Abstract
Regionalization has become a buzzword in US health care policy. Regionalization, however, has varied meanings, and definitions have lacked contextual information important to understanding its role in improving care. This concept review is a comprehensive primer and summation of 8 common core components of the national models of regionalization informed by text‐based analysis of the writing of involved organizations (professional, regulatory, and research) guided by semistructured interviews with organizational leaders. Further, this generalized model of regionalized care is applied to sepsis care, a novel discussion, drawing on existing small‐scale applications. This discussion highlights the fit of regionalization principles to the sepsis care model and the actualized and perceived potential benefits. The principal aim of this concept review is to outline regionalization in the United States and provide a roadmap and novel discussion of regionalized care integration for sepsis care.
Keywords: delivery of health care, emergency service, hospital planning, integrated, intensive care units, regional medical programs, sepsis
1. INTRODUCTION
Regionalization is the concept of organizing patients and healthcare practitioners into a system “to deliver the right resources to the right patient in the right place at the right time.” 1 The objective of this concept review was 3‐fold: (1) to provide a comprehensive summation of the available literature and established models of regionalized acute care, (2) to identify the common core components of regionalized systems, and (3) to apply lessons about regional networks to sepsis care.
2. DEFINING REGIONALIZATION
Regionalization is a geographic process of formal or informal health care policy that accentuates one or more of the following: the distribution of physicians, the distribution of equipment and facilities, and the control of patient movement within the system. 2
Regionalization is differentiated from "centralization." Centralization is the consolidation of specialized services to higher‐volume centers of care. In regionalization, systems are created to optimize the clinical capabilities of healthcare practitioners along the entire continuum of care for time‐sensitive conditions (Figure 1). 3 Regionalization seeks to capitalize on the volume–outcome relationship: the observation that outcomes are better for specialized care delivered in centers that treat more patients. 4 , 5 A volume–outcome relationship has been demonstrated in trauma, 6 burn 7 stroke, 8 ST‐elevation myocardial infarction (STEMI), 9 and neonatal ICU/obstetrics (NICU/OB) 10 , 11 care.
FIGURE 1.
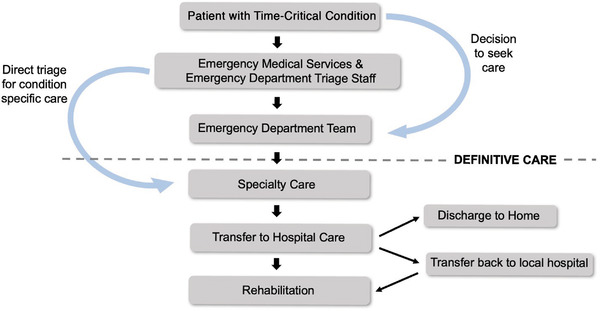
Flow diagram illustrating the standard sequencing of patient care for time‐critical conditions
Regionalization within the United States has been limited historically because of a competitive, fee‐for‐service market‐based system. Regionalization efforts instead have favored the formation of coalitions of private–public partnerships that share resources, coordinate within the existing hierarchical network, and standardize care without direct restructuring of health care organizations. 3 , 12
3. ESTABLISHED SYSTEMS
The following provides brief summaries of the existing national models of regionalized care. Table 1 details each system more extensively.
Trauma: Trauma care has been the exemplar model of regionalization efforts, stemming from military experience with a tiered model of care 13 and the idea of the “golden hour.” 14 Initial work began in the 1950–1970s with the Committee on Fractures and other Traumas that eventually amalgamated into the American College of Surgeons Committee on Trauma (ACS‐COT), 15 which published guidelines and initiated a national trauma verification program with over 1000 state‐designated and 400 ACS‐verified trauma centers. Over 70% of Americans now live within 45 minutes of a trauma center. 3
Burn: Regionalized burn care has aligned closely with the trauma model, with the initial burn care guidelines being originally published in the Resources for Optimal Care of the Injured Patient. 16 In 1995, the American Burn Association (ABA) and the American College of Surgeons developed a verification process specific to regionalized burn care. Approximately 60% of acute burn patients are hospitalized at 1 of 123 self‐designated burn centers–of which 65 are verified by the ABA and ACS. 16 , 17
Neonatal Intensive Care/Obstetrics: Perinatal and neonatal ICU care has a history of regionalization dating back to the 1960s with the advent of neonatology as a subspecialty in pediatrics. 18 , 19 In 1976, the American Academy of Pediatrics and the American College of Obstetrics and Gynecology collaborated with the March of Dimes to publish guideline recommendations for perinatal care in “toward improving the outcomes of pregnancy,” which has been subsequently revised. 20 , 21 These guidelines have been implemented in state perinatal health networks with ongoing federal and state funding. 18
Stroke: In the mid‐1990s, acute stroke therapy became time‐sensitive with the advent of systemic thrombolysis 22 and now mechanical thrombectomy. 23 The Centers for Disease Control and Prevention developed initiatives that coincided with a series of developing guidelines by the National Stroke Association Stroke Center Network Program and Brain Attack Coalition that were further adapted by the Joint Commission into a program of stroke center verification with over 153 Comprehensive Stroke Centers, 1100 Primary Stroke Centers, and 45 Acute Stroke Ready Hospitals. 8 , 24
ST‐Elevation Myocardial Infarction: STEMI regionalized care started in 2004 when the American College of Cardiology/American Heart Association (ACA/AHA) established “door‐to‐balloon” time targets in STEMI. 25 In 2007, the Mission: Lifeline program was started to provide recognition and accelerate regionalization efforts. 26 The Joint Commission as of 2019 has offered 2 voluntary certifications for STEMI care. 24
TABLE 1.
Summation of national models of regionalization
| Problem area addressed | Leadership/criteria | Generalized structure | Differentiating factors | Outcomes/database | |
|---|---|---|---|---|---|
| Trauma | 24 hours in‐house surgical coverage: specialty coverage–such as orthopedic surgery, neurosurgery, anesthesiology, emergency medicine, radiology, internal medicine, plastic surgery, oral and maxillofacial, pediatric and critical care, full lab and imaging diagnostic services, and operating room/postanesthesia care unit/ICU staff. |
American College of Surgeons–Committee on Trauma (ACS‐COT). Resources for Optimal Care of the Injured Patient, 2014. 27 Designation–State agency, generally follow ACS‐COT standards, mandated through legislative or regulatory authority for levels of trauma care, requirements of participation, and associated penalties. Verification–voluntary, evaluation process done by ACS for level 1 and 2 centers according to adult/pediatric status, lasts for 3‐year period. |
Tiered (Level I–V) according to capacity of care as by designation/verified criteria. EMS triage protocols to direct patient to most appropriate center of care–level Is for high‐acuity surgical care, through level IV and V for smaller, local facilities providing rapid evaluation, essential stabilization and transfer up the chain. |
High‐level trauma care is not profitable, 28 , 29 leading to minimal competition for providing trauma services outside of affluent urban areas. ACS verification process is active surgeon driven, self‐imposed professional mandates leading to greater acceptance and level of participation. Proposed spinoff benefits for other time‐sensitive, surgical emergencies: ruptured aortic aneurysms, etc. 30 |
From national data, risk‐adjusted mortality from trauma was 7.6% in designated Level 1 trauma centers versus 9.5% in undesignated centers. 31 Another meta‐analysis cited a 15% reduction in mortality after trauma system implementation. 32 |
| Burn | Specialized burn care is a low‐volume, high‐resource, high‐expertise condition to treat. Burn teams, led by burn surgeons, involve multidisciplinary efforts that could not exist without regionalization of care and the consolidation of patients in a geographic catchment area to maximize volume–outcome relationship. |
American Burn Association. American Burn Association–Burn Center Verification Review Program–Verification Criteria 2018. 34 Voluntary, 3‐year verification process principally evaluates survival, objective reviews of complications, emotional health and reintegration in society metrics. Additionally, the process verifies minimum guidelines for facility resources, volume, staffing, experience, continuing education, dedication to prevention, teaching and research. |
Self‐designated and American Burn Association verified burn centers:
|
Nurse staffing ratios and need for rehabilitation services unique to burn care–low turnover and high degree of multispecialty cooperation. Burn care given its resource‐intensive nature has low profitability to a hospital 35 , 36 , but given a significant catchment area can remain independently viable, even turn a profit if surrounding institutions do not "skim" insured patients. 37 Spin off benefits for highly morbid conditions such as toxic epidermal necrolysis, necrotizing fasciitis and frostbite. 38 , 39 , 40 , 41 |
Regionalization of burn care within the New York City metropolitan area was associated with care for patients in designated facilities in over 75% of the cases and a reduction in mortality by almost 50%. 42 National Burn Data Standard and National Burn Repository. 34 , 43 , 44 , 45 |
| Stroke |
Stroke became an acute care condition with intravenous tPA, thrombectomy, and neurosurgical intervention results demonstrating time‐sensitive benefits. System of care: IV tPA, computed tomography scanner, and stroke center–therapeutic, consultation, and interventional capabilities. Bypass to Primary Stroke Center/Comprehensive Stroke Center if < 15–20 minutes transport time, IV tPA at regional hospital if > 15–20 minutes transport time. |
The Joint Commission (TJC)–Stroke Program with participation of the American Heart Association (AHA) and American Stroke Association. Specifications Manual for Joint Commission National Quality Measures. 24 Voluntary verification process through a Disease‐Specific certification program. |
Four‐tiered certification system:
|
Stroke patients are primarily Medicare covered, 46 every hospital desires to treat stroke patients. 3 Telestroke is well‐received and highly effective given that stroke consult is principally cognitive based and easy to conduct via phone/imaging review. Although stroke programs are well developed and yielding successful outcomes, the EMS bypass and triage protocols are not as robust nor effective as in the trauma system–lack of state mandates. |
Organized care data from Canada and Taiwan has been shown to reduce the following risks associated with stroke: death by 14%, death or institutionalized care by 18%, and death or dependency by 18% 8 , 47 , 48 American Heart Association ‐ Get Within the Guidelines Stroke (GWTG‐S) 49 , 50 , 51 , 52 , 53 |
| STEMI PCI/Fibrinolysis |
Ongoing evidence has demonstrated a decreased chance of survival if either fibrinolysis or PCI is delayed >30 minutes 54 Push to get patients to PCI/thrombolysis capable centers as expediently as possible (door to balloon/needle times of <90 minutes and <120 minutes for inter‐hospital transfer patients). |
AHA and American College of Cardiology (ACC)–Mission Lifeline: Recognition Program. TJC–STEMI/Cardiac Program (Effective July 1, 2019). American Heart Association Mission: Lifeline ‐ Hospital Recognition Criteria v3. 26 Centers that meet certain time and guideline goals receive recognition on Gold, Silver, and Bronze levels respective to outcomes and compliance level. TJC program will feature 2 voluntary certifications for STEMI care. |
Primary PCI centers meeting ACC/AHA guidelines:
|
Cardiac PCI care is profitable and most general medical service hospitals rely upon cardiovascular care for financial viability.
Higher degree of EMS involvement with ambulance ECG and triage education for suspected STEMI. Certificate of Need laws play a significant role in establishing cardiac care facilities, minimizing procedure volume dilution. |
Multiregional study in the United States indicated all process measures demonstrating coordination between EMS and hospitals had improved–first medical contact to ECG device use time of ≤90 minutes (hospital within ≤10 minutes), first medical contact to device time to catheterization laboratory activation of ≤20 minutes, and emergency department dwell time of ≤20 minutes. These improvements in treatment times corresponded with a significant reduction in mortality (in‐hospital death 4.4%–2.3%; P = 0.001) that was not apparent in hospitals not participating in the project during the same time period. 54 American Heart Association–Coronary Artery Disease (GWTG‐CAD) databases, 49 , 50 , 51 , 52 , 53 the Cardiac Arrest Registry to Enhance Survival (CARES). 55 |
| Neonatal ICU/obstetrics (OB) | Limited numbers of pediatric subspecialists, pediatric ICU/intensivists, neonatal ICU/neonatologists, OB/Gyn capabilities coupled with pediatric focused trauma capabilities. Tiered structure of services needed to maximize ability to treat high‐risk and complicated perinatal and obstetric patients. |
American Academy of Pediatrics. Levels of Neonatal Care–American Academy of Pediatrics Policy Statement ( 21 ). 21 . Recommendations for levels of perinatal care established in consortium with the March of Dimes through “Toward Improving the Outcome of Pregnancy.” These recommendations have been adopted by state perinatal programs and networks. Federal grant money available for system. Varies state to state regarding level of integration and verification. |
Recommended levels of neonatal care: Level 1–Well newborn nursery: neonatal resuscitation, stabilize and provide care for infants born 35–37 weeks. Level 2–Special Care Nursery: Provide care for ≥32 weeks with neonatal specialists. Level 3–Neonatal ICU: Provide care <32 weeks with pediatric subspecialists. Level 4–Regional Neonatal ICU: all the above in addition to pediatric surgical subspecialists |
Long‐standing federal and state grant support. 18 Guidelines interpreted broadly and verification generally limited. No national verifying agency. Varies state to state. Neonatal ICU care has seen increasing level of profitability, with more hospital facilities interested in providing intensivist care. 56 , 57 , 58 Obstetric labor and delivery in comparison has progressively decreased in profitability, with small hospitals and facilities facing ceasing their services nationwide. 59 |
One meta‐analysis demonstrated significantly worse outcomes for very low‐birth rate and very preterm infants born at level 1 and 2 centers compared to higher levels. 60 Several studies that have looked at it from a state‐level perspective have found significant benefit for premature infants 10 , 11 and have additionally identified that deregionalization trends have negatively affected care outcomes. 61 , 62 , 63 , 64 . Vermont‐Oxford database for neonatal ICU/OB care. 65 |
Abbreviations: ABA, American Burn Association; CT, computed tomography; EMS, emergency medical services; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; tPA, tissue plasminogen activator.
4. STRUCTURAL ORGANIZATION OF REGIONALIZED SYSTEMS
The key principles behind regionalized systems are (1) standard selection, (2) infrastructure design and development, (3) data collection and surveillance, and (4) performance verification. Regionalization demands standardization through cooperation rather than competition.
Regionalization efforts vary significantly state to state and region to region, based on the level of organization, funding, professional leadership, and political prioritization. Regionalization efforts have followed a generalized, predictable pattern (Figure 2).
FIGURE 2.
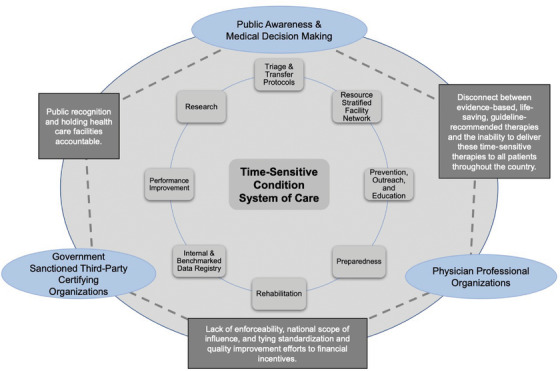
Pictorial representation of the structural generalization of regionalized systems of acute care. The interior of the figure is composed of the identified 8 core components of regionalized systems that function concurrently. The circles in the outer triangle are the 3 constituents and description of the role and structure they provide relative to one another (the text on the lines between the circles) in systems of care. The figure demonstrates the multipart and party efforts that sustain the national models of regionalized care
Professional organizations publish clinical guidelines of regionalized hierarchical systems. 21 , 24 , 26 , 27 , 34 These guidelines are adopted by governments, professional organizations, or third‐party stakeholders for verification in participating facilities. The funding for these efforts is largely sourced from fees paid by participating health care facilities for verification. Health care facilities participate for public recognition as evidence of verified efforts to improve clinical outcomes. 3 In some states, government agencies base accreditation on participation with varying degrees of legal, financial, and resource support. 3 , 21 , 24 , 26 , 27 , 34 Common to each of the systems, the guidelines and framework can be generalized to 8 core components that are discussed next.
5. COMPONENTS OF REGIONALIZED SYSTEMS
5.1. Triage and transfer
Delivering timely specialized acute care requires practical and evidence‐based prehospital triage and interhospital transfer protocols. The time from symptom onset to arrival at an emergency department is often a source of delay, and this time can cause significant morbidity and mortality. 3 , 66 , 67 , 68 Regionalized triage protocols must consider time, geography, and hospital capabilities in selection. By allowing emergency medical systems (EMS) agencies to bypass local hospitals without advanced specialty capabilities, outcome benefits can be most pronounced–the evidence is particularly strong in stroke and STEMI care. 49 , 54 , 66 , 67
Standardized severity assessment scales have been one approach to formalizing patient assessment. The Revised Trauma Score 69 , 70 and Rapid Arterial Occlusions Evaluation scores 71 have been used to identify patients for regionalized triage. Although these assessment scales have limitations, they provide a standard language for prehospital professionals to use to guide destination selection.
Advanced technology can augment prehospital triage. Notable applications have been the use of 12‐lead ECG machines by EMS personnel 54 and telemedicine triage. In some instances, a tablet computer allows doctors to speak with patients and EMS healthcare practitioners remotely to select the most appropriate destination. 72
5.2. Resource stratified‐facility network
Regional systems function in a multidirectional tiered hierarchy with the goal of matching clinical resources to patient needs (Table 1). Systems often adhere to characteristic models (Figure 3). 3 , 11 , 73 In practice, most networks have relied solely on resource‐focused stratification of existing facilities. The creation of a regionalized system of care poses several challenges: identifying the ideal geospatial organization of an ad hoc system and the state‐by‐state management of the system through designation/certification/verification processes.
Geographic Information Systems (GIS): GIS approaches have been applied to optimizing regionalized systems. These analyses propose locations for additional care facilities, typically in areas with rural or minority populations. 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 The ACS‐COT conducted and developed a Needs Based Assessment of Trauma Systems Tool for evaluating trauma service areas. The tool was designed to aid in trauma system planning and has been tested with mixed success in Tennessee and California. 82 , 83 , 84
State designation, certificates of need, and verification: The tiered structure of a regionalized system is often established by state policy. Facilities may be designated by the state or regional authorities. Thirty‐five states have Certificates of Need regulations, which artificially limit competition in providing specialized services to improve outcomes, which has been implemented with mixed success. 85
FIGURE 3.
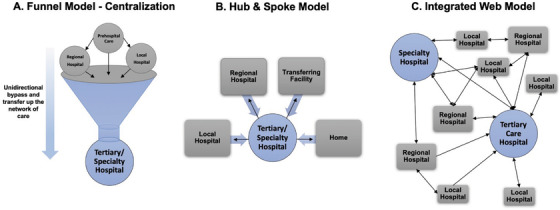
These 3 characteristic models, which have been discussed in the literature, demonstrate the degree of integration and organization within regionalized networks of care. Arrows indicate patient flow. The “integrated web” model has emerged as the desired conception of a regionalized and integrated system of care
Alternatively, outside agencies (ACS‐COT, The Joint Commission) may verify that facilities meet the criteria for specialty‐specific designation. Continuous verification ensures that the appropriate resource infrastructure remains in place and functional. 21 , 24 , 26 , 27 , 34
5.3. Prevention, outreach, and education
The educational aims of regionalized systems affect the entire continuum of care. Professional organizations and national programs take the lead in organizing and preparing public and professional educational programming. Regional and local hubs contribute to training the public in bystander response and methods for activating the acute care system (ACS‐COT's Stop the Bleed program, 86 AHA's FAST stroke symptom recognition 87 and cardiopulmonary resuscitation training 88 ). These programs view public response as the first step in reducing EMS response times and improving patient outcome. 87 , 89
Incorporating standardized disease‐specific training for healthcare practitioners has been shown to have considerable impact. 90 In the trauma and cardiac care systems, this standardization has been achieved through basic life support and advanced life support classes offered as part of regional care systems. 91
At the hospital and facility level, the verification process of every regionalized system requires some type of standardized training: advanced trauma life support), 92 advanced cardiac life support, 93 pediatric advanced life support, 94 and neonatal resuscitation program. 95 Tertiary care centers play the principal role in organizing these programs as required by verification criteria, which provides an organized dissemination pathway.
5.4. Preparedness
Regionalized systems play a vital role in disaster preparedness and mass‐casualty incidents evidenced in trauma, 96 , 97 , 98 burn, 99 , 100 , 101 and pediatric care. 102 By conducting resource assessments across the network and negotiating cooperative plans for surge events, regionalized systems can be well positioned to support the distribution of resources and care capacity that enable a rapid and organized disaster response. 3
5.5. Rehabilitation
Rehabilitation is a critical component of recovery for patients with acute care conditions–trauma, 103 burn 104 stroke, 105 STEMI, 106 and NICU/OB. 107 , 108 The outcomes currently tracked for acute care conditions are generally restricted to process measures and outcomes. 3 , 103 , 109 This neglects the many patients left with disabilities and reduced quality of life, and regional care networks allow for assessment of longer term outcomes.
5.6. Internal and benchmarked data registry
Regionalization networks sponsor national or local patient registries. Internal, hospital‐based, clinical data registries aggregate data to identify variations in care for quality improvement and have proven effective when paired with rigorous performance improvement (PI) processes. 110
Each system has established its own respective registry listed in Table 1. These registries standardize data collection by case acquisition, case definition, and coding conventions. By requiring registry compliance in the verification process, the proportion of capturing eligible cases is increased, the value of these information systems is amplified, and systemwide performance goals can be evaluated.
Unfortunately, these data systems are fragmented. They often include only paying facilities, lack linkage to EMS agencies and transferring hospitals, 111 and use proprietary software that limit public data access. 112 , 113 , 114 Additional issues include sustainability because of the resources needed to continually collect data, 110 incomplete data, 111 and control for covariates. 33 , 115
5.7. Performance improvement
Regionalization standardizes PI processes. Site visits offer an opportunity to review the PI process, ensuring that a process exists to identify cases for review and demonstrate institutional commitment to improvement. 27 The site review process functions both to independently evaluate system standards and to cross‐pollinate best practices. Each of the national models has established quality improvement programs, such as ACS's Trauma Quality Improvement Program. 115 , 116 , 117
5.8. Research
Using data from benchmarked data registries, each of the systems has analyzed metrics assessing a system's outcomes and the appropriateness of patient transfer within the system–through reporting of over and undertriage rates. 118 Published studies supporting the benefit of each of the regionalized approaches to acute care are presented in Table 1.
6. THE CASE FOR REGIONALIZING SEPSIS CARE
Systems of care drive local and regional processes, and examples of regionalization systems for other acute care conditions provide a template for how these networks can improve quality of care and outcomes. Sepsis is one example of an acute care condition for which regional care should be considered, and several small‐scale examples of regional sepsis networks have been developed. 119 , 120 , 121 , 122 Is regionalization the next frontier in sepsis quality?
6.1. Need for a sepsis regionalization network
The experience of other regional networks define criteria for diseases that can be effectively regionalized. First, regional care networks are developed for diseases that require specialized care for which triage and transfer protocols are vital to optimizing outcomes. In many cases, this is evidenced by (1) specialized technology or training (in a resource‐stratified network) or a (2) strong volume‐outcome relationship for a time‐sensitive condition in which patient choice may be impaired. Second, networks function best when they incorporate prehospital healthcare practitioners because of the importance of prehospital decision‐making in care or triage. They may also require rehabilitation as part of the continuum of care with condition‐specific considerations. Third, conditions that require significant public outreach for prevention, education, and preparedness activities are particularly amenable to regionalization. These networks may support registry‐based data collection, research activities, and local or regional standardization and protocol development.
Does sepsis meet these standards? Sepsis care follows a robust volume‐outcome relationship, 123 , 124 with care in high‐volume facilities having the best outcomes. Previous work has shown not only that interhospital transfer is associated with higher mortality 125 , 126 , 127 , 128 , 129 but also that rural bypass puts patients at increased risk of death. 130 Timely care is associated with improved clinical outcome, 131 , 132 and the conditions under which patients require sepsis care impair their ability to choose their setting for care (eg, vulnerable population). Delays in sepsis recognition, early resuscitation, and transfer continue to put patients at risk. 133 In fact, if care in top‐performing hospitals were available in lower performing centers, an estimated 20,000 US sepsis deaths could be prevented. Although sepsis care does not require advanced technology, it does require staffing and procedural resources–notably critical care and in some cases interventional radiology or surgery for source control–that are not routinely available in all hospitals. 134 Critical care services for advanced organ failure support for sepsis, however, often exist within other regionalized networks, such as trauma and stroke systems.
Prehospital care is common in sepsis, and some data suggest that prehospital recognition and management may influence outcomes. 135 Finally, sepsis care is currently poorly standardized (especially beyond initial resuscitation), with significant controversy surrounding the importance of a protocolized approach. 136 , 137 , 138 Standardizing training and outreach activities may be one strategy to expand the focus of sepsis quality improvement from one of process‐based accountability (eg, SEP‐1 measure) to one of sustained process improvement.
Financial barriers to sepsis regionalization may include a less lucrative sepsis payer mix, fewer sepsis‐associated procedures, and billing complications amid variance in clinical protocols. 139 , 140 This consideration has been recognized in stroke regionalization but has been overcome in many settings to optimize patient outcomes.
6.2. Attributes of a sepsis regional care network
Comparative analysis of regionalization networks reveals that the administrative structure and funding of these networks comes through 1 of 2 pathways: professional organizations or accrediting bodies. In many cases, these networks are driven by specialty groups who have a mission of improving access to relevant specialty care. A similar structure could be developed through a collaboration of critical care societies.
Ad hoc regional sepsis networks exist, in that critically ill patients may be triaged to more capable hospitals. Unfortunately, those triage networks have not yet been optimized, and overtriage and undertriage still plague sepsis network development. 141 , 142 , 143 , 144 , 145 , 146 These networks could be built on a hierarchical structure of patient‐focused and facility‐focused criteria that can better distribute patients among inpatient resources. In an era of resource constraints, increased regionalization, and overcrowding in tertiary care centers, formalizing risk stratification 147 , 148 , 149 , 150 to match patient needs seems a viable approach to sepsis process improvement efforts.
Such a network may play an outsized role in regional process improvement. Historically, sepsis quality measures have been narrowly focused. In contrast to the comprehensive multispecialty process‐based approach of trauma, stroke, burn, and STEMI networks, sepsis quality has been defined by adherence with a narrow set of objective early activities. Although that approach has focused efforts in ED and ICU resuscitation care, it has neglected the importance of multispecialty involvement in robust comprehensive process improvement activities, public education and outreach, and care pathways that extend beyond the first 6 hours of care. Incorporating a more global approach to sepsis management allows for more rapid dissemination of knowledge and incorporation of local factors in the development of sepsis activities. The structure of formal sepsis care networks has the capacity to significantly improve clinical outcomes, even absent innovation in diagnostics or treatment.
One regional quality improvement initiative has illustrated the utility of a regional approach to sepsis. The Kansas Sepsis Project is a collaborative based at the University of Kansas, and it has distributed a model of pragmatic care and quality improvement through midsized health systems in Kansas. 119 , 120 By connecting small hospitals with regional centers and engaging in shared process improvement, the Kansas Sepsis Project has effectively provided a structure for rural hospitals to provide high‐quality care in a standardized care environment, with criteria for screening and interhospital transfer. Early data suggest effectiveness of the program, but reports of efficacy have not yet been published.
7. CONCLUSIONS
Regionalization of care is conceptually straightforward. Between the volume–outcome relationship and the number of conditions that have time‐sensitive care interventions that need resource‐appropriate care, there are evidence‐based arguments that regionalized care should be established for many conditions. Trauma, burn, ischemic stroke, STEMI, and NICU/OB care have seen improvements in outcomes associated with regionalization. Cardiac arrest, critical care, and sepsis may benefit as well.
Regionalization is part of the modernization of medicine. Regionalization will gain importance as health care transitions to a model of population health, coinciding with increasing government involvement 151 and anticompetitive policies, where systemwide efficiencies will allow avenues for different strategies of investment to improve outcomes. Public health agencies, payers, and regional governments have a stake in the outcomes of acute care regionalization. The challenge is coordinating silos of appropriately specialized disease‐focused care into a regionally coordinated superstructure of acute care that optimizes patient outcomes throughout the United States. Understanding the framework, processes, and comparative differences behind regionalized systems will be valuable to improving existing systems and establishing new ones.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
This study was determined to not constitute human subjects research by the University of Iowa institutional review board and thus approval was waived. This manuscript does not contain any individual person's data.
CONFLICT OF INTERESTS
No financial or non‐financial competing interests for either author.
Supporting information
Supporting information
ACKNOWLEDGMENTS
This project was made possible by the University of Iowa Carver College of Medicine Summer Research Fellowship Grant (National Institutes of Health Grant # T35HL007485). Dr. Mohr is additionally supported by grant K08 HS025753 from the Agency for Healthcare Research and Quality (AHRQ), which funded the publication fees for this article. The findings and conclusions are those of the author(s), who are responsible for its content, and do not necessarily represent the views of AHRQ. The authors acknowledge the technical assistance provided by Kari Harland, PhD, and Kelli Wallace, MS, in the University of Iowa Department of Emergency Medicine and Heather Healy of the Hardin Library of Health Sciences as well as the expertise and insight provided in interviews with numerous experts and involved representatives of regionalized systems of care. The findings and conclusions are those of the author(s), who are responsible for its content, and do not necessarily represent the views of those interviewed and acknowledged.
Walton NT, Mohr NM. Concept review of regionalized systems of acute care: Is regionalization the next frontier in sepsis care? JACEP Open. 2022;3:e12631. 10.1002/emp2.12631
Presented at Great Plains Regional Society of Academic Emergency Medicine Annual conference–Springfield, Illinois–September 21, 2019
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
Supervising Editor: Faheem Guirgis, MD.
DATA AVAILABILITY STATEMENT
As listed per work cited. Digital recordings of interviews where permission to record was provided are available upon request.
REFERENCES
- 1. Martinez R, Carr B. Creating integrated networks of emergency care: from vision to value. Health Aff (Millwood). 2013;32(12):2082‐2890. [DOI] [PubMed] [Google Scholar]
- 2.Health Resources Administration , Ginzberg E. Regionalization & Health Policy. U.S. Department of Health, Education, and Welfare, Public Health Service, Health Resources Administration; 1977. [Google Scholar]
- 3.Institute of Medicine . Regionalizing Emergency Care: Workshop Summary. The National Academies Press; 2010:166. [PubMed] [Google Scholar]
- 4. Luft HS, Hunt SS, Maerki SC. The volume‐outcome relationship: practice‐makes‐perfect or selective‐referral patterns? Health Serv Res. 1987;22(2):157. [PMC free article] [PubMed] [Google Scholar]
- 5. Shahian DM, Normand S‐LT. The volume‐outcome relationship: from Luft to Leapfrog. Ann Thorac Surg. 2003;75(3):1048‐1058. [DOI] [PubMed] [Google Scholar]
- 6. Nathens AB, Jurkovich GJ, Maier RV, et al. Relationship between trauma center volume and outcomes. JAMA. 2001;285(9):1164‐1171. [DOI] [PubMed] [Google Scholar]
- 7. Pacella S, Butz D, Comstock M, Harkins D, Kuzon W, Taheri P. Hospital volume outcome and discharge disposition of burn patients. Plast Reconstr Surg. 2006;117:1296‐1305; discussion 1306. [DOI] [PubMed] [Google Scholar]
- 8. Gorelick PB. Primary and comprehensive stroke centers: history, value and certification criteria. J Stroke. 2013;15(2):78‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fanaroff AC, Zakroysky P, Dai D, et al. Outcomes of PCI in relation to procedural characteristics and operator volumes in the United States. J Am Coll Cardiol. 2017;69(24):2913‐2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Phibbs CS, Bronstein JM, Buxton E, Phibbs RH. The effects of patient volume and level of care at the hospital of birth on neonatal mortality. JAMA. 1996;276(13):1054‐1059. [PubMed] [Google Scholar]
- 11. Phibbs CS, Baker LC, Caughey AB, Danielsen B, Schmitt SK, Phibbs RH. Level and volume of neonatal intensive care and mortality in very‐low‐birth‐weight infants. N Engl J Med. 2007;356(21):2165‐2175. [DOI] [PubMed] [Google Scholar]
- 12. McKenna M. Beyond regionalization: experts grapple with research agenda in response to IOM report. Ann Emerg Med. 2010;56(2):A15‐A17. [DOI] [PubMed] [Google Scholar]
- 13. National Academy of Sciences/National Research Council . Accidental Death and Disability: The Neglected Disease of Modern Society. The National Academies Press; 1966:39. [PubMed] [Google Scholar]
- 14. Rogers FB, Rittenhouse KJ, Gross BW. The golden hour in trauma: dogma or medical folklore? Injury. 2015;46(4):525‐527. [DOI] [PubMed] [Google Scholar]
- 15. Boyd DR. Trauma systems origins in the United States. J Trauma Nurs. 2010;17(3):126‐134; quiz 135–6. [DOI] [PubMed] [Google Scholar]
- 16. Miotke SA. Burn care: regionalisation, organisation and triage. ICU Manag Pract. 2015;15(4). [Google Scholar]
- 17. Pruitt BA, Wolf SE, Mason AD. Chapter 3—Epidemiological, demographic, and outcome characteristics of burn injury*. In: Herndon DN, ed. Total Burn Care (Fourth Edition). W.B. Saunders; 2012:15‐45.e4. [Google Scholar]
- 18. Staebler S. Regionalized systems of perinatal care: health policy considerations. Adv Neonatal Care. 2011;11(1):37‐42. [DOI] [PubMed] [Google Scholar]
- 19. Hein HA. Regionalized perinatal care in North America. Semin Neonatol. 2004;9(2):111‐116. [DOI] [PubMed] [Google Scholar]
- 20. Berns SD, Kott A. Toward Improving the Outcome of Pregnancy III: Enhancing Perinatal Health through Quality, Safety and Performance Initiatives. White Plains, NY: March of Dimes National Foundation; 2010. [Google Scholar]
- 21. American Academy of Pediatrics, Committee on the Fetus and Newborn . Levels of neonatal care. Pediatrics. 2012;130(3):587‐597. [DOI] [PubMed] [Google Scholar]
- 22. Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta‐analysis. Lancet. 2012;379(9834):2364‐2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke. Stroke. 2008;39(4):1205‐1212. [DOI] [PubMed] [Google Scholar]
- 24. The Joint Commision . Specifications Manual for Joint Commission National Quality Measures. The Joint Commission; 2018:1–1074. [Google Scholar]
- 25. Rhudy JP, Jr. , Bakitas MA, Hyrkas K, et al. Effectiveness of regionalized systems for stroke and myocardial infarction. Brain Behav. 2015;5(10):e00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. American Heart Association, with American College of Cardiology . Mission: Lifeline—Hospital Recognition Criteria v3. American Heart Association; 2018. [Google Scholar]
- 27. American College of Surgeons, Committee on Trauma . Resources for Optimal Care of the Injured Patient. American College of Surgeons; 2014:1–221. [Google Scholar]
- 28. Fakhry SM, Couillard D, Liddy CT, Adams D, Norcross ED. Trauma center finances and length of stay: identifying a profitability inflection point. J Am Coll Surg. 2010;210(5):817‐821, 821–3. [DOI] [PubMed] [Google Scholar]
- 29. Knowlton LM, Morris AM, Tennakoon L, Spain DA, Staudenmayer KL. Financial stability of Level I trauma centers within safety‐net hospitals. Je Am Coll Surg. 2018;227(2):172‐180. [DOI] [PubMed] [Google Scholar]
- 30. Goldstone AB, Chiu P, Baiocchi M, et al. Interfacility transfer of Medicare beneficiaries with acute type a aortic dissection and regionalization of care in the United States. Circulation. 2019;140(15):1239‐1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. MacKenzie EJ, Rivara FP, Jurkovich GJ, et al. A national evaluation of the effect of trauma‐center care on mortality. N Engl J Med. 2006;354(4):366‐378. [DOI] [PubMed] [Google Scholar]
- 32. Celso B, Tepas J, Langland‐Orban B, et al. A systematic review and meta‐analysis comparing outcome of severely injured patients treated in trauma centers following the establishment of trauma systems. J Trauma. 2006;60(2):371‐378; discussion 378. [DOI] [PubMed] [Google Scholar]
- 33. Haider AH, Saleem T, Leow JJ, et al. Influence of the National Trauma Data Bank on the study of trauma outcomes: is it time to set research best practices to further enhance its impact? J Am Coll Surg. 2012;214(5):756‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. American Burn Association . Burn Center–Verification Criteria. American Burn Association; 2017:1–5. [Google Scholar]
- 35. Dimick AR, Potts LH, Charles ED, Jr. , Wayne J, Reed IM. The cost of burn care and implications for the future on quality of care. J Trauma. 1986;26(3):260‐266. [DOI] [PubMed] [Google Scholar]
- 36. Corpron CA, Martin AE, Roberts G, Besner GE. The pediatric burn unit: a profit center. J Pediatr Surg. 2004;39(6):961‐963; discussion 961–3. [DOI] [PubMed] [Google Scholar]
- 37. Warden GD, Heimbach D. Regionalization of burn care–A concept whose time has come. J Burn Care Rehabil. 2003;24(3):173‐174. [DOI] [PubMed] [Google Scholar]
- 38. Barillo DJ, Hallock GG, Mastropieri CJ, Troiani YM, Knowlton C. Utilization of the burn unit for nonburn patients: the “wound intensive care unit". Ann Plast Surg. 1989;23(5):426‐429. [DOI] [PubMed] [Google Scholar]
- 39. Faucher LD, Morris SE, Edelman LS, Saffle JR. Burn center management of necrotizing soft‐tissue surgical infections in unburned patients. Am J Surg. 2001;182(6):563‐569. [DOI] [PubMed] [Google Scholar]
- 40. Palmieri TL, Greenhalgh DG, Saffle JR, et al. A multicenter review of toxic epidermal necrolysis treated in U.S. burn centers at the end of the twentieth century. J Burn Care Rehabil. 2002;23(2):87‐96. [DOI] [PubMed] [Google Scholar]
- 41. Endorf FW, Supple KG, Gamelli RL. The evolving characteristics and care of necrotizing soft‐tissue infections. Burns. 2005;31(3):269‐273. [DOI] [PubMed] [Google Scholar]
- 42. Yurt RW, Bessey PQ. The development of a regional system for care of the burn‐injured patients. Surg Infect (Larchmt). 2009;10(5):441‐445. [DOI] [PubMed] [Google Scholar]
- 43. American Burn Association . ABA NBDS National Burn Data Standard: Data Dictionary. American Burn Association; http://ameriburn.org/wp‐content/uploads/2017/04/nbds_final_061615.pdf [Google Scholar]
- 44. Kahn SA, Bernal N, Mosier MJ. Pearls from the National Burn Repository. J Burn Care Res. 2018;39(4):626‐627. [DOI] [PubMed] [Google Scholar]
- 45. Taylor SL, Lee D, Nagler T, Lawless MB, Curri T, Palmieri TL. A validity review of the National Burn Repository. J Burn Care Res. 2013;34(2):274‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Medford‐Davis LN, Fonarow GC, Bhatt DL, et al. Impact of insurance status on outcomes and use of rehabilitation services in acute ischemic stroke: findings from get with the guidelines‐stroke. J Am Heart Assoc. 2016;5(11):e004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saposnik G, Fang J, O'Donnell M, Hachinski V, Kapral MK, Hill MD. Escalating levels of access to in‐hospital care and stroke mortality. Stroke. 2008;39(9):2522‐2530. [DOI] [PubMed] [Google Scholar]
- 48. Xian Y, Holloway RG, Chan PS, et al. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305(4):373‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Green Jacqueline L, Jacobs Alice K, Holmes D, et al. Taking the reins on systems of care for ST‐segment–elevation myocardial infarction patients. Circulations. 2018;11(5):e005706. [DOI] [PubMed] [Google Scholar]
- 50. O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(4):485‐510. [DOI] [PubMed] [Google Scholar]
- 51. Xian Y, Fonarow GC, Reeves MJ, et al. Data quality in the American Heart Association Get with the Guidelines‐Stroke (GWTG‐Stroke): results from a national data validation audit. Am Heart J. 2012;163(3):392‐398, e1. [DOI] [PubMed] [Google Scholar]
- 52. Ormseth CH, Sheth KN, Saver JL, Fonarow GC, Schwamm LH. The American Heart Association's Get with the Guidelines (GWTG)‐stroke development and impact on stroke care. Stroke Vasc Neurol. 2017;2(2):94‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reeves MJ, Grau‐Sepulveda MV, Fonarow GC, Olson DM, Smith EE, Schwamm LH. Are quality improvements in the get with the guidelines: stroke program related to better care or better data documentation? Circ Cardiovasc Qual Outcomes. 2011;4(5):503‐511. [DOI] [PubMed] [Google Scholar]
- 54. Jollis JG, Al‐Khalidi HR, Roettig ML, et al. Regional systems of care demonstration project: American Heart Association Mission: lifeline STEMI systems accelerator. Circulation. 2016;134(5):365‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McNally B, Stokes A, Crouch A, Kellermann AL. CARES: cardiac arrest registry to enhance survival. Ann Emerg Med. 2009;54(5):674‐683.e2. [DOI] [PubMed] [Google Scholar]
- 56. Lantos J. Cruel calculus: why saving premature babies is better business than helping them thrive. Health Affairs. 2010;29(11):2114‐2117. [DOI] [PubMed] [Google Scholar]
- 57. Freedman S. Capacity and utilization in health care: the effect of empty beds on neonatal intensive care admission. Am Econ J Econ Policy. 2016;8(2):154‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hasselbacher P. The Quest for Neonatal Intensive Care Beds. A Statewide Phenomena. Louisville, KY: Norton Kosair Children's Hospital. http://www.khpi.org/blog/the-quest-for-neonatal-intensive-care-beds-a-statewide-phenomena/ [Google Scholar]
- 59. von Gruenigen VE, Powell DM, Sorboro S, McCarroll ML, Kim U. The financial performance of labor and delivery units. Am J Obstet Gynecol. 2013;209(1):17‐19. [DOI] [PubMed] [Google Scholar]
- 60. Lasswell SM, Barfield WD, Rochat RW, Blackmon L. Perinatal regionalization for very low‐birth‐weight and very preterm infants: a meta‐analysis. JAMA. 2010;304(9):992‐1000. [DOI] [PubMed] [Google Scholar]
- 61. Menard MK, Liu Q, Holgren EA, Sappenfield WM. Neonatal mortality for very low birth weight deliveries in South Carolina by level of hospital perinatal service. Am J Obstet Gynecol. 1998;179(2):374‐381. [DOI] [PubMed] [Google Scholar]
- 62. Bode MM, O'Shea T M, Metzguer KR, Stiles AD. Perinatal regionalization and neonatal mortality in North Carolina, 1968–1994. Am J Obstet Gynecol. 2001;184(6):1302‐1307. [DOI] [PubMed] [Google Scholar]
- 63. Yeast JD, Poskin M, Stockbauer JW, Shaffer S. Changing patterns in regionalization of perinatal care and the impact on neonatal mortality. Am J Obstet Gynecol. 1998;178(1 Pt 1):131‐135. [DOI] [PubMed] [Google Scholar]
- 64. Powell SL, Holt VL, Hickok DE, Easterling T, Connell FA. Recent changes in delivery site of low‐birth‐weight infants in Washington: impact on birth weight‐specific mortality. Am J Obstet Gynecol. 1995;173(5):1585‐1592. [DOI] [PubMed] [Google Scholar]
- 65. Horbar JD. The Vermont Oxford Network: evidence‐based quality improvement for neonatology. Pediatrics. 1999;103(1 Suppl E):350‐359. [PubMed] [Google Scholar]
- 66. Hashmi ZG, Jarman MP, Uribe‐Leitz T, et al. Access delayed is access denied: relationship between access to trauma center care and pre‐hospital death. J Am Coll Surg. 2019;228(1):9‐20. [DOI] [PubMed] [Google Scholar]
- 67. Jones CM, Cushman JT, Lerner EB, et al. Prehospital trauma triage decision‐making: a model of what happens between the 9‐1‐1 call and the hospital. Prehosp Emerg Care. 2016;20(1):6‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rossnagel K, Jungehulsing GJ, Nolte CH, et al. Out‐of‐hospital delays in patients with acute stroke. Ann Emerg Med. 2004;44(5):476‐483. [DOI] [PubMed] [Google Scholar]
- 69. Sasser S, Hunt R, Sullivent E, et al. Guidelines for field triage of injured patients recommendations of the national expert panel on field triage. Morb Mortal Wkly Rep. 2009;58(RR‐1):1‐34. [PubMed] [Google Scholar]
- 70. Champion HR. Trauma scoring. Scand J Surg. 2002;91(1):12‐22. [DOI] [PubMed] [Google Scholar]
- 71. Bogle BM, Asimos AW, Rosamond WD. Regional evaluation of the severity‐based stroke triage algorithm for emergency medical services using discrete event simulation. Stroke. 2017;48(10):2827‐2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gonzalez M, Alqusairi D, Jackson A, Champagne T, Langabeer J, Persse D. Houston EMS advances mobile integrated healthcare through the ETHAN program. JEMS. 2015;2. [Google Scholar]
- 73. Carr BG, Matthew Edwards J, Martinez R. Regionalized care for time‐critical conditions: lessons learned from existing networks. Acad Emerg Med. 2010;17(12):1354‐1358. [DOI] [PubMed] [Google Scholar]
- 74. Wallace DJ, Mohan D, Angus DC, et al. Referral regions for time‐sensitive acute care conditions in the United States. Ann Emerg Med. 2018;72(2):147‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Leira EC, Fairchild G, Segre AM, Rushton G, Froehler MT, Polgreen PM. Primary stroke centers should be located using maximal coverage models for optimal access. Stroke. 2012;43(9):2417‐2422. [DOI] [PubMed] [Google Scholar]
- 76. Crandall M, Sharp D, Unger E, et al. Trauma deserts: distance from a trauma center, transport times, and mortality from gunshot wounds in Chicago. Am J Public Health. 2013;103(6):1103‐1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jarman MP, Curriero FC, Haut ER, Pollack Porter K, Castillo RC. Associations of distance to trauma care, community income, and neighborhood median age with rates of injury mortality. JAMA Surg. 2018;153(6):535‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ader J, Wu J, Fonarow GC, et al. Hospital distance, socioeconomic status, and timely treatment of ischemic stroke. Neurology. 2019;93(8):e747‐e757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tung EL, Hampton DA, Kolak M, Rogers SO, Yang JP, Peek ME. Race/ethnicity and geographic access to urban trauma care. JAMA Netw Open. 2019;2(3):e190138‐e190138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pedigo AS, Odoi A. Investigation of disparities in geographic accessibility to emergency stroke and myocardial infarction care in East Tennessee using geographic information systems and network analysis. Ann Epidemiol. 2010;20(12):924‐930. [DOI] [PubMed] [Google Scholar]
- 81. Horst MA, Gross BW, Cook AD, Osler TM, Bradburn EH, Rogers FB. A novel approach to optimal placement of new trauma centers within an existing trauma system using geospatial mapping. J Trauma Acute Care Surg. 2017;83(4):705‐710. [DOI] [PubMed] [Google Scholar]
- 82. Needs‐Based Trauma Center Designation Consensus Conference . Needs Based Assessment of Trauma Systems (NBATS) Tool. NBTCDCC ; 2015;6. [Google Scholar]
- 83. Dooley JH, Ozdenerol E, Sharpe JP, Magnotti LJ, Croce MA, Fischer PE. Location, location, location: utilizing NBATS‐2 in trauma system planning. J Trauma Acute Care Surg. 2019;88(1):94‐100. [DOI] [PubMed] [Google Scholar]
- 84. Uribe‐Leitz T, Esquivel MM, Knowlton LM, et al. The American College of Surgeons needs‐based assessment of trauma systems: estimates for the state of California. J Trauma Acute Care Surg. 2017;82(5):861‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ho V, Ku‐Goto M‐H, Jollis JG. Certificate of Need (CON) for cardiac care: controversy over the contributions of CON. Health Serv Res. 2009;44(2 Pt 1):483‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fisher AD, Bulger EM, Gestring ML. Stop the bleeding: educating the public. JAMA. 2018;320(6):589‐590. [DOI] [PubMed] [Google Scholar]
- 87. TeuschI Y, Brainin M. Stroke education: discrepancies among factors influencing prehospital delay and stroke knowledge. Int J Stroke. 2010;5(3):187‐208. [DOI] [PubMed] [Google Scholar]
- 88. Cave DM, Aufderheide TP, Beeson J, et al. Importance and implementation of training in cardiopulmonary resuscitation and automated external defibrillation in schools: a science advisory from the American Heart Association. Circulation. 2011;123(6):691‐706. [DOI] [PubMed] [Google Scholar]
- 89. Farshidi H, Rahimi S, Abdi A, Salehi S, Madani A. Factors associated with pre‐hospital delay in patients with acute myocardial infarction. Iran Red Crescent Med J. 2013;15(4):312‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Brice JH, Evenson KR, Lellis JC, et al. Emergency medical services education, community outreach, and protocols for stroke and chest pain in North Carolina. Prehosp Emerg Care. 2008;12(3):366‐371. [DOI] [PubMed] [Google Scholar]
- 91. Ryynänen O‐P, Iirola T, Reitala J, Pälve H, Malmivaara A. Is advanced life support better than basic life support in prehospital care? A systematic review. Scand J Trauma Resusc Emerg Med. 2010;18:62‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Carmont MR. The advanced trauma life support course: a history of its development and review of related literature. Postgrad Med J. 2005;81(952):87‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pozner CN. Advanced cardiac life support (ACLS) in adults.
- 94. Fleegler E, Kleinman M. Pediatric advanced life support (PALS).
- 95. Fernandes C, Weisman LE, Kim MS. Neonatal resuscitation in the delivery room. 2009.
- 96. Trunkey DD. US Trauma Center Preparation for a terrorist attack in the community. Eur J Trauma Emerg Surg. 2009;35(3):244‐264. [DOI] [PubMed] [Google Scholar]
- 97. Cryer HG, Hiatt JR, Eckstein M, et al. Improved trauma system multicasualty incident response: comparison of two train crash disasters. J Trauma. 2010;68(4):783‐739. [DOI] [PubMed] [Google Scholar]
- 98. Cryer HG, Hiatt JR. Trauma system: the backbone of disaster preparedness. J Trauma. 2009;67(2 Suppl):S111‐S113. [DOI] [PubMed] [Google Scholar]
- 99. Kearns RD, Conlon KM, Valenta AL, et al. Disaster planning: the basics of creating a burn mass casualty disaster plan for a burn center. J Burn Care Res. 2014;35(1):e1‐e13. [DOI] [PubMed] [Google Scholar]
- 100. Vandenberg V, Amara R, Crabtree J, Fruhwirth K, Rifenburg J, Garner W. Burn surge for Los Angeles County, California. J Trauma. 2009;67(2 Suppl):S143‐S146. [DOI] [PubMed] [Google Scholar]
- 101. Yurt RW, Lazar EJ, Leahy NE, et al. Burn disaster response planning: an urban region's approach. J Burn Care Res. 2008;29(1):158‐165. [DOI] [PubMed] [Google Scholar]
- 102. Barfield WD, Krug SE, Kanter RK, et al. Neonatal and pediatric regionalized systems in pediatric emergency mass critical care. Pediatr Crit Care Med. 2011;12(6 0):S128‐S134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Khan F, Amatya B, Hoffman K. Systematic review of multidisciplinary rehabilitation in patients with multiple trauma. Br J Surg. 2012;99(Suppl 1):88‐96. [DOI] [PubMed] [Google Scholar]
- 104. Serghiou M, Cowan A, Whitehead C. Rehabilitation after a burn injury. Clin Plast Surg. 2009;36(4):675‐86. [DOI] [PubMed] [Google Scholar]
- 105. Dobkin BH, Dorsch A. New evidence for therapies in stroke rehabilitation. Curr Atheroscler Rep. 2013;15(6):331‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Urbinati S, Tonet E. Cardiac rehabilitation after STEMI. Minerva Cardioangiol. 2018;66(4):464‐470. [DOI] [PubMed] [Google Scholar]
- 107. Cui LR, LaPorte M, Civitello M, et al. Physical and occupational therapy utilization in a pediatric intensive care unit. J Crit Care. 2017;40:15‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Maitre NL. Neurorehabilitation after neonatal intensive care: evidence and challenges. Arch Dis Child Fetal Neonatal Ed. 2015;100(6):F534‐F540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sleat GK, Ardolino AM, Willett KM. Outcome measures in major trauma care: a review of current international trauma registry practice. Emerg Med J. 2011;28(12):1008‐1012. [DOI] [PubMed] [Google Scholar]
- 110. Glicklick RE, Dreyer NA, Leavy MB. Registries for Evaluating Patient Outcomes: A User's Guide [Internet]. Agency for Healthcare Research and Quality. Vol 1. 3rd ed. 2014. https://www.ncbi.nlm.nih.gov/books/NBK208630/ [PubMed] [Google Scholar]
- 111. Greene J. EMS and information sharing: challenges and innovations in getting patient data from the ambulance to the emergency department and back. Ann Emerg Med. 2014;64(2):A15‐A17. [Google Scholar]
- 112. American College of Surgeons . Fees and Invoices. American College of Surgeons; 2020. [Google Scholar]
- 113. American Burn Association . Verification Program Fees. American Burn Association; 2020. [Google Scholar]
- 114. American Heart Association/American Stroke Association . 2018 Get with the Guidelines® Program Pricing Summary. American Heart Association/American Stroke Association; 2020. [Google Scholar]
- 115. Hemmila MR, Nathens AB, Shafi S, et al. The trauma quality improvement program: pilot study and initial demonstration of feasibility. J Trauma. 2010;68(2):253‐262. [DOI] [PubMed] [Google Scholar]
- 116. Shafi S, Nathens AB, Cryer HG, et al. The trauma quality improvement program of the American College of Surgeons Committee on Trauma. J Am Coll Surg. 2009;209(4):521‐530.e1. [DOI] [PubMed] [Google Scholar]
- 117. Newgard CD, Fildes JJ, Wu L, et al. Methodology and analytic rationale for the American College of Surgeons Trauma Quality Improvement Program. J Am Coll Surg. 2013;216(1):147‐157. [DOI] [PubMed] [Google Scholar]
- 118. Lossius HM, Rehn M, Tjosevik KE, Eken T. Calculating trauma triage precision: effects of different definitions of major trauma. J Trauma Manag Outcomes. 2012;6(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Simpson MD SQ . Critical care commentary: Kansas Sepsis Project. CHEST Physician. https://www.mdedge.com/chestphysician/article/77971/critical-care/critical-care-commentary-kansas-sepsis-project/page/0/1# [Google Scholar]
- 120. University of Kansas Medical Center–Simpson MD SQ . Improving Outcomes for Sepsis in Rural Kansas. University of Kansas Medical Center–Simpson MD SQ. http://www.kumc.edu/community-engagement/serving-communities/community-connections/sepsis.html [Google Scholar]
- 121. Campbell K, Vakkalanka, P. , Wittrock, A. , et al. Telemedicine is Associated with Improved Antibiotic Appropriateness in Rural Emergency Departments Society for Education and Research in Connected Health. Philadelphia: SEARCH; 2017. [Google Scholar]
- 122. Mohr NM, Campbell KD, Swanson MB, Ullrich F, Merchant KA, Ward MM. Provider‐to‐provider telemedicine improves adherence to sepsis bundle care in community emergency departments. J Telemed Telecare 2020;27(8):518‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Gaieski DF, Edwards JM, Kallan MJ, Mikkelsen ME, Goyal M, Carr BG. The relationship between hospital volume and mortality in severe sepsis. Am J Respir Crit Care Med. 2014;190(6):665‐674. [DOI] [PubMed] [Google Scholar]
- 124. Kocher KE, Haggins AN, Sabbatini AK, Sauser K, Sharp AL. Emergency department hospitalization volume and mortality in the United States. Ann Emerg Med. 2014;64(5):446‐457.e6. [DOI] [PubMed] [Google Scholar]
- 125. Mohr NM, Harland KK, Shane DM, Ahmed A, Fuller BM, Torner JC. Inter‐hospital transfer is associated with increased mortality and costs in severe sepsis and septic shock: an instrumental variables approach. J Crit Care. 2016;36:187‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Brooten JK, Buckenheimer AS, Hallmark JK, et al. Risky behavior: hospital transfers associated with early mortality and rates of goals of care discussions. West J Emer Med. 2020;21(4):935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Durairaj L, Will JG, Torner JC, Doebbeling BN. Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981‐1986. [DOI] [PubMed] [Google Scholar]
- 128. Hill AD, Vingilis E, Martin CM, Hartford K, Speechley KN. Interhospital transfer of critically ill patients: demographic and outcomes comparison with nontransferred intensive care unit patients. J Crit Care. 2007;22(4):290‐295. [DOI] [PubMed] [Google Scholar]
- 129. Arthur KR, Kelz RR, Mills AM, et al. Interhospital transfer: an independent risk factor for mortality in the surgical intensive care unit. Am Surg. 2013;79(9):909‐913. [DOI] [PubMed] [Google Scholar]
- 130. Mohr NM, Harland KK, Shane DM, et al. Rural patients with severe sepsis or septic shock who bypass rural hospitals have increased mortality: an instrumental variables approach. Criti Care Med. 2017;45(1):85‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med. 2018;46(6):997‐1000. [DOI] [PubMed] [Google Scholar]
- 132. Ferrer R, Artigas A, Levy MM, et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299(19):2294‐303. [DOI] [PubMed] [Google Scholar]
- 133. Ofoma UR, Dahdah J, Kethireddy S, Maeng D, Walkey AJ. Case volume‐outcomes associations among patients with severe sepsis who underwent interhospital transfer. Crit Care Med. 2017;45(4):615‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ilko SA, Vakkalanka JP, Ahmed A, Harland KK, Mohr NM. Central venous access capability and critical care telemedicine decreases inter‐hospital transfer among severe sepsis patients: a mixed methods design. Crit Care Med. 2019;47(5):659‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Seymour CW, Cooke CR, Heckbert SR, et al. Prehospital intravenous access and fluid resuscitation in severe sepsis: an observational cohort study. Crit Care. 2014;18(5):533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Rhee C, Filbin MR, Massaro AF, et al. Compliance with the national SEP‐1 quality measure and association with sepsis outcomes: a multicenter retrospective cohort study. Crit Care Med. 2018;46(10):1585‐1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Motzkus CA, Lilly CM. Accountability for sepsis treatment: the SEP‐1 core measure. Chest. 2017;151(5):955‐957. [DOI] [PubMed] [Google Scholar]
- 138. Gesensway D. SEP‐1: Does it improve sepsis care? Updated March. https://www.todayshospitalist.com/sep‐1‐improve‐sepsis‐care/
- 139. O'Brien JM, Jr. , Lu B, Ali NA, Levine DA, Aberegg SK, Lemeshow S. Insurance type and sepsis‐associated hospitalizations and sepsis‐associated mortality among US adults: a retrospective cohort study. Crit Care. 2011;15(3):R130‐R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Health Care Compliance Association . Payer Denials Hit Sepsis Amid Conflicting Clinical Protocols; Diagnosis is Doubted. Report on Medicare Compliance. Health Care Compliance Association; 2017. 26(18):1‐3. [Google Scholar]
- 141. Arulraja MD, Swanson MB, Mohr NM. Double inter‐hospital transfer in Sepsis patients presenting to the ED does not worsen mortality compared to single inter‐hospital transfer. J Crit Care. 2019;56:49‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Yurkova I, Wolf L. Under‐triage as a significant factor affecting transfer time between the emergency department and the intensive care unit. J Emerg Nurs. 2011;37(5):491‐496. [DOI] [PubMed] [Google Scholar]
- 143. Bledsoe JR, Allen TL, Brown SM, Peltan ID. Lower triage acuity scores are associated with delayed antibiotics in ED sepsis. D25 critical care: hard times—Resuscitating my patient: fluid, blood and other strategies. A5986‐A5986.
- 144. Coopersmith CM, De Backer D, Deutschman CS, et al. Surviving sepsis campaign: research priorities for sepsis and septic shock. Journal article. Intensive Care Med. 2018;44(9):1400‐1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wardi G, Wali AR, Villar J, et al. Unexpected intensive care transfer of admitted patients with severe sepsis. J Intensive Care. 2017;5(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Stephanie Mueller M. . Triaging Interhospital Transfers. Patient Safety Network. https://psnet.ahrq.gov/web-mm/triaging-interhospital-transfers [Google Scholar]
- 147. Bayer O, Schwarzkopf D, Stumme C, et al. An early warning scoring system to identify septic patients in the prehospital setting: the PRESEP score. Acad Emerg Med. 2015;22(7):868‐871. [DOI] [PubMed] [Google Scholar]
- 148. McLymont N, Glover GW. Scoring systems for the characterization of sepsis and associated outcomes. Ann Transl Med. 2016;4(24):527‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Sivayoham N, Holmes P, Cecconi M, Rhodes A. The simplified mortality in severe sepsis in the emergency department (missed) score to risk stratify ED sepsis. Emer Med J. 2015;32(12):986. [Google Scholar]
- 150. Shapiro NI, Wolfe RE, Moore RB, Smith E, Burdick E, Bates DW. Mortality in Emergency Department Sepsis (MEDS) score: a prospectively derived and validated clinical prediction rule. Crit Care Med. 2003;31(3):670‐675. [DOI] [PubMed] [Google Scholar]
- 151. Himmelstein DU, Woolhandler S. The current and projected taxpayer shares of US health costs. Am J Public Health. 2016;106(3):449‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lorch SA, Myers S, Carr B. The regionalization of pediatric health care. Pediatrics. 2010;126(6):1182‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
As listed per work cited. Digital recordings of interviews where permission to record was provided are available upon request.


