ABSTRACT.
This prospective cohort study aimed to assess incidence and etiology of adult-onset epilepsy in previously seizure-free Atahualpa residents aged ≥ 20 years. Persons with adult-onset epilepsy occurring over 5 years were identified from annual door-to-door surveys and other overlapping sources. Those who emigrated or declined consent were excluded at the administrative censoring date of the last survey when these subjects were interviewed. Persons who died and those who developed incident epilepsy were censored at the time of these outcomes. Poisson regression models adjusted for demographics, education, alcohol intake, and the length of observation time, were used to estimate annual adult-onset epilepsy incidence rate ratio and cumulative incidence. Systematic neuroimaging screening was offered to participants to get insights on the etiology of epilepsy. Individuals enrolled in this cohort (N = 1,480) contributed to 6,811.6 years of follow-up. Seventeen developed incident adult-onset epilepsy, for an annual incident rate of 249.2 per 100,000 persons-year (95% CI: 130.7–367.7). Cumulative incidence was 1,245.9 per 100,000 persons (95% CI: 653.7–1,838.3) after a mean of 4.6 (SE: 0.06) years of follow-up. Six persons with incident epilepsy had neurocysticercosis (35%). Individuals with neurocysticercosis were six times more likely to develop adult-onset epilepsy than those without this disease (IRR: 6.01; 95% CI: 2.16–16.7), after adjusting for relevant covariates. The attributable fraction of incident adult-onset epilepsy due to neurocysticercosis was 30.9% (95% CI: 25.6–46.2%). This rural Ecuadorian population has a high incidence of adult-onset epilepsy, with neurocysticercosis being an important contributory cause.
INTRODUCTION
According to current estimates, about 46 million people have active epilepsy, most of whom live in Low-and-Middle-Income Countries (LMIC).1 A sizable proportion of these individuals have adult-onset epilepsy (also known as late-onset epilepsy), which is defined as epilepsy starting after the age of 20 years.2 Although old estimates suggested that 700 individuals per million population per year develop adult-onset epilepsy, and that about 20–25% of all persons with epilepsy (PWE) have their first seizure in adult life,3 there is no robust evidence addressing the global incidence of adult-onset epilepsy. Most studies came from patients admitted to hospitals or evaluated at epilepsy clinics,4–7 and others were confined to older adults or just assessed the risk of seizures in patients with specific diseases, that is, strokes, brain tumors, or encephalitides.8–13 These studies are not representative of the population of individuals with adult-onset epilepsy at large.
Several population-based studies on the incidence of adult-onset epilepsy have been carried out in developed countries.14–16 These may not reflect the reality of PWE living in LMIC, where traumatic brain injuries and infections of the central nervous system are more common than in the developed world, explaining up to one-third of the excess fraction of adult-onset epilepsy observed in these regions.17,18 In addition, people in remote settings still links epilepsy to supernatural forces, which causes substantial stigma that, in turn, often results in deprived education, reduced marriage options, and unemployment.19 Stigma has also been associated with denial and underreporting, likely explaining the heterogeneity observed in epidemiological studies on epilepsy conducted in LMIC.20–22
Accurate estimations of the burden of adult-onset epilepsy will provide useful information to health authorities for the implementation of region-specific strategies directed to recognize symptomatic cases and to reduce the treatment gap, which is a major health issue for PWE living in many underserved communities.23–25 Taking the opportunity of the well-established Atahualpa Project Cohort, this 5-year prospective study aimed to assess the incidence and etiology of adult-onset epilepsy in a rural community of Ecuador that is representative of the region.26
MATERIAL AND METHODS
Study population.
Atahualpa is a rural village located in coastal Ecuador, where previous epidemiological studies on the prevalence and etiology of epilepsy have been conducted.21,27 Atahualpa is a small rural village with a low migration rate, which makes it an optimal setting for the conduction of population-based cohort studies; the population is homogeneous regarding ethnicity (Amerindian ancestry), socioeconomic status and overall living conditions, as detailed elsewhere.28
Background data.
A door-to-door survey was conducted in October 2015, to assess epilepsy prevalence—defined as history of at least two unprovoked seizures occurring > 24 hours apart—in community-dwellers aged ≥ 20 years living in Atahualpa. In that survey, 41 out of 1,530 participants had epilepsy, for a crude prevalence rate of 26.8 (95% CI: 19.8–36.2) per 1,000 population.21 Surveyed participants, excluding the 41 with histories of epilepsy and nine with a history of a single seizure, were followed in a cohort study to assess epilepsy incidence.
Cohort study design.
This prospective cohort aimed to assess the annual incidence rate and the 5-year cumulative incidence of adult-onset epilepsy. In addition, the study attempted to get insights into the etiology of epilepsy. It commenced on November 1, 2015, and started with 1,480 seizure-free individuals aged ≥ 20 years identified during the above-mentioned prevalence survey. To identify incident epilepsy cases, all participants were screened yearly by means of door-to-door surveys using a validated field instrument developed by Placencia et al.,29 and the diagnosis was confirmed by a certified neurologist following the same protocol and operational definitions as those used for detection of prevalent cases.21 These subsequent surveys (2016–2020) were conducted at the same time of the year as the aforementioned prevalence assessment. Over the study years, Atahualpa residents were periodically visited at their homes to recall information about several disorders of interest, allowing our field personnel to be in continuous contact with villagers.30 In addition, we used the method of capture-recapture to enhance the detection and provide full ascertainment of all possible cases during the follow-up.31 Therefore, besides annual door-to-door surveys and frequent home visits, medical records of cohort participants were searched and reviewed after each annual survey from the single health center of Atahualpa.
All individuals who were confirmed to have adult-onset epilepsy in the year before each of the surveys were excluded from the cohort and followed up by study physicians (administrative censoring was the date of the second unprovoked seizure). Individuals with a single unprovoked seizure continued in the cohort until the end of the study or the date when they develop a second unprovoked seizure (incident adult-onset epilepsy case). Persons with acute symptomatic seizures (alcohol intoxication, head trauma without demonstrable brain injury, severe dehydration, and—more recently—associated with the acute phase of COVID-19) were not considered to have epilepsy and continued into the cohort. Those who emigrated or declined further consent were taken out of the cohort at the administrative censoring date of the last annual survey when the individual was interviewed. Persons who died were censored at the time of death.
The present study followed the recommendations of the standards for reporting of observational studies in epidemiology guidelines,32 and both the study protocol and a comprehensive informed consent were approved by the I.R.B. of Hospital-Clínica Kennedy (FWA 00030727) up to 2019, and by that of Universidad Espiritu Santo—Ecuador (FWA: 00028878) in 2020.
Complementary exams.
All participants were offered a nonenhanced CT of the head, performed with a Philips Brilliance 64 CT scanner (Philips Medical Systems, Eindhoven, The Netherlands). Women of childbearing age had a pregnancy test before the scan and those who were pregnant were rescheduled after delivery. Brain MRIs, performed with a Philips Intera 1.5T (Philips Medical Systems, Eindhoven, The Netherlands), were also offered to all individuals aged ≥ 60 years, to those who had abnormalities on computed tomography (CT) scans (irrespective of age), and to those with newly diagnosed adult-onset epilepsy (incident cases) during the follow-up. Neuroimaging exams were independently reviewed by an experienced neuroradiologist and a neurologist, blinded to clinical information. Kappa coefficients for inter-rater agreement were higher than 0.85 for all lesions of interest, and disagreements were resolved by consensus.
In addition, a 1-hour scalp electroencephalograph (EEG) using a Nihon Kohden EEG-1200 digital machine (Nihon Kohden Corporation, Tokyo, Japan), which was collected using the international 10–20 electrode configuration with the addition of T1 and T2 electrodes, was offered to individuals who developed seizures during the follow-up (performed at least 2 weeks after the seizure) as well as to a group of healthy individuals that were randomly selected as controls in other studies conducted by our group.33 Missing one or more complementary exams did not represent a criterion for exclusion.
Statistical analysis.
Data analyses are carried out by using STATA version 17 (College Station, TX). In univariate analyses, continuous variables were compared by linear models and categorical variables by χ2 or Fisher exact test as appropriate. For computing persons per year of follow-up, we considered the time under surveillance starting the day of first visit and ending at the administrative censoring date (October 31) of the last annual survey when the individual was interviewed, or the date of adult-onset epilepsy diagnosis or death. Poisson regression models adjusted for age, gender, level of education, history of alcohol intake, and the length of observation time, were used to estimate adult-onset epilepsy incidence rate ratio (IRR), as well as covariates influencing such incidence. Likewise, the attributable fraction of epilepsy due to neurocysticercosis, the most common cause of symptomatic epilepsy in the village,27 and the likelihood of this parasitic disease to cause incident adult-onset epilepsy were computed.
RESULTS
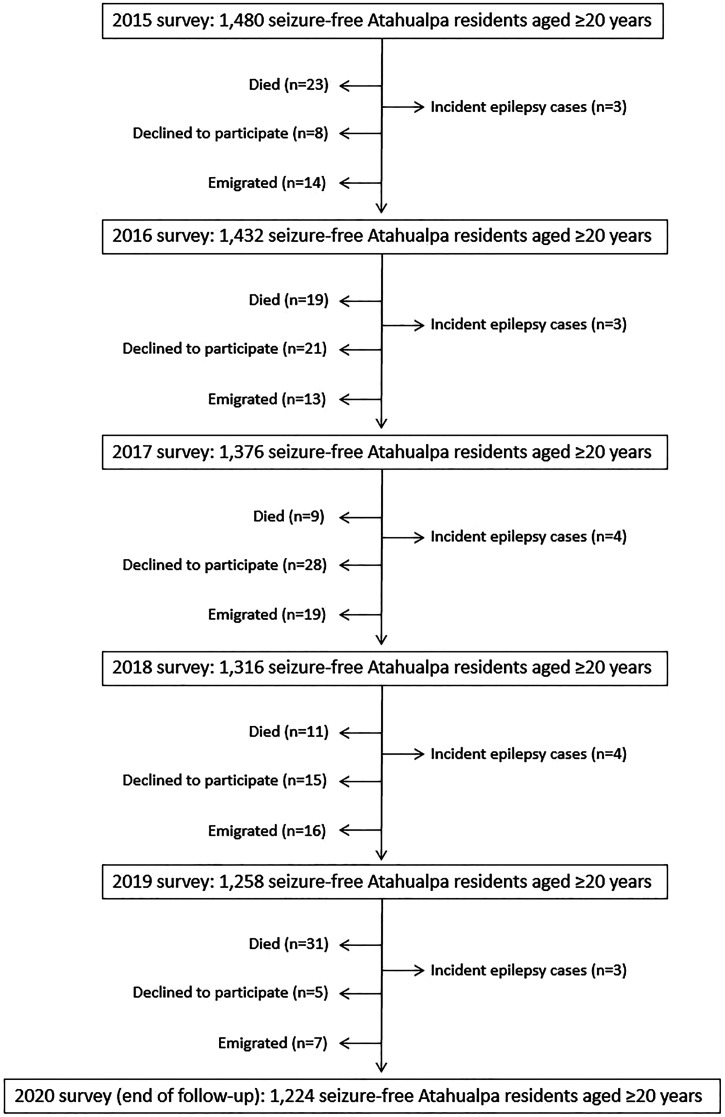
Figure 1 is a flow diagram depicting the reasons for excluding individuals at each of the annual door-to-door surveys. The low number of subjects who left the study after the 2019 survey as well as the high mortality during 2020, were related to the SARS-CoV-2 pandemic (that severely struck the village from March 2020).34 The mean (±SD) age of the 1,480 subjects enrolled in the naive cohort was 43.7 ± 18.4 years (age range: 20–97 years), 791 (53%) were women, 596 (40%) had primary school education only, and 298 (20%) admitted moderate-to-severe alcohol intake (all of them were men). CTs of the head were performed in 1,248 (84%) participants, brain magnetic resonance imaging (MRI) in 484 (33%), and EEG recordings in 181 (12%).
Figure 1.
Flow diagram depicting the reasons for censoring individuals at each of the annual door-to-door surveys and the raw number of incident adult-onset epilepsy cases per year.
A total of 93 individuals died, 77 declined consent, and 69 emigrated during the study years. These subjects were censored before the end of the study, as previously mentioned. In addition, 17 individuals developed adult-onset epilepsy and were also censored before the end of the study. The remaining 1,224 participants were followed-up until the last censoring date (October 31, 2020). The latter included three subjects who only experienced a single unprovoked seizure and five who had acute symptomatic seizures (during the acute phase of COVID-19 in two, as the result of alcohol intoxication in two, and related to minor traumatic brain injury in one). Individuals in this cohort contributed to 6,811.6 years of follow-up (95% CI: 6,733.7–6,889.5).
Table 1 depicts the characteristics of individuals enrolled up to the end of the study as well as of those who were censored earlier due to each of the abovementioned reasons. When compared with people who finished the cohort, individuals who died during the study years were older, more often male and less educated, individuals who declined consent were older, and those who emigrated were younger and more educated. There were no differences in any of the covariates investigated across participants who finished the cohort and those who developed incident epilepsy (univariate analyses).
Table 1.
Differences in Atahualpa residents enrolled up to the end of the study (referent category) as well as of those who were censored earlier due to several reasons (univariate analysis)
| Variable | Finished the cohort (N = 1,224) | Censored before the end of the study (N = 256) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Death (N = 93) | P value* | Declined (N = 77) | P value* | Emigrated (N = 69) | P value* | Epilepsy (N = 17) | P value* | ||
| Age, years (mean ± SD) | 41.6 ± 16.9 | 70.9 ± 12.7 | < 0.001† | 50.8 ± 19 | < 0.001† | 37 ± 16.7 | 0.028† | 42.4 ± 18.8 | 0.847 |
| Female gender, n (%) | 666 (54) | 40 (43) | 0.034† | 43 (56) | 0.807 | 30 (43) | 0.076 | 12 (71) | 0.183 |
| Primary school education, n (%) | 472 (39) | 72 (77) | < 0.001† | 34 (44) | 0.329 | 14 (20) | 0.002† | 4 (24) | 0.315 |
| Mod-severe alcohol intake, n (%) | 257 (21) | 14 (15) | 0.172 | 16 (21) | 0.964 | 10 (14) | 0.194 | 1 (6) | 0.223 |
P values were calculated by comparing characteristics of individuals censored before the end of the study (for each of the different causes) and those who finished the cohort.
Statistically significant result.
The most common abnormality identified in neuroimaging studies was neurocysticercosis in 110 out of 1,248 individuals evaluated with CT (8.8%; 95% CI: 7.4–10.5%). All subjects with neurocysticercosis had typical parenchymal brain calcifications,35 which were also identified by MRI in 70 out of 109 individuals (roughly two-thirds of cases). Typical parenchymal brain calcifications were defined as single or multiple solid calcified nodules that measure less than 10 mm in diameter.35 There were no other forms of neurocysticercosis (live or degenerating parenchymal brain cysts or extra-parenchymal lesions) even after MRI readings. Calcifications were single in 79 (72%) cases, from two to three in 24, and ≥ 4 in the remaining seven. Hippocampal atrophy—visually rated according to the Scheltens’ medial temporal atrophy scale (as previously described by our group36)—was noticed in 29/109 (27%) individuals with neurocysticercosis and in 38/375 (10%) of those without neurocysticercosis evaluated with MRI (P < 0.001). Other potentially relevant abnormalities identified by either CT or MRI included cortical infarctions in 15 cases, supratentorial brain hemorrhages in five, congenital arachnoid cysts in three, brain tumors in two, and an old cerebral contusion in one. Electroencephalogram recordings were normal in 157 (87%) out of 181 individuals. Electroencephalogram abnormalities—in 24 cases—included focal or generalized slowing in 19 and sharp waves in five.
A total of 17 out of 1,480 individuals developed incident adult-onset epilepsy, for an annual incidence rate of 249.2 per 100,000 persons-year (95% CI: 130.7–367.7). Cumulative incidence was 1,245.9 per 100,000 persons (95% CI: 653.7–1,838.3) after a mean of 4.6 (SE: 0.06) years of follow-up. Incident adult-onset epilepsy was not related to age, gender, level of education, or severity of alcohol intake in an adjusted Poisson regression model (Table 2). Age was not only modeled as a continuous variable, but also stratified in quartiles (20–28, 29–39, 40–58, 59–91 years) and no differences were found (data not shown).
Table 2.
Poisson regression model fitted to assess the relationship between age, gender, level of education and severity of alcohol intake, and incident epilepsy (as the dependent variable)
| Incident epilepsy | β coefficient | 95% CI | P value |
|---|---|---|---|
| Age | 0.006 | −0.024–0.037 | 0.693 |
| Female sex | 0.367 | −0.764–1.497 | 0.525 |
| Primary school education | −0.887 | −2.176–0.403 | 0.178 |
| Mod-severe alcohol intake | −1.108 | −3.298–1.082 | 0.321 |
Statistically significant result.
All the 17 patients with incident adult-onset epilepsy were studied with both CT and MRI (and 16 of them also had scalp EEG recordings). Six (35.3%; 95% CI: 17.3–58.7%) of these patients had calcified neurocysticercosis (a single calcification in four, and two and six calcifications in one patient each); only one of these patients had hippocampal atrophy and two others had interictal focal paroxysmal abnormalities in the EEG. The time elapsed between the first and the second unprovoked seizures varied from a few days to 20 months. Seizures were generalized in four cases and focal in the remaining two. EEG recordings showed sharp waves ipsilateral to a single calcification in one patient with focal seizures, generalized sharp waves in one patient with a single calcification and tonic-clonic generalized seizures, diffuse slowing in another patient with one calcification, ipsilateral hippocampal atrophy and focal seizures, and were normal in the remaining three (two with a single calcification and the other with two calcifications; all with generalized tonic-clonic seizures). None of these patients had any other potentially epileptogenic lesion on neuroimaging studies, with the exception of the above-described patient with focal epilepsy who also had asymmetrical hippocampal atrophy, which has been related to neurocysticercosis.37
Individuals with neurocysticercosis were six times more likely to develop incident adult-onset epilepsy than those without neurocysticercosis on baseline CT scans (6/110 versus 11/1,138; IRR: 6.01; 95% CI: 2.16–16.7; P = 0.001), after adjusting for demographics, levels of education, and severity of alcohol intake (Table 3). The attributable fraction of incident adult-onset epilepsy due to neurocysticercosis was 30.9% (95% CI: 25.6–46.2%).
Table 3.
Poisson regression model showing the incident rate risk of individuals with neurocysticercosis to develop adult-onset epilepsy when compared with those without neurocysticercosis, after adjusting for demographics, levels of education, and severity of alcohol intake
| Incident epilepsy | IRR | 95% CI | P value |
|---|---|---|---|
| Neurocysticercosis | 6.01 | 2.16–16.7 | 0.001* |
| Age | 0.99 | 0.96–1.03 | 0.865 |
| Female sex | 1.21 | 0.39–3.79 | 0.741 |
| Primary school education | 0.49 | 0.14–1.75 | 0.274 |
| Mod-severe alcohol intake | 0.32 | 0.04–2.87 | 0.309 |
Statistically significant result.
Of the remaining 11 patients with incident adult-onset epilepsy, nine had normal CT/MRI and were classified as of unknown etiology (EEG recording showed diffuse slowing in only one case), one patient had an old ischemic stroke in the cortical territory of the middle cerebral artery, and the other had a congenital arachnoid cyst in the temporal lobe, which may or may not be the cause of the epilepsy. The patient with the old cortical infarction had focal slowing in the corresponding cerebral hemisphere and the one with the arachnoid cyst had a normal EEG. One of the subjects with epilepsy of unknown etiology had systemic lupus erythematous as a comorbidity. Six of nine patients with epilepsy of unknown etiology had generalized (tonic-clonic) seizures and the remaining three had focal seizures (simple partial with motor symptoms in two can complex partial in one). The patient with a cortical infarction and the one with a congenital arachnoid cyst had focal seizures (simple partial with motor symptoms and complex partial, respectively).
DISCUSSION
This prospective cohort study, conducted in community dwellers aged ≥ 20 years living in rural Ecuador, demonstrates a high incidence rate of adult-onset epilepsy, providing further support to previous pooled data disclosing that incidence rates of all epilepsies—per 100,000 persons per year—is higher in LMIC (139.0; 95% CI: 69.4–278.2) than in the developed world (48.9; 95% CI: 39.0–61.1).38
There are some high-quality studies on the incidence of epilepsy conducted in LMIC. A recent systematic review and meta-analysis addressing epilepsy prevalence and incidence in Latin America and the Caribbean,39 compiled six incidence studies that included only children,40 or both children and adults.41–45 Two of them evaluated retrospective incidence, three prospective incidence, and the other prospective incidence after an intervention. The pooled incidence rate of epilepsy in the six studies was 111.2 (95% CI: 64.9–169.5) per 100,000 persons per year. However, all these studies included children and some have different designs and results may not be totally comparable with our findings.
In another population-based study, Placencia et al.,46 evaluated the retrospective 1-year incidence of epilepsy in inhabitants of the Andean region of Ecuador (all ages), and found incidence rates ranging from 122 to 190 cases per 100,000 population. The lower incidence rate of epilepsy in that study when compared with the present cohort could have been related to the retrospective design, as it is well-known that the effects of recall biases or denial when individuals from remote settings are interviewed about seizures.21 Another study from low-resource settings in India disclosed an annual epilepsy incidence rate of 58 per 100,000 population, but the study was limited to children aged 5 to 15 years old and cannot be compared with our cohort.47 In another systematic review and meta-analysis of studies addressing epilepsy incidence, Fiest et al.,22 found a pooled annual incidence rate of epilepsy in LMIC of 138.99 per 100,000 population. In that study, the 95% confidence interval was 69.45 to 278.16 per 100,000. The upper end of the range of the incidence rate (278.16) is quite similar to the findings of the present study.
The importance of the independent assessment of adult-onset epilepsy in different geographical settings lies on the identification of regions with a high burden that, in turn, may help to recognize causes that can be prevented and treated to achieve a better seizure control and overall prognosis.4–8
While designs of studies aimed to assess incidence and etiology of adult-onset epilepsy at the population level in LMIC may not be comparable with the current cohort, data from other studies conducted in developed countries may serve for comparison purposes. A large population study in Sweden disclosed an incidence rate of 76 per 100,000 persons per year among individuals aged ≥ 17 years (close to the minimum age for considering adult-onset epilepsy); in this cohort, acquired epilepsy occurred more often with advancing age, reaching a prevalence of 77% in older adults (aged ≥ 60 years), and the most common etiologies were cerebrovascular diseases and brain tumors.14 In another French study limited to older adults (age ≥ 60 years), the incidence rate of all seizures was 127.2 per 100,000 persons-year, but most enrolled individuals had single seizures or acute symptomatic seizures and only less than one-third had recurrent unprovoked seizures.16 In that study, cerebrovascular diseases were the most common cause of this condition. Another study in individuals aged 17–64 years living in Ohio, and based on Medicaid claims, disclosed a rate of recently diagnosed (past 5 years) epilepsy of 362 per 100,000 persons per year.15 According to the authors, this high incidence rate was likely related to the poor socioeconomic status and premorbid conditions of people covered by Medicaid. While this high rate may reflect the situation of people living in LMIC, the design made it impossible to determine whether PWE enrolled in the study also had seizures during childhood.
Hospital-based and cross-sectional studies on adult-onset epilepsy reported from developed countries have shown a peak in older adults and a male predominance.48 This is not the case of the present cohort, where the mean age of incident cases was about 40 years, and women predominated (71% of cases). Such disparities may be related to the younger age of the cohort, the female predominance, and the most common etiology of symptomatic adult-onset epilepsy.
Our results also disclose that neurocysticercosis is an important etiology of adult-onset epilepsy—at the population level—in rural Ecuador, as has been previously shown in hospital-based cross-sectional studies in Latin America.5,6 In Atahualpa, this parasitic disease accounted for about one-third of incident cases and increased six times the risk for developing this condition than in those without neurocysticercosis, after adjusting for several covariates. Of interest, all neurocysticercosis patients who had a first unprovoked seizure during the study period developed a second seizure during the study years, providing proof of concept that calcified cysticerci represent enduring epileptogenic foci that should not be seen as lesions associated with acute symptomatic seizures.49,50 This may not be true for some patients with a single colloidal cysticercus located in the brain parenchyma, but only in the event that this lesion disappears either spontaneously or as the result of cysticidal drug therapy.51 Nevertheless, patients with colloidal cysticercus should not be a priori considered to have acute symptomatic seizures since many of these lesions ended up into a calcified nodule, which represent a permanent epileptogenic foci with a high rate of seizure relapses.52 Results from the present cohort are consistent with our previous prevalence study in the same population, where PWE had three times the odds of having neurocysticercosis than those without epilepsy.27 It would be a serious omission to deprive these patients from therapy based on the incorrect assumption that they will not developed seizure relapses.
The present study has limitations that include the relatively small number of persons with incident epilepsy (limiting more robust conclusions on etiology) and the fact that the cohort was restricted to one village, which is, nevertheless, representative of neighboring villages in terms of living conditions and risk factors,26 as well as in the prevalence of neurocysticercosis (O. H. Del Brutto et al., unpublished data). In addition, a sizable proportion of the studied population did not receive MRI, thus limiting the detection of some small subarachnoid or ventricular cysts not readily visualized on CT. However, missing MRI information did not modify the results of this study since the diagnosis of epilepsy is done on clinical grounds alone. Likewise, small subarachnoid or ventricular cysticercal lesions not detected by CT (in patients without MRI) were unlikely to have changed the contributory role of neurocysticercosis on epilepsy incidence since all patients with newly diagnosed epilepsy received a brain MRI.
While it is theoretically possible that epilepsy and neurocysticercosis occur by chance alone, this is unlikely. Evidence suggest that this parasitic disease is indeed associated with epilepsy, and it is possible that the scenario of Atahualpa is similar to that of other Latin American populations where neurocysticercosis is a leading cause of acquired epilepsy.18,45
On the other hand, this study has several strengths such as the population-based design with unbiased enrollment of participants, the length of the cohort (5 years), the close follow-up by field personnel acquainted with the population (reducing the bias of denial related to stigma), the use of a validated field instrument for seizures detection, the neurological interview for confirming suspected cases, and the practice of neuroimaging studies in most participants (including all with newly diagnosed epilepsy).
In conclusion, this study demonstrates a high annual incidence rate and cumulative incidence of adult-onset epilepsy in a rural setting of a LMIC, as well as the importance of neurocysticercosis as an etiology of this condition. The high incidence is likely similar in many villages endemic for neurocysticercosis, where this parasitic disease significantly contributes to the global burden of adult-onset epilepsy.
REFERENCES
- 1. Global Burden of Disease 2016 Epilepsy Collaborators , 2019. Global, regional, and national burden of epilepsy, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18: 357–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daras M, Tuchman AJ, Strobos RJ, 1987. Computed tomography in adult onset epileptic seizures in a city hospital population. Epilepsia 28: 519–522. [DOI] [PubMed] [Google Scholar]
- 3.Dam AM, Fuglsang-Frederiksen A, Svarre-Olsen U, Dam M, 1985. Late-onset epilepsy: etiologies, types of seizures, and value of clinical investigation, EEG, and computerized tomography scan. Epilepsia 26: 227–231. [DOI] [PubMed] [Google Scholar]
- 4.Caprana ALF, Rissardo JP, Leite MTB, Silveira JOF, Jauris PGM, Arend J, Kegler A, Freire Royes LF, Rechi Fighera M, 2020. Course and prognosis of adult-onset epilepsy in Brazil: a cohort-study. Epilepsy Behav 105: 106969. [DOI] [PubMed] [Google Scholar]
- 5.Del Brutto OH, Noboa CA, 1991. Late-onset epilepsy in Ecuador: aetiology and clinical features in 225 patients. J Trop Geogr Neurol 1: 31–34. [Google Scholar]
- 6.Medina MT, Rosas E, Rubio-Donnadieu F, Sotelo J, 1990. Neurocysticercosis as the main cause of late-onset epilepsy in Mexico. Arch Intern Med 150: 323–325. [PubMed] [Google Scholar]
- 7.Paradowski B, Zagrajek MM, 2005. Epilepsy in middle-aged and elderly people: a three-year observation. Epileptic Disord 7: 91–95. [PubMed] [Google Scholar]
- 8.Burneo JG, Tellez-Zenteno J, Steven DA, Niaz N, Hader W, Pillay N, Wiebe S, 2008. Adult-onset epilepsy associated with dysembryoplastic neuroepithelial tumors. Seizure 17: 498–504. [DOI] [PubMed] [Google Scholar]
- 9.Graham NS, Crichton S, Koutroumanidis M, Wolfe CD, Rudd AG, 2013. Incidence and associations of poststroke epilepsy: the prospective South London Stroke Register. Stroke 44: 605–611. [DOI] [PubMed] [Google Scholar]
- 10.Lezaic N, Roussy J, Masson H, Jetté N, Keezer MR, 2020. Epilepsy in the elderly: unique challenges in an increasing prevalent population. Epilepsy Behav 102: 106724. [DOI] [PubMed] [Google Scholar]
- 11.Luhdorf K, Jensen LK, Plesner AM, 1986. Etiology of seizures in the elderly. Epilepsia 27: 458–463. [DOI] [PubMed] [Google Scholar]
- 12.Szaflarski JP, Rackley AY, Kleindorfer DO, Khoury J, Woo D, Miller R, Alwell K, Broderick JP, Kissela M, 2008. Incidence of seizures in the acute phase of stroke: a population-based study. Epilepsia 49: 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Podewils F, Suesse M, Geithner J, Gaida B, Wang ZI, Lange J, 2017. Prevalence and outcome of late-onset seizures due to autoimmune etiology: a prospective observational population-based cohort study. Epilepsia 58: 1542–1550. [DOI] [PubMed] [Google Scholar]
- 14.Forsgren L, Buth G, Eriksson S, Bregmark L, 1996. Incidence and clinical characterization of unprovoked seizures in adults: a prospective population-based study. Epilepsia 37: 224–229. [DOI] [PubMed] [Google Scholar]
- 15.Kaiboriboon K, Bakaki PM, Lhatoo SD, Koroukian S, 2013. Incidence and prevalence of treated epilepsy among poor health and low-income Americans. Neurology 80: 1942–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loiseau J, Loiseau P, Duché B, Guyot M, Daretigues JF, Aublet B, 1990. A survey of epileptic disorders in southwest France: seizures in elderly patients. Ann Neurol 27: 232–237. [DOI] [PubMed] [Google Scholar]
- 17.Bruno E, Bartoloni A, Zammarchi L, Strohmeyer M, Bartalesi F, Bustos JA, Santivañez S, García HH, Nicoletti A, COHEMI Project Study Group , 2013. Epilepsy and neurocysticercosis in Latin America: a systematic review and meta-analysis. PLoS Negl Trop Dis 7: e2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debacq G, Moyano LM, Garcia HH, Boumediene F, Marin B, Ngoungou EB, Preux PM, 2017. Systematic review and meta-analysis estimating association of cysticercosis and neurocysticercosis with epilepsy. PLoS Negl Trop Dis 11: e0005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiest KM, Birbeck GL, Jacoby A, Jette N, 2014. Stigma in epilepsy. Curr Neurol Neurosci Rep 14: 444. [DOI] [PubMed] [Google Scholar]
- 20.Burneo JG, Tellez-Zenteno J, Wiebe S, 2005. Understanding the burden of epilepsy in Latin America: a systematic review of its prevalence and incidence. Epilepsy Res 66: 63–74. [DOI] [PubMed] [Google Scholar]
- 21.Del Brutto OH, Mera RM, for the Atahualpa Project Investigators , 2016. The importance of people compliance (social desirability bias) in the assessment of epilepsy in rural areas of developing countries. Results of the Atahualpa Project. Epilepsia 57: e221–e224. [DOI] [PubMed] [Google Scholar]
- 22.Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, Pringsheim T, Lorenzetti DL, Jetté N, 2017. Prevalence and incidence of epilepsy. A systematic review and meta-analysis of international studies. Neurology 88: 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu J. et al. 2014. Prevalence and treatment gap of active convulsive epilepsy: a large community-based survey in rural West China. Seizure 23: 333–337. [DOI] [PubMed] [Google Scholar]
- 24.Hunter E. et al. 2016. The epilepsy treatment gap in rural Tanzania: a community-based study in adults. Seizure 36: 49–56. [DOI] [PubMed] [Google Scholar]
- 25.Mogal Z, Aziz H, 2020. Epilepsy treatment gap and stigma reduction in Pakistan: a tested public awareness model. Epilepsy Behav 102: 106637. [DOI] [PubMed] [Google Scholar]
- 26.Del Brutto OH, Mera RM, Peralta LD, Hill JP, Generale LM, Torpey AP, Sedler MJ, 2020. Cardiovascular health status among community-dwelling Ecuadorian natives living in neighboring rural communities: the Three Villages Study. J Community Health 45: 154–160. [DOI] [PubMed] [Google Scholar]
- 27.Del Brutto OH, Arroyo G, Del Brutto VJ, Zambrano M, Garcia HH, 2017. On the relationship between calcified neurocysticercosis and epilepsy in an endemic village: a large-scale, computed tomography-based population study in rural Ecuador. Epilepsia 58: 1955–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Brutto OH, Zambrano M, 2017. Atahualpa, una población rural ideal para la práctica de estudios epidemiológicos. Rev Ecuat Neurol 26: 88–94. [Google Scholar]
- 29.Placencia M, Sander JW, Shorvon SD, Ellison RH, Cascante SM, 1992. Validation of a screening questionnaire for the detection of epileptic seizures in epidemiological studies. Brain 115: 783–794. [DOI] [PubMed] [Google Scholar]
- 30.Del Brutto OH, Castillo PR, Sedler MJ, Del Brutto VJ, Zambrano M, Mera RM, Wright CB, Rundek T, 2018. Reasons for declining consent in a population-based cohort study conducted in a rural South American community. J Environ Public Health 2018: 8267948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. International Working Group for Disease Monitoring and Forecasting , 1995. Capture-recapture and multiple-record systems estimation I: history and theoretical development. Am J Epidemiol 142: 1047–1058. [PubMed] [Google Scholar]
- 32.von Elm E, Altman G, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, STROBE Initiative , 2007. The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370: 1437–1457. [DOI] [PubMed] [Google Scholar]
- 33.Issa NP, Sedler MJ, Del Brutto VJ, Darsan E, Milla L, Montes J, Zambrano M, Del Brutto OH, 2018. EEG patterns in patients with calcified neurocysticercosis with and without hippocampal atrophy. J Clin Neurophysiol 35: 332–338. [DOI] [PubMed] [Google Scholar]
- 34.Del Brutto OH, Costa AF, Mera RM, Recalde BY, Bustos JA, Garcia HH, 2021. SARS-CoV-2 in rural Latin America. A population-based study in coastal Ecuador. Clin Infect Dis 73: 314–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Brutto OH, Nash TE, White AC, Jr., Rajshekhar V, Wilkins PP, Singh G, Vasquez CM, Salgado P, Gilman RH, García HH, 2017. Revised diagnostic criteria for neurocysticercosis. J Neurol Sci 372: 202–210. [DOI] [PubMed] [Google Scholar]
- 36.Del Brutto OH, Salgado P, Lama J, Del Brutto VJ, Campos X, Zambrano M, García HH, 2015. Calcified neurocysticercosis associates with hippocampal atrophy: a population-based study. Am J Trop Med Hyg 92: 64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Brutto OH, Engel J, Jr, Eliashiv DS, Salamon N, García HH, 2014. Hippocampal sclerosis: the missing link of cysticercosis epileptogenesis? Epilepsia 55: 2077–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beghi E, 2020. The epidemiology of epilepsy. Neuroepidemiology 54: 185–191. [DOI] [PubMed] [Google Scholar]
- 39.Alva-Díaz C. et al. 2021. Prevalence and incidence of Epilepsy in Latin America and the Caribbean: a systematic review and meta-analysis of population-based studies. Epilepsia 62: 984–996. [DOI] [PubMed] [Google Scholar]
- 40.Nunes ML, Cosalter Geib LT, Apego G, 2011. Incidence of epilepsy and seizure disorders in childhood and association with social determinants: a birth cohort stud. J Pediatr 87: 50–56. [DOI] [PubMed] [Google Scholar]
- 41.Bruno E, Quattrocchi G, Crespo Gomes EB, Sofia V, Padilla S, Camargo M, Zappia M, Bartoloni A, Nicoletti A, 2015. Prevalence and incidence of epilepsy associated with convulsive seizures in rural Bolivia. A Global Campaign Against Epilepsy Project. PLoS One 10: e0139108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lavados J, Germain L, Morales A, Campero M, Lavados P, 1992. A descriptive study of epilepsy in the district of El Salvador, Chile, 1984–1988. Acta Neurol Scand 85: 249–256. [DOI] [PubMed] [Google Scholar]
- 43.Medina MT. et al. 2011. Reduction in rate of epilepsy from neurocysticercosis by community interventions: the Salamá, Honduras study. Epilepsia 52: 1177–1185. [DOI] [PubMed] [Google Scholar]
- 44.Placencia M, 1992. Incidencia de crisis epilépticas en la Sierra Norte Ecuatoriana: un perfil epidemiológico, 1984–1989. Rev Ecuat Neurol 1: 72–76. [Google Scholar]
- 45.Villarán MV, Montano SM, Gonzalvez G, Moyano LM, Chero JC, Rodriguez S, Gonzalez AE, Pan W, Tsang VCW, Gilman RH, García HH, Cysticercosis Working Group in Perú , 2009. Epilepsy and neurocysticercosis: an incidence study in a Peruvian rural population. Neuroepidemiology 33: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Placencia M, Shorvon SD, Paredes V, Bimos C, Sander JW, Suarez J, Cascante SM, 1992. Epileptic seizures in an Andean region of Ecuador. Incidence and prevalence and regional variation. Brain 115: 771–782. [DOI] [PubMed] [Google Scholar]
- 47.Murthy JMK, Jaiswal SK, Reddy MP, Srikrishna S, 2020. Incidence study of epilepsy using the ILAE 2017 classification of epilepsies in a cohort of school children accessing education in government primary schools in south India. Neurol India 68: 1389–1393. [DOI] [PubMed] [Google Scholar]
- 48.Abramovici S, Bagic A, 2016. Epidemiology of epilepsy. Handb Clin Neurol 138: 159–171. [DOI] [PubMed] [Google Scholar]
- 49.Nash TE. et al. 2004. Calcific neurocysticercosis and epileptogenesis. Neurology 62: 1934–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nash TE. et al. 2015. Neurocysticercosis: a natural human model of epileptogenesis. Epilepsia 56: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh G, Singh P, Singh I, Rani A, Kaushal S, Avasthi G, 2006. Epidemiologic classification of seizures associated with neurocysticercosis: observations from a sample of seizure disorders in neurologic care in India. Acta Neurol Scand 113: 233–240. [DOI] [PubMed] [Google Scholar]
- 52.Bustos JA. et al. 2020. Frequency and determinant factors for calcification in neurocysticercosis. Clin Infect Dis. doi: 10.1093/cid/ciaa784. [DOI] [PMC free article] [PubMed] [Google Scholar]