 0.5, P = 0.6) or fed rat blood from both sites (β = −0.4, SE = 0.5, P = 0.8). We detected differences by replicate (P = 0.007). Daily survival curves averaged over the three replicates are presented in Figure 4.
Figure 4.
Survival (days) of Baltimore and NY Aedes albopictus fed human (N = 94 and 91, respectively) and rat (N = 95 and 97, respectively) blood. Curves are averaged over three replicates. This figure appears in color at www.ajtmh.org.
The mean (±SE) basic reproductive rate (R0) (averaged over three replicates) of Baltimore Ae. albopictus fed human blood was 20.4 (±1.2), 19.7 (±4.6) for Baltimore rat, 29.3 (±5.7) for NY human, and 24.5 (±4.5) for NY rat. No significant differences were found for R0 among any of the blood/colony combinations.
DISCUSSION
Mosquito feeding behavior plays a vital role in disease transmission, however, it can be difficult to quantify and predict because there are diverse factors that influence the feeding behavior in nature. We investigated the feeding patterns of the globally invasive vector, Ae. albopictus, from eight sampling sites, categorized as farm and residential habitats at the northern edge of its range in the United States. In tandem, we addressed two factors that may influence these patterns: host availability and variation in mosquito fitness from different host blood sources. We detected 10 host species, some of which were over- or underused compared with their availability as measured by HFIs and FR. Host blood source had a limited impact on mosquito survival, egg production, and basic reproductive rate, indicating that fitness does not play a significant role in predicting Ae. albopictus feeding patterns in the northeastern United States. The 10 host species we detected in Ae. albopictus blood meals from Long Island, NY, are hosts previously reported for Ae. albopictus elsewhere in the world. The proportion of human blood meals (32.2%) identified in Long Island was lower than reported in many other locations worldwide,6,7,13–18,20–24 but was higher than in some other studies from the United States (Hawaii, Missouri, North Carolina, Maryland, and Virginia).8–12 More Ae. albopictus fed on cats in our study on Long Island than in any other location previously reported, with the exception of Virginia.10 The third most common host for this mosquito species on Long Island, the horse, has only been detected in four of 18 previous Ae. albopictus blood meal studies and at lower levels.9,12,14,16 Similarly, the fourth most common host, opossum, has been reported in five previous studies, also at lower levels, with the exception of Virginia.10–12,15,17 Long Island Aedes albopictus fed less frequently on dogs compared with the representative proportion in numerous other studies.9,11,12,14–18 Notably absent from the Long Island blood meals were cows, deer, and birds, all of which were present on at least one site in our study and have been detected in at least six previous blood meal studies. It is possible that a larger sampling of blood meals may have revealed these hosts, however, birds have also been absent from most other studies in northeastern United States.8,15,17 Notably, only about half of collected blood meals were successfully identified to species, but the reason for the low success rate is unknown. It is possible that this may have biased the species that were identified, however, tests of primer versatility performed by Reeves et al. (2018) showed amplification for the majority of vertebrate species (90/93).37
This is only the third study of Ae. albopictus blood feeding biology that quantitatively assessed host availability, and the first to do so with wild animals. Abundance and time-weighted HFIs calculated using household interview data revealed disproportionately high levels of feeding on cats and dogs compared with humans. Richards et al. (2006) reported a similar trend for HFIs based on host abundance in North Carolina, but when time-weighted, found that humans were fed upon disproportionately often compared with cats and dogs.12 In Brazil, HFIs based on host abundance showed the opposite trend to ours, suggesting that Ae. albopictus fed disproportionately often on humans compared with cats and dogs.14 These results highlight the need for additional studies that measure host availability and also suggest a need for caution when extrapolating these results to make conclusions about innate mosquito preference. In both Long Island and North Carolina, collections were only conducted at a subset of houses per neighborhood, allowing for the movement of blood fed mosquitoes from properties where interviews were not conducted. Flight range for engorged blood fed Ae. albopictus is not known, but it is likely that movement between properties is possible after feeding according to the reported range of other blood fed species and records of Ae. albopictus dispersal between blood feeding and oviposition.48–51 Furthermore, household interview data depend on accurate self-reporting of outdoor activity, which may be unreliable.52 This inaccuracy of outdoor time estimates is compounded if the interview is only administered once for the entire sampling period, such as in Richards et al. (2006).12 Another potential source of bias is insecticide/endectocide use for domesticated animals53—we did not gather data on this and therefore cannot determine whether this may have impacted observed feeding patterns by limiting domestic animal blood meals.
We also assessed host availability through camera traps to calculate FR for free-roaming animals, which suggested a tendency to feed on cats and opossums and to avoid raccoons, squirrels, and birds compared with their relative abundance in residential sites. Although camera traps do not provide a perfect measure of host abundance, it is considered a robust method for mammal inventories.54 Camera traps may be less useful in estimating bird abundance,55 however, birds were one of the most frequently photographed groups of animals in our study, but were not fed upon, so improved accuracy in estimating bird abundance would not have altered conclusions drawn from FR calculations. Forage ratio calculations were limited to animals that tend to cross freely between yards despite fences, including all wild animals and cats, but excluding humans and dogs. Camera traps were only placed in two properties per site, limiting the utility of camera traps to assess the site-wide availability of these animals with high property-line fidelity. Furthermore, camera traps were not operated for the full collection period—20 blood meals were collected before camera trap deployment. Host availability can shift over the season,56 so this may have impacted our results.
For both household interviews and camera trap host census methods, heterogeneity in host availability between sampled households can lead to uneven exposure of mosquitoes to a given host. When analyses are conducted across many households, as in this study, this heterogeneity can be lost. The level at which host availability measures and blood meals are grouped can impact the interpretation.12 This may be particularly relevant when considering hosts with a high level of variation in time spent outdoors, such as cats in this study.
Despite limitations, estimating host availability and abundance in conjunction with blood meal studies is much more informative than studies that lack such data. By understanding more about the context in which a certain feeding pattern arose, more general conclusions can be drawn about the feeding behavior. However, the patterns revealed after accounting for host availability can be caused by numerous factors, such as host defenses. This may explain the high number of opossum blood meals because this nocturnal marsupial would likely be asleep, with decreased self-defense, during Ae. albopictus daytime biting activity. However, raccoons are also nocturnal and in contrast, were fed upon less often than expected, suggesting that innate preferences or other factors could potentially also be at play. Only two preference studies have been conducted for Ae. albopictus; in La Reunion Island, a no-choice blood feeding experiment on 12 host types found chicken, human, dog, and cow were fed upon more often than duck, shrew, rat, pig, mouse, goat, gecko, and chameleon.26 Subsequently, a choice experiment showed higher attraction to humans compared with chickens, dogs, cows, and goats.26 However, large and small animals were treated differently and were not given equal opportunities for self-defense, potentially affecting the results. In Thailand, landing catches demonstrated preference for humans compared with pigs, buffalo, dogs, and chickens; however, the use of a second human to catch mosquitoes from the nonhuman animals may have impacted results. It therefore remains unclear whether Ae. albopictus has innate host preference.
One mechanism by which host preferences may evolve is through natural selection whereby feeding on a certain host enhances reproductive fitness, leading selection to favor genetic variants with preference for that host. This is known to be the case for other species, such as Ae. aegypti.4,29 We investigated the potential role of fitness in driving Ae. albopictus feeding patterns by assessing survival and egg production of mosquitoes after feeding on blood from several host blood sources in the Northeastern United States. Within the NY Ae. albopictus population, we found that host blood source had very limited impact on survival, egg production, or basic reproductive rate. The only significant differences were lower egg production after feeding on cats compared with humans and opossums, and no significant differences in survival. Interestingly, the reduced fecundity on cat blood is opposite to what we might expect based on the feeding index, which suggested a tendency to feed more often on cats compared with humans. There are many reasons that these contrasting results may have occurred, including possible under-estimation of cat availability in host censuses leading to an inflated HFI, potentially lower levels of host defenses among cats compared with other animals leading to higher feeding success rate, and the possible evolution of preference via selection on other traits. Additionally, eggs used to establish the NY colony included some sites with a wider geographic spread (∼78 km) than that studied for feeding patterns in the field (∼40 km). This broader geographic origin may have impacted the results if variability for this trait exists within southern New York. This may have obscured more location-specific effects of blood type if they existed.
A previous report from Baltimore of high feeding rates on rats, led us to compare the fitness of NY and Baltimore Ae. albopictus after feeding on human and rat blood. Specifically, we investigated whether differences in fitness may be driving the striking differences in feeding patterns between the two locations. However, the only significant difference was higher egg production by NY mosquitoes fed human blood than all three other groups. If egg production was driving this difference, we would expect to also see higher egg production for Baltimore mosquitoes fed rat compared with human blood, but this was not the case. Furthermore, survival of mosquitoes fed on human blood was longer than those fed on rat blood for both Baltimore and NY Ae. albopictus. Together, these results suggest that a fitness advantage does not drive different feeding patterns in these two locations. The Baltimore study did not quantitatively assess host availability; however, the authors suggest that the percentage of abandoned properties and time spent in by residents in backyards (unpublished data) varied by neighborhood and corresponded with human blood meal proportion.8 In the absence of detected fitness benefits, it is possible that host availability was the driver of feeding pattern differences.
The impact of host blood source on Ae. albopictus egg production has only been assessed twice before. Gubler (1970) found greater fecundity for mouse-fed females, followed by guinea pig, rat, and chicken; however, the study was not replicated and no statistical analyses were conducted.31 In another study, chicken-fed Ae. albopictus were less fecund than those offered guinea pig or human blood and, consistent with our results, no differences between the two mammals were found.30 These results do not demonstrate a selective pressure for Ae. albopictus to evolve preferences within mammalian hosts. However, preference can evolve through other pathways and should be assessed directly. Other specialist feeders lack apparent fitness advantages for their preferred host. For example, Anopheles gambiae has a well-established preference for humans, but in a single study conducted to date, there is no fitness advantage provided by a human-only diet compared with a generalist diet.57
It is also possible that when assessed under different conditions, differences in fitness by host blood source may be revealed. For instance, we did not provide the mosquitoes with sugar after blood feeding; the presence of sugar has been shown to reduce reproductive fitness in Ae. albopictus compared with human blood alone and mosquitoes on Long Island feed frequently on sugar.43,58 For Ae. aegypti, the addition of sugar changed the directionality of host blood source effects on fitness, shifting the fitness benefits from human to mouse blood.29 If a similar phenomenon exists for Ae. albopictus, the absence of sugar in our experiments would maximize the fitness of human blood compared with other host types. We also only provided the mosquitoes with one blood meal. Providing a series of blood meals may have influenced our results.30
Aedes albopictus is often referred to as anthropophilic because of the high percentage of human blood meals in numerous field studies and the preference assessments conducted by Delatte et al. (2010).26 However, this classification remains unproven. In fact, our results are more indicative of a generally mammalophilic feeding behavior for Ae. albopictus. It is important to understand the underlying blood feeding behavior and physiology of Ae. albopictus because it influences and modulates the feeding patterns in the field, which will ultimately influence pathogen transmission.25 In Long Island, the diverse utilization of hosts in residential and farm settings demonstrates that Ae. albopictus could serve as an enzootic bridge vector. However, the absence of bird blood meals suggests that Ae. albopictus may be of limited concern as a vector of West Nile and Eastern equine encephalitis viruses in the northeastern United States. Populations of Ae. albopictus in this region have sufficient vector competence to transmit numerous anthroponotic viruses,59–61 but transmission of these pathogens may be limited as a result of lower rates of human feeding compared with other regions.62
Our results provide insight into blood feeding hosts that may influence disease transmission risk by Ae. albopictus in northeastern United States. Additionally, our observations reveal that integrating host availability measures into mosquito blood meal studies is important to interpret feeding patterns, but does not fully explain blood meal distribution. Fitness benefits did not explain the feeding patterns observed in NY or Baltimore, highlighting the need for further research on determinants of Ae. albopictus feeding behavior.
Supplemental tables
ACKNOWLEDGMENTS
We thank Paul Leisnham at the University of Maryland and Shannon LaDeau at the Cary Institute for providing the Baltimore Aedes albopictus eggs, Alex Amaro for assistance with blood meal identification, Dan Gilrein at Cornell Cooperative Extension, Moses Cucura, and Scott Campbell at Suffolk County Government for providing laboratory space and logistical support, Erika Mudrak for providing statistical expertise, and Talya Shragai for assistance with field site selection.
Note: Supplemental files appear at www.ajtmh.org.
Footnotes
Data availability: Data will be deposited in Cornell University Library's institutional repository, eCommons (https://ecommons.cornell.edu), for preservation and access. Datasets will be available via the world wide web without restriction at this DOI: https://doi.org/10.7298/84ky-sv64. eCommons provides each item with a persistent identifier and is committed to preserving the binary form of the digital object.
REFERENCES
- 1.Gratz N, 2004. Critical review of the vector status of Aedes albopictus. Med Vet Entomol 18: 215–227. [DOI] [PubMed] [Google Scholar]
- 2.Kraemer MU. et al. , 2015. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 4: e08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orsborne J, Mohammed AR, Jeffries CL, Kristan M, Afrane YA, Walker T, Yakob L, 2020. Evidence of extrinsic factors dominating intrinsic blood host preferences of major African malaria vectors. Sci Rep 10: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyimo IN, Ferguson HM, 2009. Ecological and evolutionary determinants of host species choice in mosquito vectors. Trends Parasitol 25: 189–196. [DOI] [PubMed] [Google Scholar]
- 5.Fikrig K, Harrington LC, 2021. Understanding and interpreting mosquito blood feeding studies: the case of Aedes albopictus. Trends in Parasitology. Available at: 10.1016/j.pt.2021.07.013. [DOI] [PubMed]
- 6.Ponlawat A, Harrington LC, 2005. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol 42: 844–849. [DOI] [PubMed] [Google Scholar]
- 7.Kamgang B, Nchoutpouen E, Simard F, Paupy C, 2012. Notes on the blood-feeding behavior of Aedes albopictus (Diptera: Culicidae) in Cameroon. Parasit Vectors 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodman H, Egizi A, Fonseca DM, Leisnham PT, LaDeau SL, 2018. Primary blood-hosts of mosquitoes are influenced by social and ecological conditions in a complex urban landscape. Parasit Vectors 11: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tempelis CH, Hayes RO, Hess AD, Reeves WC, 1970. Blood-feeding habits of 4 species of mosquito found in Hawaii. Am J Trop Med Hyg 19: 335–341. [DOI] [PubMed] [Google Scholar]
- 10.Little EA, Harriott OT, Akaratovic KI, Kiser JP, Abadam CF, Shepard JJ, Molaei G, 2021. Host interactions of Aedes albopictus, an invasive vector of arboviruses, in Virginia, USA. PLoS Neglect Trop D 15: e0009173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savage HM, Niebylski ML, Smith GC, Mitchell CJ, Craig GB, 1993. Host-feeding patterns of Aedes albopictus (Diptera, Culicidae) at a temperate North American site. J Med Entomol 30: 27–34. [DOI] [PubMed] [Google Scholar]
- 12.Richards SL, Ponnusamy L, Unnasch TR, Hassan HK, Apperson CS, 2006. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in relation to availability of human and domestic animals in suburban landscapes of central North Carolina. J Med Entomol 43: 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawabe K. et al. , 2010. Host-feeding habits of Culex pipiens and Aedes albopictus (Diptera: Culicidae) collected at the urban and suburban residential areas of Japan. J Med Entomol 47: 442–450. [DOI] [PubMed] [Google Scholar]
- 14.Gomes AC, Silva NN, Marques G, Brito M, 2003. Host-feeding patterns of potential human disease vectors in the Paraiba Valley Region, State of Sao Paulo, Brazil. J Vector Ecol 28: 74–78. [PubMed] [Google Scholar]
- 15.Faraji A, Egizi A, Fonseca DM, Unlu I, Crepeau T, Healy SP, Gaugler R, 2014. Comparative host feeding patterns of the Asian tiger mosquito, Aedes albopictus, in urban and suburban northeastern USA and implications for disease transmission. Plos Neglect Trop D 8: e3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valerio L, Marini F, Bongiorno G, Facchinelli L, Pombi M, Caputo B, Maroli M, della Torre A, 2010. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in urban and rural contexts within Rome province, Italy. Vector-Borne Zoonot 10: 291–294. [DOI] [PubMed] [Google Scholar]
- 17.Egizi A, Healy SP, Fonseca DM, 2013. Rapid blood meal scoring in anthropophilic Aedes albopictus and application of PCR blocking to avoid pseudogenes. Infect Genet Evol 16: 122–128. [DOI] [PubMed] [Google Scholar]
- 18.Guo XX, Li CX, Wang G, Zheng Z, Dong YD, Zhang YM, Xing D, Zhao TY, 2014. Host feeding patterns of mosquitoes in a rural malaria-endemic region in Hainan Island, China. J Am Mosquito Contr 30: 309–311. [DOI] [PubMed] [Google Scholar]
- 19.Sivan A, Shriram AN, Sunish IP, Vidhya PT, 2015. Host-feeding pattern of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in heterogeneous landscapes of South Andaman, Andaman and Nicobar Islands, India. Parasitol Res 114: 3539–3546. [DOI] [PubMed] [Google Scholar]
- 20.Stenn T, Peck KJ, Pereira GR, Burkett-Cadena ND, 2019. Vertebrate hosts of Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus (Diptera: Culicidae) as potential vectors of Zika virus in Florida. J Med Entomol 56: 10–17. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Yu HM, Lim HW, Yang SC, Roh JY, Chang KS, Shin EH, Ju YR, Lee WG, 2017. Host-feeding pattern and dengue virus detection of Aedes albopictus (Diptera: Culicidae) captured in an urban park in Korea. J Asia Pac Entomol 20: 809–813. [Google Scholar]
- 22.Niebylski ML, Savage HM, Nasci RS, Craig GB, 1994. Blood hosts of Aedes albopictus in the United States. J Am Mosquito Contr 10: 447–450. [PubMed] [Google Scholar]
- 23.Sivan A, Shriram AN, Sunish IP, Vidhya PT, 2015. Host-feeding pattern of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in heterogeneous landscapes of South Andaman, Andaman and Nicobar Islands, India. Parasitol Res 114: 3539–3546. [DOI] [PubMed] [Google Scholar]
- 24.Kek R. et al. , 2014. Feeding host range of Aedes albopictus (Diptera: Culicidae) demonstrates its opportunistic host-seeking behavior in rural Singapore. J Med Entomol 51: 880–884. [DOI] [PubMed] [Google Scholar]
- 25.Takken W, Verhulst NO, 2013. Host preferences of blood-feeding mosquitoes. Annu Rev Entomol 58: 433–453. [DOI] [PubMed] [Google Scholar]
- 26.Delatte H, Desvars A, Bouétard A, Bord S, Gimonneau G, Vourc’h G, Fontenille D, 2010. Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Réunion. Vector-Borne Zoonot 10: 249–258. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan MF, Gould DJ, Maneechai S, 1971. Observations on host range and feeding preferences of Aedes albopictus (Skuse). J Med Entomol 8: 713–716. [DOI] [PubMed] [Google Scholar]
- 28.McBride CS, Baier F, Omondi AB, Spitzer SA, Lutomiah J, Sang R, Ignell R, Vosshall LBJN, 2014. Evolution of mosquito preference for humans linked to an odorant receptor. Nature 515: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrington LC, Edman JD, Scott TW, 2001. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J Med Entomol 38: 411–422. [DOI] [PubMed] [Google Scholar]
- 30.Xue RD, Barnard DR, Ali A, 2009. Influence of multiple blood meals on gonotrophic dissociation and fecundity in Aedes albopictus. J Am Mosquito Contr 25: 504–507. [DOI] [PubMed] [Google Scholar]
- 31.Gubler D, 1970. Comparison of reproductive potentials of Aedes (Stegomyia) albopictus Skuse and Aedes (Stegomyia) polynesiensis Marks. Mosq News 30: 201–209. [Google Scholar]
- 32.Harrington LC, Shragai T, 2016. New York State Tiger Mosquito Education Network (Tiger NET). Ithaca, NY: eCommons, Cornell University.
- 33.Shragai T, Harrington LC, 2019. Aedes albopictus (Diptera: Culicidae) on an invasive edge: abundance, spatial distribution, and habitat usage of larvae and pupae across urban and socioeconomic environmental gradients. J Med Entomol 56: 472–482. [DOI] [PubMed] [Google Scholar]
- 34.Andreadis TG, Thomas MC, Shepard JJ, 2005. Identification Guide to the Mosquitoes of Connecticut. New Haven, CT: Connecticut Agricultural Experiment Station.
- 35.Ledermann JP, Powers AM, 2016. Analysis of CHIKV in mosquitoes infected via artificial blood meal. Methods in Molecular Biology 1426: 129–142. [DOI] [PubMed] [Google Scholar]
- 36.Molaei G, Oliver J, Andreadis TG, Armstrong PM, Howard JJ, 2006. Molecular identification of blood-meal sources in Culiseta melanura and Culiseta morsitans from an endemic focus of eastern equine encephalitis virus in New York. Am J Trop Med 75: 1140–1147. [PubMed] [Google Scholar]
- 37.Reeves LE, Gillett-Kaufman JL, Kawahara AY, Kaufman PE, 2018. Barcoding blood meals: new vertebrate-specific primer sets for assigning taxonomic identities to host DNA from mosquito blood meals. PLoS Negl Trop Dis 12: e0006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kent RJ, Norris DE, 2005. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am J Trop Med 73: 336–342. [PMC free article] [PubMed] [Google Scholar]
- 39.Linske MA, Williams SC, Stafford KC, III, Ortega IM, 2018. Ixodes scapularis (Acari: Ixodidae) reservoir host diversity and abundance impacts on dilution of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) in residential and woodland habitats in Connecticut, United States. J Med Entomol 55: 681–690. [DOI] [PubMed] [Google Scholar]
- 40.Shragai T, 2020. Aedes albopictus invasions: how an invasive mosquito vector adapts and behaves in novel environments. In: Cornell Theses and Dissertations. Ithaca, NY: eCommons, Cornell University. Available at: 10.7298/md10-0v54. [DOI]
- 41.Ledesma N, Harrington L, 2015. Fine-scale temperature fluctuation and modulation of Dirofilaria immitis larval development in Aedes aegypti. Vet Parasitol 209: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nasci RS, 1990. Relationship of wing length to adult dry weight in several mosquito species (Diptera: Culicidae). J Med Entomol 27: 716–719. [DOI] [PubMed] [Google Scholar]
- 43.Fikrig K, Peck S, Deckerman P, Dang SR, St Fleur K, Goldsmith H, Qu S, Rosenthal H, Harrington LC, 2020. Sugar feeding patterns of New York Aedes albopictus mosquitoes are affected by saturation deficit, flowers, and host seeking. Plos Neglect Trop D 14: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kay BH, Boreham PFL, Edman JD, 1979. Application of the feeding index concept to studies of mosquito host-feeding patterns. Mosq News 39: 68–72. [Google Scholar]
- 45.Hess A, Hayes RO, Tempelis C, 1968. The use of the forage ratio technique in mosquito host preference studies. Mosq News 28: 386–389. [Google Scholar]
- 46.Lenth R, 2019. Emmeans: estimated marginal means, aka least-squares means. R package v. 1.3. 4.
- 47.Southwood T, 1978. Introduction to the study of animal populations. Ecological Methods. Springer, 1–6. [Google Scholar]
- 48.Tuten HC, Bridges WC, Paul KS, Adler PH, 2012. Blood-feeding ecology of mosquitoes in zoos. Med Vet Entomol 26: 407–416. [DOI] [PubMed] [Google Scholar]
- 49.Greenberg JA, DiMenna MA, Hanelt B, Hofkin BV, 2012. Analysis of post‐blood meal flight distances in mosquitoes utilizing zoo animal blood meals. J Vector Ecol 37: 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marini F, Caputo B, Pombi M, Tarsitani G, Della Torre A, 2010. Study of Aedes albopictus dispersal in Rome, Italy, using sticky traps in mark–release–recapture experiments. Med Vet Entomol 24: 361–368. [DOI] [PubMed] [Google Scholar]
- 51.Honório NA, Silva Wd C, Leite PJ, Gonçalves JM, Lounibos LP, Lourenço-de-Oliveira R, 2003. Dispersal of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in an urban endemic dengue area in the State of Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 98: 191–198. [DOI] [PubMed] [Google Scholar]
- 52.Alshurafa N, Jain J, Stump TK, Spring B, Robinson JK, 2019. Assessing recall of personal sun exposure by integrating UV dosimeter and self-reported data with a network flow framework. PLoS One 14: e0225371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laidoudi Y, Tahir D, Medkour H, Varloud M, Mediannikov O, Davoust B, 2020. Effect of dinotefuran, permethrin, and pyriproxyfen (Vectra® 3D) on the foraging and blood-feeding behaviors of Aedes albopictus using laboratory rodent model. Insects 11: 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silveira L, Jacomo AT, Diniz-Filho JAF, 2003. Camera trap, line transect census and track surveys: a comparative evaluation. Biol Conserv 114: 351–355. [Google Scholar]
- 55.O’Brien TG, Kinnaird MF, 2008. A picture is worth a thousand words: the application of camera trapping to the study of birds. Bird Conserv Int 18: S144–S162. [Google Scholar]
- 56.Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P, 2006. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol 4: e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lyimo I, Keegan S, Ranford‐Cartwright L, Ferguson H, 2012. The impact of uniform and mixed species blood meals on the fitness of the mosquito vector Anopheles gambiae ss: does a specialist pay for diversifying its host species diet? J Evol Biol 25: 452–460. [DOI] [PubMed] [Google Scholar]
- 58.Braks MAH, Juliano SA, Lounibos LP, 2006. Superior reproductive success on human blood without sugar is not limited to highly anthropophilic mosquito species. Med Vet Entomol 20: 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanchez-Vargas I, Harrington LC, Black WC, Olson KE, 2019. Analysis of salivary glands and saliva from Aedes albopictus and Aedes aegypti infected with chikungunya viruses. Insects 10: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dieme C, Ciota AT, Kramer LD, 2020. Transmission potential of Mayaro virus by Aedes albopictus, and Anopheles quadrimaculatus from United States. Parasit Vectors 13: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaczmarek ME, Herzog NL, Noval MG, Zuzworsky J, Shah Z, Bajwa WI, Stapleford KA, 2020. Distinct New York City Aedes albopictus mosquito populations display differences in salivary gland protein D7 diversity and chikungunya virus replication. Viruses 12: 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olson MF, Ndeffo-Mbah ML, Juarez JG, Garcia-Luna S, Martin E, Borucki MK, Frank M, Estrada-Franco JG, Rodríguez-Pérez MA, Fernández-Santos NA, 2020. High rate of non-human feeding by Aedes aegypti reduces Zika virus transmission in south Texas. Viruses 12: 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.