Abstract
Background
Infants born very preterm often receive multiple red blood cell (RBC) transfusions during their initial hospitalisation. However, there is an increasing awareness of potential adverse effects of RBC transfusions in this vulnerable patient population. Modification of RBCs prior to transfusion, through washing with 0.9% saline, may reduce these adverse effects and reduce the rate of significant morbidity and mortality for preterm infants and improve outcomes for this high‐risk group.
Objectives
To determine whether pre‐transfusion washing of RBCs prevents morbidity and mortality in preterm infants.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 7), MEDLINE via PubMed (31 July 2015), EMBASE (31 July 2015), and CINAHL (31 July 2015). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
Randomised, cluster randomised, and quasi‐randomised controlled trials including preterm infants (less than 32 weeks gestation) or very low birth weight infants (less than 1500 g), or both, who received one or more washed packed RBC transfusions.
Data collection and analysis
Two review authors independently assessed the eligibility of the trials. We identified four studies from the initial search. After further review of the full‐text studies, we found one study meeting the selection criteria.
Main results
We included a single study enrolling a total of 21 infants for analysis in this review and reported on all‐cause mortality during hospital stay, length of initial neonatal intensive care unit (NICU) stay (days), and duration of mechanical ventilation (days). There was no significant difference in mortality between the washed versus the unwashed RBCs for transfusion groups (risk ratio 1.63, 95% confidence interval (CI) 0.28 to 9.36; risk difference 0.10, 95% CI ‐0.26 to 0.45). There was no significant difference in the length of initial NICU stay between the washed versus the unwashed RBCs for transfusion groups (mean difference (MD) 25 days, 95% CI ‐21.15 to 71.15) or the duration of mechanical ventilation between the washed versus the unwashed RBCs for transfusion groups (MD 9.60 days, 95% CI ‐1.90 to 21.10).
Authors' conclusions
We identified a single small study. The results from this study show a high level of uncertainty, as the confidence intervals are consistent with both a large improvement or a serious harm caused by the intervention. Consequently, there is insufficient evidence to support or refute the use of washed RBCs to prevent the development of significant neonatal morbidities or mortality. Further clinical trials are required to assess the potential effects of pre‐transfusion washing of RBCs for preterm or very low birth weight infants, or both, on short‐ and long‐term outcomes.
Plain language summary
Does pre‐transfusion washing of red blood cells for preterm babies improve their health outcomes?
Background: Babies born preterm or with a low birth weight may be given blood transfusions for a number of reasons. For example, they are sometimes unable to make their own blood well yet; they may need several blood tests to monitor their condition; or they may need extra blood if they become critically unwell.
Studies in older children and in adults have found that a process of ‘washing’ blood cells before transfusion improved short‐ and longer‐term outcomes. Washing blood removes almost all plasma proteins and most white blood cells, which may help reduce the side effects of a blood transfusion. We wanted to learn if preterm babies might experience these same positive effects.
Review question: We wanted to learn whether washing blood cells before transfusion reduces the chance of illnesses that tend to occur in preterm babies. Some of the outcomes that we looked at were illnesses affecting eyes (retinopathy of prematurity), lungs (chronic lung disease), brain (intraventricular haemorrhages or cysts), and long‐term developmental problems. We also wanted to look at other outcomes like length of hospital stay and acute transfusion reactions.
Key results: This review found only one study that evaluated the effects of washing blood cells before transfusion in preterm babies. This study included small numbers of babies. The outcomes the study reported that were relevant to our review were mortality, duration of mechanical ventilation, and length of initial hospitalisation. The results for all these outcomes were very uncertain. Washing blood cells might be helpful or harmful, but we cannot make a determination.
Quality of the evidence: It was hard from the available evidence to draw any conclusions about whether washing blood would be helpful or not for preterm babies. As of now, there is no strong evidence showing that washing blood makes any difference to the outcomes of preterm babies.
Background
Anaemia of prematurity (AOP) is a common multifactorial complication of preterm birth. Contributing causes include reduced levels of plasma erythropoietin (EPO) in response to anaemia and hypoxia, diminished red blood cell (RBC) life span, phlebotomy losses for laboratory testing, limited transplacental transfer of iron due to premature birth, and dependence on hepatic EPO production (Venkatesh 2012). Small‐volume RBC transfusions are used to manage AOP, with over 90% of preterm neonates with a birth weight at less than 1000 g receiving at least one RBC transfusion during their initial hospitalisation (Baer 2011; Mohamed 2012). While it is assumed that these transfusions are beneficial in preterm infants, the evidence available to support this is limited (Venkatesh 2012).
There is an increasing awareness of potential adverse effects related to RBC transfusions in the neonatal population. Transfusion may be associated with necrotising enterocolitis (NEC) (Mohamed 2012), intraventricular haemorrhage (IVH) (Baer 2011), retinopathy of prematurity (ROP) (Giannantonio 2012), chronic lung disease (CLD) (Cooke 1997), and mortality (dos Santos 2011; Valieva 2009). Furthermore, there is emerging evidence that other transfusion‐specific morbidities, such as transfusion‐related acute lung injury, may be under‐reported and under‐recognised in preterm infants (Gauvin 2012; Rashid 2013).
Several biologically plausible mechanisms to explain these associations have been proposed, including a 'two‐hit' model of post‐transfusion injury (Aiboshi 2001). This model hypothesises that an underlying inflammatory state primes the recipient's immune system with subsequent RBC transfusion triggering immune cell activation and related immunomodulation, resulting in frank inflammation (Tinmouth 2006). Transfusion‐related immunomodulation is proposed to underlie much of the increased transfusion‐associated morbidity and mortality observed in adult populations (Tinmouth 2006). A similar mechanism may exist in the vulnerable preterm population and could explain many of the associations observed between RBC transfusion and significant neonatal morbidities such as NEC, ROP, and CLD.
Research in paediatric and adult populations suggest that modifications to blood‐product processing prior to transfusion may improve both inflammatory responses and important clinical outcomes, including mortality in the recipient (Bilgin 2004; Blumberg 2004; Blumberg 2010; Fergusson 2003). In vivo studies in preterm infants suggest that allogeneic leukodepleted RBCs, used in many parts of the world as the standard blood product for preterm infants, are biologically active and result in endothelial activation, inflammation, and oxidative stress in preterm infants (Keir 2013; Stark 2013). Modification of RBCs prior to transfusion, through washing with 0.9% saline, may reduce these deleterious effects and improve outcomes for all populations, including preterm infants.
Description of the condition
Significant morbidities, including NEC, IVH, ROP, CLD, as well as increased mortality, have been associated with receipt of RBC transfusion in preterm infants. No direct causal relationship has been established, but transfusion‐related immunomodulation may underlie this increased transfusion‐associated morbidity and mortality. Modifications of the RBC product prior to transfusion may ameliorate some of these potential effects and lead to better outcomes for preterm infants.
Description of the intervention
Saline‐washed RBCs are units of whole blood or RBCs that have been washed with 1 to 2 L of saline prior to transfusion. Pre‐transfusion washing of RBCs occurs through a manual 'open' system technique (Grabmer 2006), an automated cell washer (O'Leary 2011), or via an auto‐transfusion device (de Vroege 2007). Washed units contain 10% to 20% fewer RBCs than the original units. These units are depleted of 99% of plasma proteins and 85% of white blood cells. It is important to consider the clinical implications of this time‐ and resource‐intensive processing step.
How the intervention might work
Transfusion‐related inflammation and poor clinical outcomes may be caused by RBCs themselves, time‐dependent accumulation of bioactive substances in the supernatant (storage lesion), or both (Lannan 2013). Transfusions can alter the immune system of recipients, and it is possible that saline washing of RBCs prior to transfusion may reduce these deleterious effects and improve outcomes for all patient populations, including preterm infants. Animal models using washed red cells have demonstrated blunting of the pro‐inflammatory response posthaemorrhage when compared with unwashed RBCs (Belizaire 2012). The use of washed RBC transfusions in paediatric cardiac surgery reduced pro‐inflammatory biomarkers and number of transfusions, and demonstrated a trend towards reduced mortality, when compared with unwashed RBCs (Cholette 2012). There is additional evidence in both adult and paediatric populations that washing RBCs prior to transfusion significantly reduces both mortality and morbidity (Blumberg 2004; Blumberg 2010; Cholette 2012). If a similar beneficial effect of equivalent magnitude exists in transfused preterm infants, this would represent a major advantage for this vulnerable patient population.
Why it is important to do this review
RBC transfusions are almost unavoidable in infants less than 1000 g birth weight, despite increasingly restrictive transfusion practice. These infants carry the highest mortality risk and heaviest burden of outcome‐changing morbidities compared with late preterm and term infants. Consequently, there is increasing interest in methods to reduce any adverse effects attributable to RBC transfusion. This can be accomplished by minimising the number of RBC transfusions, using alternatives to RBC transfusions such as EPO, and by making the transfused products potentially safer through pre‐transfusion modifications to the product itself. In this review, we focused on one method to make transfused blood products potentially safer, that is pre‐transfusion washing of RBCs with saline.
Objectives
To determine whether pre‐transfusion washing of RBCs prevents morbidity and mortality in preterm infants.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, cluster randomised, and quasi‐randomised controlled trials.
Types of participants
Preterm infants (less than 32 weeks' gestation) or very low birth weight infants (less than 1500 g birth weight), or both, who received one or more packed RBC transfusions during their initial hospitalisation.
Types of interventions
Transfusions of washed (through a manual 'open' system technique, in an automated cell washer, or via an auto‐transfusion device) packed RBCs versus unwashed packed RBCs in emergent and non‐emergent situations, excluding exchange transfusion, massive transfusion, or placental‐infant (delayed cord clamping) transfusion.
Types of outcome measures
Primary outcomes
Mortality: before discharge from initial hospital or before a defined period of follow‐up (28 days, 12 months, or 18 months postnatal age, or a combination).
ROP, grade 3 or more prior to discharge home (ICCRP 2005).
Severe adverse findings at ultrasound (grades 3 to 4 IVH (Papile 1983), hydrocephalus, cortical atrophy, or periventricular leukomalacia) during first hospitalisation (Pinto‐Martin 1995).
CLD requiring additional oxygen at 36 weeks' postmenstrual age or prior to discharge home (Shennan 1988).
NEC, stage 2 or greater (Bell 1978).
Cerebral palsy by physician assessment.
Developmental delay (developmental quotient more than two standard deviations below the mean on validated assessment tool of cognitive function (e.g. Bayley Score of Infant Development).
Blindness (visual acuity less than 20/200 in best eye).
Deafness (hearing loss requiring amplification or cochlear implantation).
Secondary outcomes
-
Composite outcome of death or severe adverse outcomes:
-
mortality or severe morbidity (or its complement, survival without severe morbidity) at initial hospital discharge, where significant morbidity is defined as:
ROP, grade 3 or more prior to discharge home (ICCRP 2005);
severe adverse findings at ultrasound (grades 3 to 4 IVH (Papile 1983), hydrocephalus, cortical atrophy, or periventricular leukomalacia) during first hospitalisation (Pinto‐Martin 1995);
CLD requiring additional oxygen at 36 weeks' postmenstrual age or prior to discharge home (Shennan 1988); or
NEC, stage 2 or greater (Bell 1978).
-
-
Composite outcome of mortality or severe adverse neurosensory outcome (or its complement, survival without serious adverse neurosensory outcome) at a defined period of follow‐up at age 18 to 24 months' adjusted gestational age or older, where adverse neurosensory outcome is defined as:
cerebral palsy by physician assessment;
developmental quotient (more than two standard deviations below the mean on validated assessment tool of cognitive function (e.g. Bayley Score of Infant Development);
blindness (visual acuity less than 20/200 in best eye); or
deafness (hearing loss requiring amplification or cochlear implantation).
-
Other outcomes:
late‐onset sepsis (sepsis diagnosed more than 72 hours after birth)
length of mechanical ventilation (days)
donor exposure
numbers of RBC transfusions
length of initial neonatal intensive care unit stay (days)
markers of inflammation or oxidative stress (if available), or both, including tumour necrosis factor (TNF)‐α, monocyte chemoattractant protein‐1, interleukin (IL)‐1, IL‐6, IL‐8, and total oxidant load
-
Transfusion reactions as defined by the Serious Hazards of Transfusion (SHOT) scheme (Stainsby 2008):
acute transfusion reaction;
delayed transfusion reaction;
transfusion‐related acute lung injury;
transfusion‐associated graft‐versus‐host disease;
post‐transfusion purpura; or
transfusion‐transmitted infection.
Search methods for identification of studies
We used the standard search method of the Cochrane Neonatal Review Group.
Electronic searches
We searched the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 7) (Appendix 1);
MEDLINE (January 1996 to 31 July 2015) (Appendix 2);
EMBASE (January 1980 to 31 July 2015) (Appendix 3);
CINAHL (1982 to 31 July 2015) (Appendix 4).
We searched for completed or ongoing clinical trials through major clinical trial registration websites: ClinicalTrials.gov (clinicaltrials.gov), Australian New Zealand Clinical Trials Registry (anzctr.org.au), Current Controlled Trials (controlled‐trials.com), European Union Clinical Trials Register (clinicaltrialsregister.eu), ISRCTN registry (isrctn.org), and National Institute of Public Health Clinical Trials Search (rctportal.niph.go.jp/en/index) (Appendix 5). We applied no language restrictions.
Searching other resources
We searched the reference lists of existing reviews and studies included in the review. We contacted experts in the field for suggestions of relevant unidentified studies (published and unpublished). We searched abstracts and conference proceedings (Pediatric Academic Societies at www.abstracts2view.com/pas/, European Society for Paediatric Research (1990 to current); American Society of Hematology Annual Meeting).
Data collection and analysis
Selection of studies
Two review authors initially screened all electronically derived citations and abstracts of papers identified by the review search strategy for relevance. A second review author initially screened the same citations and abstracts for relevance. We excluded clearly irrelevant studies at this stage.
Two review authors then formally assessed the full texts of all potentially relevant trials for eligibility. If necessary, we requested further information from the authors where articles contained insufficient data to make a decision about eligibility. Two review authors assessed the papers and recorded reasons for exclusion in the Characteristics of excluded studies table. Disagreements between the review authors were resolved by consensus.
Data extraction and management
Two review authors independently conducted data extraction using a data extraction form designed (and piloted) specifically for use in this systematic review. Disagreements between the review authors were resolved by consensus. The review authors were not blinded to names of authors, institutions, journals, or outcomes of the trials.
Assessment of risk of bias in included studies
We employed the standard methods of the Cochrane Neonatal Group.
We assessed risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Inventions (Higgins 2011). We reported the following domains: selection bias (random sequence generation and allocation concealment), performance bias, detection bias, attrition bias, and other bias. We assessed these domains and entered them into the 'Risk of bias' table.
Selection bias (random sequence generation and allocation concealment)
Random sequence generation
For each included study, we categorised the risk of random sequence generation as:
low risk ‐ adequate (any truly random process, e.g. random number table, computer random number generator);
high risk ‐ inadequate (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number);
unclear risk ‐ no or unclear information provided.
Allocation concealment
For each included study, we categorised the risk of bias regarding allocation concealment as:
low risk ‐ adequate (e.g. telephone or central randomisation, consecutively numbered, sealed, opaque envelopes);
high risk ‐ inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk ‐ no or unclear information provided.
Performance bias
For each included study, we categorised the methods used to blind study personnel from the knowledge of which intervention a participant received as:
low risk ‐ adequate for personnel (a placebo that could not be distinguished from the active drug was used in the control group);
high risk ‐ inadequate (personnel aware of group assignment);
unclear risk ‐ no or unclear information provided.
Detection bias (blinding)
For each included study, we categorised the methods used to blind outcome assessors from knowledge of which intervention a participant received. We categorised the methods used for detection bias as:
low risk ‐ adequate (follow‐up was performed with assessors blinded to group assignment);
high risk ‐ inadequate (assessors at follow‐up were aware of group assignment);
unclear risk ‐ no or unclear information provided.
Attrition bias (outcome data)
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we included the missing data in the analyses. We categorised the methods with respect to the risk attrition bias as:
low risk ‐ adequate;
high risk ‐ inadequate;
unclear risk ‐ no or unclear information provided.
Reporting bias (selective outcome reporting)
For each included study, we described how we investigated the risk of selective outcome reporting bias and what we found. We assessed the methods as:
low risk ‐ adequate (where it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk ‐ inadequate (where not all of the study's prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study did not include results of a key outcome that would have been expected to have been reported);
unclear risk ‐ no or unclear information provided (the study protocol was not available).
Other bias
For each included study, we described any important concerns that we had about other possible sources of bias. We assessed whether each study was free of other issues that could put it at risk of bias as:
low risk ‐ no concerns of other bias raised;
high risk ‐ concerns raised after multiple looks at the data with the results made known to the investigators; difference in number of participants enrolled in abstract and final publications of the paper;
unclear ‐ concerns raised about potential sources of bias that could not be verified by contacting the authors.
Two review authors independently made judgements about risk of bias. We resolved discrepancies through consensus.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as risk ratio, risk difference, and mean difference where appropriate using Review Manager 5 software (RevMan 2014). We calculated 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome but used different methods.
Unit of analysis issues
We included studies with two, or more than two, treatment groups and dealt with analyses as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When a multi‐arm study contributes more than one comparison to a particular meta‐analysis, we planned to either combine treatment groups or divide the control group, to avoid inclusion of data from the same infant more than once in the same analysis. If we had identified any cluster trials and deemed the data were not appropriately analysed, we would have adjusted for correlation using an effective sample size based on the design effect for each study (Higgins 2011).
Dealing with missing data
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis. The denominator for each outcome in each trial was the number of participants randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
As we identified and analysed only one study, assessments of heterogeneity were not appropriate and were therefore not performed.
Assessment of reporting biases
If there were 10 or more studies in the meta‐analysis, we would have investigated reporting biases using funnel plots. We would have assessed funnel plot asymmetry visually. If a visual assessment suggested asymmetry, we would have performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (RevMan 2014). We reported the mean difference where appropriate using Review Manager 5 software and calculated 95% confidence intervals (RevMan 2014). We did not carry out further analysis as we identified and analysed one study only.
Subgroup analysis and investigation of heterogeneity
Where possible, we planned to undertake predefined subgroup analyses for the different washing techniques (manual 'open’ system technique, an automated cell washer, or an auto‐transfusion device). We planned to examine additional subgroups depending on whether the RBCs were irradiated or not prior to washing.
Sensitivity analysis
We planned sensitivity analysis on primary outcomes to determine what effect the exclusion of studies with high risk of bias (for allocation concealment and incomplete outcome data) might have on the overall result of the meta‐analysis.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies
Results of the search
The preliminary electronic database search (Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 10, 2014), MEDLINE (1966 to November 2014), CINAHL (1982 to November 2014), and EMBASE (1980 to November 2014) yielded 546 results. After review of the titles, we selected four records for detailed abstract review and then full‐text review. One of these studies met the selection criteria and one study was an abstract of a full‐text record already identified (Cholette 2012). A search of online registers of clinical trials revealed one additional study meeting the inclusion criteria; we have included this study in the table Characteristics of ongoing studies. We undertook handsearching of conference abstracts, which did not yield any relevant studies. We reviewed a previous Cochrane review in a relevant area (Wilkinson 2014), which yielded one study not previously identified through prior searches; after full‐text review, we excluded this study. We have detailed the characteristics of the single included study and the three excluded studies in the tables Characteristics of included studies and Characteristics of excluded studies, respectively.
We updated the above search in July 2015 and identified no new eligible studies.
Included studies
Lee 1995 was the single study that met the inclusion criteria. It was a single‐centre randomised control trial conducted in the United States of America of dedicated donor (unwashed) RBC packs versus split donor (washed) RBC packs. The population consisted of inborn and outborn infants with a birth weight of less than 1500 g admitted to a neonatal intensive care unit in San Francisco (California Pacific Medical Center) and their families. Twenty‐three infants were randomised, with two infants withdrawn before undertaking the study intervention at parental request, within seven days of birth (see Characteristics of included studies). The inclusion criteria were: birth weight less than 1500 g and having an initial RBC transfusion ordered during the first week of age. The only stated exclusion criterion was no previous RBC transfusion prior to enrolment in the study.
One group of infants (unwashed RBC group) were randomised to receive type‐specific packed RBCs from dedicated donor (either community or directed donation) units equipped with seven satellite bags. The other group of infants (washed RBC group) received packed RBCs from units divided into three split packs shared with other infants receiving transfusions. The packed RBCs used in the washed RBC group were washed with an automated cell washer (IBM‐COBE Blood Processor 2991; COBE Laboratories, Inc., Lakewood, Colorado) and re‐suspended in a saline solution to a hematocrit value of 80% to 85%. All packed RBCs used in the study were cytomegalovirus antibody negative and were irradiated prior to use. Infants in both groups could receive RBCs from individuals nominated by their families donating blood specifically for the infant (directed donation). These directed donations were collected in citrate‐phosphate‐dextrose anticoagulant preservative and stored in adenine in saline anticoagulant preservation and had an expiration date of 42 days. Community donations were collected in citrate‐phosphate‐dextrose‐adenine anticoagulant preservation and had an expiration date of 35 days.
The stated primary outcome of the study was number of donor exposures per infant. Infants were monitored until one hour post‐transfusion for acute transfusion reactions. Data regarding demographics, length of hospital stay, days of mechanical ventilation, days of supplemental oxygen use, and RBC transfusion details were collected. Infants received 86 RBC transfusions in total across both groups (6.0 versus 7.5 per infant; median, control versus study). Infants were able to receive directed donations from family members; these were either unwashed or washed depending on the group to which the infant was assigned. Fewer donor exposures occurred in the unwashed group (2.0 versus 5.5 per infant; median, control versus study).
The study was stopped prematurely after enrolment of 21 infants (planned sample size 34 infants) as there was a significant difference in the primary outcome (number of donor exposures) between the two groups.
Excluded studies
We excluded Cholette 2012, Hosking 1990, and Swindell 2007 after full‐text review, as they did not include infants less than 32 weeks' gestation or infants with a birth weight of less than 2500 g, or both.
Risk of bias in included studies
We assessed the overall risk of bias for the included study as low. We have included a detailed ‘Risk of bias’ table under Characteristics of included studies.
Allocation
The sequence generation was unclear based on the published methods in Lee 1995, but allocation was concealed from the bedside healthcare team and families.
Blinding
There was no description of how or by whom the outcomes were collected; however, the outcomes assessed (for example mortality) were at low risk of being affected by lack of blinding.
Incomplete outcome data
Two infants withdrew from the study after randomisation, but this level of missing data was unlikely to affect observed results.
Selective reporting
No published study protocol was available.
Other potential sources of bias
We identified no other potential sources of bias.
Effects of interventions
Washed versus unwashed RBCs for transfusion (Comparison I)
Primary outcomes
Mortality
One study (n = 21 infants) reported on all‐cause mortality during hospital stay. There was no significant difference in mortality between the washed versus the unwashed RBCs for transfusion groups (risk ratio 1.63, 95% confidence interval (CI) 0.28 to 9.36; risk difference 0.10, 95% CI ‐0.26 to 0.45) (Analysis 1.1; Figure 1). Tests for heterogeneity were not applicable.
1.1. Analysis.
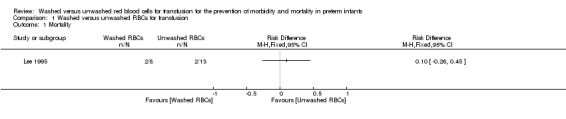
Comparison 1 Washed versus unwashed RBCs for transfusion, Outcome 1 Mortality.
1.

Forest plot of comparison: 1 Washed versus unwashed RBCs for transfusion, outcome: 1.1 Mortality.
Secondary outcomes
Length of initial neonatal intensive care unit stay (days)
One study (n = 21 infants) reported on length of initial neonatal intensive care unit (NICU) stay (days). There was no significant difference in the length of initial NICU stay between the washed versus the unwashed RBCs for transfusion groups; mean difference 25 days (95% CI ‐21.15 to 71.15) (Analysis 1.2; Figure 2). Tests for heterogeneity were not applicable.
1.2. Analysis.
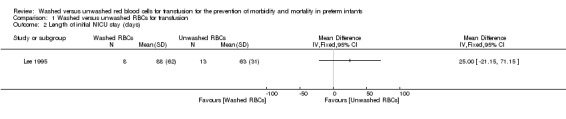
Comparison 1 Washed versus unwashed RBCs for transfusion, Outcome 2 Length of initial NICU stay (days).
2.

Forest plot of comparison: 1 Washed versus unwashed RBCs for transfusion, outcome: 1.2 Length of initial NICU stay (days).
Duration of mechanical ventilation (days)
One study (n = 21 infants) reported on duration of mechanical ventilation. There was no significant difference in duration of mechanical ventilation between the washed versus the unwashed RBCs for transfusion groups; mean difference 9.60 days (95% CI ‐1.90 to 21.10) (Analysis 1.3; Figure 3). Tests for heterogeneity were not applicable.
1.3. Analysis.
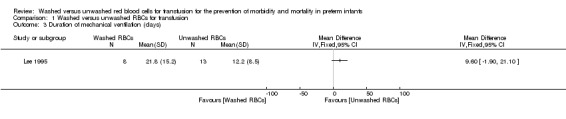
Comparison 1 Washed versus unwashed RBCs for transfusion, Outcome 3 Duration of mechanical ventilation (days).
3.

Forest plot of comparison: 1 Washed versus unwashed RBCs for transfusion, outcome: 1.3 Duration of mechanical ventilation (days).
Discussion
Summary of main results
The single included study only assessed one method of washing, by an automated cell washer, and was a dual intervention study, with the unwashed RBC group receiving blood from a dedicated donor split into eight packs.
Due to the small sample size, 21 included infants, estimates for the three reported outcomes relevant to our review (mortality during initial hospitalisation, duration of mechanical ventilation, and length of initial NICU hospitalisation) had very wide confidence intervals.
No outcome data was available for our other primary outcomes including retinopathy of prematurity (stage 3 or greater), necrotising enterocolitis (stage 2 or greater), chronic lung disease, adverse findings on head ultrasound screening, or adverse neurodevelopmental outcome (cerebral palsy by physician assessment, development delay, blindness or deafness). No data was available for most of our secondary outcomes, including previously discussed composite outcomes, late onset sepsis or markers of inflammation and/or oxidative stress.
Although we assessed the study as at low risk of bias, the imprecision of the estimates it provides made this study unhelpful in answering the review questions.
Overall completeness and applicability of evidence
We performed an extensive search of published and unpublished literature, including searches of trial registries for ongoing studies. We have no reason believe that there are any additional studies relevant to our review at this time. The one study we did identify examined only one method of RBC washing. The blood product processing and storage, including routine irradiation, choice of storage media and anticoagulant, that occurred in this study may not be applicable to all healthcare settings that care for preterm infants.
Quality of the evidence
The quality of the evidence provided by this single study was reasonable, however the study included only a very small number of infants.
Potential biases in the review process
It is possible that the exclusion of studies including more mature infants (more than 32 weeks' gestation) may have resulted in potentially relevant studies being missed. However, infants of this gestational age are not usually at risk of developing the primary outcomes identified by our review. The results reported by this review for the included study were straightforward, and no re‐analysis or selective reporting occurred.
Agreements and disagreements with other studies or reviews
Based on the current evidence, it is unclear whether there is a benefit or risk to washing RBCs prior to transfusion for preterm infants. Studies undertaken in older infants and children (Cholette 2012), as well as in the adult population (Blumberg 2004), suggest there may be a benefit in outcomes addressed within this review. However, no such evidence is available for preterm infants, and so further clinical trials are needed.
Authors' conclusions
Implications for practice.
There is insufficient evidence to support or refute the use of washed RBCs for transfusion in preterm infants to prevent morbidity or mortality.
Implications for research.
As have been conducted in adult medicine, Blumberg 2004, and in paediatrics, Cholette 2012, randomised controlled trials are needed in neonatology to assess the potential benefits and the effects on short‐term outcomes, neonatal morbidities, and mortality of pre‐washing RBCs for preterm or low birth weight infants, or both. When designing a future study to determine whether washing RBCs prior to transfusion benefits preterm infants or not, a randomised, multicentre, controlled trial design is recommended. Included infants would be those born with a gestational age up to and including 28 weeks and 6 days, who receive a packed RBC transfusion as per a standardised clinical guideline, for example the restrictive transfusion thresholds used in the Premature Infants in Need of Transfusion (PINT) (Kirpalani 2006). Eligible infants would be randomly allocated to receive either washed or non‐washed standard non‐irradiated, leukodepleted allogeneic packed RBCs. At least 448 infants would be required to detect a decrease in the composite outcome from 51% to 36% (two‐sided alpha 0.05, 90% power). Infants in the washed (study) group would receive 15 ml/kg non‐irradiated, leukodepleted washed packed RBCs. Infants in the standard‐therapy (control) group would receive 15 ml/kg non‐irradiated, leukodepleted packed RBCs. All subsequent transfusions would comply with the initial randomisation. An additional component of this trial would be to assess the cost‐effectiveness, safety in terms of acute adverse transfusion effects, and the practicalities of providing washed packed RBCs for routine neonatal RBC transfusions.
Acknowledgements
We would like to acknowledge the Australasian Cochrane Group for their significant support provided at the Review Completion Workshop in Melbourne, Australia, in November 2014.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
Search terms: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW) AND (transfusion OR hemotransfus* OR haemotransfus* OR hemotherap*) AND (erythrocyte OR red blood cell OR RBC)
Appendix 2. MEDLINE search strategy
((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])) AND (transfusion OR hemotransfus* OR haemotransfus* OR hemotherap*) AND (erythrocyte OR red blood cell OR RBC) AND (Humans[Mesh] AND infant[MeSH])
Appendix 3. EMBASE search strategy
((infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW) and (human not animal) and (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial) and (transfusion or hemotransfus* or haemotransfus* or hemotherap*) and (erythrocyte or red blood cell or RBC)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
limit 1 to human
1 and 2
limit 3 to infant <to one year>
Appendix 4. CINAHL search strategy
(infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW) AND ( randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) AND (transfusion OR hemotransfus* OR haemotransfus* OR hemotherap*) AND (erythrocyte OR red blood cell OR RBC)
Appendix 5. Online clinical trial registries search strategy
(transfusion OR hemotransfus* OR haemotransfus* OR hemotherap*) AND (erythrocyte OR red blood cell OR RBC) AND infant
Data and analyses
Comparison 1. Washed versus unwashed RBCs for transfusion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Length of initial NICU stay (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Duration of mechanical ventilation (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lee 1995.
| Methods | Single‐centre randomised control trial | |
| Participants | Inborn and outborn infants with a birth weight < 1500 g | |
| Interventions | One group of infants (unwashed RBC group) were randomised to receive type‐specific packed RBCs from dedicated donor (either community or directed donation) units equipped with 7 satellite bags. The other group of infants (washed RBC group) received packed RBCs from units divided into 3 split packs shared with other infants receiving transfusions | |
| Outcomes | The primary outcome of the study was number of donor exposures per infant | |
| Notes | Infants were monitored until 1‐hour post‐transfusion for acute transfusion reactions. Data regarding demographics, length of hospital stay, days of mechanical ventilation, days of supplemental oxygen use, and RBC transfusion details were collected | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Study infants were randomly assigned by hospital blood bank personnel into 1 of 2 groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Transfusions were ordered by primary caretakers who were masked to study group assignments. RBCs were processed by hospital blood bank personnel and sent to the neonatal intensive care unit in syringes unmarked as to study group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Low risk for mortality; unclear risk for other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two infants withdrew from the study after randomisation, but this level of missing data is unlikely to affect observed results |
| Selective reporting (reporting bias) | Unclear risk | No published study protocol was available to review |
| Other bias | Low risk | No other risks of bias were identified |
RBC: red blood cell
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cholette 2012 | Infants not in gestational age or birth weight range |
| Hosking 1990 | Infants not in gestational age or birth weight range |
| Swindell 2007 | Infants not in gestational age or birth weight range |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12613000237785.
| Trial name or title | Effect of transfusion of washed red blood cells on neonatal outcome: a randomised controlled trial |
| Methods | Randomised, multicentre, controlled trial |
| Participants | Infants born with a gestational age up to and including 28 weeks and 6 days |
| Interventions | Eligible infants will be randomly allocated to receive either washed or non‐washed standard non‐irradiated, leukodepleted allogeneic packed red blood cells. |
| Outcomes | The primary outcome is a composite of mortality (defined as death of a live born infant > 48 hours of age) and/or major neonatal morbidities associated with organ dysfunction or failure following transfusion, until discharge from neonatal intensive care unit. Major neonatal morbidity is defined as one or more of bronchopulmonary dysplasia (oxygen and/or respiratory support ‐ intubation/continuous positive airway pressure/high‐flow nasal cannula oxygen ≥ 2 L/min) for any portion of the day at 36 weeks and 0 days corrected gestational age), brain injury defined as intraventricular haemorrhage (grades 3 and 4), retinopathy of prematurity (> stage 2), and necrotising enterocolitis (based on a grading of stage 2 or greater). Secondary outcomes include nosocomial infection (blood culture positive sepsis diagnosed > 48 hours after birth), length of mechanical ventilation, and length of primary admission |
| Starting date | Not yet recruiting |
| Contact information | michael.stark@adelaide.edu.au |
| Notes | Trial ID: ACTRN12614000419662 |
Differences between protocol and review
We included one additional online clinical register in our search strategy, the World Health Organization (WHO) International Clinical Trials Registry Platform.
Contributions of authors
Amy Keir (AK) screened the titles and abstracts of all studies identified by the search strategy. AK and Dominic Wilkinson (DW) screened the full text of each study identified as of potential relevance. AK and DW extracted the data separately, compared data, and resolved any differences by consensus. AK, DW, Chad Andersen and Michael Stark completed the final review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
Declarations of interest
Associate Professor Michael Stark and Dr. Chad Andersen are undertaking a clinical trial to examine the effect of pre‐transfusion washing of red blood cells on neonatal outcomes.
Amy Keir and Dominic Wilkinson have no conflicts of interest to declare.
New
References
References to studies included in this review
Lee 1995 {published data only}
References to studies excluded from this review
Cholette 2012 {published data only}
- Cholette JM, Henrichs KF, Alfieris GM, Powers KS, Phipps R, Spinelli SL, et al. Washing red blood cells and platelets transfused in cardiac surgery reduces postoperative inflammation and number of transfusions: results of a prospective, randomized, controlled clinical trial. Pediatric Critical Care Medicine 2012; Vol. 13, issue 3:290‐9. [PUBMED: 21926663] [DOI] [PMC free article] [PubMed]
Hosking 1990 {published data only}
- Hosking MP, Beynen FM, Raimundo HS, Oliver WC Jr, Williamson KR. A comparison of washed red blood cells versus packed red blood cells (AS‐1) for cardiopulmonary bypass prime and their effects on blood glucose concentration in children. Anesthesiology 1990; Vol. 72, issue 6:987‐90. [PUBMED: 2112346] [DOI] [PubMed]
Swindell 2007 {published data only}
- Swindell CG, Barker TA, McGuirk SP, Jones TJ, Barron DJ, Brawn WJ, et al. Washing of irradiated red blood cells prevents hyperkalaemia during cardiopulmonary bypass in neonates and infants undergoing surgery for complex congenital heart disease. European Journal of Cardio‐thoracic Surgery 2007; Vol. 31, issue 4:659‐64. [PUBMED: 17291775] [DOI] [PubMed]
References to ongoing studies
ACTRN12613000237785 {published data only}
- Effect of transfusion of washed red blood cells on neonatal outcome: a randomised controlled trial. Ongoing study Not yet recruiting.
Additional references
Aiboshi 2001
- Aiboshi J, Moore EE, Ciesla DJ, Silliman CC. Blood transfusion and the two‐insult model of post‐injury multiple organ failure. Shock 2001;15(4):302‐6. [PUBMED: 11303730] [DOI] [PubMed] [Google Scholar]
Baer 2011
- Baer VL, Lambert DK, Henry E, Snow GL, Butler A, Christensen RD. Among very‐low‐birth‐weight neonates is red blood cell transfusion an independent risk factor for subsequently developing a severe intraventricular hemorrhage?. Transfusion 2011;51(6):1170‐8. [PUBMED: 21166684] [DOI] [PubMed] [Google Scholar]
Belizaire 2012
- Belizaire RM, Makley AT, Campion EM, Sonnier DI, Goodman MD, Dorlac WC, et al. Resuscitation with washed aged packed red blood cell units decreases the proinflammatory response in mice after hemorrhage. Journal of Trauma and Acute Care Surgery 2012;73(2 Suppl 1):S128‐33. [PUBMED: 22847082] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978; Vol. 187, issue 1:1‐7. [PUBMED: 413500] [DOI] [PMC free article] [PubMed]
Bilgin 2004
- Bilgin YM, Watering LM, Eijsman L, Versteegh MI, Brand R, Oers MH, et al. Double‐blind, randomized controlled trial on the effect of leukocyte‐depleted erythrocyte transfusions in cardiac valve surgery. Circulation 2004;109(22):2755‐60. [PUBMED: 15148271] [DOI] [PubMed] [Google Scholar]
Blumberg 2004
- Blumberg N, Heal JM, Rowe JM. A randomized trial of washed red blood cell and platelet transfusions in adult acute leukemia. BMC Blood Disorders 2004;4(1):6. [PUBMED: 15588315] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blumberg 2010
- Blumberg N, Heal JM, Gettings KF, Phipps RP, Masel D, Refaai MA, et al. An association between decreased cardiopulmonary complications (transfusion‐related acute lung injury and transfusion‐associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion 2010;50(12):2738‐44. [PUBMED: 20561296] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooke 1997
- Cooke RW, Drury JA, Yoxall CW, James C. Blood transfusion and chronic lung disease in preterm infants. European Journal of Pediatrics 1997;156(1):47‐50. [PUBMED: 9007491] [DOI] [PubMed] [Google Scholar]
de Vroege 2007
- Vroege R, Wildevuur WR, Muradin JA, Graves D, Oeveren W. Washing of stored red blood cells by an autotransfusion device before transfusion. Vox Sanguinis 2007;92(2):130‐5. [PUBMED: 17298575] [DOI] [PubMed] [Google Scholar]
dos Santos 2011
- dos Santos AM, Guinsburg R, Almeida MF, Procianoy RS, Leone CR, Marba ST, et al. Red blood cell transfusions are independently associated with intra‐hospital mortality in very low birth weight preterm infants. Journal of Pediatrics 2011;159(3):371‐6.e1‐3. [PUBMED: 21489555] [DOI] [PubMed] [Google Scholar]
Fergusson 2003
- Fergusson D, Hebert PC, Lee SK, Walker CR, Barrington KJ, Joseph L, et al. Clinical outcomes following institution of universal leukoreduction of blood transfusions for premature infants. JAMA 2003;289(15):1950‐6. [PUBMED: 12697797] [DOI] [PubMed] [Google Scholar]
Gauvin 2012
- Gauvin F, Robillard P, Hume H, Grenier D, Whyte RK, Webert KE, et al. Transfusion‐related acute lung injury in the Canadian paediatric population. Paediatrics & Child Health 2012;17(5):235‐9. [PUBMED: 23633895] [PMC free article] [PubMed] [Google Scholar]
Giannantonio 2012
- Giannantonio C, Papacci P, Cota F, Vento G, Tesfagabir MG, Purcaro V, et al. Analysis of risk factors for progression to treatment‐requiring ROP in a single neonatal intensive care unit: is the exposure time relevant?. Journal of Maternal‐Fetal & Neonatal Medicine 2012;25(5):471‐7. [PUBMED: 22280305] [DOI] [PubMed] [Google Scholar]
Grabmer 2006
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICCRP 2005
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Archives of Ophthalmology 2005;123(7):991‐9. [PUBMED: 16009843] [DOI] [PubMed] [Google Scholar]
Keir 2013
- Keir AK, McPhee AJ, Andersen CC, Stark MJ. Plasma cytokines and markers of endothelial activation increase after packed red blood cell transfusion in the preterm infant. Pediatric Research 2013;73(1):75‐9. [PUBMED: 23095979] [DOI] [PubMed] [Google Scholar]
Kirpalani 2006
- Kirpalani H, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. Journal of Pediatrics 2006; Vol. 149, issue 3:301‐7. [0022‐3476: (Print)] [DOI] [PubMed]
Lannan 2013
- Lannan KL, Sahler J, Spinelli SL, Phipps RP, Blumberg N. Transfusion immunomodulation‐‐the case for leukoreduced and (perhaps) washed transfusions. Blood Cells, Molecules & Diseases 2013;50(1):61‐8. [PUBMED: 22981700] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohamed 2012
- Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: a meta‐analysis of observational data. Pediatrics 2012;129(3):529‐40. [PUBMED: 22351894] [DOI] [PubMed] [Google Scholar]
O'Leary 2011
- O'Leary MF, Szklarski P, Klein TM, Young PP. Hemolysis of red blood cells after cell washing with different automated technologies: clinical implications in a neonatal cardiac surgery population. Transfusion 2011;51(5):955‐60. [PUBMED: 21091957] [DOI] [PubMed] [Google Scholar]
Papile 1983
- Papile LA, Munsick‐Bruno G, Schaefer A. Relationship of cerebral intraventricular hemorrhage and early childhood neurologic handicaps. Journal of Pediatrics 1983;103(2):273‐7. [PUBMED: 6875724] [DOI] [PubMed] [Google Scholar]
Pinto‐Martin 1995
- Pinto‐Martin JA, Riolo S, Cnaan A, Holzman C, Susser MW, Paneth N. Cranial ultrasound prediction of disabling and nondisabling cerebral palsy at age two in a low birth weight population. Pediatrics 1995;95(2):249‐54. [PUBMED: 7838643] [PubMed] [Google Scholar]
Rashid 2013
- Rashid N, Al‐Sufayan F, Seshia MM, Baier RJ. Post transfusion lung injury in the neonatal population. Journal of Perinatology 2013;33(4):292‐6. [PUBMED: 22955289] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shennan 1988
- Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82(4):527‐32. [PUBMED: 3174313] [PubMed] [Google Scholar]
Stainsby 2008
- Stainsby D, Jones H, Wells AW, Gibson B, Cohen H, SHOT Steering Group. Adverse outcomes of blood transfusion in children: analysis of UK reports to the serious hazards of transfusion scheme 1996‐2005. British Journal of Haematology 2008;141(1):73‐9. [PUBMED: 18324969] [DOI] [PubMed] [Google Scholar]
Stark 2013
- Stark MJ, Keir AK, Andersen CC. Does non‐transferrin bound iron contribute to transfusion related immune‐modulation in preterms?. Archives of Disease in Childhood. Fetal and Neonatal Edition 2013;98(5):F424‐9. [PUBMED: 23475935] [DOI] [PubMed] [Google Scholar]
Tinmouth 2006
- Tinmouth A, Fergusson D, Yee IC, Hebert PC, ABLE Investigators, Canadian Critical Care Trials Group. Clinical consequences of red cell storage in the critically ill. Transfusion 2006;46(11):2014‐27. [PUBMED: 17076859] [DOI] [PubMed] [Google Scholar]
Valieva 2009
- Valieva OA, Strandjord TP, Mayock DE, Juul SE. Effects of transfusions in extremely low birth weight infants: a retrospective study. Journal of Pediatrics 2009;155(3):331‐7.e1. [PUBMED: 19732577] [DOI] [PMC free article] [PubMed] [Google Scholar]
Venkatesh 2012
- Venkatesh V, Khan R, Curley A, Hopewell S, Doree C, Stanworth S. The safety and efficacy of red cell transfusions in neonates: a systematic review of randomized controlled trials. British Journal of Haematology 2012;158(3):370‐85. [PUBMED: 22639894] [DOI] [PubMed] [Google Scholar]
Wilkinson 2014
- Wilkinson KL, Brunskill SJ, Doree C, Trivella M, Gill R, Murphy MF. Red cell transfusion management for patients undergoing cardiac surgery for congenital heart disease. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD009752.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


