Highlights
-
•
Activated platelets are associated with AS.
-
•
Platelets are a player in bioprosthetic valve dysfunction.
-
•
Randomized trials have assessed clinical outcomes with various antiplatelet/anticoagulation strategies in patients undergoing TAVR.
Key Words: aortic stenosis, calcified aortic valves, platelets, TAVR, thrombosis
Abbreviations and Acronyms: AS, aortic stenosis; AV, aortic valve; AVR, aortic valve replacements; COX, cyclooxygenase; ECM, extracellular matrix protein; HALT, hypoattenuating leaflet thickening; HMW, high molecular weight; MK, megakaryocyte; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacements; TGF, transforming growth factor; VEC, vascular endothelial cell; VHD, valvular heart disease; VIC, valve interstitial cell; vWF, Von Willebrand factor; WSS, wall shear stress
Summary
Aortic stenosis (AS) is the most common heart valve disease requiring surgery in developed countries, with a rising global burden associated with aging populations. The predominant cause of AS is believed to be driven by calcific degeneration of the aortic valve and a growing body of evidence suggests that platelets play a major role in this disease pathophysiology. Furthermore, platelets are a player in bioprosthetic valve dysfunction caused by their role in leaflet thrombosis and thickening. This review presents the molecular function of platelets in the context of recent and rapidly evolving understanding the role of platelets in AS, both of the native aortic valve and bioprosthetic valves, where there remain concerns about the effects of subclinical leaflet thrombosis on long-term prosthesis durability. This review also presents the role of antiplatelet and anticoagulation therapies on modulating the impact of platelets on native and bioprosthetic aortic valves, highlighting the need for further studies to determine whether these therapies are protective and may increase the life span of surgical and transcatheter aortic valve implants. By linking molecular mechanisms through which platelets drive disease of native and bioprosthetic aortic valves with studies evaluating the clinical impact of antiplatelet and antithrombotic therapies, we aim to bridge the gaps between our basic science understanding of platelet biology and their role in patients with AS and ensuing preventive and therapeutic implications.
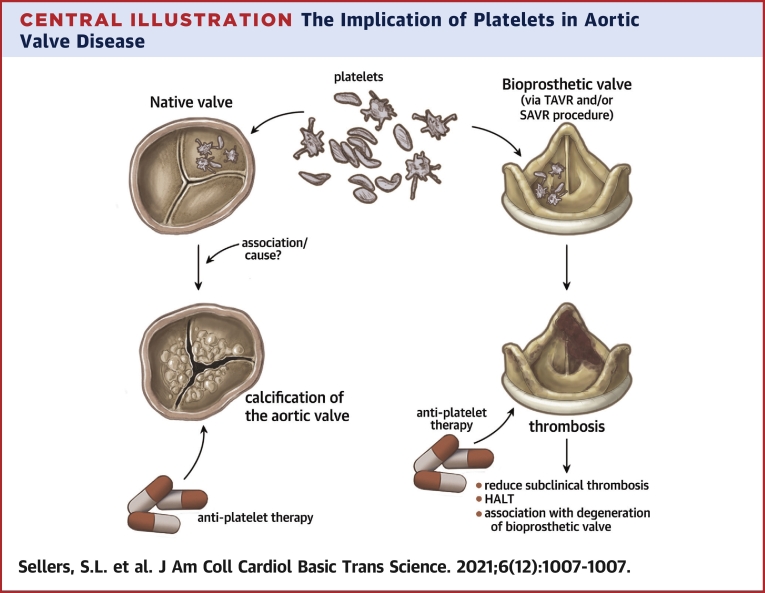
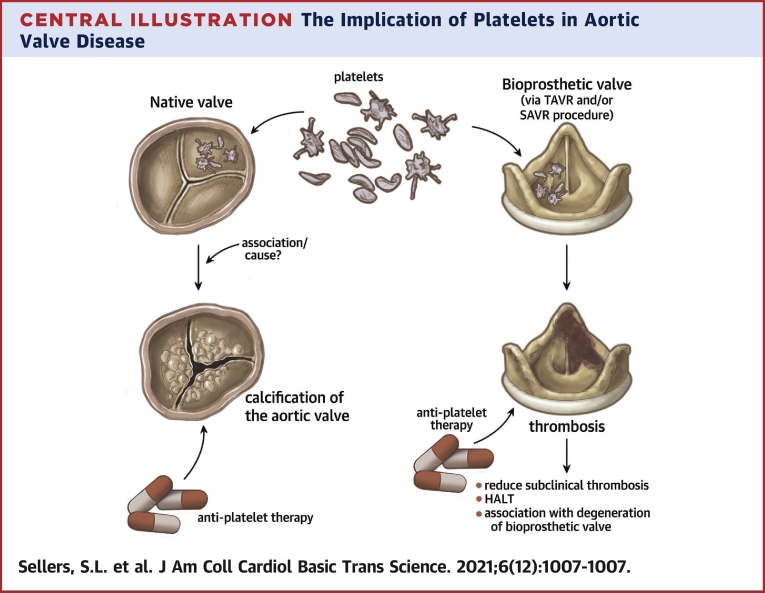
Central Illustration
Valvular heart disease (VHD) remains a life-threatening and growing problem in the aging population. Aortic stenosis (AS), narrowing of the aortic valve (AV) restricting blood flow from the left ventricle caused by fibrotic and calcific degeneration, is the one of the most common forms of valve disease, with a significant rate of morbidity and mortality. Unfortunately no known medical therapy has been identified to prevent or slow the development or progression of AS despite decades of study. This leaves valve replacement, with either a surgical or transcatheter technique as the treatment option for AS. However, most valve replacements are with use of bioprosthetic heart valves, which by their nature are subject to eventual dysfunction and failure.
From the translational perspective, this life span of valves of many patients with AS, from presentation and progression of native AS to valve replacement and eventual failure of the bioprosthetic valve, presents a complex interplay that involves understanding both native and bioprosthetic valve disease. In approaching this scope of the “lifetime” of a patient’s valve, we propose there are important disease players that it is vital to understand from cellular pathophysiology through to clinical care that have roles in both native AS and bioprosthetic valve disease. As such, we present a review on one such player: platelets.
Produced in the bone marrow by megakaryocytes, platelets are anucleate cells that circulate in blood plasma for 7 to 10 days before being removed by the spleen. They generally play a role in hemostasis maintenance, thrombosis formation, innate and active immunity, and inflammation responses (1). In VHD, there is growing evidence for the study and targeting of platelets in both native AS and bioprosthetic valve dysfunction. Therefore, this review presents an overview of the formation and function of platelets and their role in VHD with consideration for the specific roles of platelets in AS and bioprosthetic heart valves, including antiplatelet therapies that are being investigated. This review highlights discoveries about platelet involvement in AS pathogenesis that suggest further research is warranted to investigate the potential role of antiplatelet therapies in attenuating AS progression and increasing bioprosthetic valve life span.
Platelet Formation and Function: An Overview
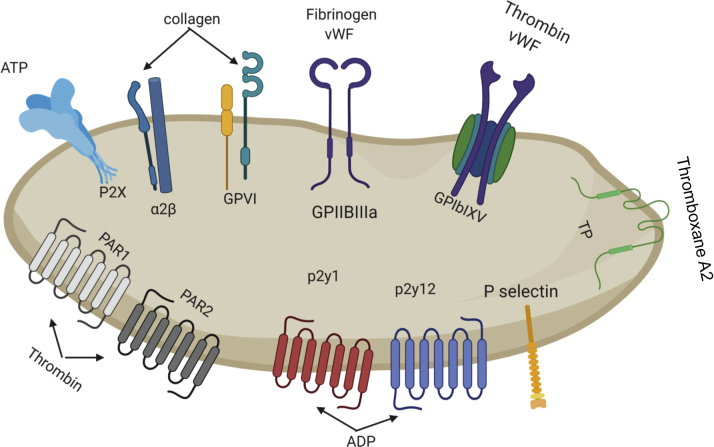
An understanding of platelet formation and general function from the fundamental/basic science perspective is central to understanding the role platelets play in VHD and therapeutic targets. Overall, platelets are the result of megakaryocytes (MK) fragmentation, which are differentiated hematopoietic stem cells that reside primarily in bone marrow (2). The generation of platelets first involves maturation, where MKs undergo endomitosis to make a polyploidy nucleus, growth of their cytoplasmic contents, and creation of an invaginated membrane system throughout their cytoplasm (3). MKs migrate to the vascular sinusoids within the bone marrow to form proplatelets, long cytoplasmic tubular extensions that are platelet precursors (4). To generate proplatelets, MKs undergo branching processes, powered by their cytoskeleton, through sinusoidal blood vessel endothelial cells that extend to form proplatelets in the sinusoidal blood vessel (3). Platelets are then formed at proplatelet ends, within the blood vessel (5). As a result of this generation, platelets are irregularly shaped and lack a nucleus and typically measure 2 to 3 μm in diameter. A number of factors can promote or inhibit proplatelet formation, but no current therapeutics in use target these or are directly linked to the VHD. Instead, key links to VHD and patient treatment are derived from the fundamental scientific understanding of the expression and function of platelet receptors that mediate hemostasis and respond to vascular damage and inflammation (4). Figure 1 highlights the receptors that mediate signaling within platelets, an important consideration for a translational understanding of VHD. Briefly, clotting is achieved when platelets bind to exposed extracellular matrix (ECM) on the vascular sub-endothelium at the sites of vascular damage. They then aggregate and coagulate to form a plug with the aid of fibrin and other inflammatory factors such as collagen and von Willebrand Factor (vWF) (3). Activated platelets release the content of their alpha-granules and recruit other platelets and proinflammatory cells, such as leukocytes and monocytes, through paracrine signaling to the site of injury to help in the inflammation process and hemostasis (3). vWF, P-selectin, and glycoprotein IIb-IIIa are adhesion molecules that promote interaction and aggregation with leukocytes and vascular endothelial cells (VECs) to promote coagulation (3). Further descriptions of platelet receptors are detailed in Figure 1 as well as in conjunction with discussion of their targeting by antiplatelet therapies that follows.
Figure 1.
Major Platelet Receptors
Graphic representation of the main platelet receptors and their ligands. P2Y1 and P2Y12 receptors are both activated by ADP and they are essential for platelet aggregation. The collagen receptors play an important role in both platelet adhesion and activation. vWF facilitates the adhesion of platelets to damaged arteries to stimulate platelet activation and the activation of clotting cascade. This figure was made using BioRender. ADP = adenosine diphosphate; vWF = von Willebrand Factor.
Antiplatelet Therapies
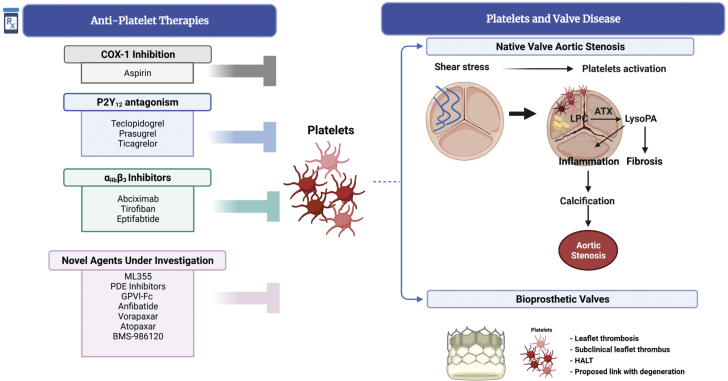
Targeting of platelet receptors and signaling provide the molecular basis for current and in development antiplatelet therapies that come into focus when considering VHD. Most antiplatelet therapies in clinical use target platelet receptors cyclooxygenase (COX)-1, the G-coupled protein P2Y12 receptor, or integrin IIb3 receptor (Figure 2); COX-1 inhibitors, such as aspirin, have been used to irreversibly bind COX-1 to decrease prothrombotic activity in platelets. P2Y12 receptor inhibitors prevent P2Y12 activation that leads to platelet activation, including granule secretion and integrin IIb3 activation through the PI3K/Akt pathway (6). Irreversible P2Y12 receptor inhibitors approved by the Food and Drug Administration include clopidogrel, prasugrel, and ticlopidine, whereas ticagrelor and cangrelor are reversible P2Y12 receptor inhibitors (6). Finally, blockade of the integrin IIb3 receptor is achieved by inhibitors that include Abciximab, Tirofiban, and Eptifibatide, which achieve effective anticoagulation, as IIb3 is the most highly expressed glycoprotein on platelet surfaces (60,000 to 80,000 receptors per platelet), and binds to fibrinogen to form blood clots in activated platelets (6,7). These inhibitors have shown to be effective, although have increased risk of bleeding and acute thrombocytopenia (6,7).
Figure 2.
The Role of Platelets in Cardiovascular Disease
Platelets, inhibited by treatment of patients with a variety of antiplatelet therapeutics, play a key role in many aspects of native cardiovascular pathology, including development of aortic stenosis, venous thrombosis, and arterial thrombosis often associated with atherosclerosis. Platelets also play a notable role in thrombosis and subclinical thrombus formation on bioprosthetic valves. This figure was made using BioRender. COX = cyclooxygenase; HALT = hypoattenuating leaflet thickening.
Targeting of COX-1, P2Y12, and IIb3 as described previously is most commonly used clinically; however, other strategies are being explored and developed. For example, GPVI-Fc is a collagen receptor inhibitor that prevents collagen-induced platelet aggregation that would normally occur following a vascular injury. GPVI-Fc is still being investigated, but has shown to improve vascular and endothelial abnormalities in atherosclerotic rabbits (6,7). Protease-activated receptors (PARs) 1 and 4 are expressed on platelets, recognize thrombin, and induce platelet activation (6,7). Atopaxar, Vorapaxar, and BMS-986120 are all therapies targeting PAR platelet receptors to decrease arterial thrombosis by preventing platelet granule secretion (6). Anfibatide is being researched as a potential glycoprotein Ib-IX-V receptor inhibitor on platelets, which normally binds to vWF initiating platelet activation, adhesion (6,7). Anfibatide has been shown to prevent thrombus formation and platelet adhesion with a lower risk of bleeding in mice models (6,7). 12-Lipoxygenases (12-LOX) is a protein that regulates platelet activity and is thus a suggested antiplatelet target (6). ML355 is an antiplatelet therapy that specifically inhibits 12-LOX in vivo, and has shown to be effective at preventing thrombosis with a limited effect on bleeding (7). Last, phosphodiesterase inhibitors increase intracellular cyclic nucleotides (cCAMP and cGMP) in platelets, which ultimately prevents platelet activation and integrin IIb3 receptor activation (6,7) (Figure 2).
Implications of Platelets in Native Aortic Valve Stenosis
Platelet function in patients with AS
Calcification and fibrosis of the AV leaflets causing AS results in high wall shear stress (WSS) of the valvular tissue (8). The AV is composed of leaflet structures, each containing 3 layers of tissue (fibrosa, spongiosa, ventricularis) densely populated with ECM, and are coated in VECs (9). Each layer contains valve interstitial cells (VICs), which are the primary cellular component comprising the AV (8). Both in vivo and in vitro research has revealed that increased platelet activity is involved in the pathogenesis of AS (Figure 2). In AS, the WSS and accompanying turbulent blood flow through the AV can activate the endothelium, which activates platelets and stimulates their adhesion to the leaflets (10). This review explores the following ways that platelets are involved in accelerating the pathogenesis of AS: 1) activated platelets’ release of transforming growth factor (TGF)- that promotes the conversion of VECs and VICs to collagen-producing-myofibroblasts, which increases fibrosis and mineralization (11); 2) the role of Dickkopf-1 (DKK-1) glycoprotein and lymphangiogenesis in AS; 3) release of ADP from activated platelets, which promotes the osteogenic transition of VICs and leads to AV mineralization (10); 4) retention and oxidation of lipids that play an active role in VIC osteogenic transition by activating the nuclear factor-kappa B (NF-B) inflammation pathway (8).
Increased TGF-1 released from platelets causes VEC and VIC transition to myofibroblasts that contributes to AS progression
TGF-1 is a cytokine that plays a role in the pathogenesis of multiple fibrotic diseases by inducing the activation of canonical SMAD signaling, which leads to matrix protein production (11). Although all cells can produce TGF-1, platelets are accountable for producing 40% to 50% of all circulating TGF-1 (11). Increased WSS of the AV causes increased activation of platelets, which triggers the release of their granule contents including TGF-1, and also activates TGF-1 (12). WSS also causes surface P-selectin exposure on activated platelets leading to the formation of platelet leukocyte aggregates (PLAs), where increased levels of TGF-1 were shown to be involved in the pathogenesis of AS by assessing levels of phosphorylated SMAD2 signaling in circulating leukocytes and PLAs (12). Further research by Varshney et al. (11) showed that locally released platelet TGF-1 has a more significant effect in VEC signaling than circulating TGF-1. Platelets can adhere VECs and trigger their inward migration and VEC-to-mesenchymal transition to form collagen-producing myofibroblasts that promote AS progression (11). TGF-1 has also been shown to cause VIC transformation into secretory myofibroblast cells, although serotoninergic receptor 5-HT inhibition prevents this transformation (8). In addition to contributing to collagen production and fibrosis, myofibroblasts also cause contraction of the ECM, which leads to apoptosis in cell aggregates within the matrix, and contributes to mineralization through calcium sedimentation (9). In summary, platelets are involved in AS pathogenesis through TGF-1 release, which reveals that inhibiting platelet TGF-1 activation or signaling at VECs could be a potential therapeutic agent to attenuate AS progression (11).
Release of DKK-1 in AS and observations on lymphangiogenesis
Platelets might also play a role in AV calcification through the regulation of the Wnt pathway (13). Indeed, platelets release DKK-1 glycoprotein that acts as a potent antagonist to the Wnt-β-catenin signaling pathway. As a consequence, runt-related transcription factor 2 (Runx2), which is a canonical Wnt target and an osteogenic marker, gets activated (14,15). The upregulation of Runx2 by DKK-1 enhances the fibrotic mineralization of aortic endothelial cells under osteogenic conditions (13). Furthermore, the inhibition of DKK-1 with a neutralizing antibody, abolished interleukin (IL)-8 and MCP-1 in human umbilical vein endothelial cells that were activated by platelets (16,17). Moreover, a recent clinical showed that DKK1 was associated with calcific deposits on cardiac valves in hemodialysis patients (15). In addition to the role of platelets in the blood vascular network, they mediate the formation lymphatic vessels during embryonic development and prevent the mixing of blood to the lymphatic network (18). Studies by the Kovanen group (19) showed the presence of lymphatic vessels in heart valves affected by infective endocarditis and degenerative calcified stenosis (19,20); however, it is still unclear whether platelets play role in the development of lymphatic vessels in calcified AVs. Altogether, the preceding studies suggest the possible implication of platelets in stimulating inflammation and in the activation of osteogenic transition of valve cells.
Activated platelets contribute to AV mineralization via promotion of VIC osteogenic transition
Platelet activation and subsequent secretion of TGF-1 and ADP contribute to the mineralization of AVs. TGF-1 enhances glycosaminoglycan elongation, primarily located in the spongiosa layer of AVs, which increases lipid retention in the ECM (8). Lipid retention leads to the oxidation of lipid species that can activate the NF-B pathway, leading to VIC osteogenic transition (7). Simultaneously, ADP released from activated platelets causes increased autotaxin (ATX) expression and release from VICs. ATX associates with platelets and ultimately assists in the conversion of lysophosphatidylcholine (LPC), a product of oxidized lipoproteins (Lp) and activated platelets, to lysophosphatidic acid (LPA) (10). LPA binds to surface receptors on VICs, causing signaling that activates the NF-B pathway, leading to VIC osteogenic transition (21). Therefore, ADP released by activated platelets leads to LPA formation, enhanced by the availability of lipids retained in the ECM caused by released TGF-1, which activates the NF-B pathway in VICs promoting osteogenic transition and thus mineralization of AVs.
Molecular mechanism of platelet-assisted VIC osteogenic transition
Bouchareb et al. (10) showed the presence of activated platelets in explanted AVs of patients with AS (Figure 2), and revealed that activated platelets release ADP, which binds to the metabotropic receptor P2Y1 receptors on VIC plasma membranes. Platelet VIC interaction leads to increased ATX expression and release by VICs (10). Released ATX associates with glycoprotein IIb-IIIa on platelets, where it gains access to its substrate LPC (10). ApoB, oxidized-low-density-lipoproteins (Ox-LDL), and lipoprotein (a) (Lp[a]) infiltrate the AV and are retained by various molecules, including decorin associated with lipoprotein lipase, increased levels of proteoglycans, biglycan, and TGF-1 assisted glycosaminoglycan elongation (22). Oxidation of infiltrating lipids creates oxidized lipid species with the ability to activate toll-like receptors (TLR) and lysophosphatidic acid receptor 1 (LPAR1) on VIC plasma membranes and both receptor types can activate the NF-B pathway and contribute to VIC osteogenic transition.
Lp(a) is a carrier of oxidized-phospholipids (Ox-PLs) that can be metabolized by phospholipases to LPC in AVs (10). Platelets release phospholipase A1 (PLA1) into the AV, which converts Ox-PLs into LPC (10). LPC serves as a substrate for platelet-associated ATX, which has lysophospholipase D activity used to produce LPA (10) (Figure 2). LPA binds to LPAR1 on VIC plasma membrane, and has been shown to initiate the RhoA/ROCK cascade that activates the NF-B pathway and leads to mineralization (23). Ox-LDL, LPA, and other oxidized lipid species can bind to TLRs on VIC plasma membranes that also initiate the NF-B pathway (8).
The canonical NF-B pathway requires the activation of the inhibitor of IB kinase (IKK), which can be activated by tumor necrosis factor-alpha (TNF-), IL-1 and others molecules (8). IKK is a multisubunit complex that phosphorylates IB on Ser residues 32 and 36, targeting IB for degradation (8). Unphosphorylated IB is bound to the inactive p65 (RelA) and p50 heterodimer, in the VIC cytosol (8). Phosphorylated IB releases the heterodimer which can translocate to the nucleus and control the expression of target genes such as upregulating IL-6 and TNF-α (24). Evidence shows that IL-6 leads to VIC osteogenic transition through activation of the osteogenic gene bone morphogenetic protein-2 (BMP2) pathway, leading to high levels of BMP2 gene expression (24). BMP2 promotes mineralization because it increases the expression of RUNX2, an osteogenic transcription factor, which increases the expression of BGLAP and upregulates the mineralization process (10). RUNX2 also positively regulates the expression of alkaline phosphatase, which, along with other ectonucleotidases, generates inorganic phosphates (22) that are associated with lower levels of Akt, a kinase that prevents NF-B pathway activation in VICs (8). In summary, platelets promote VIC osteogenic transition by releasing ADP, TGF-1, and PLA1, which all play a role in assisting the activation of the NF-B pathway, which upregulates osteogenic genes that promote mineralization and thus accelerate the progression of AS. Inhibitors of LPAR1, LPA, and ATX are proposed targets to be investigated for potential treatments to AS progression (10). IL-6 or TNF- monoclonal antibodies could also be tested in preclinical animal models for AS treatment (8).
Platelets and Bioprosthetic Valves
Bioprosthetic valve thrombosis
Transcatheter and surgical AV replacements (TAVR and SAVR, respectively) are the primary treatments for AS, as there are currently no pharmacologic therapies to attenuate AS pathogenesis. Both techniques involve implanting a bioprosthetic valve in the aorta with the goal of simulating normal native AV function. However, clinical research has revealed that even these bioprosthetic valves can experience thrombosis, fibrosis, and calcification, leading to valve dysfunction and failure (22,25). Leaflet thrombosis, which may be subclinical, is posited to be a potential nidus for early valve calcification and degeneration in some cases (26) (Figure 2). The makeup of most bioprosthetic valves, with collagen making up large portions of porcine and pericardial leaflets, make platelet activation a key potential mechanisms involved in response to bioprosthetic valve implantation. The formation of bioprosthetic valve thrombus, also has a potential proinflammatory impact thought to drive valve degeneration. Detailed mechanistic studies of platelet interactions with bioprosthetic valve leaflets similar to those of native valves are currently lacking and require future study. However, clinical analysis, using imaging and outcomes studies, guides our understanding of the role of platelets both directly and indirectly in bioprosthetic valve implantation (Central Illustration).
Central Illustration.
The Implication of Platelets in Aortic Valve Disease
Anti-platelet therapy reduces subclinical thrombosis, HALT and the delay the degeneration of bioprosthetic valve. In native valve activated platelets might have a causal role in the progression of the calcification. More evidence is needed to show the efficiency of antiplatelet therapy in slowing the progression of aortic stenosis.
vWF and bioprosthetic valve dysfunction
A key interaction of platelets is with vWF, a multimeric glycoprotein that aids in hemostasis in blood circulation and commonly measured in blood plasma. MKs and endothelial cells synthesize, store, and secrete vWF as ultra-large multimers that are cleaved on release by a-disintegrin and metalloprotease with a thrombospondin type-1 motif family (ADAMTS13) (25,27). The resulting vWF proteins range in size from free dimers to large multimers that circulate in plasma and be distinguished from one and other (27). High-molecular-weight (HMW) multimers contain more than 20 to 30 vWF subunits, and are the most hemostatically efficient (27). With high WSS conditions, these HMW multimers become activated then elongated, which increases collagen and platelet binding and activation, but also exposes ADAMTS13 binding domain (27). Ultimately, these HMW multimers are deactivated and cleaved by circulating ADAMTS13, producing relatively smaller and inefficient multimers (27, 28, 29). Patients with AS with calcified and fibrotic valve leaflets subject all circulating blood to high WSS. Consequently, many circulating HMW multimers are proteolyzed and circulate in low concentration in patients with AS in proportional concentrations to the amount of WSS (27). Clinically this leads to the observation that in severe AS, acquired vWF syndrome is also an important consideration and that severity of AS (assessed by mean transvalvular gradient) is linearly related to loss of circulating HMW vWF multimers measured in blood tests. Thus vWF has been shown to correlate with AS severity. Following clinical AV replacements from more than 60 reports and 1,801 patients, the concentration of HMW multimers after TAVRs increased instantaneously (27,30, 31, 32). This is attributed to correction of shear stress levels and thus prevention of proteolysis and an acute release of vWF from the endothelium. vWF levels are also an important consideration in patients with TAVR, as deficiency is potentially linked to increased risk of procedural bleeding and proposed as a means of risk stratification, but there are conflicting data regarding the evidence for these patients (27). For example, Sedaghat et al. (30) showed that levels of HMW vWF multimers were not associated with bleeding, but decreased vWF activity to antigen ratios were, Kibler et al. (33) showed an association of vWF and late bleeding events post-TAVR, and Grodecki et al. (34) showed no predictive value for vWF analysis in patients with TAVR. Studies evaluating vWF and per-operative and post-discharge bleeding are summarized extensively in a recent review, but overall support the need to evaluate the utility of vWF analysis for bleeding risk further. This will be a key factor to balancing the risk-reward of anticoagulation to prevent valve thrombosis versus risk of bleeding risk impacting patient outcomes.
In addition to procedural bleeding, aortic regurgitation post-TAVR remains a risk in terms of patient outcomes and transcatheter heart valve (THV) dysfunction. Relatively recently, the rapid analysis of HMW vWF multimers has come to the forefront for evaluating patients for aortic regurgitation, most commonly in the form of paravalvular leak/regurgitation (PVL) post-TAVR. Van Belle et al. (35) showed an association of loss of HMW vWF multimers with PVL and also demonstrated the application of rapid point of care testing to evaluate vWF activity as represented by closure time with adenosine diphosphate (CT-ADP). Overall, there was a negative predictive value of >96% for PVL in both cohorts evaluated with CT-ADP. HMW multimer and CT-ADP analyses also showed association with outcomes at 1 year. Moreover, Kibler et al. (33) showed use of CT-ADP testing to be a predictor of PVL 30 days post-TAVR, and Blackshear et al. (36) showed that vWF abnormalities were seen in 3.8% versus 83.3% of patients with dysfunctional bioprostheses when evaluating a mixed cohort of TAVR and SAVR patients. Notably, CT-ADP is not impacted by anticoagulation or aspirin but this should be tempered by the fact that CT-ADP can be cofounded by coexisting states such as mitral regurgitation and low platelet count.
Resolution of vWF abnormalities is also proposed to be dependent on patient prosthesis mismatch (PPM); evaluation of patients after surgical valve replacement by Vincentelli et al. (31) demonstrated those with PPM had significantly lower levels of HMW vWF multimers than those without 6 months postimplant when comparing 10 patients with PPM with 28 without. This was similar to findings by Yamashita et al. (37) who demonstrated persisting low levels of HMW vWF multimers in patients with PPM post-SAVR and is somewhat related to observations of the inverse relationship between transvalvular gradient and HMW vWF multimers. To our knowledge, however, vWF activity or HMW multimers have not been formally evaluated in TAVR patients comparing those with and without PPM postimplant.
Much remains to be studied with regard to the role of vWF in THV dysfunction and degeneration, as well as its potential as a biomarker. Most studies evaluate vWF in the context of valve dysfunction associated with PVL and/or PPM, as discussed previously. This presents a research gap where considering factors that may relate to long-term degeneration could be useful to guide bench investigations and short-term clinical studies while long-term follow-up of TAVR patients is being gathered in ongoing trials. In this respect, based on known features of valve dysfunction and/or degeneration, such as thrombus, fibrosis, and calcification, there are studies that point the way. For example, lack of anticoagulation was recently associated with hemodynamic valve deterioration, suggesting a role of thrombus in this process (38). At the same time, it is known that the A1 and A3 domains of vWF bind to collagen, the main component of pericardial bioprosthetic leaflets that constitute the vast majority of TAVR valves in use. Certainly this potential link requires further investigation, but suggests that TAVR could potentially result in a more prothrombotic host caused by restoration of vWF. In fact, Yamashita et al. (37) did observe a significant increase in thrombus formation to collagen from whole blood taken from patients post-AVR. vWF may also be a factor to consider in THV thrombus, as it is a central component of endothelial cells, which have been suggested to be dysfunction on the surface of explanted TAVRs.
Clinical imaging of bioprosthetic valve leaflet thrombosis
As a prominent component of thrombus, the role of platelets with regard to leaflet thrombosis on bioprosthetic valves and the associated impact on clinical outcomes and durability has received growing interest. Subclinical bioprosthetic leaflet thrombosis and associated restricted leaflet motion was first described by Makkar et al. in 2015 (39). In a pooled analysis of 187 patients who underwent bioprosthetic AV replacement (AVR, either transcatheter [n = 160] or surgical [n = 27]), restricted leaflet opening was evident on computed tomography (CT) imaging postimplantation in 39 (21%) subjects and corresponded with hypoattenuating opacities predominantly at the base of the valve leaflets (39). The use of therapeutic anticoagulation with warfarin was associated with a lower incidence of leaflet restriction. Restoration of leaflet motion was evident on follow-up CT in all 11 patients with leaflet restriction who were subsequently treated with anticoagulation, suggesting that thrombosis could be a key driver of restricted leaflet motion in bioprosthetic AVRs. No clear association of restricted leaflet motion with stroke or transient ischemic attack was observed. Nevertheless, these observations have prompted several studies characterizing hypoattenuating leaflet thickening (HALT) and the association of this phenomenon with clinical outcomes following TAVR and SAVR (Table 1).
Table 1.
Key Studies Evaluating the Incidence and Clinical Outcomes of Hypoattenuating Leaflet Thickening or Leaflet Thrombosis Following Surgical or Transcatheter Bioprosthetic Aortic Valve Replacement
| Study, Year (Ref. #) | Sample Size (N) | Subjects | Assessment(s) | Key finding(s) |
|---|---|---|---|---|
| Hansson et al, 2016 (25) | 405 | Post-TAVR (Edwards Sapien XT or Sapien 3), age 83 yrs, 54% women. Mixture of antiplatelet regimens postimplant. | Follow-up CT and TTE 1-3 months post-TAVR (median 42 d). | THV thrombosis in 28 (7%) patients. 29 mm THV (RR: 2.89; 95% CI: 1.44–5.80) and no post-TAVR warfarin (RR: 5.46; 95% CI: 1.68 to 17.7) independently associated with THV thrombosis. NS difference in stroke or mortality between patients with and without THV thrombosis. |
| Pache et al, 2016 (59) | 156 | Post-TAVR (Edwards Sapien), age 82 yrs, 54% women. Single (aspirin 21%) or dual (aspirin and clopidogrel 71%) antiplatelet therapy postimplant. | Follow-up CT post-TAVR (median 5 d). | HALT present in 16 (10%) patients. No association of HALT with procedural characteristics nor with antiplatelet regime. No association of HALT with symptoms. Resolution of HALT with anticoagulation in 13 patients. |
| Vollema et al, 2017 (60) | 434 | Post-TAVR (91% balloon or 9% self-expandable), age 80 yrs, 49% women, 3% VIV. Aspirin 52%, clopidogrel 33%, warfarin 37%. | Follow-up: TTE at discharge (n = 431), 6 mo (n = 350), 1 y (n = 229), 2 y (n = 116), and 3 y (n=61); CT at median 35 d (n = 128). | HALT present in 16 (12.5%) patients who underwent CT. Presence of HALT associated with higher mean aortic valve gradient (12.4 ± 8.0 vs 9.4 ± 4.3 mm Hg, P = 0.026) and smaller AVA (1.49 ± 0.39 vs 1.78 ± 0.45 cm2, P = 0.017). No association of HALT with stroke/TIA. |
| Chakravarty et al, 2017 (46) | 890 | Post-TAVR (n = 752) or SAVR (n = 138), age 79 yrs, 44% women. Anticoagulation 25%, single antiplatelet 26%, dual-antiplatelet therapy 49%. | Follow-up CT post-AVR (median 83 d). | Subclinical leaflet thrombosis present in 12% (n = 106) overall (n = 5 post-SAVR and n = 101 post-TAVR). AVR thrombosis less frequent in patients receiving anticoagulation (4%) vs dual-antiplatelet therapy (15%). AVR thrombosis resolved in all patients treated with anticoagulation (n = 36) and persisted in 91% not treated with anticoagulation. AVR thrombosis associated with increased aortic valve gradients. NS association of AVR thrombosis with stroke, but increased rate of TIA (4.18 vs 0.60 TIAs per 100 person-years, P < 0.001). |
| Ruile et al, 2018 (41) | 754 | Post-TAVR (80% balloon-expandable, 15% self-expandable, 5% other), age 82 yrs, 55% women. Anticoagulation at discharge 41%. | Follow-up CT after 3 mo. Median patient follow-up duration 406 d. | Leaflet thrombosis present in 16% (n = 120) patients. NS difference in survival in patients with vs without leaflet thrombosis (18-mo survival 86.4% vs 85.4%, P = 0.912). NS difference in stroke or TIA in patients with vs without leaflet thrombosis (18-mo stroke/TIA-survival 98.5% vs 96.8%, P = 0.331). |
| Makkar et al, 2020 (61) | 435 | PARTNER 3 CT Substudy: patients with low-risk AS undergoing TAVR (Sapien 3, n =179) vs SAVR (n = 125), age 72 yrs, 37% women. Anticoagulation at discharge: 4.7% in TAVR and 21.0% in SAVR. | Follow-up CT in 346 (30-d) and 312 (1-y) | Incidence of HALT increased from 10% at 30 d to 24% at 1 y. Incidence of HALT at 30 d higher in TAVR vs SAVR group (13 vs 5%, RR: 2.64; 95% CI: 1.11–6.32). NS difference in HALT at 1 y between groups. NS difference in valve hemodynamics between patients with and without HALT. HALT resolved spontaneously in 54% of patients at 1 y, and new HALT appeared in 21% of patients at 1 y. NS association of HALT with individual death, MI, or stroke. Presence of HALT associated with the composite of death, stroke, TIA, and retinal artery occlusion at 30 d and 1 y. |
| Blanke et al, 2020 (50) | 375 | Evolut Low-Risk trial LTI substudy: patients with low-risk severe AS undergoing TAVR (self-expanding, n = 198) vs SAVR (n = 178), age 73 yrs, 32% women. Patients on oral anticoagulation excluded from primary analyses. | Follow-up CT at 30 d (n = 318) and 1 y (n = 268) post-procedure. | HALT present in 17.3% for TAVR and 16.5% for SAVR at 30 d (P = 0.856), and 30.9% for TAVR and 28.4% for SAVR at 1 y (P = 0.661). RLM present in 14.6% for TAVR and 14.3% for SAVR at 30 d (P = 0.93), and 30.9% for TAVR and 27.0% for SAVR at 1 y (P = 0.485). Spontaneous resolution of HALT by 1 y in 27% overall. No clear association between HALT and valve hemodynamics or clinical outcomes at 30 d or 1 y. |
AS = aortic stenosis; CT = computed tomography; HALT = hypoattenuated leaflet thickening; MI = myocardial infarction; NS = not significant; RLM = reduced leaflet mobility; TAVR = transcatheter aortic valve replacement; THV = transcatheter heart valve; TIA = transient ischemic attack; TTE = transthoracic echocardiogram; VIV = valve-in-valve.
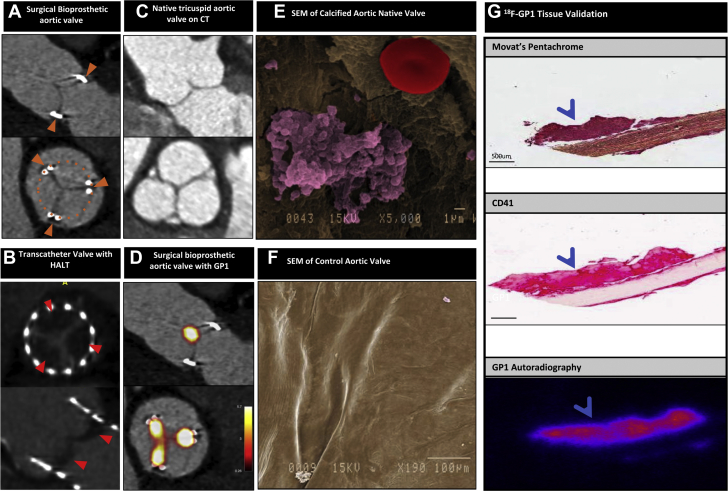
HALT describes the CT finding of a curvilinear meniscoid thickening extending from the base of the bioprosthetic valve leaflet, which can occur in the absence of any hemodynamic disturbance of valve function on echocardiography (Figure 3), as early as 3 to 5 days after valve implantation (40,41). Severity is graded according to the extent of leaflet involvement (<25%, 25%-50%, 50%-75% or >75%) (42) and may be accompanied by restricted leaflet motion. The presence and extent of HALT has been associated with modest increases in AV gradients (Table 1), in which case it is regarded as a form of mild valve dysfunction related to leaflet thrombus. It is noteworthy that no systematic histologic examination of HALT has been performed to confirm whether this phenomenon truly represents thrombosis or other pathology (eg, pannus), although the reversibility with anticoagulation is highly suggestive that thrombus is the major component.
Figure 3.
Bioprosthetic and Native Aortic Valves
(A) Surgical bioprosthetic aortic valve imaged by computed tomography (CT). Orange arrowheads note the location of the valve stent posts and orange dotted circle outlines the circumference of the valve (not visible) (B) Transcatheter bioprosthetic aortic valve demonstrating hypoattenuating leaflet thickening (HALT, red arrowheads) on CT. (C) Normal tricuspid aortic valve imaged by CT. (D) Thrombus (platelets and red blood cells) on a native calcified aortic valve seen on scanning electron microscopy (SEM). (E) Bioprosthetic aortic valve showing avidity for 18F-GP1 tracer on positron emission tomography/CT imaging. (F) Movat’s pentachrome staining and immunohistochemistry for CD41 on an explanted bioprosthetic valve showing a small accumulation of platelets corresponding to autoradiography of 18F-GP1.
Clinical imaging of bioprosthetic valve leaflet thrombosis
Recent advancements in positron emission tomography (PET)-CT radiotracer developments targeting activated platelets may herald unique insights to the pathophysiology and detection of leaflet thrombosis. 18F-GP1 is a novel small molecule fiban-class ligand that binds with high affinity to the glycoprotein IIb/IIIa receptor, which is expressed in high density on activated platelets (43). This binding is not affected by heparin or aspirin. Two recent observational studies have demonstrated the feasibility of in vivo thrombus detection using 18F-GP1 while confirming its favorable safety and radiation dosimetry profile. Kim et al. (44) performed 18F-GP1 PET-CT in patients with known deep vein thrombosis (n = 10) or pulmonary embolism (n = 10) and demonstrated uptake in 89% of vessels with deep vein thrombosis and 60% with pulmonary embolism, as well as uptake in 32 vessels that were not detected by conventional imaging. These findings may represent potential temporal effects on the structure and composition of thrombus, as well as highlighting the ability of 18F-GP1 to detect foci of activated platelets beyond the resolution of CT. Chae et al. (45) studied 10 patients with thrombosis across several arterial beds (n = 10) and detected focal 18F-GP1 uptake in all patients. Crucially, both studies demonstrated very high signal-to-background ratios.
These preliminary data have formed the basis for several ongoing 18F-GP1 studies that aim to establish whether the technique can be used to detect thrombosis beyond current modalities. Early data from in vivo valve imaging have demonstrated consistent 18F-GP1 uptake in bioprosthetic valve leaflets (but not valve frames), in comparison to patients with normal native AVs (Figures 3C and 3D). Ex vivo autoradiography of explanted bioprosthetic valves with thrombus demonstrate specific, focal uptake of 18F-GP1 in regions of thrombus that correlate with histology as well as immunohistochemistry (Figure 3G). These are early findings and their clinical implications are unknown, but they provide insight into long-term in vivo platelet biology and activation in valve prostheses. Further research is required to contextualize these findings with regard to clinical sequelae, and whether there is potential to guide targeted therapy to improve valve durability.
Although the adoption and testing of new PET/CT tracers is a great translational scientific step forward in understanding platelets and their interplay with the vasculature including bioprosthetic valves, other techniques and approaches are continuing to shed light on leaflet thrombus formation. Histologically, thrombus formation has been studied in explanted valves, modeled in vitro, and in silico. In the case of transcatheter valves, explant analysis supports the formation of early thrombus formation within hours of implantation and the accumulation of platelets. In vitro, analysis of differences in surface treatments and flow dynamics have been studied, and in silico, the application of computational modeling provides insight to factors that may drive platelet activation, but much remains to be determined about how to prevent this and the clinical significance.
The debate on clinical significance of HALT and role of anticoagulation
The clinical significance of HALT remains incompletely understood. Risk factors, triggers, and clinical outcomes following HALT are poorly defined. This is largely because of the heterogeneous patient groups studied, variations in AVR type (eg, surgical versus transcatheter, self-expanding versus balloon-expanded valves) and differences in timing of CT scanning post-procedure. The vast majority of studies examining HALT have been in patients undergoing TAVR rather than SAVR, with only recent substudies of randomized trials and registry studies comparing prevalence in surgical versus transcatheter valves (Table 1). Observational data from 2 contemporary registries (comprising 890 patients; 138 SAVR and 752 TAVR) suggest the occurrence of HALT is greater following TAVR compared with SAVR (4% versus 13%, respectively; P = 0.001) (46). Studies have reported a lower incidence of HALT with self-expanding transcatheter valves (ranging between 6% and 8%) compared with balloon-expanded valves (incidence between 14% and 28%) (40,46,47). However, differences in patient selection and anticoagulation strategies likely confound these observations and further studies are needed to evaluate the impact of valve type on the incidence of HALT. Other predictors of increased risk for HALT include larger prosthesis size, smaller aortic annulus, female sex, obesity, valve-in-valve procedure, and single antiplatelet therapy post-procedure (48, 49, 50). It remains unclear whether HALT is associated with worse clinical outcomes, although there may be an association with increased thromboembolic events (Table 1).
The optimal strategy of antiplatelet and anticoagulation therapy following TAVR for stroke risk reduction and prevention or treatment of leaflet thrombosis also remains a point of debate. Antiplatelet therapies developed to help prevent thrombotic events (51) are not yet known because of wide variations in clinical practice and heterogeneous trial populations. It is also not known whether thromboembolic complications and leaflet thrombosis following TAVR occur predominantly because of platelet- or thrombin-mediated clot formation, which could also alter the strategy of anticoagulation post-procedure (52). Current U.S. guidelines advocate for dual antiplatelet therapy (aspirin and clopidogrel) for 6 months post-TAVR followed by lifelong aspirin monotherapy in patients with no other indication for anticoagulation (such as atrial fibrillation), or 3 months of anticoagulation with warfarin for patients with low bleeding risk to prevent valve thrombosis (53). Three large, randomized trials have assessed clinical outcomes with various antiplatelet/anticoagulation strategies in patients undergoing TAVR (Table 2). In the large (n = 1,644, mean age 80 years, 50% females) GALILEO (Global Study Comparing a revaroxaban-based Antithrombotic Strategy to an antiplatelet-based Strategy After Transcatheter aortic valve replacement to Optimize Clinical Outcomes) trial, patients following TAVR (46% balloon-expandable, 46% self-expandable, 8% other) without another clinical indication for oral anticoagulation were randomly assigned to receive 3 months of treatment with either the direct factor Xa inhibitor rivaroxaban plus aspirin or dual antiplatelet therapy with aspirin and clopidogrel. After a median follow-up duration of 17 months, treatment with rivaroxaban was associated with a higher risk of death and thromboembolic events (HR: 1.35, 95% CI: 1.01-1.81), as well as major, disabling, or life-threatening bleeding (HR: 1.50, 95% CI: 0.95-2.37) (54). However, in a substudy of 231 patients from the trial who underwent CT approximately 90 days after randomization, the incidence of HALT was significantly lower in the rivaroxaban arm of the trial compared with those who received dual antiplatelet therapy (HALT identified in 2.1% versus 10.9%, respectively) (55). In the recent POPULAR TAVR (Antiplatelet Therapy for Patients Undergoing Transcatheter Aortic Valve Implantation) trial, patients without a clinical indication for anticoagulation were randomized to receive 3 months of aspirin monotherapy versus dual antiplatelet therapy with aspirin and clopidogrel following TAVR. The incidence of bleeding and the composite of bleeding or thromboembolic events at 1 year were lower with aspirin monotherapy (56). Similarly, in patients with a preexisting clinical indication for anticoagulation receiving either vitamin K antagonist or a direct oral anticoagulant, the addition of clopidogrel for 3 months following TAVR was associated with higher rates of bleeding without conferring any benefit on stroke or myocardial infarction at 12 months. Last, in a small (n = 94, mean age 73 years, 30% female) randomized trial in low-risk patients undergoing TAVR (50% balloon-expandable, 42% self-expandable, 7% other), the combination of aspirin plus warfarin was associated with a lower prevalence of HALT and/or valve dysfunction at 30 days, compared with aspirin monotherapy (57). To date no studies have evaluated the impact of P2Y12-receptor antagonists other than clopidogrel, such as ticagrelor and prasugrel, which may have more potent antiplatelet effects (Central Illustration). However, these agents are themselves associated with increased risks of major bleeding when used in patients following acute coronary syndromes (58) and may pose the same challenges with regard to safety as anticoagulants. At present, it is reasonable to reserve anticoagulation following TAVR only for those patients with HALT and restricted leaflet motion with elevated AV gradients on echocardiography, unless otherwise indicated.
Table 2.
Key Studies Evaluating the Impact of Various Antiplatelet and/or Anticoagulation Strategies on Clinical or Radiological Outcomes Following Transcatheter Aortic Valve Replacement
| Study, Year (Ref. #) | Total sample size (N) | Subjects | Antiplatelet / anticoagulation | Key finding(s) |
|---|---|---|---|---|
| Dangas et al, 2020 (62) | 1,644, mean age 81 yrs, 50% female. | Post-TAVR (46% balloon expandable, 46% self-expandable, 8% other), without an established indication for anticoagulation. | Randomized to: 1) rivaroxaban 10 mg OD plus aspirin 75-100 mg OD for 3 months, then long-term rivaroxaban; or 2) aspirin 75-100 mg OD plus clopidogrel 75 mg OD for 3 months, then long-term aspirin. Median time to randomization: 2 days (IQR 0-8), follow-up duration 17 months (IQR 13-21). | Primary outcome (composite of death or thromboembolic event): HR with rivaroxaban 1.35; 95% CI: 1.01 to 1.81; p = 0.04). NS difference in symptomatic valve thrombosis between trial arms: HR with rivaroxaban 0.43; 95% CI: 0.11 to 1.66). NS difference in rates of stroke or MI between groups. HALT and reduced leaflet motion sub-study (n = 231)55: lower incidence of HALT (12.4% vs. 32.4%) and reduced leaflet motion (2.1% vs. 10.9%) in rivaroxaban group. |
| Nijenhuis et al, 2020 (63) | 313, mean age 81 yrs, 45% female. | Undergoing TAVR (50% balloon expandable, 42% self-expanding, 8% other), already on anticoagulation (73% warfarin, 27% NOAC). | Randomized to 3 months: 1) clopidogrel 75 mg OD, or 2) no clopidogrel, in addition to pre-existing anticoagulation. Minimum 12 months follow-up. | Primary outcome (bleeding rate): lower in group not receiving clopidogrel: 21.7% vs. 34.6%, RR: 0.63; 95% CI: 0.43 to 0.90; p = 0.01). Secondary outcome (composite of CV death, non-procedure bleeding, stroke, MI): lower in group not receiving clopidogrel (RR: 0.69; 95% CI: 0.51 to 0.92). |
| Brouwer et al, 2020 (56) | 665, mean age 80 yrs, 49% female. | Undergoing TAVR (46% balloon expandable, 49% self-expanding, 5% other), with no indication for anticoagulation. | Randomized to 3 months: 1) aspirin 80-100 mg OD monotherapy; or 2) aspirin 80-100 mg OD plus clopidogrel 75 mg OD. | Primary outcome (bleeding rate): lower in aspirin monotherapy group (15.1% vs. 26.6%, RR: 0.57, 95% CI: 0.42 to 0.77, p = 0.001). Secondary outcome (composite of bleeding, CV death, stroke, MI): lower in aspirin monotherapy group (23.0% vs. 31.1%, RR: 0.74, 95% CI: 0.57 to 0.95, p = 0.04). |
| Rogers et al, 2021 (57) | 94, mean age 73 yrs, 30% female. | Low-risk patients undergoing transfemoral TAVR (43% balloon expandable, 57% self-expanding), with no indication for anticoagulation. | Randomized to 30 days: 1) aspirin 80-100 mg OD monotherapy; or 2) aspirin 80-100 mg OD plus warfarin (target INR 2.5). CT scanning in n = 90 at 30 days. | Primary composite effectiveness endpoint at 30 days (HALT, at least moderate restricted leaflet motion, aortic valve gradient ≥20 mmHg, effective orifice area ≤1.0 cm2, valve index <0.35, moderate or worse aortic regurgitation): lower in aspirin plus warfarin group (26.5% vs. 7.0%, p = 0.014). Trend towards lower prevalence of HALT (4.7 vs. 16.3%, p = 0.07) in aspirin plus warfarin group. |
CI = confidence interval; CT = computed tomography; CV = cardiovascular; HALT = hypoattenuation leaflet thickening; HR = hazard ratio; INR = international normalized ratio; MI = myocardial infarction; NS = no significant; OD = once daily; RR = risk ratio; TAVR = transcatheter aortic valve replacement.
In summary, it appears that HALT: 1) is identified in 10% to 15% of patients following AVR, with a higher prevalence following TAVR as compared with SAVR; 2) is likely to represent bioprosthetic leaflet thrombosis; 3) does not clearly correspond with worse clinical outcomes, although may be associated with increased risk of thromboembolic events (eg, stroke/transient ischemic attack) and increased AV gradients; and 4) is a dynamic phenomenon, which appears consistently to regress with anticoagulation therapy but may also resolve spontaneously.
Conclusions
Platelets have a complex biology with a diverse array of associated signaling pathways that have relied heavily on detailed basic science investigation to elucidate. Although much has been learned, there remain many key mechanistic questions as well as those related to how to translate this fundamental knowledge to the clinic and improve patient care. This is a challenge in many vascular diseases, including VHD. As reviewed, evidence supports an active role of platelets in pathogenesis of native AS and the formation of thrombus on bioprosthetic AV replacements. From the native valve pathology and treatment of AS perspective, further research is needed to understand more of the mechanism by which platelets drive valve disease and how we may target them. For example, although there is a well-established role of platelet-derived growth factor on VICs, how this impacts overall AV pathophysiology is a point of future investigation. Similarly, although some platelet-associated pathways may seem highly likely to have a therapeutic effect, no current therapy has shown utility in slowing AS. Moreover, targets such as COX-2 inhibition and warfarin may have adverse effects, such as the recent finding that Celecoxib is associated with valve calcification. From the bioprosthetic valve perspective, the establishment of a mechanistic link between bioprosthetic valve thrombus, platelet function, and valve degeneration or adverse outcomes remains elusive and is needed to guide decision making, and further imaging tools are needed to monitor patients.
Overall, the current state of knowledge provides integration of the molecular interactions and signaling of platelets and their biology and the current clinical evidence, and antiplatelet treatment options. An appreciation of these aspects of the role of platelets in AS and in aortic bioprosthetic heart valves is key to guiding the next steps of both clinical and basic science investigation and improving outcomes for patients.
Funding Support and Author Disclosures
Dr Sellers has been supported by fellowships from the Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research. Dr Pibarot has provided core lab services for Edwards Lifesciences and Medtronic, makers of bioprosthetic valves, for which he receives no direct compensation. Dr Leipsic has been supported by a Canadian Research Chair in Advanced Cardiopulmonary Imaging and has consulted for Edwards Lifesciences, Heartflow Inc, and Circle Cardiovascular Imaging and provides CT core lab services for Edwards Lifesciences, Medtronic, Neovasc, Boston Scientific, and Tendyne Holdings, for which no direct compensation is received. Dr Sathananthan has received speaker fees from Edwards Lifesciences; and has been a consultant for Edwards Lifesciences, Boston Scientific, and Medtronic. Dr Gulsin has been supported by a British Heart Foundation Travel Fellowship.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Thon J.N., Italiano J.E. Platelets: production, morphology and ultrastructure. Handb Exp Pharmacol. 2012;210:3–22. doi: 10.1007/978-3-642-29423-5_1. [DOI] [PubMed] [Google Scholar]
- 2.Patel S.R., Hartwig J.H., Italiano J.E., Jr. The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest. 2005;115:3348–3354. doi: 10.1172/JCI26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machlus K.R., Italiano J.E., Jr. The incredible journey: from megakaryocyte development to platelet formation. J Cell Biol. 2013;201:785–796. doi: 10.1083/jcb.201304054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leiva O., Leon C., Kah Ng S., Mangin P., Gachet C., Ravid K. The role of extracellular matrix stiffness in megakaryocyte and platelet development and function. Am J Hematol. 2018;93:430–441. doi: 10.1002/ajh.25008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Italiano J.E., Jr., Lecine P., Shivdasani R.A., Hartwig J.H. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J Cell Biol. 1999;147:1299–1312. doi: 10.1083/jcb.147.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeung J., Li W., Holinstat M. Platelet signaling and disease: targeted therapy for thrombosis and other related diseases. Pharmacol Rev. 2018;70:526–548. doi: 10.1124/pr.117.014530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mangin P.H., Onselaer M.B., Receveur N., et al. Immobilized fibrinogen activates human platelets through glycoprotein VI. Haematologica. 2018;103:898–907. doi: 10.3324/haematol.2017.182972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathieu P., Bouchareb R., Boulanger M.C. Innate and adaptive immunity in calcific aortic valve disease. J Immunol Res. 2015;2015:851945. doi: 10.1155/2015/851945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutkovskiy A., Malashicheva A., Sullivan G., et al. Valve interstitial cells: the key to understanding the pathophysiology of heart valve calcification. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouchareb R., Boulanger M.C., Tastet L., et al. Activated platelets promote an osteogenic programme and the progression of calcific aortic valve stenosis. Eur Heart J. 2019;40:1362–1373. doi: 10.1093/eurheartj/ehy696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varshney R., Murphy B., Woolington S., et al. Inactivation of platelet-derived TGF-beta1 attenuates aortic stenosis progression in a robust murine model. Blood Adv. 2019;3:777–788. doi: 10.1182/bloodadvances.2018025817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W., Burg N., Vootukuri S., Coller B.S. Increased Smad2/3 phosphorylation in circulating leukocytes and platelet-leukocyte aggregates in a mouse model of aortic valve stenosis: evidence of systemic activation of platelet-derived TGF-beta1 and correlation with cardiac dysfunction. Blood Cells Mol Dis. 2016;58:1–5. doi: 10.1016/j.bcmd.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng S.L., Shao J.S., Behrmann A., Krchma K., Towler D.A. Dkk1 and MSX2-Wnt7b signaling reciprocally regulate the endothelial-mesenchymal transition in aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:1679–1689. doi: 10.1161/ATVBAHA.113.300647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albanese I., Yu B., Al-Kindi H., et al. Role of noncanonical Wnt signaling pathway in human aortic valve calcification. Arterioscler Thromb Vasc Biol. 2017;37:543–552. doi: 10.1161/ATVBAHA.116.308394. [DOI] [PubMed] [Google Scholar]
- 15.Younis D., Bahie A., Elzehery R., El-Kannishy G., Wahab A.M. Association between serum Dickkopf-1 (DKK-1) glycoprotein and calcific deposits on cardiac valves and carotid intimal-medial thickness in hemodialysis patients. Cardiorenal Med. 2020;10:313–322. doi: 10.1159/000507183. [DOI] [PubMed] [Google Scholar]
- 16.Askevold E.T., Gullestad L., Aakhus S., et al. Secreted Wnt modulators in symptomatic aortic stenosis. J Am Heart Assoc. 2012;1 doi: 10.1161/JAHA.112.002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueland T., Otterdal K., Lekva T., et al. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1228–1234. doi: 10.1161/ATVBAHA.109.189761. [DOI] [PubMed] [Google Scholar]
- 18.Hess P.R., Rawnsley D.R., Jakus Z., et al. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J Clin Invest. 2014;124:273–284. doi: 10.1172/JCI70422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Syvaranta S., Helske S., Lappalainen J., Kupari M., Kovanen P.T. Lymphangiogenesis in aortic valve stenosis--novel regulatory roles for valvular myofibroblasts and mast cells. Atherosclerosis. 2012;221:366–374. doi: 10.1016/j.atherosclerosis.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 20.Bertozzi C.C., Hess P.R., Kahn M.L. Platelets: covert regulators of lymphatic development. Arterioscler Thromb Vasc Biol. 2010;30:2368–2371. doi: 10.1161/ATVBAHA.110.217281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun W., Yang J. Molecular basis of lysophosphatidic acid-induced NF-kappaB activation. Cell Signal. 2010;22:1799–1803. doi: 10.1016/j.cellsig.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathieu P., Boulanger M.C. Autotaxin and lipoprotein metabolism in calcific aortic valve disease. Front Cardiovasc Med. 2019;6:18. doi: 10.3389/fcvm.2019.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nsaibia M.J., Boulanger M.C., Bouchareb R., et al. OxLDL-derived lysophosphatidic acid promotes the progression of aortic valve stenosis through a LPAR1-RhoA-NF-kappaB pathway. Cardiovasc Res. 2017;113:1351–1363. doi: 10.1093/cvr/cvx089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akdag S., Akyol A., Asker M., Duz R., Gumrukcuoglu H.A. Platelet-to-lymphocyte ratio may predict the severity of calcific aortic stenosis. Med Sci Monit. 2015;21:3395–3400. doi: 10.12659/MSM.894774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansson N.C., Grove E.L., Andersen H.R., et al. Transcatheter aortic valve thrombosis: incidence, predisposing factors, and clinical implications. J Am Coll Cardiol. 2016;68:2059–2069. doi: 10.1016/j.jacc.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Cartlidge T.R.G., Doris M.K., Sellers S.L., et al. Detection and prediction of bioprosthetic aortic valve degeneration. J Am Coll Cardiol. 2019;73:1107–1119. doi: 10.1016/j.jacc.2018.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Belle E., Vincent F., Rauch A., et al. von Willebrand Factor and management of heart valve disease: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;73:1078–1088. doi: 10.1016/j.jacc.2018.12.045. [DOI] [PubMed] [Google Scholar]
- 28.Springer T.A. von Willebrand factor, Jedi knight of the bloodstream. Blood. 2014;124:1412–1425. doi: 10.1182/blood-2014-05-378638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai H.M., Sussman, Nagel R.L. Shear stress enhances the proteolysis of von Willebrand factor in normal plasma. Blood. 1994;83:2171–2179. [PubMed] [Google Scholar]
- 30.Sedaghat A., Kulka H., Sinning J.M., et al. Transcatheter aortic valve implantation leads to a restoration of von Willebrand factor (VWF) abnormalities in patients with severe aortic stenosis - Incidence and relevance of clinical and subclinical VWF dysfunction in patients undergoing transfemoral TAVI. Thromb Res. 2017;151:23–28. doi: 10.1016/j.thromres.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 31.Vincentelli A., Susen S., Le Tourneau T., et al. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med. 2003;349:343–349. doi: 10.1056/NEJMoa022831. [DOI] [PubMed] [Google Scholar]
- 32.Spangenberg T., Budde U., Schewel D., et al. Treatment of acquired von Willebrand syndrome in aortic stenosis with transcatheter aortic valve replacement. J Am Coll Cardiol Intv. 2015;8:692–700. doi: 10.1016/j.jcin.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Kibler M., Marchandot B., Messas N., et al. CT-ADP point-of-care assay predicts 30-day paravalvular aortic regurgitation and bleeding events following transcatheter aortic valve replacement. Thromb Haemost. 2018;118:893–905. doi: 10.1055/s-0038-1639352. [DOI] [PubMed] [Google Scholar]
- 34.Grodecki K., Zbronski K., Przybyszewska-Kazulak E., et al. Pre-procedural abnormal function of von Willebrand Factor is predictive of bleeding after surgical but not transcatheter aortic valve replacement. J Thromb Thrombolysis. 2019;48:610–618. doi: 10.1007/s11239-019-01917-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Belle E., Rauch A., Vincent F., et al. Von Willebrand factor multimers during transcatheter aortic-valve replacement. N Engl J Med. 2016;375:335–344. doi: 10.1056/NEJMoa1505643. [DOI] [PubMed] [Google Scholar]
- 36.Blackshear J.L., McRee C.W., Safford R.E., et al. von Willebrand factor abnormalities and Heyde syndrome in dysfunctional heart valve prostheses. JAMA Cardiol. 2016;1:198–204. doi: 10.1001/jamacardio.2016.0075. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita K., Yagi H., Hayakawa M., et al. Rapid restoration of thrombus formation and high-molecular-weight von Willebrand factor multimers in patients with severe aortic stenosis after valve replacement. J Atheroscler Thromb. 2016;23:1150–1158. doi: 10.5551/jat.34421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Del Trigo M., Munoz-Garcia A.J., Latib A., et al. Impact of anticoagulation therapy on valve haemodynamic deterioration following transcatheter aortic valve replacement. Heart. 2018;104:814–820. doi: 10.1136/heartjnl-2017-312514. [DOI] [PubMed] [Google Scholar]
- 39.Makkar R.R., Fontana G., Jilaihawi H., et al. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med. 2015;373:2015–2024. doi: 10.1056/NEJMoa1509233. [DOI] [PubMed] [Google Scholar]
- 40.Yanagisawa R., Tanaka M., Yashima F., et al. Early and late leaflet thrombosis after transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2019;12 doi: 10.1161/CIRCINTERVENTIONS.118.007349. [DOI] [PubMed] [Google Scholar]
- 41.Ruile P., Minners J., Breitbart P., et al. Medium-term follow-up of early leaflet thrombosis after transcatheter aortic valve replacement. J Am Coll Cardiol Intv. 2018;11:1164–1171. doi: 10.1016/j.jcin.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Blanke P., Weir-McCall J.R., Achenbach S., et al. Computed tomography imaging in the context of transcatheter aortic valve replacement (TAVR)/transcatheter aortic valve replacement (TAVR): an expert consensus document of the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol Img. 2019;12:1–24. doi: 10.1016/j.jcmg.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Lohrke J., Siebeneicher H., Berger M., et al. 18)F-GP1, a novel PET tracer designed for high-sensitivity, low-background detection of thrombi. J Nucl Med. 2017;58:1094–1099. doi: 10.2967/jnumed.116.188896. [DOI] [PubMed] [Google Scholar]
- 44.Kim C., Lee J.S., Han Y., et al. Glycoprotein IIb/IIIa receptor imaging with (18)F-GP1 positron emission tomography for acute venous thromboembolism: an open-label, non-randomized, first-in-human phase 1 study. J Nucl Med. 2018;60:244–249. doi: 10.2967/jnumed.118.212084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chae S.Y., Kwon T.W., Jin S., et al. A phase 1, first-in-human study of (18)F-GP1 positron emission tomography for imaging acute arterial thrombosis. EJNMMI Res. 2019;9:3. doi: 10.1186/s13550-018-0471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chakravarty T., Sondergaard L., Friedman J., et al. RESOLVE, SAVORY Investigators Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. 2017;389:2383–2392. doi: 10.1016/S0140-6736(17)30757-2. [DOI] [PubMed] [Google Scholar]
- 47.Khan J.M., Rogers T., Waksman R., et al. Hemodynamics and subclinical leaflet thrombosis in low-risk patients undergoing transcatheter aortic valve replacement. Circ Cardiovasc Imaging. 2019;12 doi: 10.1161/CIRCIMAGING.119.009608. [DOI] [PubMed] [Google Scholar]
- 48.D'Ascenzo F., Salizzoni S., Saglietto A., et al. Incidence, predictors and cerebrovascular consequences of leaflet thrombosis after transcatheter aortic valve implantation: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2019;56:488–494. doi: 10.1093/ejcts/ezz099. [DOI] [PubMed] [Google Scholar]
- 49.Kefer J. Hypoattenuated leaflet thickening in transcatheter and surgical aortic valves. J Am Coll Cardiol. 2020;75:2443–2445. doi: 10.1016/j.jacc.2020.03.048. [DOI] [PubMed] [Google Scholar]
- 50.Blanke P., Leipsic J.A., Popma J.J., et al. Bioprosthetic aortic valve leaflet thickening in the Evolut Low Risk Sub-Study. J Am Coll Cardiol. 2020;75:2430–2442. doi: 10.1016/j.jacc.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 51.Zou H., Li Y., Xu G. Management of anticoagulation and antiplatelet therapy in patients with primary membranous nephropathy. BMC Nephrol. 2019;20:442. doi: 10.1186/s12882-019-1637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lugo L.M., Romaguera R., Gomez-Hospital J.A., Ferreiro J.L. Antithrombotic therapy after transcatheter aortic valve implantation. Eur Cardiol. 2020;15:1–8. doi: 10.15420/ecr.2019.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishimura R.A., Otto C.M., Bonow R.O., et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252–289. doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 54.Dangas G.D., De Backer O., Windecker S. A controlled trial of rivaroxaban after transcatheter aortic-valve replacement. Reply. N Engl J Med. 2020;383:e8. doi: 10.1056/NEJMc2017351. [DOI] [PubMed] [Google Scholar]
- 55.De Backer O., Dangas G.D., Jilaihawi H., et al. Reduced leaflet motion after transcatheter aortic-valve replacement. N Engl J Med. 2020;382:130–139. doi: 10.1056/NEJMoa1911426. [DOI] [PubMed] [Google Scholar]
- 56.Brouwer J., Nijenhuis V.J., Delewi R., et al. Aspirin with or without clopidogrel after transcatheter aortic-valve implantation. N Engl J Med. 2020;383:1447–1457. doi: 10.1056/NEJMoa2017815. [DOI] [PubMed] [Google Scholar]
- 57.Rogers T., Shults C., Torguson R., et al. Randomized trial of aspirin versus warfarin after transcatheter aortic valve replacement in low-risk patients. Circ Cardiovasc Interv. 2021;14 doi: 10.1161/CIRCINTERVENTIONS.120.009983. [DOI] [PubMed] [Google Scholar]
- 58.Navarese E.P., Khan S.U., Kolodziejczak M., et al. Comparative efficacy and safety of oral P2Y12 inhibitors in acute coronary syndrome: network meta-analysis of 52 816 patients from 12 randomized trials. Circulation. 2020;142:150–160. doi: 10.1161/CIRCULATIONAHA.120.046786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pache G., Schoechlin S., Blanke P., et al. Early hypo-attenuated leaflet thickening in balloon-expandable transcatheter aortic heart valves. Eur Heart J. 2016;37:2263–2271. doi: 10.1093/eurheartj/ehv526. [DOI] [PubMed] [Google Scholar]
- 60.Vollema E.M., Kong W.K.F., Katsanos S., et al. Transcatheter aortic valve thrombosis: the relation between hypo-attenuated leaflet thickening, abnormal valve haemodynamics, and stroke. Eur Heart J. 2017;38:1207–1217. doi: 10.1093/eurheartj/ehx031. [DOI] [PubMed] [Google Scholar]
- 61.Makkar R.R., Blanke P., Leipsic J., et al. Subclinical leaflet thrombosis in transcatheter and surgical bioprosthetic valves: PARTNER 3 Cardiac Computed Tomography Substudy. J Am Coll Cardiol. 2020;75:3003–3015. doi: 10.1016/j.jacc.2020.04.043. [DOI] [PubMed] [Google Scholar]
- 62.Dangas G.D., Tijssen J.G.P., Wohrle J., et al. A controlled trial of rivaroxaban after transcatheter aortic-valve replacement. N Engl J Med. 2020;382:120–129. doi: 10.1056/NEJMoa1911425. [DOI] [PubMed] [Google Scholar]
- 63.Nijenhuis V.J., Brouwer J., Delewi R., et al. Anticoagulation with or without clopidogrel after transcatheter aortic-valve implantation. N Engl J Med. 2020;382:1696–1707. doi: 10.1056/NEJMoa1915152. [DOI] [PubMed] [Google Scholar]