Key Points
Question
Does the risk of subsequent stroke differ by care setting among patients with transient ischemic attack (TIA) or minor stroke?
Findings
In this systematic review and meta-analysis of 226 683 unique patients in 71 unique studies, patients cared for in a TIA clinic vs as inpatients had similar risks of subsequent stroke. Patients who were treated in emergency departments without further follow-up had a higher risk of subsequent stroke than those treated as inpatients or in TIA clinics.
Meaning
In this study, the risk of subsequent stroke among patients who received treatment in a TIA clinic was not higher than those who were hospitalized.
This systematic review and meta-analysis compares the risk of subsequent ischemic stroke among patients with transient ischemic attack (TIA) or minor ischemic stroke who received care at rapid-access TIA or neurology clinics, inpatient units, emergency departments, and unspecified or multiple settings.
Abstract
Importance
Transient ischemic attack (TIA) often indicates a high risk of subsequent cerebral ischemic events. Timely preventive measures improve the outcome.
Objective
To estimate and compare the risk of subsequent ischemic stroke among patients with TIA or minor ischemic stroke (mIS) by care setting.
Data Sources
MEDLINE, Web of Science, Scopus, Embase, International Clinical Trials Registry Platform, ClinicalTrials.gov, Trip Medical Database, CINAHL, and all Evidence-Based Medicine review series were searched from the inception of each database until October 1, 2020.
Study Selection
Studies evaluating the occurrence of ischemic stroke after TIA or mIS were included. Cohorts without data on evaluation time for reporting subsequent stroke, with retrospective diagnosis of the index event after stroke occurrence, and with a report of outcomes that were not limited to patients with TIA or mIS were excluded. Two authors independently screened the titles and abstracts and provided the list of candidate studies for full-text review; discrepancies and disagreements in all steps of the review were addressed by input from a third reviewer.
Data Extraction and Synthesis
The study was prepared and reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses, Meta-analysis of Observational Studies in Epidemiology, Methodological Expectations of Cochrane Intervention Reviews, and Enhancing the Quality and Transparency of Health Research guidelines. The Risk of Bias in Nonrandomized Studies—of Exposures (ROBINS-E) tool was used for critical appraisal of cohorts, and funnel plots, Begg-Mazumdar rank correlation, Kendall τ2, and the Egger bias test were used for evaluating the publication bias. All meta-analyses were conducted under random-effects models.
Main Outcomes and Measures
Risk of subsequent ischemic stroke among patients with TIA or mIS who received care at rapid-access TIA or neurology clinics, inpatient units, emergency departments (EDs), and unspecified or multiple settings within 4 evaluation intervals (ie, 2, 7, 30, and 90 days).
Results
The analysis included 226 683 patients from 71 articles recruited between 1981 and 2018; 5636 patients received care at TIA clinics (mean [SD] age, 65.7 [3.9] years; 2291 of 4513 [50.8%] men), 130 139 as inpatients (mean [SD] age, 78.3 [4.0] years; 49 458 of 128 745 [38.4%] men), 3605 at EDs (mean [SD] age, 68.9 [3.9] years; 1596 of 3046 [52.4%] men), and 87 303 patients received care in an unspecified setting (mean [SD] age, 70.8 [3.8] years, 43 495 of 87 303 [49.8%] men). Among the patients who were treated at a TIA clinic, the risk of subsequent stroke following a TIA or mIS was 0.3% (95% CI, 0.0%-1.2%) within 2 days, 1.0% (95% CI, 0.3%-2.0%) within 7 days, 1.3% (95% CI, 0.4%-2.6%) within 30 days, and 2.1% (95% CI, 1.4%-2.8%) within 90 days. Among the patients who were treated as inpatients, the risk of subsequent stroke was to 0.5% (95% CI, 0.1%-1.1%) within 2 days, 1.2% (95% CI, 0.4%-2.2%) within 7 days, 1.6% (95% CI, 0.6%-3.1%) within 30 days, and 2.8% (95% CI, 2.1%-3.5%) within 90 days. The risk of stroke among patients treated at TIA clinics was not significantly different from those hospitalized. Compared with the inpatient cohort, TIA clinic patients were younger and had had lower ABCD2 (age, blood pressure, clinical features, duration of TIA, diabetes) scores (inpatients with ABCD2 score >3, 1101 of 1806 [61.0%]; TIA clinic patients with ABCD2 score >3, 1933 of 3703 [52.2%]).
Conclusions and Relevance
In this systematic review and meta-analysis, the risk of subsequent stroke among patients who were evaluated in a TIA clinic was not higher than those hospitalized. Patients who received treatment in EDs without further follow-up had a higher risk of subsequent stroke. These findings suggest that TIA clinics can be an effective component of the TIA care component pathway.
Introduction
Studies have shown up to an 80% reduction in the risk of stroke after a transient ischemic attack (TIA) with early implementation of secondary stroke prevention strategies.1,2,3 Our study4 examining the trends in TIA outcome during the past 5 decades indicated that the risk of subsequent stroke has remained unchanged since 1999.
Despite the need for an urgent investigation of the etiology and initiation of preventive measures for patients with TIA, there is no consensus on the care pathway protocol. The evaluation and hospitalization rates after TIA vary widely among practitioners, hospitals, and regions.5,6,7,8 Several TIA care pathway models have been proposed mainly to reduce the hospital length of stay and admission costs and to improve outcomes.9,10,11 Several studies have indicated that the outpatient management of TIA among selected patients can be safe and cost-effective.1,9,10,12,13,14,15 Nevertheless, in many instances outpatient care for selected patients with TIA is avoided.
There is no comprehensive study comparing the outcome of patients with TIA who received care in different settings. The goal of the current meta-analysis was to estimate and compare the risk of subsequent ischemic stroke among patients with TIA or minor ischemic stroke (mIS) who received care at rapid access TIA or neurology clinics, inpatient units, emergency departments (EDs), and unspecified or multiple settings within 4 evaluation intervals (2, 7, 30, and 90 days).
Methods
We prepared and reported the present study according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA),16 Meta-analysis of Observational Studies in Epidemiology (MOOSE),17 Methodological Expectations of Cochrane Intervention Reviews (MECIR),18 and Enhancing the Quality and Transparency of Health Research (EQUATOR)19 guidelines.
Search Strategy
We identified potentially eligible studies by systematically searching the databases Medline, Web of Science, Scopus, Embase, International Clinical Trials Registry Platform (ICTRP), ClinicalTrials.gov, Trip Medical Database, CINAHL, and all Evidence-Based Medicine review series (Cochrane Database of Systematic Reviews, ACP Journal Club, Database of Abstracts of Reviews of Effects, Cochrane Clinical Answers, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, Health Technology Assessment, and NHS Economic Evaluation Database) (eAppendix in the Supplement). The search queries were primarily conducted from the inception of each database until October 1, 2020, without restriction on study design, document type, language, or socioeconomic and health-expenditure indices of the publishing institute. To minimize the risk of publication bias, peer-reviewed publications, unpublished studies, and gray literature sources were evaluated. We augmented the search results by manually forward and backward citation tracking (in Google Scholar) and communication with selected authors.
Eligibility Criteria
All studies providing information on the occurrence of ischemic stroke after TIA or mIS (index event) were recorded. We included retrospective and prospective cohorts of adult patients, with both the time-based20 and the tissue-based21 definitions of TIA as well as alternative definitions of mIS, as National Institutes of Health Stroke Scale (NIHSS) score of 3 or less,22 persistence of symptoms for at least 24 hours, or positive diffusion-weighted imaging within 24 hours of symptom onset.21 We excluded cohorts (1) without available evaluation time for reporting subsequent stroke, (2) with retrospective diagnosis of index event after stroke occurrence, (3) with a report of outcomes for all triaged patients not limited to TIA or mIS, and (4) duplicate reports.
Outcome Measure
The outcome of the study was the proportion of early ischemic strokes after the index TIA or mIS among patients who received acute care management in 4 settings: (1) TIA clinic, defined as rapid-access TIA or neurology clinics in which a patient was evaluated within 2 weeks of symptom onset; (2) inpatient, defined as medical-surgical units, stroke units, or observation units; (3) ED, defined as cohorts of patients receiving care in an ED without referral to the TIA clinic or hospitalization; and (4) unspecified setting, including combined reports of outcome from different settings when they could not be differentiated and multicenter studies without a unique protocol. We considered the comparison between the outcomes of patients treated in a TIA clinic vs as inpatients as our main interest. Admissions to in-hospital observation units (ie, <24 hours), although often seen as an outpatient visit (for billing purposes), were considered inpatient due to similarities in the protocols. We reported the outcomes of each setting within 2, 7, 30, and 90 days.
Screening and Data Extraction
Two reviewers (S.S. and E.K.) independently screened the titles and abstracts and provided the list of candidate studies for full-text review. We addressed the discrepancies and disagreements in all steps of the review by input from a third reviewer (R.Z.). The output of the search was compiled in Mendeley version 1.19.6. Duplicate sets were removed. Records in languages other than English were screened by native speakers. For each study, the data regarding each cohort of patients who received acute care in a similar setting were recorded separately.
Risk-of-Bias and Publication Bias Assessment
We applied the Risk of Bias in Nonrandomized Studies—of Exposures (ROBINS-E) tool23,24 for critical appraisal of the cohorts. The assessment was recorded as low, moderate, or high risk of bias or no information. The degree of bias was measured by the Begg-Mazumdar rank correlation Kendall τ2 and the Egger bias test.25
Statistical Analysis
To explore the differences among the estimators, we used (1) moment estimators, ie, DerSimonian and Laird (DL), Hunter and Schmidt (HS), and Hedges (HE); (2) maximum likelihood estimators, ie, maximum likelihood (ML) and restricted maximum likelihood (REML); (3) model error variance estimator, ie, Sidik and Jonkman (SJ); and (4) Bayes estimator, ie, empirical Bayes (EB).25
We explored the possible moderator effect of (1) acute-care setting, (2) evaluation intervals, (3) study design of each cohort, (4) recruitment interval (ie, before 2000, 2000-2007, and after 2007, based on the pioneer guidelines in TIA care1,13,26,27), and (5) age, blood pressure, clinical features, duration of TIA, diabetes (ABCD2) score (percentage of patients in each cohort with ABCD2 score of <4 vs ≥4) on the outcome (risk of subsequent stroke) through mixed-effect models using REML as an estimator.28 Omnibus test was used to compare the models vs null hypothesis. We compared the outcome of each moderator by calculating the risk of subsequent stroke, between-group I2, residual heterogeneity, and P value (eTable 1 in the Supplement). We assessed the subsequent stroke risk estimates for each evaluation interval separately and considered the setting of care as a subclass under each evaluation interval. We reevaluated the association of ABCD2 score with the outcome under each setting-of-care strata (eTable 2 in the Supplement). We performed sensitivity analysis for evaluating the impact of recruitment interval and study design.
We considered a 2-tailed P < .05 as statistically significant in all tests. The difference among subgroups was evaluated by pairwise comparisons and adjusted α level, when applicable. Meta-analyses were performed using R version 4.0.2, metafor package (R Project for Statistical Computing).28 Forest plots were reproduced in Python version 3.8 for further validation and better visualization.
Results
Literature Review and Study Selection
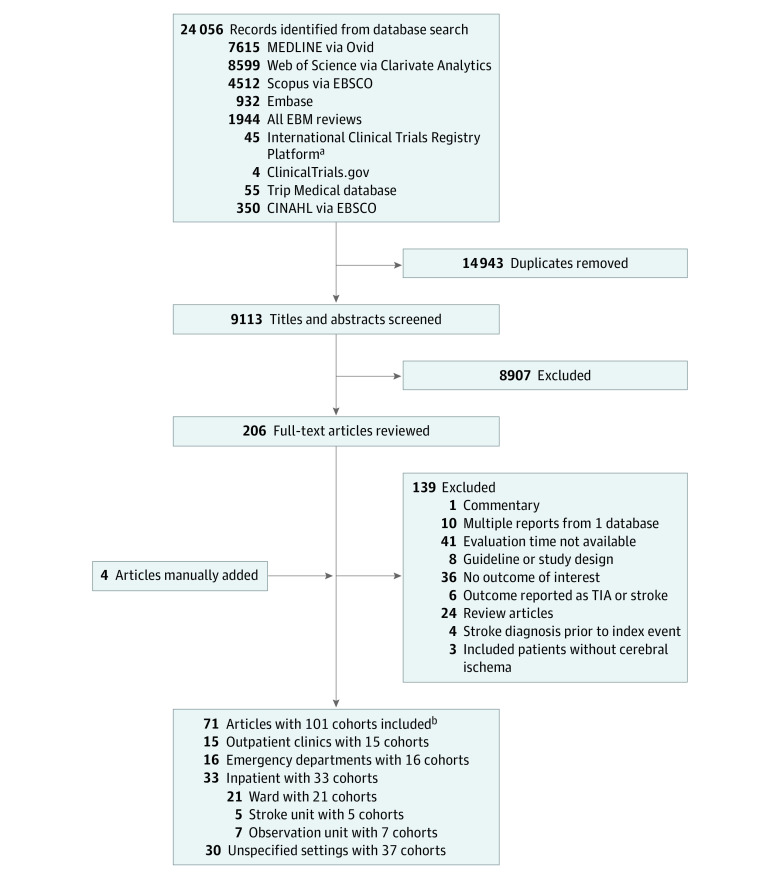
The search protocol resulted in 24 056 records (Figure 1). After the removal of 14 943 duplicate records, the titles and abstracts of 9113 discrete search results were screened. Of the 206 potentially eligible studies, 139 articles were excluded after full-text review (eTable 3 in the Supplement).
Figure 1. Study Flowchart.
TIA indicates transient ischemic attack.
aEBM reviews include Cochrane Database of Systematic Reviews, ACP Journal Club, Database Abstracts of Reviews and Effects, Cochrane Clinical Answers; Cochrane Central Register of Controlled Trials, Cochrane Methodological Register, Health Technology, and NHS Economic Evaluation Database.
bArticles could include multiple settings.
Review of the reference lists, citation tracking, and communication with authors led to inclusion of four additional studies. A total of 71 studies were included (A. Mowla, MD, unpublished data, 2020).9,15,27,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96
Patient Characteristics
This review includes 226 683 patients recruited between 1981 and 2018. Patients were studied prospectively in 24 cohorts (23.8%).29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49 By considering the health care setting for the index event, we recorded 101 distinct cohorts. Out of 101 cohorts, 16 (15.8%) included patients with TIA and mIS.27,29,30,32,50,51,52,53,97 TIA was defined based on a tissue-based definition in 7 studies (9.8%)33,52,54,55,56,57 (and A. Mowla, MD, unpublished data, 2020). The Table includes the summary of baseline characteristics and vascular risk factors.
Table. Baseline Characteristics and Vascular Risk Factors Among Patients Receiving Care at Each Setting.
| Characteristic | Patients by acute-care setting, No./total No. available (%) | |||
|---|---|---|---|---|
| TIA clinic (n = 5636)a | Inpatient (n = 130 136) | Emergency department (n = 3605) | Unspecified (n = 87 303) | |
| Age, mean (SD), y | 65.7 (3.9) | 78.3 (4.0) | 68.9 (3.9) | 70.8 (3.8) |
| Men | 2291/4513 (50.8) | 49 458/128 745 (38.4) | 1596/3046 (52.4)b | 43 495/87 303 (49.8) |
| Women | 2222/4513 (49.2) | 79 287/128 745 (61.6) | 1450/3046 (47.6) | 43 808/87 303 (50.2) |
| ABCD2 score >3 | 1933/3703 (52.2) | 1101/1806 (61.0) | 984/1735 (56.7) | 6610/9440 (70.0) |
| Hypertension | 2694/4729 (57.0) | 84 677/128 933 (65.7) | 2402/3605 (66.6) | 36 938/86 081 (42.9) |
| Diabetes | 667/4729 (14.1) | 33 651/128 933 (26.1) | 722/3605 (20.0) | 12 508/85 364 (14.7) |
| Dyslipidemia | 146/3934 (3.7) | 314/2772 (11.3) | 106/2250 (4.7)b | 385/60 795 (0.6) |
| Ischemic heart disease | 406/3476 (11.7) | 265/1504 (17.6) | 106/447 (23.7) | 318/1635 (19.4) |
| Peripheral vascular disease | 83/1868 (4.5) | 82/4624 (1.8) | 46/737 (6.3)b | 491/8973 (5.5) |
| Atrial fibrillation | 360/3934 (9.2) | 20 260/130 139 (15.6) | 279/1987 (14.0) | 11 266/80 757 (14) |
| Carotid stenosis | 879/3566 (24.7) | 165/1086 (15.2) | 271/1419 (19.1) | 2655/53 905 (4.9) |
| Prior TIA | 436/2188 (19.9) | 214/1349 (15.9) | 164/880 (18.6) | 3225/17 332 (18.6) |
| Prior stroke | 227/3309 (6.9) | 14 784/126 332 (11.7) | 311/1674 (18.6) | 6486/30 880 (21.0) |
| Prior TIA or stroke | 663/3327 (19.9) | 15 293/127 629 (12.0) | 738/2680 (27.5) | 7467/18 240 (40.9) |
| Smoking | 772/3633 (21.2) | 8134/124 447 (6.5) | 488/2423 (20.2)b | 10 689/80 031 (13.4) |
Abbreviations: ABCD2, age, blood pressure, clinical features, duration of transient ischemic attack, diabetes; TIA, transient ischemic attack.
All comparisons between the TIA clinic cohort and inpatient cohort are significantly different (P < .001). Unless otherwise noted, all comparisons between the TIA clinic cohort and emergency department cohort are significantly different (P < .001).
Indicates P > .05 in comparison between TIA clinic cohort and emergency department cohort.
In 15 cohorts (5636 patients; mean [SD] age, 65.7 [3.9] years; 2291 of 4513 [50.8%] men) acute care was delivered in TIA clinics (A. Mowla, MD, unpublished data, 2020).9,29,30,31,32,51,52,53,54,55,56,57,58,59 Among the inpatients (33 cohorts; 130 139 patients; mean [SD] age, 78.3 [4.0] years; 49 458 of 128 745 [38.4%] men), 21 cohorts (125 719 patients) received care in medical-surgical units (A. Mowla, MD, unpublished data, 2020),9,15,27,32,38,39,40,41,51,52,53,55,58,59,64,65,79,81,83,84 5 cohorts (2487 patients) in stroke units,49,63,79,80,92 and 7 cohorts (1933 patients) in observation units.48,65,66,82,83,84,95 In 16 cohorts (3605 patients; mean [SD] age, 68.9 [3.9] years; 1596 of 3046 [52.4%] men), the acute care was offered at the ED (A. Mowla, MD, unpublished data, 2020).15,33,34,35,36,37,40,61,67,68,81,85,87,93 The setting of care was not fully described or the study included the patients who received treatment in various care settings and multiple centers in 37 cohorts (87 303 patients; mean [SD] age, 70.8 [3.8] years, 43 495 of 87 303 [49.8%] men).27,42,43,44,45,46,47,50,51,53,59,60,62,69,70,71,72,73,74,75,76,77,78,79,81,86,88,89,90,92,94 Eight studies9,32,51,52,53,58,59 (and A. Mowla, MD, unpublished data, 2020) provided the outcome of the patients in both inpatient and TIA clinic cohorts. The risk of subsequent stroke was reported for 35 356 patients within 2 days, 36 134 patients within 7 days, 142 185 patients within 30 days, and 94 731 patients within 90 days.
Among the patients who were referred to TIA clinics, 3 studies29,52,56 reported a clinic no-show rate of 36.0% (447 of 1241 referred patients with suspected cerebral ischemia). The evaluation window at the TIA clinics was within 24 hours in 101 patients,29 within 72 hours in 22 patients,32 within 1 week in 828 patients,51,52,54,59 and within 2 weeks among 857 patients.31,56 One study58 with 982 patients determined the appropriate interval according to ABCD2 score. Three studies51,52,54 reported the complication risk while the patients were waiting to be seen in the outpatient clinic after being discharged from the ED. This risk was zero in 2 studies (165 patients)53,59 and 0.6% in one study (1 of 157).51
Final diagnosis of TIA and mIS was made in 2895 out 4302 patients (67.3%) evaluated in the TIA clinics and 689 of 1055 patients (65.3%) of inpatients (P = .22). ABCD2 score of 4 or greater was reported in 1933 of 3703 patients (52.2%) treated at a TIA clinic and 1101 of 1806 patients (61.0%) treated as inpatients (P < .001). Although patients treated at a TIA clinic had lower ABCD2 scores compared to inpatients (TIA clinic patients with ABCD2 score >3, 1933 of 3703 [52.2%]; inpatients with ABCD2 score >3, 1101 of 1806 [61.0%]) (Table), this score did not seem to affect the risk estimation under different setting of care when we considered all cohorts or when we estimated the risk within each evaluation time (eTable 2 in the Supplement). More patients treated in TIA clinics had carotid stenosis than those treated as inpatients (879 of 3566 [24.7%] vs 214 of 1349 [15.9%]).
Outcome of Meta-analyses
As presented in the eTable 4 in the Supplement, the difference among estimated risk of subsequent stroke measured by seven estimators (DL, HE, HS, ML, REML, SJ, and EB) was negligible. The forest plots based on the REML estimator43,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,96,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123 are shown Figure 2 and Figure 3 and eFigure 2 and eFigure 3 in the Supplement. Among the patients who were treated in a TIA clinic, the risk of subsequent stroke following a TIA or mIS was 0.3% (95% CI, 0.0%-1.2%) within 2 days, 1.0% (95% CI, 0.3%-2.0%) within 7 days, 1.3% (95% CI, 0.4%-2.6%) within 30 days, and 2.1% (95% CI, 1.4%-2.8%) within 90 days. Among the patients who were treated as inpatients, the risk of subsequent stroke was 0.5% (95% CI, 0.1%-1.1%) within 2 days, 1.2% (95% CI, 0.4%-2.2%) within 7 days, 1.6% (95% CI, 0.6%-3.1%) within 30 days, and 2.8% (95% CI, 2.1%-3.5%) within 90 days. At the EDs, the risk was 1.9% (95% CI, 1.2%-2.7%) within 2 days, 3.4% (95% CI, 2.3%-4.7%) within 7 days, 3.5% (95% CI, 1.5%-6.3%) within 30 days, and 3.5% (95% CI, 2.5%-4.5%) within 90 days. In the cohort of patients from unspecified settings the risk of subsequent stroke was reported under considerable heterogeneity in all intervals as 2.2% (95% CI, 1.3%-3.1%) within 2 days, 3.4% (95% CI, 2.3%-4.5%) within 7 days, 4.2% (95% CI, 2.8%-5.9%) within 30 days, and 6.0% (95% CI, 4.5%-7.8%) within 90 days.
Figure 2. Risk of Subsequent Ischemic Stroke Within 7 Days of the Index Event by Care Setting.
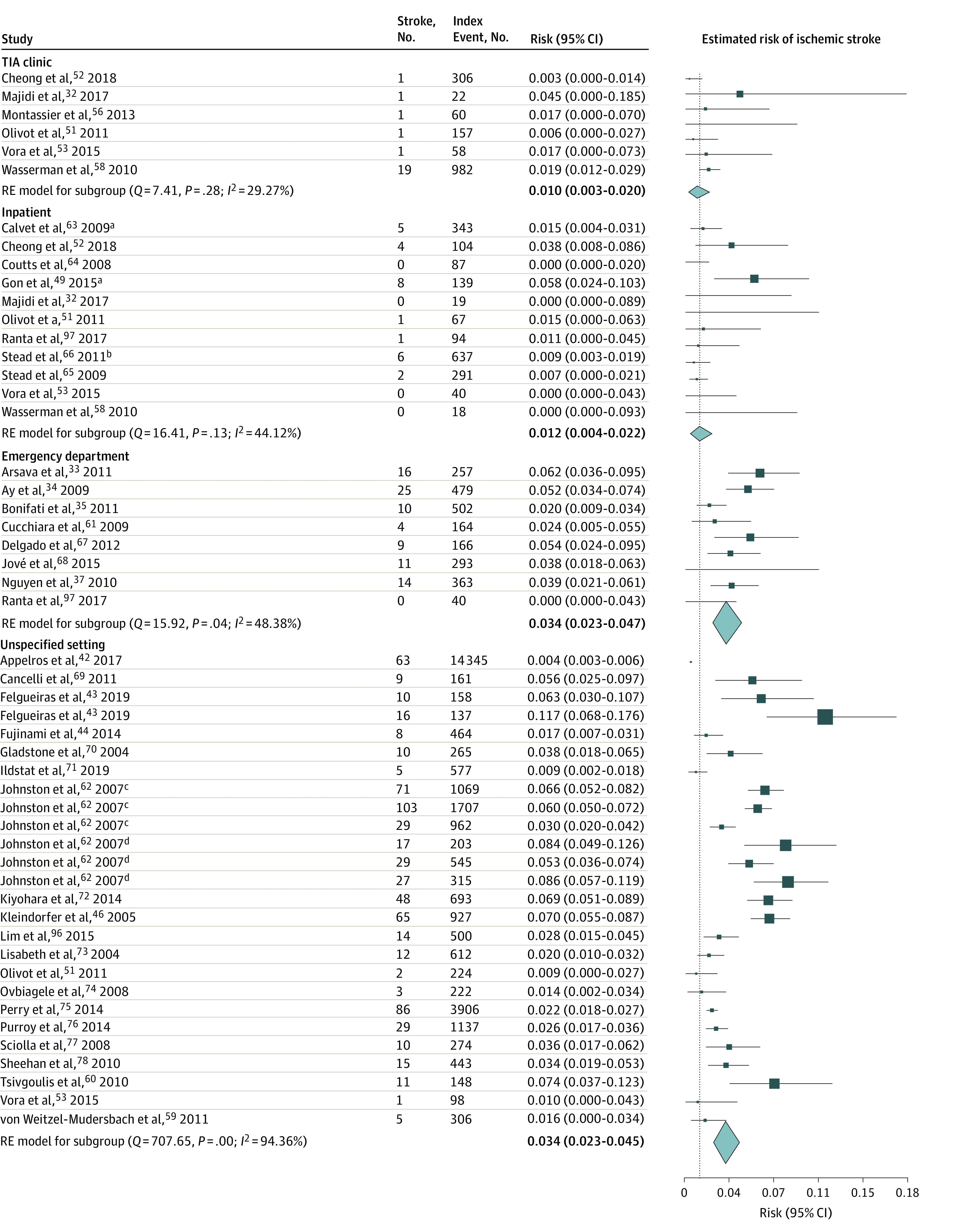
The risk estimate for inpatients was considered as the reference line.
aIndicates the stroke unit.
bIndicates the observation unit.
cKaiser Permanente Medical Care Program.
dOxfordshire Community Stroke Project.
Figure 3. Risk of Subsequent Ischemic Stroke Within 90 Days of the Index Event by Care Setting.
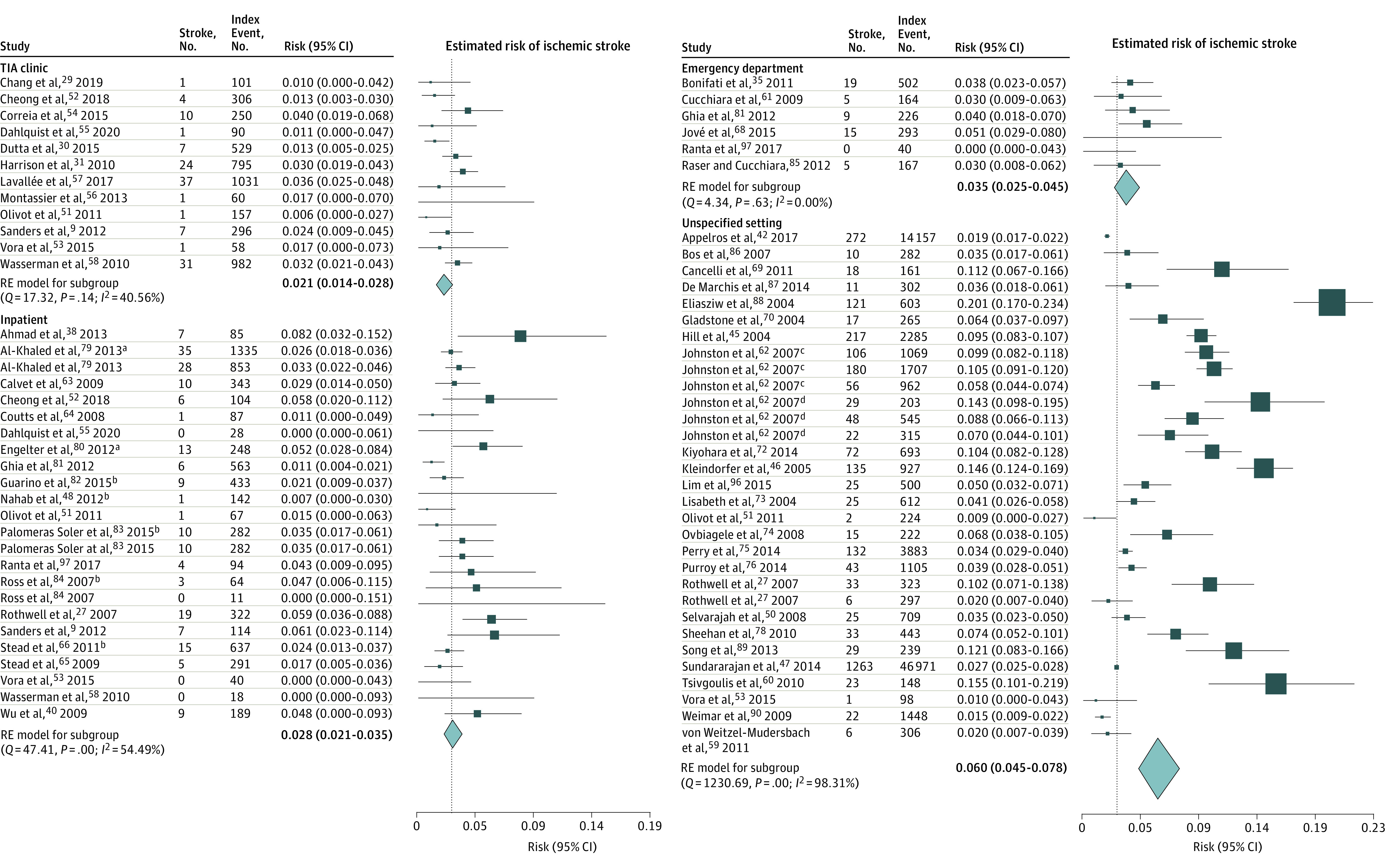
The risk estimate for inpatients was considered as the reference line.
aIndicates the stroke unit.
bIndicates the observation unit.
cKaiser Permanente Medical Care Program.
dOxfordshire Community Stroke Project.
Comparing the subsequent stroke risk estimates in the cohort of patients treated in the TIA clinics vs inpatient settings did not reveal a significant difference in any of the 4 evaluation intervals (eTable 5 in the Supplement). In comparison with patients referred to TIA clinics and hospitalized patients, those who received care in the ED had a significantly higher risk of subsequent stroke at 2 and 7 days (for inpatients) and 2, 7, and 90 days (for patients referred to TIA clinics) (eTable 5 in the Supplement).
In the sensitivity analyses in which only prospective cohorts recruited after 2000 were included (eFigures 4-7 and eTable 5 in the Supplement), we did not find significant differences in risk among patients treated in the TIA clinics and inpatients at 2 days (0.2% [95% CI, 0-1.0%] vs 0.3% [95% CI, 0-0.8%] among inpatients; P = .94, I2 < 0.001) (eFigure 4 in the Supplement), 7 days (0.8% [95% CI, 0.2%-1.8%] vs 0.7% [95% CI, 0.3%-1.3%] among inpatients; P = .81, I2 < 0.001) (eFigure 5 in the Supplement), 30 days (1.3% [95% CI, 0.4%-2.5%] vs 1.3% [0.3%-2.7%] among inpatients; P > .99; I2 < 0.001) (eFigure 6 in the Supplement), and 90 days (2.2% [95% CI, 1.5%-3.0%] vs 2.6% [95% CI, 1.9%-3.3%] among inpatients; P = .46; I2 < 0.001) (eFigure 7 in the Supplement).
Quality Assessment of the Included Cohorts
Publication Bias Assessment
Funnel plots presenting the publication bias of studies within 2, 7, 30, and 90 days under each setting of care (TIA clinic, inpatient, ED, and unspecified setting) are available in eFigure 1 in the Supplement. Neither the Begg-Mazumdar rank correlation, Kendall τ2 statistic, or the Egger bias test could detect publication bias among included cohorts (eTable 6 in the Supplement).
Risk-of-Bias Assessment
eTable 7 in the Supplement summarizes the results of the risk of bias assessment according to ROBINS-E. Among the 63 cohorts with specified settings (ie, TIA clinic, inpatient, and ED) 59 cohorts (90.6%) had low risk of bias,9,13,30,31,32,33,34,35,36,37,38,40,41,42,43,44,45,48,49,50,51,53,54,55,56,57,58,59,60,61,62,63,64,67,68,69,70,71,72,73,74,75,76,77,79,81,82,83,84,85,86,87,88,90,91,92,93,94,95,96 and 4 cohorts (9.4%) had a moderate overall risk of bias.39,46,52,80
Heterogeneity Assessment
We considered 7 different estimators (DL, HS, HE, ML, REML, SJ, and EB) to assess the heterogeneity in the risk of stroke after TIA within 2, 7, 30, and 90 days under each care setting (eTable 4 in the Supplement). Overall, the HE resulted in lower I2, and the SJ estimator resulted in higher I2 values in comparison with other estimators. The heterogeneity among the cohort of patients treated in TIA clinics was minimal, regardless of the estimator or evaluation time.
Discussion
Comparing the risk of subsequent stroke at 2, 7, 30, and 90 days after a TIA or mIS suggested that offering rapid management at TIA clinics is not inferior to inpatient care models. Among the identified cohort, patients who received care at TIA clinics were younger, had a higher rate of carotid stenosis, but a lower ABCD2 score.
Our results also suggest an increased risk of subsequent stroke in patients who were treated and discharged from ED without assigned follow-up care. Previous studies have reported that patients with TIA who were discharged from ED were less likely to receive guideline-concordant care and underwent fewer timely brain and carotid imaging, monitoring for arrhythmia, and administration of preventive medications such as antithrombotic, antihypertensive, and lipid-lowering agents.98,99 The risk of recurrence can be stratified by the clinical scales (such as ABCD2) and risk factor profiles37,60,61,100,101,102,103,104,105,106; however, many practitioners, especially in community hospitals, rely on a one-size-fits-all approach.5,107,108
However, there is growing evidence that suggests TIA clinics can be considered an alternative to hospitalization.1,9,10,12,13,14 Despite the very different structures of risk stratification and patient selection, referral patterns, and diagnostic and therapeutic protocols in these TIA clinic models, the risk of cerebral ischemia in patients treated at a TIA clinic did not exceed those treated in an inpatient setting.32,51,53,55,58,59
Many practice guidelines also endorse outpatient TIA management and recommend hospitalization of high-risk patients. The American Heart/American Stroke Association (AHA/ASA) guideline21 recommends an urgent evaluation and hospitalization of TIA patients if they present within 72 hours and have an ABCD2 score of 3 or greater.62 The Australian National Stroke Foundation includes a set of high-risk indicators beside the ABCD2 scoring into the triaging criteria. This guideline recommends urgent and comprehensive management of TIA by use of a local TIA pathway covering primary care, emergency, and stroke specialist teams within locally available resources.109 The United Kingdom national guideline110 and Canadian Stroke Best Practice Recommendations111 consider the time elapsed from symptoms onset and risk of early recurrence. The United Kingdom guideline recognizes outpatient clinics to provide care for TIA patients. The Canadian guideline also states that while high-risk patients should be seen within 24 hours, providing care for other patients can be slightly delayed based on their risk scoring.
Challenges of TIA Outpatient Care
Differentiation between vascular and nonvascular causes of TIA-like presentations is challenging, especially for nonneurologists.2,112,113,114 Our previous study113 and others112,115,116,117,118 indicate that the percentage of TIA misdiagnosis can be as high as 60% in EDs and primary care offices. Approximately half of the patients with clinical presentations of cerebral ischemia have the final diagnosis of a TIA mimic, and many of them may present with a high ABCD2 score.100,119 A meta-analysis found that 20% of patients with an ABCD2 score of less than 4 had atrial fibrillation or more than 50% had carotid stenosis.100 One step toward the timely and efficient management of TIA is reducing diagnostic errors by providing education and using advanced diagnostic and management-assistive tools by leveraging electronic health records and advanced predictive tools.15,120,121 Moreover, providing timely outpatient care to TIA patients is challenging. It is critically important to understand the potential delays through the clinical care pathways from symptom onset to a specialist.
Value of TIA Clinic
Although several studies have found TIA clinics could substantially reduce the cost of care,9,122 evaluation of the cost-effectiveness of TIA clinics remains limited in the literature. Beyond clinical management, the benefits of TIA clinics could also include a more accurate diagnosis for patients with suspected TIA compared with inpatient and ED settings, fast-track access to specialists, and appropriate patient education and follow-up,123 which could depend on the infrastructure and resources of the existing health service system.
Strengths and Limitations
In this study, we attempted to systematically review the available literature on TIA care models. However, our study has several limitations. We did not consider the details of diagnostic and therapeutic measures in each care setting, variability in the definition of TIA or minor stroke, and the health care system and referral rules in each country in the meta-analyses. Although data were sparse in terms of patients with ABCD2 scores of less than 4, we observed that patients with lower ABCD2 scores were more likely to be referred to outpatient clinics, and hospitals considered different thresholds of cerebral ischemia severity for discharging the patients. Although we were able to calculate the rate of TIA overdiagnosis in TIA clinic and inpatient settings, we did not have enough detailed information to calculate the outcome among patients who had the final diagnosis of TIA or mIS. Nevertheless, the rates of overdiagnosis were similar between inpatient and TIA clinic cohorts. In addition, we observed a discrepancy in the size of patient cohorts under each setting of care, which can explain some of the residual confounding. These assumptions may affect the conclusion regarding the safety of TA clinics compared with other care settings. However, they may propose a practical algorithm for the triage of patients with TIA and safely offer outpatient care for those at a lower risk. We were not able to further clarify and subcategorize patients in unspecified settings. However, by including the cohorts from national or multicentric registries (unspecified settings), we highlighted the variability of outcomes among patients treated in the absence of defined care protocols.
Conclusions
This systematic review and meta-analysis of the outcome of 226 683 patients who experienced a transient ischemic stroke or minor stroke suggest the risk of subsequent stroke among patients who were evaluated in a TIA clinic was not higher than that among those hospitalized. Patients who were treated in EDs without further follow-up had a higher risk of subsequent stroke.
eAppendix. Search Protocols
eTable 1. Mixed-Effect Models Considering Different Possible Moderators
eTable 2. Mixed-Effect Model Considering ABCD2 Scores
eTable 3. Excluded Studies
eTable 4. Heterogeneity and Risk of Stroke Assessment Based on Different Estimators
eTable 5. Comparison of Risk Estimates
eTable 6. Publication Bias Assessment
eTable 7. Risk-of-Bias Assessment Based on ROBINS-E Tool
eFigure 1. Funnel Plots
eFigure 2. Risk of Subsequent Ischemic Stroke Within 2 Days
eFigure 3. Risk of Subsequent Ischemic Stroke Within 30 Days
eFigure 4. Sensitivity Analysis: Risk of Subsequent Ischemic Stroke Within 2 Days
eFigure 5. Sensitivity Analysis: Risk of Subsequent Ischemic Stroke Within 7 Days
eFigure 6. Sensitivity Analysis: Risk of Subsequent Ischemic Stroke Within 30 Days
eFigure 7. Sensitivity Analysis: Risk of Subsequent Ischemic Stroke Within 90 Days
eReferences.
References
- 1.Lavallée PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6(11):953-960. doi: 10.1016/S1474-4422(07)70248-X [DOI] [PubMed] [Google Scholar]
- 2.Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis. 2008;26(6):630-635. doi: 10.1159/000166839 [DOI] [PubMed] [Google Scholar]
- 3.Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke. 2007;38(6):1881-1885. doi: 10.1161/STROKEAHA.106.475525 [DOI] [PubMed] [Google Scholar]
- 4.Shahjouei S, Sadighi A, Chaudhary D, et al. A 5-decade analysis of incidence trends of ischemic stroke after transient ischemic attack: a systematic review and meta-analysis. JAMA Neurol. 2020;17822:1-11. doi: 10.1001/jamaneurol.2020.3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edlow JA, Kim S, Pelletier AJ, Camargo CAJ Jr. National study on emergency department visits for transient ischemic attack, 1992-2001. Acad Emerg Med. 2006;13(6):666-672. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez L, Kim-Tenser MA, Sanossian N, et al. Trends in transient ischemic attack hospitalizations in the United States. J Am Heart Assoc. 2016;5(9):1-9. doi: 10.1161/JAHA.116.004026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khare S. Risk factors of transient ischemic attack: an overview. J Midlife Health. 2016;7(1):2-7. doi: 10.4103/0976-7800.179166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranta A, Barber PA. Transient ischemic attack service provision: a review of available service models. Neurology. 2016;86(10):947-953. doi: 10.1212/WNL.0000000000002339 [DOI] [PubMed] [Google Scholar]
- 9.Sanders LM, Srikanth VK, Jolley DJ, et al. Monash transient ischemic attack triaging treatment: safety of a transient ischemic attack mechanism-based outpatient model of care. Stroke. 2012;43(11):2936-2941. doi: 10.1161/STROKEAHA.112.664060 [DOI] [PubMed] [Google Scholar]
- 10.Jarhult SJ, Howell ML, Barnaure-Nachbar I, et al. Implementation of a rapid, protocol-based TIA management pathway. West J Emerg Med. 2018;19(2):216-223. doi: 10.5811/westjem.2017.9.35341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benavente L, Calleja S, Larrosa D, et al. Long term evolution of patients treated in a TIA unit. Int Arch Med. 2013;6(1):19. doi: 10.1186/1755-7682-6-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webster F, Saposnik G, Kapral MK, Fang J, O’Callaghan C, Hachinski V. Organized outpatient care: stroke prevention clinic referrals are associated with reduced mortality after transient ischemic attack and ischemic stroke. Stroke. 2011;42(11):3176-3182. doi: 10.1161/STROKEAHA.111.621524 [DOI] [PubMed] [Google Scholar]
- 13.Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet. 2005;366(9479):29-36. doi: 10.1016/S0140-6736(05)66702-5 [DOI] [PubMed] [Google Scholar]
- 14.Sadighi A, Abedi V, Stanciu A, et al. Six-month outcome of transient ischemic attack and its mimics. Front Neurol. 2019;10:294. doi: 10.3389/fneur.2019.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranta A, Dovey S, Weatherall M, O’Dea D, Gommans J, Tilyard M. Cluster randomized controlled trial of TIA electronic decision support in primary care. Neurology. 2015;84(15):1545-1551. doi: 10.1212/WNL.0000000000001472 [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Shamseer L, Clarke M, et al. ; PRISMA-P Group . Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 18.Chandler J, Churchill R, Lasserson T, Tovey D, Higgins J. Methodological standards for the conduct of new Cochrane Intervention Reviews. Accessed December 1, 2021. http://www.editorial-unit.cochrane.org/mecir
- 19.Equator Network . Enhancing the quality and transparency of health research. Accessed December 1, 2021. https://www.equator-network.org
- 20.Panuganti KK, Tadi P, Lui F. Transient Ischemic Attack. StatPearls Publishing; 2019. [PubMed] [Google Scholar]
- 21.Easton JD, Saver JL, Albers GW, et al. ; American Heart Association; American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Interdisciplinary Council on Peripheral Vascular Disease . Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardio. Stroke. 2009;40(6):2276-2293. doi: 10.1161/STROKEAHA.108.192218 [DOI] [PubMed] [Google Scholar]
- 22.Fischer U, Baumgartner A, Arnold M, et al. What is a minor stroke? Stroke. 2010;41(4):661-666. doi: 10.1161/STROKEAHA.109.572883 [DOI] [PubMed] [Google Scholar]
- 23.Morgan RL, Thayer KA, Santesso N, et al. ; GRADE Working Group . A risk of bias instrument for non-randomized studies of exposures: a users’ guide to its application in the context of GRADE. Environ Int. 2019;122(122):168-184. doi: 10.1016/j.envint.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan RL, Thayer KA, Santesso N, et al. Evaluation of the risk of bias in non-randomized studies of interventions (ROBINS-I) and the ‘target experiment’ concept in studies of exposures: Rationale and preliminary instrument development. Environ Int. 2018;120:382-387. doi: 10.1016/j.envint.2018.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreiliger G. Statistical Assessment and Adjustment of Publication Bias in the Cochrane Database of Systematic Reviews. Master’s Thesis. University of Zurich; 2019.
- 26.Albers GW, Hart RG, Lutsep HL, Newell DW, Sacco RL. AHA scientific statement: supplement to the guidelines for the management of transient ischemic attacks: a statement from the Ad Hoc Committee on Guidelines for the Management of Transient Ischemic Attacks, Stroke Council, American Heart Association. Stroke. 1999;30(11):2502-2511. doi: 10.1161/01.STR.30.11.2502 [DOI] [PubMed] [Google Scholar]
- 27.Rothwell PM, Giles MF, Chandratheva A, et al. ; Early use of Existing Preventive Strategies for Stroke (EXPRESS) study . Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370(9596):1432-1442. doi: 10.1016/S0140-6736(07)61448-2 [DOI] [PubMed] [Google Scholar]
- 28.Wolfgang V. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 29.Chang BP, Rostanski S, Willey J, et al. Safety and feasibility of a rapid outpatient management strategy for transient ischemic attack and minor stroke: the Rapid Access Vascular Evaluation-Neurology (RAVEN) approach. Ann Emerg Med. 2019;74(4):562-571. doi: 10.1016/j.annemergmed.2019.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutta D, Bowen E, Foy C. Four-year follow-up of transient ischemic attacks, strokes, and mimics: a retrospective transient ischemic attack clinic cohort study. Stroke. 2015;46(5):1227-1232. doi: 10.1161/STROKEAHA.114.008632 [DOI] [PubMed] [Google Scholar]
- 31.Harrison JK, Sloan B, Dawson J, Lees KR, Morrison DS. The ABCD and ABCD2 as predictors of stroke in transient ischemic attack clinic outpatients: a retrospective cohort study over 14 years. QJM. 2010;103(9):679-685. doi: 10.1093/qjmed/hcq108 [DOI] [PubMed] [Google Scholar]
- 32.Majidi S, Leon Guerrero CR, Burger KM, Rothrock JF. Inpatient versus outpatient management of TIA or minor stroke: clinical outcome. J Vasc Interv Neurol. 2017;9(4):49-53. [PMC free article] [PubMed] [Google Scholar]
- 33.Arsava EM, Furie KL, Schwamm LH, Sorensen AG, Ay H. Prediction of early stroke risk in transient symptoms with infarction: relevance to the new tissue-based definition. Stroke. 2011;42(8):2186-2190. doi: 10.1161/STROKEAHA.110.604280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ay H, Arsava EM, Johnston SC, et al. Clinical- and imaging-based prediction of stroke risk after transient ischemic attack: the CIP model. Stroke. 2009;40(1):181-186. doi: 10.1161/STROKEAHA.108.521476 [DOI] [PubMed] [Google Scholar]
- 35.Bonifati DM, Lorenzi A, Ermani M, et al. Carotid stenosis as predictor of stroke after transient ischemic attacks. J Neurol Sci. 2011;303(1-2):85-89. doi: 10.1016/j.jns.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 36.Geil K, González-Concepción JJ, Jiménez-Velázquez IZ, Medina B, Velazco X. Management and outcome of transient ischemic attacks in Ponce, Puerto Rico. Bol Asoc Med P R. 2008;100(3):11-14. [PubMed] [Google Scholar]
- 37.Nguyen H, Kerr D, Kelly A-M. Comparison of prognostic performance of scores to predict risk of stroke in ED patients with transient ischaemic attack. Eur J Emerg Med. 2010;17(6):346-348. doi: 10.1097/MEJ.0b013e328337b1c6 [DOI] [PubMed] [Google Scholar]
- 38.Ahmad O, Penglase RG, Chen MS, Harvey I, Hughes AR, Lueck CJ. A retrospective analysis of inpatient compared to outpatient care for the management of patients with transient ischaemic attack. J Clin Neurosci. 2013;20(7):988-992. doi: 10.1016/j.jocn.2012.09.016 [DOI] [PubMed] [Google Scholar]
- 39.Lichtman JH, Jones SB, Watanabe E, et al. Elderly women have lower rates of stroke, cardiovascular events, and mortality after hospitalization for transient ischemic attack. Stroke. 2009;40(6):2116-2122. doi: 10.1161/STROKEAHA.108.543009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu CM, Manns BJ, Hill MD, Ghali WA, Donaldson C, Buchan AM. Rapid evaluation after high-risk TIA is associated with lower stroke risk. Can J Neurol Sci. 2009;36(4):450-455. doi: 10.1017/S0317167100007770 [DOI] [PubMed] [Google Scholar]
- 41.Vigen T, Thommessen B, Rønning OM. Stroke risk is low after urgently treated transient ischemic attack. J Stroke Cerebrovasc Dis. 2018;27(2):291-295. doi: 10.1016/j.jstrokecerebrovasdis.2017.08.037 [DOI] [PubMed] [Google Scholar]
- 42.Appelros P, Háls Berglund M, Ström JO. Long-term risk of stroke after transient ischemic attack. Cerebrovasc Dis. 2017;43(1-2):25-30. doi: 10.1159/000451061 [DOI] [PubMed] [Google Scholar]
- 43.Felgueiras R, Magalhães R, Silva MR, Silva MC, Correia M. Transient ischemic attack: incidence and early risk of stroke in northern Portugal from 1998–2000 to 2009–2011. Int J Stroke. 2019;0(0):1-11. [DOI] [PubMed] [Google Scholar]
- 44.Fujinami J, Uehara T, Kimura K, et al. Incidence and predictors of ischemic stroke events during hospitalization in patients with transient ischemic attack. Cerebrovasc Dis. 2014;37(5):330-335. doi: 10.1159/000360757 [DOI] [PubMed] [Google Scholar]
- 45.Hill MD, Yiannakoulias N, Jeerakathil T, Tu JV, Svenson LW, Schopflocher DP. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology. 2004;62(11):2015-2020. doi: 10.1212/01.WNL.0000129482.70315.2F [DOI] [PubMed] [Google Scholar]
- 46.Kleindorfer D, Panagos P, Pancioli A, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36(4):720-723. doi: 10.1161/01.STR.0000158917.59233.b7 [DOI] [PubMed] [Google Scholar]
- 47.Sundararajan V, Thrift AG, Phan TG, Choi PM, Clissold B, Srikanth VK. Trends over time in the risk of stroke after an incident transient ischemic attack. Stroke. 2014;45(11):3214-3218. doi: 10.1161/STROKEAHA.114.006575 [DOI] [PubMed] [Google Scholar]
- 48.Nahab F, Leach G, Kingston C, et al. Impact of an emergency department observation unit transient ischemic attack protocol on length of stay and cost. J Stroke Cerebrovasc Dis. 2012;21(8):673-678. doi: 10.1016/j.jstrokecerebrovasdis.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 49.Gon Y, Sakaguchi M, Okazaki S, Mochizuki H, Kitagawa K. Prevalence of positive diffusion-weighted imaging findings and ischemic stroke recurrence in transient ischemic attack. J Stroke Cerebrovasc Dis. 2015;24(5):1000-1007. doi: 10.1016/j.jstrokecerebrovasdis.2014.12.023 [DOI] [PubMed] [Google Scholar]
- 50.Selvarajah JR, Smith CJ, Hulme S, Georgiou RF, Vail A, Tyrrell PJ; NORTHSTAR Collaborators . Prognosis in patients with transient ischaemic attack (TIA) and minor stroke attending TIA services in the North West of England: the NORTHSTAR Study. J Neurol Neurosurg Psychiatry. 2008;79(1):38-43. doi: 10.1136/jnnp.2007.129163 [DOI] [PubMed] [Google Scholar]
- 51.Olivot JM, Wolford C, Castle J, et al. Two aces: transient ischemic attack work-up as outpatient assessment of clinical evaluation and safety. Stroke. 2011;42(7):1839-1843. doi: 10.1161/STROKEAHA.110.608380 [DOI] [PubMed] [Google Scholar]
- 52.Cheong E, Toner P, Dowie G, Jannes J, Kleinig T. Evaluation of a CTA-triage based transient ischemic attack service: a retrospective single center cohort study. J Stroke Cerebrovasc Dis. 2018;27(12):3436-3442. doi: 10.1016/j.jstrokecerebrovasdis.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 53.Vora N, Tung CE, Mlynash M, et al. TIA triage in emergency department using acute MRI (TIA-TEAM): a feasibility and safety study. Int J Stroke. 2015;10(3):343-347. doi: 10.1111/ijs.12390 [DOI] [PubMed] [Google Scholar]
- 54.Correia M, Fonseca AC, Canhão P. Short-term outcome of patients with possible transient ischemic attacks: a prospective study. BMC Neurol. 2015;15(1):78. doi: 10.1186/s12883-015-0333-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dahlquist RT, Young JM, Reyner K, et al. Initiation of the ABCD3-I algorithm for expediated evaluation of transient ischemic attack patients in an emergency department. Am J Emerg Med. 2020;38(4):741-745. doi: 10.1016/j.ajem.2019.06.018 [DOI] [PubMed] [Google Scholar]
- 56.Montassier E, Lim TX, Goffinet N, et al. Results of an outpatient transient ischemic attack evaluation: a 90-day follow-up study. J Emerg Med. 2013;44(5):970-975. doi: 10.1016/j.jemermed.2012.09.145 [DOI] [PubMed] [Google Scholar]
- 57.Lavallée PC, Sissani L, Labreuche J, et al. Clinical significance of isolated atypical transient symptoms in a cohort with transient ischemic attack. Stroke. 2017;48(6):1495-1500. doi: 10.1161/STROKEAHA.117.016743 [DOI] [PubMed] [Google Scholar]
- 58.Wasserman J, Perry J, Dowlatshahi D, et al. Stratified, urgent care for transient ischemic attack results in low stroke rates. Stroke. 2010;41(11):2601-2605. doi: 10.1161/STROKEAHA.110.586842 [DOI] [PubMed] [Google Scholar]
- 59.von Weitzel-Mudersbach P, Johnsen SP, Andersen G. Low risk of vascular events following urgent treatment of transient ischaemic attack: the Aarhus TIA study. Eur J Neurol. 2011;18(11):1285-1290. doi: 10.1111/j.1468-1331.2011.03452.x [DOI] [PubMed] [Google Scholar]
- 60.Tsivgoulis G, Stamboulis E, Sharma VK, et al. Multicenter external validation of the ABCD2 score in triaging TIA patients. Neurology. 2010;74(17):1351-1357. doi: 10.1212/WNL.0b013e3181dad63e [DOI] [PubMed] [Google Scholar]
- 61.Cucchiara BL, Messe SR, Sansing L, et al. D-dimer, magnetic resonance imaging diffusion-weighted imaging, and ABCD2 score for transient ischemic attack risk stratification. J Stroke Cerebrovasc Dis. 2009;18(5):367-373. doi: 10.1016/j.jstrokecerebrovasdis.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 62.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283-292. doi: 10.1016/S0140-6736(07)60150-0 [DOI] [PubMed] [Google Scholar]
- 63.Calvet D, Touzé E, Oppenheim C, Turc G, Meder JF, Mas JL. DWI lesions and TIA etiology improve the prediction of stroke after TIA. Stroke. 2009;40(1):187-192. doi: 10.1161/STROKEAHA.108.515817 [DOI] [PubMed] [Google Scholar]
- 64.Coutts SB, Hill MD, Campos CR, et al. ; VISION study group . Recurrent events in transient ischemic attack and minor stroke: what events are happening and to which patients? Stroke. 2008;39(9):2461-2466. doi: 10.1161/STROKEAHA.107.513234 [DOI] [PubMed] [Google Scholar]
- 65.Stead LG, Bellolio MF, Suravaram S, et al. Evaluation of transient ischemic attack in an emergency department observation unit. Neurocrit Care. 2009;10(2):204-208. doi: 10.1007/s12028-008-9146-z [DOI] [PubMed] [Google Scholar]
- 66.Stead LG, Suravaram S, Bellolio MF, et al. An assessment of the incremental value of the ABCD2 score in the emergency department evaluation of transient ischemic attack. Ann Emerg Med. 2011;57(1):46-51. doi: 10.1016/j.annemergmed.2010.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delgado P, Chacón P, Penalba A, et al. Lipoprotein-associated phospholipase A(2) activity is associated with large-artery atherosclerotic etiology and recurrent stroke in TIA patients. Cerebrovasc Dis. 2012;33(2):150-158. doi: 10.1159/000334193 [DOI] [PubMed] [Google Scholar]
- 68.Jové M, Mauri-Capdevila G, Suárez I, et al. Metabolomics predicts stroke recurrence after transient ischemic attack. Neurology. 2015;84(1):36-45. doi: 10.1212/WNL.0000000000001093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cancelli I, Janes F, Gigli GL, et al. Incidence of transient ischemic attack and early stroke risk: validation of the ABCD2 score in an Italian population-based study. Stroke. 2011;42(10):2751-2757. doi: 10.1161/STROKEAHA.110.612705 [DOI] [PubMed] [Google Scholar]
- 70.Gladstone DJ, Kapral MK, Fang J, Laupacis A, Tu JV. Management and outcomes of transient ischemic attacks in Ontario. CMAJ. 2004;170(7):1099-1104. doi: 10.1503/cmaj.1031349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ildstad F, Ellekjær H, Wethal T, et al. Stroke risk after transient ischemic attack in a Norwegian prospective cohort. BMC Neurol. 2019;19(1):2-9. doi: 10.1186/s12883-018-1225-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kiyohara T, Kamouchi M, Kumai Y, et al. ; Fukuoka Stroke Registry Investigators . ABCD3 and ABCD3-I scores are superior to ABCD2 score in the prediction of short- and long-term risks of stroke after transient ischemic attack. Stroke. 2014;45(2):418-425. doi: 10.1161/STROKEAHA.113.003077 [DOI] [PubMed] [Google Scholar]
- 73.Lisabeth LD, Ireland JK, Risser JMH, et al. Stroke risk after transient ischemic attack in a population-based setting. Stroke. 2004;35(8):1842-1846. doi: 10.1161/01.STR.0000134416.89389.9d [DOI] [PubMed] [Google Scholar]
- 74.Ovbiagele B, Cruz-Flores S, Lynn MJ, Chimowitz MI; Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Study Group . Early stroke risk after transient ischemic attack among individuals with symptomatic intracranial artery stenosis. Arch Neurol. 2008;65(6):733-737. doi: 10.1001/archneur.65.6.733 [DOI] [PubMed] [Google Scholar]
- 75.Perry JJ, Sharma M, Sivilotti MLA, et al. A prospective cohort study of patients with transient ischemic attack to identify high-risk clinical characteristics. Stroke. 2014;45(1):92-100. doi: 10.1161/STROKEAHA.113.003085 [DOI] [PubMed] [Google Scholar]
- 76.Purroy F, Jiménez Caballero PE, Gorospe A, et al. How predictors and patterns of stroke recurrence after a TIA differ during the first year of follow-up. J Neurol. 2014;261(8):1614-1621. doi: 10.1007/s00415-014-7390-z [DOI] [PubMed] [Google Scholar]
- 77.Sciolla R, Melis F; SINPAC Group . Rapid identification of high-risk transient ischemic attacks: prospective validation of the ABCD score. Stroke. 2008;39(2):297-302. doi: 10.1161/STROKEAHA.107.496612 [DOI] [PubMed] [Google Scholar]
- 78.Sheehan OC, Kyne L, Kelly LA, et al. Population-based study of ABCD2 score, carotid stenosis, and atrial fibrillation for early stroke prediction after transient ischemic attack: the North Dublin TIA study. Stroke. 2010;41(5):844-850. doi: 10.1161/STROKEAHA.109.571844 [DOI] [PubMed] [Google Scholar]
- 79.Al-Khaled M, Matthis C, Eggers J. The prognostic impact of the stroke unit care versus conventional care in treatment of patients with transient ischemic attack: a prospective population-based German study. J Vasc Interv Neurol. 2013;5(2):22-26. [PMC free article] [PubMed] [Google Scholar]
- 80.Engelter ST, Amort M, Jax F, et al. Optimizing the risk estimation after a transient ischaemic attack - the ABCDE⊕ score. Eur J Neurol. 2012;19(1):55-61. doi: 10.1111/j.1468-1331.2011.03428.x [DOI] [PubMed] [Google Scholar]
- 81.Ghia D, Thomas P, Cordato D, et al. Low positive predictive value of the ABCD2 score in emergency department transient ischaemic attack diagnoses: the South Western Sydney transient ischaemic attack study. Intern Med J. 2012;42(8):913-918. doi: 10.1111/j.1445-5994.2011.02564.x [DOI] [PubMed] [Google Scholar]
- 82.Guarino M, Rondelli F, Favaretto E, et al. Short-and long-term stroke risk after urgent management of transient ischaemic attack: the Bologna TIA clinical pathway. Eur Neurol. 2015;74(1-2):1-7. doi: 10.1159/000430810 [DOI] [PubMed] [Google Scholar]
- 83.Palomeras Soler E, Fossas Felip P, Cano Orgaz AT, Sanz Cartagena P, Casado Ruiz V, Muriana Batista D. Rapid assessment of transient ischaemic attack in a hospital with no on-call neurologist. Neurologia. 2015;30(6):325-330. doi: 10.1016/j.nrl.2013.12.021 [DOI] [PubMed] [Google Scholar]
- 84.Ross MA, Compton S, Medado P, Fitzgerald M, Kilanowski P, O’Neil BJ. An emergency department diagnostic protocol for patients with transient ischemic attack: a randomized controlled trial. Ann Emerg Med. 2007;50(2):109-119. doi: 10.1016/j.annemergmed.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 85.Raser JM, Cucchiara BL. Modifications of the ABCD2 score do not improve the risk stratification of transient ischemic attack patients. J Stroke Cerebrovasc Dis. 2012;21(6):467-470. doi: 10.1016/j.jstrokecerebrovasdis.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 86.Bos MJ, van Rijn MJE, Witteman JCM, Hofman A, Koudstaal PJ, Breteler MM. Incidence and prognosis of transient neurological attacks. JAMA. 2007;298(24):2877-2885. doi: 10.1001/jama.298.24.2877 [DOI] [PubMed] [Google Scholar]
- 87.De Marchis GM, Weck A, Audebert H, et al. Copeptin for the prediction of recurrent cerebrovascular events after transient ischemic attack: results from the CoRisk study. Stroke. 2014;45(10):2918-2923. doi: 10.1161/STROKEAHA.114.005584 [DOI] [PubMed] [Google Scholar]
- 88.Eliasziw M, Kennedy J, Hill MD, Buchan AM, Barnett HJM; North American Symptomatic Carotid Endarterectomy Trial Group . Early risk of stroke after a transient ischemic attack in patients with internal carotid artery disease. CMAJ. 2004;170(7):1105-1109. doi: 10.1503/cmaj.1030460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song B, Fang H, Zhao L, et al. Validation of the ABCD3-I score to predict stroke risk after transient ischemic attack. Stroke. 2013;44(5):1244-1248. doi: 10.1161/STROKEAHA.113.000969 [DOI] [PubMed] [Google Scholar]
- 90.Weimar C, Benemann J, Huber R, et al. ; German Stroke Study Collaboration . Long-term mortality and risk of stroke after transient ischemic attack: a hospital-based cohort study. J Neurol. 2009;256(4):639-644. doi: 10.1007/s00415-009-0150-9 [DOI] [PubMed] [Google Scholar]
- 91.Al-Khaled M, Eggers J. Early hospitalization of patients with TIA: a prospective, population-based study. J Stroke Cerebrovasc Dis. 2014;23(1):99-105. [DOI] [PubMed] [Google Scholar]
- 92.Ohara T, Uehara T, Sato S, et al. ; PROMISE-TIA Study Investigators . Small vessel occlusion is a high-risk etiology for early recurrent stroke after transient ischemic attack. Int J Stroke. 2019;14(9):871-877. doi: 10.1177/1747493019840931 [DOI] [PubMed] [Google Scholar]
- 93.Ottaviani M, Vanni S, Moroni F, Peiman N, Boddi M, Grifoni S. Urgent carotid duplex and head computed tomography versus ABCD2 score for risk stratification of patients with transient ischemic attack. Eur J Emerg Med. 2016;23(1):19-23. doi: 10.1097/MEJ.0000000000000165 [DOI] [PubMed] [Google Scholar]
- 94.Ricci S, Celani MG, La Rosa F, et al. A community-based study of incidence, risk factors and outcome of transient ischaemic attacks in Umbria, Italy: the SEPIVAC study. J Neurol. 1991;238(2):87-90. doi: 10.1007/BF00315687 [DOI] [PubMed] [Google Scholar]
- 95.Raposo N, Albucher JF, Rousseau V, Acket B, Chollet F, Olivot JM. ED referral dramatically reduces delays of initial evaluation in a French TIA clinic. Front Neurol. 2018;9:914. doi: 10.3389/fneur.2018.00914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lim J-S, Hong K-S, Kim G-M, et al. Cerebral microbleeds and early recurrent stroke after transient ischemic attack: results from the Korean Transient Ischemic Attack Expression Registry. JAMA Neurol. 2015;72(3):301-308. doi: 10.1001/jamaneurol.2014.3958 [DOI] [PubMed] [Google Scholar]
- 97.Ranta A, Weatherall M, Gommans J, Tilyard M, Odea D, Dovey S. Appropriateness of general practitioner imaging requests for transient ischaemic attack patients: secondary analysis of a cluster randomised controlled trial. J Prim Health Care. 2017;9(2):131-135. doi: 10.1071/HC17005 [DOI] [PubMed] [Google Scholar]
- 98.Hosier GW, Phillips SJ, Doucette SP, Magee KD, Gubitz GJ. Transient ischemic attack: management in the emergency department and impact of an outpatient neurovascular clinic. CJEM. 2016;18(5):331-339. doi: 10.1017/cem.2016.3 [DOI] [PubMed] [Google Scholar]
- 99.Kapral MK, Hall R, Fang J, et al. Association between hospitalization and care after transient ischemic attack or minor stroke. Neurology. 2016;86(17):1582-1589. doi: 10.1212/WNL.0000000000002614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wardlaw JM, Brazzelli M, Chappell FM, et al. ABCD2 score and secondary stroke prevention: meta-analysis and effect per 1,000 patients triaged. Neurology. 2015;85(4):373-380. doi: 10.1212/WNL.0000000000001780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Merwick A, Albers GW, Amarenco P, et al. Addition of brain and carotid imaging to the ABCD2 score to identify patients at early risk of stroke after transient ischaemic attack: a multicentre observational study. Lancet Neurol. 2010;9(11):1060-1069. doi: 10.1016/S1474-4422(10)70240-4 [DOI] [PubMed] [Google Scholar]
- 102.Ong MEH, Chan YH, Lin WP, Chung WL. Validating the ABCD(2) score for predicting stroke risk after transient ischemic attack in the ED. Am J Emerg Med. 2010;28(1):44-48. doi: 10.1016/j.ajem.2008.09.027 [DOI] [PubMed] [Google Scholar]
- 103.Holzer K, Feurer R, Sadikovic S, et al. Prognostic value of the ABCD2 score beyond short-term follow-up after transient ischemic attack (TIA)—a cohort study. BMC Neurol. 2010;10:50. doi: 10.1186/1471-2377-10-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Asimos AW, Johnson AM, Rosamond WD, et al. A multicenter evaluation of the ABCD2 score’s accuracy for predicting early ischemic stroke in admitted patients with transient ischemic attack. Ann Emerg Med. 2010;55(2):201-210.e5. doi: 10.1016/j.annemergmed.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 105.Acosta I, Bloch S, Morales M, Bornstein NM, Savitz SI, Hallevi H. Predicting the need for hospital admission of TIA patients. J Neurol Sci. 2014;336(1-2):83-86. doi: 10.1016/j.jns.2013.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koton S, Rothwell PM, Study OV. Performance of the ABCD and ABCD2 scores in TIA patients with carotid stenosis and atrial fibrillation. Cerebrovasc Dis. 2007;24(2-3):231-235. doi: 10.1159/000104483 [DOI] [PubMed] [Google Scholar]
- 107.Ford AL, Williams JA, Spencer M, et al. Reducing door-to-needle times using Toyota’s lean manufacturing principles and value stream analysis. Stroke. 2012;43(12):3395-3398. doi: 10.1161/STROKEAHA.112.670687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Coben JH, Owens PL, Steiner CA, Crocco TJ. Hospital and demographic influences on the disposition of transient ischemic attack. Acad Emerg Med. 2008;15(2):171-176. doi: 10.1111/j.1553-2712.2008.00041.x [DOI] [PubMed] [Google Scholar]
- 109.Australian Stroke Foundation . Clinical guidelines for stroke management—chapter 2: early assessment and diagnosis. Accessed December 1, 2021. https://app.magicapp.org/#/guideline/3993/section/45730
- 110.Frank A, Bowen A, Young GR, James MA. The latest national clinical guideline for stroke. Clin Med (Lond). 2017;17(5):478. doi: 10.7861/clinmedicine.17-5-478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wein T, Lindsay MP, Cote R, et al. Canadian stroke best practice recommendations: secondary prevention of stroke, sixth edition practice guidelines, update 2017. Int J Stroke. 2018;13(4):420-443. doi: 10.1177/1747493017743062 [DOI] [PubMed] [Google Scholar]
- 112.Ferro JM, Falcão I, Rodrigues G, et al. Diagnosis of transient ischemic attack by the nonneurologist: a validation study. Stroke. 1996;27(12):2225-2229. doi: 10.1161/01.STR.27.12.2225 [DOI] [PubMed] [Google Scholar]
- 113.Sadighi A, Stanciu A, Banciu M, et al. Rate and associated factors of transient ischemic attack misdiagnosis. eNeurologicalSci. 2019;15:100193. doi: 10.1016/j.ensci.2019.100193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nadarajan V, Perry RJ, Johnson J, Werring DJ. Transient ischaemic attacks: mimics and chameleons. Pract Neurol. 2014;14(1):23-31. doi: 10.1136/practneurol-2013-000782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Castle J, Mlynash M, Lee K, et al. Agreement regarding diagnosis of transient ischemic attack fairly low among stroke-trained neurologists. Stroke. 2010;41(7):1367-1370. doi: 10.1161/STROKEAHA.109.577650 [DOI] [PubMed] [Google Scholar]
- 116.Tomasello F, Mariani F, Fieschi C, et al. Assessment of inter-observer differences in the Italian multicenter study on reversible cerebral ischemia. Stroke. 1982;13(1):32-35. doi: 10.1161/01.STR.13.1.32 [DOI] [PubMed] [Google Scholar]
- 117.Kraaijeveld CL, van Gijn J, Schouten HJ, Staal A, Staal A. Interobserver agreement for the diagnosis of transient ischemic attacks. Stroke. 1984;15(4):723-725. doi: 10.1161/01.STR.15.4.723 [DOI] [PubMed] [Google Scholar]
- 118.Koudstaal PJ, Gerritsma JG, van Gijn J. Clinical disagreement on the diagnosis of transient ischemic attack: is the patient or the doctor to blame? Stroke. 1989;20(2):300-301. doi: 10.1161/01.STR.20.2.300 [DOI] [PubMed] [Google Scholar]
- 119.Dutta D. Diagnosis of TIA (DOT) score—design and validation of a new clinical diagnostic tool for transient ischaemic attack. BMC Neurol. 2016;16(1):20. doi: 10.1186/s12883-016-0535-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Abedi V, Goyal N, Tsivgoulis G, et al. Novel screening tool for stroke using artificial neural network. Stroke. 2017;48(6):1678-1681. doi: 10.1161/STROKEAHA.117.017033 [DOI] [PubMed] [Google Scholar]
- 121.Stanciu A, Banciu M, Sadighi A, et al. A predictive analytics model for differentiating between transient ischemic attacks (TIA) and its mimics. BMC Med Inform Decis Mak. 2020;20(1):112. doi: 10.1186/s12911-020-01154-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Paul NLM, Koton S, Simoni M, Geraghty OC, Luengo-Fernandez R, Rothwell PM. Feasibility, safety and cost of outpatient management of acute minor ischaemic stroke: a population-based study. J Neurol Neurosurg Psychiatry. 2013;84(3):356-361. doi: 10.1136/jnnp-2012-303585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lavallée P, Amarenco P. TIA clinic: a major advance in management of transient ischemic attacks. Front Neurol Neurosci. 2014;33:30-40. doi: 10.1159/000351890 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Search Protocols
eTable 1. Mixed-Effect Models Considering Different Possible Moderators
eTable 2. Mixed-Effect Model Considering ABCD2 Scores
eTable 3. Excluded Studies
eTable 4. Heterogeneity and Risk of Stroke Assessment Based on Different Estimators
eTable 5. Comparison of Risk Estimates
eTable 6. Publication Bias Assessment
eTable 7. Risk-of-Bias Assessment Based on ROBINS-E Tool
eFigure 1. Funnel Plots
eFigure 2. Risk of Subsequent Ischemic Stroke Within 2 Days
eFigure 3. Risk of Subsequent Ischemic Stroke Within 30 Days
eFigure 4. Sensitivity Analysis: Risk of Subsequent Ischemic Stroke Within 2 Days
eFigure 5. Sensitivity Analysis: Risk of Subsequent Ischemic Stroke Within 7 Days
eFigure 6. Sensitivity Analysis: Risk of Subsequent Ischemic Stroke Within 30 Days
eFigure 7. Sensitivity Analysis: Risk of Subsequent Ischemic Stroke Within 90 Days
eReferences.