Key Points
Question
How do the incidence and timing of the diagnosis of neurodevelopmental disorders among children in the US differ by population characteristics?
Findings
In this cohort study of more than 3.3 million children, approximately 1 in 4 publicly and 1 in 9 privately insured children had received a diagnosis of a neurodevelopmental disorder by 8 years of age; the risk was considerably higher among boys and White children. For most disorders, privately insured children tended to receive a diagnosis earlier than publicly insured children.
Meaning
Because neurodevelopmental disorders are common and risks may be ameliorated with early intervention, identifying modifiable risk factors and opportunities for early intervention is needed.
Abstract
Importance
Neurodevelopmental disorders are associated with poor health and social outcomes. Population-based data on incidence, age at diagnosis, and demographic variations are essential to identify modifiable risk factors and inform the planning of services and interventions.
Objectives
To assess the incidence and timing of diagnosis of neurodevelopmental disorders during childhood in the US and to evaluate differences by population characteristics.
Design, Setting, and Participants
This population-based cohort study used nationwide data on birth cohorts nested in the 2000-2014 Medicaid Analytic eXtract and the 2003-2015 IBM MarketScan Research Database on 2 070 541 publicly and 1 309 900 privately insured children enrolled at birth. Data were analyzed between May 1, 2020, and June 30, 2021.
Main Outcomes and Measures
Neurodevelopmental disorders, autism spectrum disorders, attention-deficit/hyperactivity disorder, learning disabilities, speech or language disorders, developmental coordination disorders, intellectual disabilities, and behavioral disorders were identified based on validated algorithms. Kaplan-Meier analyses were used to estimate the incidence and timing of diagnosis, stratified by child’s sex, birth year, maternal age at delivery, and race and ethnicity.
Results
The cohorts comprised 2 070 541 publicly insured children (1 045 426 boys [50.5%]) and 1 309 900 privately insured children (667 607 boys [51.0%]) enrolled at birth. By 8 years of age, 23.9% of publicly insured children and 11.0% of privately insured children received a diagnosis of 1 or more neurodevelopmental disorders (autism spectrum disorder, 1.6% and 1.3%; attention-deficit/hyperactivity disorder, 14.5% and 5.8%; learning disability, 1.2% and 0.6%; speech or language disorder, 8.4% and 4.5%; developmental coordination disorder, 0.9% and 0.7%; intellectual disability, 0.7% and 0.1%; and behavioral disorder, 8.4% and 1.5%). Risks were substantially higher among boys (incidence of ≥1 neurodevelopmental disorder by age 8 years for boys vs girls: 30.7% vs 16.7% among publicly insured children and 15.0% vs 6.7% among privately insured children) and White children (30.2% vs 9.1% among Asian children, 23.0% among Black children, 15.4% among Hispanic children, and 22.7% among children of unknown race or ethnicity; information on race and ethnicity was available only for publicly insured children). The association of maternal age and birth year with incidence of neurodevelopmental disorders varied by outcome. Except for attention-deficit/hyperactivity disorder, the diagnosis tended to be established somewhat earlier for privately insured children. The association of race and ethnicity with age at diagnosis varied by outcome. Co-occurring neurodevelopmental disorders were common, especially among children with autism spectrum disorder and intellectual disability (>70% had ≥1 other disorder).
Conclusions and Relevance
In this population-based cohort study, a relatively high incidence of and co-occurrence of neurodevelopmental disorders as well as the disparity in incidence and timing of diagnosis by insurance type and race and ethnicity were found. These findings represent important public health concerns and underscore the need for timely and accessible developmental assessments and educational services to help reduce the burden of these disorders.
This cohort study of a nationwide sample of publicly and privately insured US children assesses the incidence and timing of diagnosis of neurodevelopmental disorders during childhood and evaluates differences by population characteristics.
Introduction
Neurodevelopmental disorders (NDDs) are characterized by deficits in the development of the central nervous system that can affect a child’s memory, language, motor function, and ability to learn, socialize, and maintain self-control. They frequently co-occur and may be associated with severe impairments of functioning in adulthood. Neurodevelopmental disorders are clinically diagnosed by evaluating the presence of aberrant behaviors or delays in achieving developmental milestones and by comparing such behaviors with those of children of the same age, sex, and culture.1
Existing data on the occurrence of NDDs are primarily from cross-sectional studies, surveys, or small prospective studies.2,3,4,5,6,7,8,9,10,11,12,13 Although cross-sectional studies provide snapshots of a single moment, they cannot easily define temporal trends and associations. Surveys can help quickly gather large data but are susceptible to limitations, such as survey fatigue, nonobjective responses, and low completion rates, raising concerns regarding the representativeness of the sample. Prospective cohort studies allow for close follow-up over time and are well suited to document subtle manifestations of NDDs (eg, abnormal cognitive test scores or behavior assessments), but they tend to be too small to evaluate the risk of more debilitating conditions, such as autism spectrum disorder (ASD), and can be challenged by substantial and potentially informative losses to follow-up, especially when cohorts are followed up from birth.
Because NDDs encompass a variety of phenotypes with multiple symptoms and severity levels, measurement varies considerably across studies, making comparisons difficult, especially because many studies do not report or insufficiently report on the validity of the diagnostic criteria and the algorithms used.14 Between-study comparisons are further hampered by differences in the study population, including the selected age range of children under study, the study period, and sociodemographic variations that could serve as barriers to diagnosis (eg, because of lack of access to specialized health services).2
Neurodevelopmental disorders are an important public health concern, and studies assessing risk factors at the population level are needed to help identify opportunities for prevention, early diagnosis, and intervention. Large-scale studies using data on nationwide health care use are well suited for this purpose. Such data sources provide prospectively collected, comprehensive real-world information on demographic characteristics and medical conditions for population-based cohorts and are free of some of the problems that affect other types of data (eg, response bias, small size, and lack of generalizability). Nevertheless, data are collected for administrative purposes. Thus, when used for research, algorithms need to be developed and applied to measure health conditions (such as NDDs) that might not perfectly reflect the patient’s actual status owing to variability in the quality of clinical diagnosis and recording. Using information from 2 large population-based health care databases and validated outcome algorithms, we sought to assess the nationwide incidence and timing of the diagnosis of specific NDDs and their co-occurrence in publicly and privately insured children in the US and to evaluate differences by population characteristics.
Methods
Data Source and Study Design
We conducted a cohort study of publicly and privately insured children nested in the 2000-2014 Medicaid Analytic eXtract (MAX) (the most recent years available at the time of study conduct) and the 2003-2015 IBM MarketScan Research Database (MarketScan). The development of the MAX mother-child cohort has been described previously.15 A similar approach was used for the development of the MarketScan cohort (for a detailed description of the cohorts, see eAppendix 1 in the Supplement). Linkage to maternal records allowed for the examination of the association of maternal characteristics, such as age, diabetes, and hypertension, with NDDs in offspring. Both data sources include rich patient-level information on demographic factors; diagnosis and procedure claims recorded during inpatient, outpatient, or emergency department visits; and outpatient prescription medication dispensings. Children were followed up from birth until their continuous eligibility ended, the study period ended, they developed the specific NDD of interest, they died, or they reached their 12th birthday (to harmonize the maximum follow-up in both cohorts), whichever came first. The research was approved by the institutional review board at Brigham and Women’s Hospital, which granted a waiver of informed consent because the data were deidentified. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Identification of NDDs
Outcomes of interest were the most common NDDs: (1) ASD, (2) attention-deficit/hyperactivity disorder and other hyperkinetic syndromes of childhood (ADHD), (3) learning disability, (4) developmental speech or language disorder, (5) developmental coordination disorder (DCD), (6) intellectual disability, (7) behavioral disorder (including disturbance of conduct and disturbance of emotions), and (8) any NDD (defined based on the presence of any of the specific 7 preceding disorders) (eTable 1 in the Supplement). The presence of the individual outcomes was assessed using validated claims-based algorithms.16
Patient Characteristics
We considered the presence of co-occurring NDDs, as well as demographic factors (including child’s sex, geographic region, birth year, and race and ethnicity). No information on race and ethnicity was available in MarketScan; in MAX, race and ethnicity were determined on the basis of information submitted to the Centers for Medicare & Medicaid Services by individual states, which was based on information that had been collected and coded from Medicaid applications. We also considered selected maternal and birth conditions previously reported to be associated with NDDs to evaluate whether such crude associations could be replicated in our data, thus supporting the validity of these data sources for studying NDDs. Maternal conditions included maternal age at delivery, preexisting hypertension, preexisting diabetes, gestational diabetes, and preeclampsia. Maternal age was assessed because mothers of older age are more likely to experience pregnancy or delivery complications that in turn might increase the risk of NDDs, their age might reflect a longer cumulative exposure to potential risk factors for NDDs (eg, environmental factors), and they might also be more likely to seek professional medical advice to evaluate their child’s behavior. Birth conditions included low birth weight, small size for gestational age, preterm birth (>28 and <37 weeks’ gestation), very preterm birth (≤28 weeks’ gestation), and neonatal hypoxia or asphyxia. The specific variables and their respective assessment periods are presented in the Table17 and eTable 2 in the Supplement.
Table. Select Patient Characteristics After 1:4 Matching by State and Follow-upa.
| Characteristic | Any specific NDD, No. (%) | ASD, No. (%) | ADHD, No. (%) | |||
|---|---|---|---|---|---|---|
| Children with the outcome of interestb | Children without any NDDc | Children with the outcome of interestb | Children without any NDDc | Children with the outcome of interestb | Children without any NDDc | |
| Public insurance | ||||||
| Total | 71 604 | 286 416 | 10 093 | 40 372 | 33 259 | 133 036 |
| Follow-up, mean (SD), y | 5.3 (2.1) | 5.2 (2.1) | 6.8 (2.8) | 6.7 (2.9) | 7.3 (2.0) | 7.23 (2.0) |
| Maternal age at delivery, mean (SD), y | 24.8 (5.9) | 24.7 (6.0) | 25.8 (6.2) | 24.7 (6.0) | 23.9 (5.5) | 24.6 (6.0) |
| Sex | ||||||
| Male | 47 193 (65.9) | 137 567 (48.0) | 7703 (76.3) | 18 869 (46.7) | 23 171 (69.7) | 61 979 (46.6) |
| Female | 23 409 (32.7) | 146 556 (51.2) | 2207 (21.9) | 21 181 (52.5) | 9678 (29.1) | 70 045 (52.7) |
| Unknown | 1002 (1.4) | 2293 (0.8) | 183 (1.8) | 322 (0.8) | 410 (1.2) | 1012 (0.8) |
| Region | ||||||
| Northeast | 16 899 (23.6) | 67 596 (23.6) | 2586 (25.6) | 10 344 (25.6) | 6010 (18.1) | 24 040 (18.1) |
| Midwest | 19 721 (27.5) | 78 884 (27.5) | 2997 (29.7) | 11 988 (29.7) | 9852 (29.6) | 39 408 (29.6) |
| South | 18 597 (26.0) | 74 388 (26.0) | 2689 (26.6) | 10 756 (26.6) | 11 066 (33.3) | 44 264 (33.3) |
| West | 16 387 (22.9) | 65 548 (22.9) | 1821 (18.0) | 7284 (18.0) | 6331 (19.0) | 25 324 (19.0) |
| Year of birth | ||||||
| Before 2006 | 16 913 (23.6) | 84 379 (29.5) | 3928 (38.9) | 18 752 (46.5) | 14 640 (44.0) | 61 943 (46.6) |
| 2006-2009 | 39 143 (54.7) | 149 064 (52.0) | 4353 (43.1) | 15 923 (39.4) | 17 675 (53.1) | 67 192 (50.5) |
| After 2009 | 15 548 (21.7) | 52 973 (18.5) | 1812 (18.0) | 5697 (14.1) | 944 (2.8) | 3901 (2.9) |
| Race and ethnicityd | ||||||
| Asian or Pacific Islander | 2084 (2.9) | 13 488 (4.7) | 322 (3.2) | 1688 (4.2) | 398 (1.2) | 5254 (4.0) |
| Black or African American | 19 963 (27.9) | 97 518 (34.1) | 2660 (26.4) | 15 036 (37.2) | 10 263 (30.9) | 52 515 (39.5) |
| Hispanic or Latino | 9452 (13.2) | 40 893 (14.3) | 1106 (11.0) | 4748 (11.8) | 3295 (9.9) | 16 655 (12.5) |
| White | 33 071 (46.2) | 10 8112 (37.8) | 4848 (48.0) | 15 402 (38.2) | 17 283 (52.0) | 49 933 (37.5) |
| Other or unknowne | 7034 (9.8) | 26 405 (9.2) | 1157 (11.5) | 3498 (8.7) | 2020 (6.1) | 8679 (6.5) |
| Maternal conditions | ||||||
| Preexisting hypertension | 2286 (3.2) | 6519 (2.3) | 385 (3.8) | 933 (2.3) | 1012 (3.0) | 3077 (2.3) |
| Preeclampsia | 4595 (6.4) | 14 408 (5.0) | 653 (6.5) | 2008 (5.0) | 2037 (6.1) | 6601 (5.0) |
| Preexisting diabetes | 2642 (3.7) | 7238 (2.5) | 444 (4.4) | 988 (2.5) | 1078 (3.2) | 3121 (2.4) |
| Gestational diabetes | 136 (0.2) | 422 (0.2) | 20 (0.2) | 57 (0.1) | 53 (0.2) | 202 (0.2) |
| Obstetric conditions | ||||||
| Low birth weight | 5805 (8.1) | 12 016 (4.2) | 762 (7.6) | 1724 (4.3) | 2107 (6.3) | 5765 (4.3) |
| Small for gestational age | 2943 (4.1) | 8406 (2.9) | 364 (3.6) | 1134 (2.8) | 1104 (3.3) | 3626 (2.7) |
| Neonatal hypoxia or asphyxia | 756 (1.1) | 2191 (0.8) | 108 (1.1) | 358 (0.9) | 337 (1.0) | 1187 (0.9) |
| Preterm birth (>28 and <37 wk gestation) | 10 846 (15.2) | 27 244 (9.5) | 1521 (15.1) | 3788 (9.4) | 4306 (13.0) | 12 809 (9.6) |
| Very preterm birth (≤28 wk gestation) | 1296 (1.8) | 1444 (0.5) | 146 (1.5) | 210 (0.5) | 318 (1.0) | 670 (0.5) |
| No. of specific NDDs, mean (SD) | 1.3 (0.6) | NA | 2.3 (1.1) | NA | 1.7 (0.8) | NA |
| Private insurancef | ||||||
| Total | 32 426 | 129 704 | 3847 | 15 388 | 8260 | 33 040 |
| Follow-up, mean (SD), y | 5.7 (2.7) | 5.6 (2.7) | 5.9 (2.4) | 5.9 (2.5) | 8.5 (1.9) | 8.4 (1.9) |
| Maternal age at delivery, mean (SD), y | 32.7 (4.7) | 32.3 (4.6) | 33.1 (4.8) | 32.4 (4.6) | 32.1 (4.8) | 32.2 (4.5) |
| Sex | ||||||
| Male | 22 229 (68.6) | 64 094 (49.4) | 3002 (78.0) | 7534 (49.0) | 5942 (71.9) | 16 045 (48.6) |
| Female | 9395 (29.0) | 64 461 (49.7) | 726 (18.9) | 7695 (50.0) | 2168 (26.3) | 16 710 (50.6) |
| Unknown | 802 (2.5) | 1149 (0.9) | 119 (3.1) | 159 (1.0) | 150 (1.8) | 285 (0.9) |
| Region | ||||||
| Northeast | 6075 (18.7) | 24 300 (18.7) | 973 (25.3) | 3892 (25.3) | 934 (11.3) | 3736 (11.3) |
| Midwest | 8649 (26.7) | 34 596 (26.7) | 825 (21.5) | 3300 (21.5) | 2140 (25.9) | 8560 (25.9) |
| South | 12 901 (39.8) | 51 604 (39.8) | 1398 (36.3) | 5592 (36.3) | 4440 (53.8) | 17 760 (53.8) |
| West | 4582 (14.1) | 18 328 (14.1) | 620 (16.1) | 2480 (16.1) | 699 (8.5) | 2796 (8.5) |
| Year of birth | ||||||
| Before 2006 | 4491 (13.9) | 20 395 (15.7) | 525 (13.7) | 2408 (15.7) | 2692 (32.6) | 10 629 (32.2) |
| 2006-2009 | 13 670 (42.2) | 57 992 (44.7) | 1731 (45.0) | 7227 (47.0) | 5019 (60.8) | 20 101 (60.8) |
| After 2009 | 14 265 (44.0) | 51 317 (39.6) | 1591 (41.4) | 5753 (37.4) | 549 (6.7) | 2310 (7.0) |
| Maternal conditions | ||||||
| Preexisting hypertension | 943 (2.9) | 2797 (2.2) | 121 (3.2) | 332 (2.2) | 225 (2.7) | 703 (2.1) |
| Preeclampsia | 2807 (8.7) | 7458 (5.8) | 361 (9.4) | 886 (5.8) | 694 (8.4) | 1835 (5.6) |
| Preexisting diabetes | 978 (3.0) | 2910 (2.2) | 141 (3.7) | 340 (2.2) | 270 (3.3) | 713 (2.2) |
| Gestational diabetes | 40 (0.1) | 169 (0.1) | 4 (0.1) | 14 (0.1) | 5 (0.1) | 45 (0.1) |
| Obstetric conditions | ||||||
| Low birth weight | 2512 (7.8) | 3958 (3.1) | 287 (7.5) | 451 (2.9) | 457 (5.5) | 980 (3.0) |
| Small for gestational age | 1372 (4.2) | 3345 (2.6) | 149 (3.9) | 374 (2.4) | 222 (2.7) | 626 (1.9) |
| Neonatal hypoxia or asphyxia | 291 (0.9) | 895 (0.7) | 38 (1.0) | 102 (0.7) | 58 (0.7) | 238 (0.7) |
| Preterm birth (>28 and <37 wk gestation) | 4847 (15.0) | 10 316 (8.0) | 577 (15.0) | 1239 (8.1) | 1009 (12.2) | 2575 (7.8) |
| Very preterm birth (≤28 wk gestation) | 674 (2.1) | 632 (0.5) | 77 (2.0) | 67 (0.4) | 96 (1.2) | 188 (0.6) |
| No. of specific NDDs, mean (SD) | 1.2 (0.5) | NA | 2.0 (0.8) | NA | 1.5 (0.8) | NA |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder or hyperkinetic syndrome of childhood; ASD, autism spectrum disorder or pervasive developmental disorder; LMP, last menstrual period; NA, not available; NDD, neurodevelopmental disorder.
The following assessment periods were used for the respective covariates: first date of LMP minus 90 days to LMP plus 90 days: preexisting hypertension; LMP minus 90 days to LMP plus 140 days: preexisting diabetes; LMP plus 140 days to delivery: gestational diabetes; LMP plus 140 days to delivery plus 30 days: preeclampsia; delivery to delivery plus 30 days: preterm or very preterm birth, low birth weight, and small for gestational age; and delivery hospitalization: neonatal hypoxia or asphyxia. Last menstrual period and gestational age at birth were estimated using a previously validated algorithm that is based on diagnostic codes for preterm birth recorded in either the maternal or infant claims within the first month after delivery or birth.17
Cohort includes all children with the specific NDD of interest.
Cohort consists of a sample of children without any NDD, which is defined using an extended definition that also includes other developmental, neurotic, and personality disorders (eTable 1 in the Supplement). Children with the specific NDD of interest and those without any NDD were 1:4 matched on state and duration of follow-up after birth (±60 days).
Race and ethnicity were determined on the basis of information submitted to the Centers for Medicare & Medicaid Services by individual states, which was based on information that had been collected and coded from Medicaid applications.
Race and ethnicity category “other or unknown” includes the following races and ethnicities: American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, Hispanic or Latino and 1 or more races, more than 1 race, and unknown. The category “Hispanic or Latino” includes Hispanic or Latino with no race information available, whereas “other or unknown” includes Hispanic or Latino with 1 or more races.
Information on race and ethnicity not available in the privately insured cohort (MarketScan).
Statistical Analysis
Data were analyzed between May 1, 2020, and June 30, 2021. All analyses were conducted separately for publicly (MAX) and privately (MarketScan) insured children. We used Kaplan-Meier analyses to estimate the risk of each outcome throughout childhood, plotted unadjusted cumulative incidence curves with 95% CIs accounting for losses to follow-up (ie, end of enrollment or end of data) (Figure 1 and Figure 2), and stratified analyses by demographic factors (eFigure 1 in the Supplement). To explore variations in age at diagnosis, we calculated the mean age and 95% CI at which the respective outcomes of interest (ie, NDDs) were first diagnosed, and we assessed differences in mean age by insurance type, child’s sex, maternal age, and race and ethnicity (Figure 3; eFigure 2 in the Supplement). Details on how these parameters were estimated are shown in eAppendix 2 in the Supplement.
Figure 1. Cumulative Incidence Curves for Any Neurodevelopmental Disorder (A), Autism Spectrum Disorder (B), Attention-Deficit/Hyperactivity Disorder (C), and Learning Disability (D) by Insurance Type.
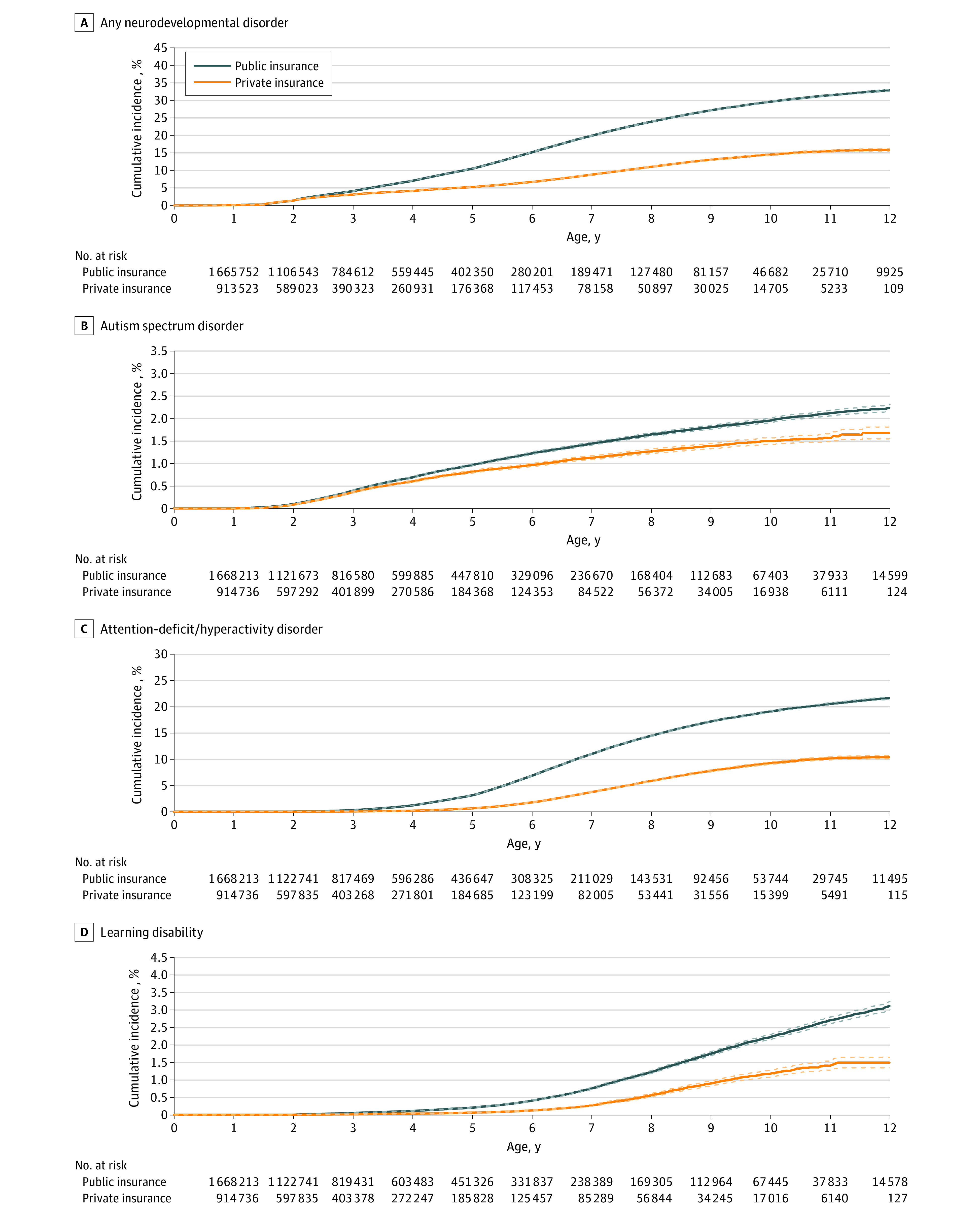
Solid lines indicate cumulative incidences, and dashed lines indicate 95% CIs.
Figure 2. Cumulative Incidence Curves for Developmental Speech or Language Disorder (A), Developmental Coordination Disorder (B), Intellectual Disability (C), and Behavioral Disorder (D) by Insurance Type.
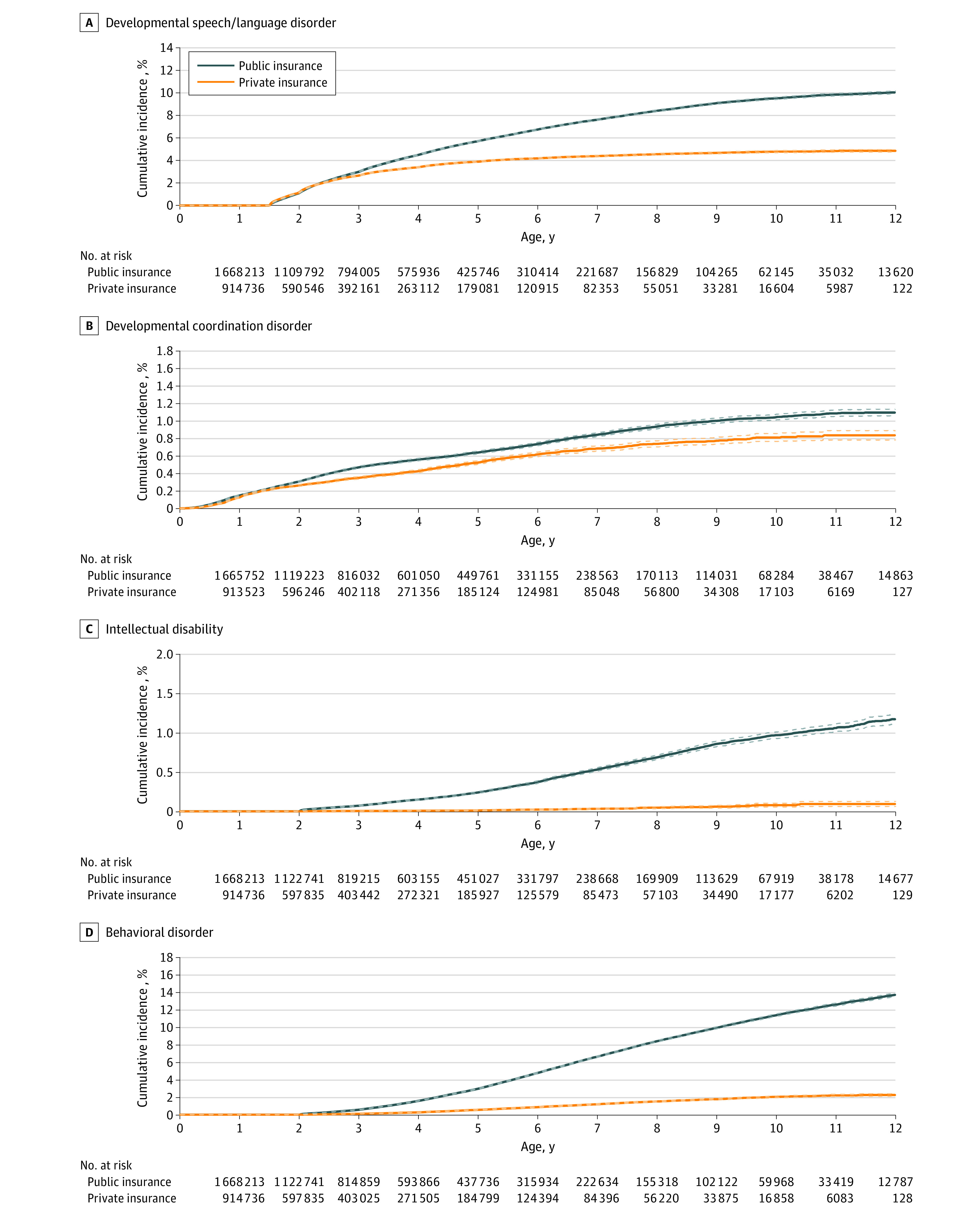
Solid lines indicate cumulative incidences, and dashed lines indicate 95% CIs.
Figure 3. Mean Age at Diagnosis of Specific Neurodevelopmental Disorder by Insurance Type.
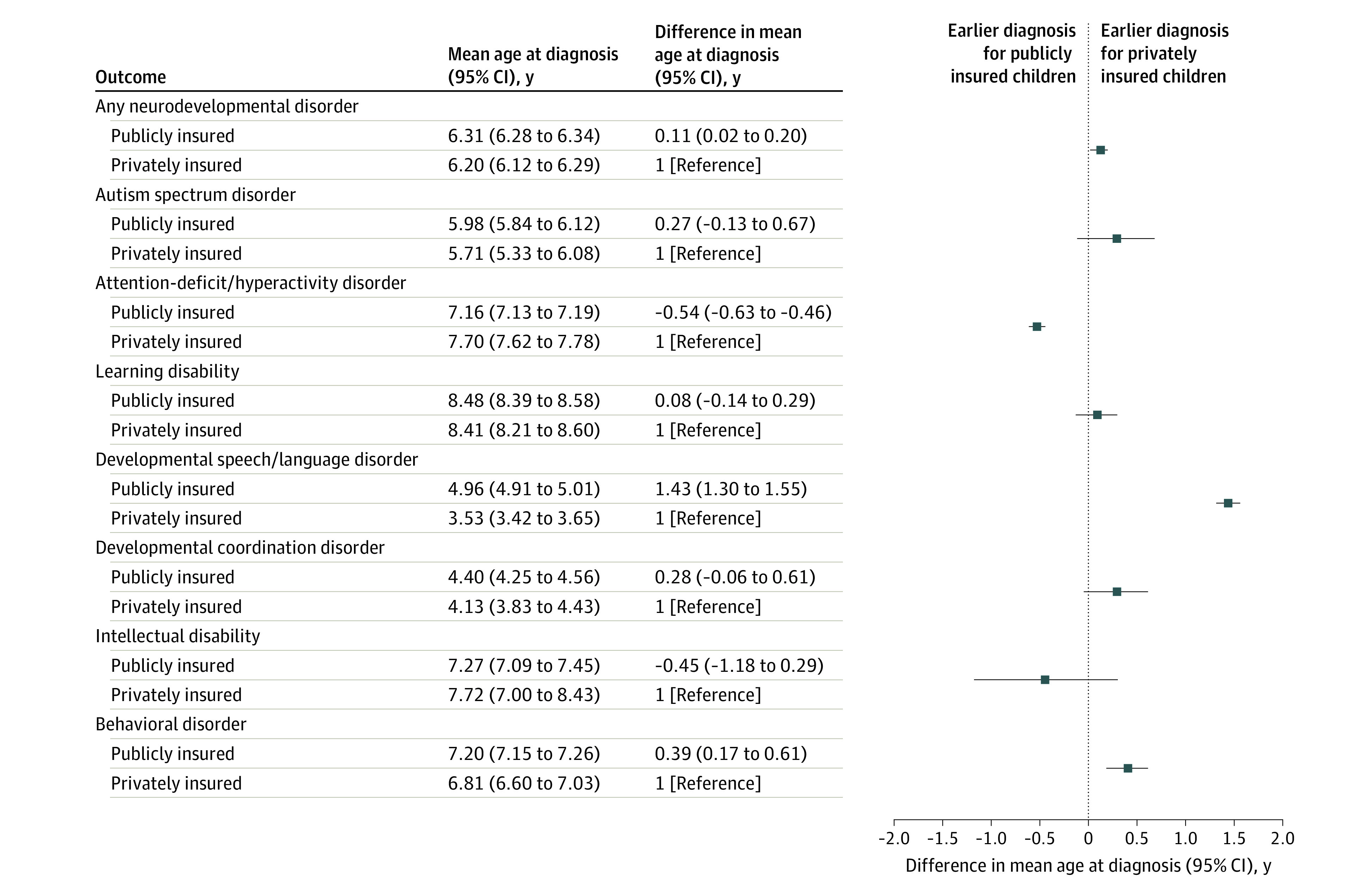
Characteristics were compared between children with the specific NDD of interest and children without any NDD after 1:4 matching by state (because Medicaid eligibility requirements can vary by state) (Table17; eTable 1 in the Supplement). Children without any NDD were identified using an extended definition that also included other neurodevelopmental, mental, or behavioral disorders (eTable 1 in the Supplement). They were further required to still be under follow-up (±60 days) at the age of first diagnosis of the matching case because children with shorter follow-up have less opportunity to receive a diagnosis of an NDD. We also evaluated the frequency of co-occurring NDDs.
There is potential for informative censoring because individuals who disenroll might be different from those who remain enrolled. Health policy and economic changes may affect who qualifies for public and private insurances and for how long people stay enrolled. In addition, children with more health issues and/or who are born to mothers with more comorbidities might be eligible for Medicaid for a longer period than healthier children. Thus, to assess whether such potentially informative loss to follow-up was present and might have been associated with the generalizability of our findings to the broader pediatric US population, we explored potential variations in selected patient characteristics by length of the child’s enrollment after birth in supplementary analyses (eFigure 3 and eTable 3 in the Supplement). All analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
The cohorts consisted of 2 070 541 publicly insured children (MAX; 1 045 426 boys [50.5%]) and 1 309 900 privately insured children (MarketScan; 667 607 boys [51.0%]) enrolled at birth. Of these, 54.2% (MAX) and 45.6% (MarketScan) were continuously followed up for at least the first 2 years of life. The corresponding proportions were 21.8% (MAX) and 14.2% (MarketScan) for 5 years and 8.3% (MAX) and 4.4% (MarketScan) for 8 years. There was no evidence of informative censoring due to loss of insurance coverage over time (eFigure 3 and eTable 3 in the Supplement).
Incidence of NDDs
By 8 years of age, 23.9% of publicly insured children and 11.0% of privately insured children received a diagnosis of 1 or more NDDs (Figure 1 and Figure 2). Incidences were 1.6% and 1.3% for ASD, 14.5% and 5.8% for ADHD, 1.2% and 0.6% for learning disability, 8.4% and 4.5% for speech or language disorder, 0.9% and 0.7% for DCD, 0.7% and 0.1% for intellectual disability, and 8.4% and 1.5% for behavioral disorder, respectively.
For all outcomes of interest, the incidence was much higher in boys than in girls (incidence of ≥1 NDD by age 8 years, 30.7% vs. 16.7% among publicly insured children and 15.0% vs. 6.7% among privately insured children), with the strongest discrepancy observed for ASD in both cohorts (2.5% vs. 0.7% among publicly insured children and 2.0% vs. 0.5% among privately insured children; eFigure 1 in the Supplement). Compared with children born in the early 2000s, those born in later years had slightly steeper incidence curves for ASD, speech or language disorder, and DCD (incidence of ASD by age 4 years [the highest age available in the data for those born in the more recent years] among children born before or during 2005 vs those born during or after 2010: 0.5% vs 1.0% among publicly insured children and 0.5% vs 0.7% among privately insured children), suggesting more and/or earlier diagnoses; they had slightly more shallow incidence curves for ADHD and intellectual disability (incidence of ADHD at age 4 years, 1.4% vs. 0.7% among publicly insured children and 0.2% vs. 0.1% among privately insured children), suggesting fewer and/or later diagnoses. We found no temporal trends for diagnoses of learning disability and behavioral disorder. These birth cohort patterns held up for both publicly and privately insured children (eFigure 1 in the Supplement). For publicly insured children, the incidence of any NDD was higher for those born to younger mothers, (incidence of any NDD by age 8 years among those born to mothers aged ≤24 years vs ≥35 years, 25.7% vs 18.9%), which was largely associated with ADHD and behavioral disorder. A similar difference by maternal age for any NDD was not observed among privately insured children (incidence of any NDD by age 8 years among those born to mothers aged ≤24 years vs ≥35 years, 10.4% vs. 11.7%). Autism spectrum disorder and DCD were diagnosed more commonly among the offspring of mothers aged 35 years or older in both cohorts (incidence of ASD by age 8 years among those born to mothers aged ≤24 years vs ≥35 years, 1.4% vs 2.3% among publicly insured children and 1.1% vs 1.6% among privately insured children). Speech or language disorders were more commonly diagnosed among privately insured children born to older mothers, but a similar pattern was not seen among publicly insured children (incidence by age 8 years among those born to mothers aged ≤24 years vs ≥35 years, 3.3% vs 5.3% among privately insured children and 8.3% vs 8.1% among publicly insured children). No clear differences by maternal age were seen for the other outcomes (eFigure 1 in the Supplement). We further found strong differences by race and ethnicity in the public insurance cohort, with all outcomes being least common among Asian children and most common among White children (incidence of any NDD by age 8 years among Asian vs White children, 9.1% vs. 30.2%), except for intellectual disability, which was most frequently diagnosed among children of Asian origin (1.5% among Asian children vs ≤0.9% among children of other races and ethnicities) (eFigure 1 in the Supplement).
Age at Diagnosis
Diagnoses were typically made earlier among privately insured children compared with publicly insured children, but differences were generally small and nonsignificant. The exception was ADHD, which was diagnosed, on average, half a year earlier, and speech or language disorders, which were diagnosed 1.5 years later among publicly insured children (Figure 3).
Attention-deficit/hyperactivity disorder and speech or language disorders tended to be diagnosed slightly earlier (3-4 months) among boys than girls, whereas DCD was diagnosed later, particularly among privately insured boys (1.4 years) (eFigure 2 in the Supplement). Overall, NDDs were diagnosed somewhat earlier in offspring of older mothers, but no consistent and clear pattern was observed across specific NDDs (eFigure 2 in the Supplement). Last, when assessing variations by race and ethnicity among publicly insured children, we found that ASD was consistently diagnosed later and ADHD was consistently diagnosed earlier in White children compared with children with other racial and ethnic backgrounds. Furthermore, Asian children were typically diagnosed earlier than White children for most outcomes, with the strongest discrepancy for DCD (mean, 2.7 years). Except for ADHD, there was no substantial difference in age at diagnosis between Black and White children for other specific NDDs (eFigure 2 in the Supplement).
Patient Characteristics
Compared with children without NDDs, those who received a diagnosis of an NDD were more likely to be born to mothers with preexisting diabetes (3.7% vs 2.5%), hypertension (3.2% vs 2.3%), and preeclampsia (6.4% vs 5.0%) (Table17; eTable 2 in the Supplement). Children who received a diagnosis of an NDD were more often White (MAX; 46.2% vs 37.8%), male (65.9% vs 48.0%), and born preterm (15.2% vs 9.5%), small for gestational age (4.1% vs 2.9%), and with low birth weight (8.1% vs 4.2%) compared with children without NDDs. These associations were observed for all specific disorders and were most pronounced for DCD.
Co-occurring NDDs
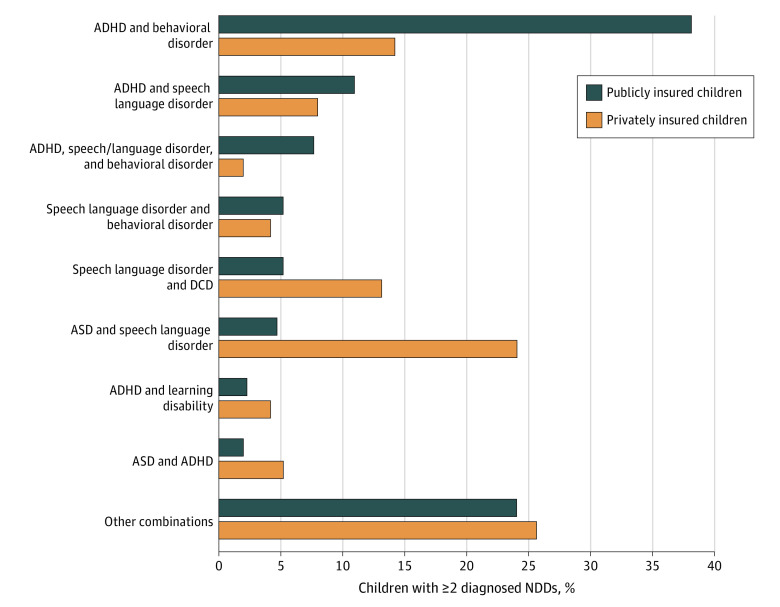
Co-occurring NDD diagnoses were frequent; 23.0% of the publicly insured and 17.9% of the privately insured children with NDDs had 1 or more other NDD. The highest frequency (>70%) of co-occurring NDDs was observed for children with either ASD or intellectual disability, with about half the children also receiving a diagnosis of speech or language disorder. Similarly, nearly half of all children with either a learning disability or a behavioral disorder also received a diagnosis of ADHD in both cohorts (eTable 2 in the Supplement). The most common combinations of NDDs are presented in Figure 4.
Figure 4. Most Common Combinations of Specific Neurodevelopmental Disorders (NDDs) Among Publicly and Privately Insured Children With 2 or More Diagnosed Disorders.
ADHD indicates attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; and DCD, developmental coordination disorder.
Discussion
Using large health care databases of private and public insurance beneficiaries, we described the incidence, age at first diagnosis, demographic variation, and co-occurrence of specific NDDs among children. By the age of 8 years, approximately 1 in 4 publicly insured and 1 in 9 privately insured children in the US received a diagnosis of an NDD, the risk of which was considerably higher among boys (both cohorts) and White children (public insurance cohort). The risk was largely associated with ADHD, speech or language disorders, and behavioral disorders. Although the incidence was lower for privately insured children, diagnoses were typically made somewhat earlier among privately insured children compared with publicly insured children. Co-occurring NDDs were common, with the highest frequency observed among children with ASD and intellectual disability (with >70% of these children having ≥1 other NDD).
Zablotsky and colleagues2 recently investigated the prevalence and patterns of a range of NDDs among children 3 to 17 years of age using data from the 2009-2017 National Health Interview Survey (NHIS), an annual survey intended to provide nationally representative estimates. The authors reported an overall prevalence of any NDD (defined as ASD, ADHD, learning disability, intellectual disability, blindness, cerebral palsy, hearing loss, seizures, stuttering or stammering, and other developmental delay) of 16.9% and, similar to our results, found that boys, non-Hispanic White children, children with low birth weight, and children with public insurance were more likely to receive a diagnosis of an NDD. They reported a prevalence of 13.8% for any NDD among privately insured children (vs an incidence of 11.0% by 8 years of age in our study) and of 21.8% among publicly insured children (vs 23.9% in our study). However, direct comparisons should be made with caution. The NHIS data were used to estimate the prevalence among 3- to 17-year-old children at the time of the survey, whereas we report the incidence up to a certain age for a cohort followed up from birth. The NHIS includes disabilities that can be caused by general medical conditions (eg, blindness and hearing loss) but do not specifically contain information about DCD, behavioral disorder, and speech or language disorder (other than stuttering or stammering).
Beyond the NHIS, other data on NDDs are typically derived from surveys (eg, the US National Survey of Children’s Health3 and the National Center for Learning Disabilities survey4), network programs (eg, the Centers for Disease Control and Prevention–funded Autism and Developmental Disabilities Monitoring Network5 and the Metropolitan Atlanta Developmental Disabilities Surveillance Program6,7,8), and prospective longitudinal or cross-sectional studies.9,10,11,12,13 Corroboration of the observed NDD patterns is challenging owing to differences in study design, outcome ascertainment, assessment period, and population characteristics. For instance, with respect to outcome ascertainment, DCD has previously been reported in 1.4% to 19% of all children, depending on whether the definition is restricted to severe coordination difficulties that interfere with activities of daily living or based on failed standardized tests of motor coordination.9,10,11,12 Authors of a recent systematic review reported that, among 110 included studies on child neurodevelopment after in utero exposure to psychotropics or analgesics, outcomes were assessed with 47 different psychometric instruments and 13 diagnostic algorithms, with most studies not reporting or insufficiently reporting on the validity of these measures.14 Although administrative databases have previously been used to assess NDDs during childhood, such studies usually focused on prevalence rather than cumulative incidence or on a single or a small group of conditions.18,19,20,21,22
Strengths and Limitations
This study has some strengths. We used large established nationwide population-based birth cohorts of publicly and privately insured children (linked to their mothers), which are broadly representative of the entire pediatric population in the US. This allowed us to directly compare the incidence of specific NDDs ascertained using previously validated algorithms between these 2 insurance types and to explore diagnosis patterns up to 12 years of age, given the longitudinal nature of the data.
Despite these strengths, our study is subject to some limitations. Owing to a significant lag time in the availability of Medicaid data, nationwide data were available up to 2014 at the time the study was conducted. Our data therefore represent the most recent nationwide estimates available for the Medicaid-insured population. We chose a similar study period for the MarketScan cohort to facilitate the comparison between publicly and privately insured children. Second, when conducting research using data on health care use, we infer absence of the condition based on absence of a recorded diagnosis. Neurodevelopmental disorders that were not recorded or did not meet our highly specific definition were missed, which could have resulted in an underestimation of the true incidence. On the other hand, relying on diagnostic codes alone might have led to inclusion of children with diagnostic codes for NDDs who would not have been classified as having the outcome based on clinical guidelines.23 However, the use of stricter algorithm requirements with high positive predictive values reduced the potential for the inclusion of coding errors, ruled-out diagnoses, and minor developmental issues and allowed us to focus on more clinically significant conditions. Finally, while we examined co-occurrence of multiple NDDs, we were not able to assess whether diagnoses of different NDDs were perhaps manifestations of the same underlying disorder.
Conclusions
Our findings have research, clinical, and policy implications. This study demonstrates the feasibility and advantages of using birth cohorts nested in health care databases to study NDDs. It has laid the groundwork that will enable research to increase our understanding of the role of factors such as maternal morbidities and advanced paternal age (which has been suggested to be associated with higher risk of ASD24) and to identify potential modifiable risk factors such as nutrition, drugs of abuse, and other in utero drug exposures. The available information on environmental factors during critical periods of development and growth, coupled with information on the timing of the diagnosis of health outcomes, should also support research related to the Developmental Origins of Health and Disease hypothesis.25 Our findings that publicly insured children tended to receive a diagnosis of NDDs somewhat later than privately insured children and that there were substantial racial and ethnic discrepancies in both incidence and age at diagnosis—even within a cohort where all children are publicly insured, and thus, expected to be quite similar in terms of socioeconomic status—point toward disparities in health service access and use, as has previously been reported for ASD,26,27 as well as to the overdiagnosis and/or underdiagnosis of these disorders in certain racial and ethnic groups. As a next step, it will therefore be important to explore potential associated factors, such as culturally different perspectives on child behavior, communication barriers, and differences in access to care. The difference in age at diagnosis across cohorts could also point to differences in the distribution of underlying causes and in the severity of NDDs between publicly and privately insured cohorts. For instance, genetic causes might be associated with potentially more severe NDDs that are apparent early in life, whereas environmental causes (such as childhood infections, malnutrition, and maltreatment) could be associated with NDDs that are less severe and diagnosed later in life. Understanding the exact mechanisms associated with the discrepancies in the timing of diagnosis will be an important focus for future work. The substantially higher incidence and the tendency to earlier diagnosis in boys vs girls is not necessarily associated with higher susceptibility of boys to these disorders but might be partially due to symptoms presenting differently in boys and girls, which may be associated with both parental health care–seeking behaviors and clinician diagnostic behaviors. For instance, girls tend to have more subtle symptoms of ADHD that are harder to identify. Consequently, these girls might be underdiagnosed and treated insufficiently, which could further cause additional issues in the future (eg, development of mental health disorders).28,29
The high incidence of NDDs, coupled with racial and sociodemographic disparities, underscores the importance of raising awareness of providing universal and timely access to psychological and educational services to ensure that diagnosis, intervention, and support can start as soon as possible for all children with NDDs, with the goal of preventing and/or mitigating long-term developmental deficits and economic or other burdens on the family and society.
eTable 1. Definition of Neurodevelopmental Disorders of Interest
eTable 2. Patient Characteristics Among Children With the Specific Neurodevelopmental Disorder of Interest vs Children Without Any Neurodevelopmental Disorders, After 1:4 Matching on State and Follow-up, Separately for Publicly and Privately Insured Children
eTable 3. Patient Characteristics Among Children, Stratified by Length of Continuous Enrollment, Separately for A) Publicly Insured Children and B) Privately Insured Children
eFigure 1. Cumulative Incidence Curves for Each of the Specific Neurodevelopmental Disorders of Interest, Stratified by Insurance Type, Child’s Sex, Year of Birth, Maternal Age, and Race/Ethnicity
eFigure 2. Children’s Mean Age in Years (95% Confidence Interval) at Diagnosis of Each of the Specific Neurodevelopmental Disorders of Interest, Separately for Publicly and Privately Insured Children, Stratified by A) Child’s Sex, B) Maternal Age, and C) Race/Ethnicity (Public Insurance Only)
eFigure 3. Length of Continuous Enrollment Stratified by Birth Year, Among A) Publicly Insured and B) Privately Insured Children
eAppendix 1. Description and Patient Characteristics of the Publicly and Privately Insured Mother-Child Linked Cohorts
eAppendix 2. Calculation of Mean Age at Diagnosis and 95% Confidence Intervals Accounting for Loss to Follow up
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 2.Zablotsky B, Black LI, Maenner MJ, et al. Prevalence and trends of developmental disabilities among children in the United States: 2009-2017. Pediatrics. 2019;144(4):e20190811. doi: 10.1542/peds.2019-0811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kogan MD, Vladutiu CJ, Schieve LA, et al. The prevalence of parent-reported autism spectrum disorder among US children. Pediatrics. 2018;142(6):e20174161. doi: 10.1542/peds.2017-4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortiella C, Horowitz SH. The State of Learning Disabilities: Facts, Trends and Emerging Issues. National Center for Learning Disabilities; 2014. [Google Scholar]
- 5.Maenner MJ, Shaw KA, Baio J, et al. Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2016. MMWR Surveill Summ. 2020;69(4):1-12. doi: 10.15585/mmwr.ss6904a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyle CA, Yeargin-Allsopp M, Doernberg NS, Holmgreen P, Murphy CC, Schendel DE. Prevalence of selected developmental disabilities in children 3-10 years of age: the Metropolitan Atlanta Developmental Disabilities Surveillance Program, 1991. MMWR CDC Surveill Summ. 1996;45(2):1-14. [PubMed] [Google Scholar]
- 7.Bhasin TK, Brocksen S, Avchen RN, Van Naarden Braun K. Prevalence of four developmental disabilities among children aged 8 years—Metropolitan Atlanta Developmental Disabilities Surveillance Program, 1996 and 2000. MMWR Surveill Summ. 2006;55(1):1-9. [PubMed] [Google Scholar]
- 8.Van Naarden Braun K, Christensen D, Doernberg N, et al. Trends in the prevalence of autism spectrum disorder, cerebral palsy, hearing loss, intellectual disability, and vision impairment, metropolitan Atlanta, 1991-2010. PLoS One. 2015;10(4):e0124120. doi: 10.1371/journal.pone.0124120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lingam R, Hunt L, Golding J, Jongmans M, Emond A. Prevalence of developmental coordination disorder using the DSM-IV at 7 years of age: a UK population-based study. Pediatrics. 2009;123(4):e693-e700. doi: 10.1542/peds.2008-1770 [DOI] [PubMed] [Google Scholar]
- 10.Tsiotra GD, Flouris AD, Koutedakis Y, et al. A comparison of developmental coordination disorder prevalence rates in Canadian and Greek children. J Adolesc Health. 2006;39(1):125-127. doi: 10.1016/j.jadohealth.2005.07.011 [DOI] [PubMed] [Google Scholar]
- 11.Kadesjö B, Gillberg C. Developmental coordination disorder in Swedish 7-year-old children. J Am Acad Child Adolesc Psychiatry. 1999;38(7):820-828. doi: 10.1097/00004583-199907000-00011 [DOI] [PubMed] [Google Scholar]
- 12.Wright HC, Sugden DA. A two-step procedure for the identification of children with developmental co-ordination disorder in Singapore. Dev Med Child Neurol. 1996;38(12):1099-1105. doi: 10.1111/j.1469-8749.1996.tb15073.x [DOI] [PubMed] [Google Scholar]
- 13.de Milander M, Coetzee F, Venter A. Developmental coordination disorder in grade 1 learners. S Afr J Res Health Phys Education Recreation. 2014;2031(2031):1075-1085. [Google Scholar]
- 14.Hjorth S, Bromley R, Ystrom E, Lupattelli A, Spigset O, Nordeng H. Use and validity of child neurodevelopment outcome measures in studies on prenatal exposure to psychotropic and analgesic medications—a systematic review. PLoS One. 2019;14(7):e0219778. doi: 10.1371/journal.pone.0219778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmsten K, Huybrechts KF, Mogun H, et al. Harnessing the Medicaid Analytic eXtract (MAX) to evaluate medications in pregnancy: design considerations. PLoS One. 2013;8(6):e67405. doi: 10.1371/journal.pone.0067405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Straub L, Bateman BT, Hernandez-Diaz S, et al. Validity of claims-based algorithms to identify neurodevelopmental disorders in children. Pharmacoepidemiol Drug Saf. 2021;30(12):1635-1642. doi: 10.1002/pds.5369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margulis AV, Setoguchi S, Mittleman MA, Glynn RJ, Dormuth CR, Hernández-Díaz S. Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiol Drug Saf. 2013;22(1):16-24. doi: 10.1002/pds.3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Department of Education . 41st Annual report to Congress on the implementation of the Individuals with Disabilities Education Act, parts B and C: 2019. Accessed November 21, 2021. https://www2.ed.gov/about/reports/annual/osep/2019/parts-b-c/index.html
- 19.Centers for Disease Control and Prevention . Autism data visualization tool. Accessed October 11, 2020. https://www.cdc.gov/ncbddd/autism/data/index.html
- 20.Visser SN, Danielson ML, Wolraich ML, et al. Vital signs: national and state-specific patterns of attention deficit/hyperactivity disorder treatment among insured children aged 2-5 years—United States, 2008-2014. MMWR Morb Mortal Wkly Rep. 2016;65(17):443-450. doi: 10.15585/mmwr.mm6517e1 [DOI] [PubMed] [Google Scholar]
- 21.Committee to Evaluate the Supplemental Security Income Disability Program for Children with Mental Disorders; Board on the Health of Select Populations; Board on Children, Youth, and Families; Institute of Medicine; Division of Behavioral and Social Sciences and Education; The National Academies of Sciences, Engineering, and Medicine . Mental Disorders and Disabilities Among Low-Income Children. National Academies Press; 2015. [PubMed] [Google Scholar]
- 22.Qiu C, Lin JC, Shi JM, et al. Association between epidural analgesia during labor and risk of autism spectrum disorders in offspring. JAMA Pediatr. 2020;174(12):1168-1175. doi: 10.1001/jamapediatrics.2020.3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolraich ML, Bard DE, Stein MT, Rushton JL, O’Connor KG. Pediatricians’ attitudes and practices on ADHD before and after the development of ADHD pediatric practice guidelines. J Atten Disord. 2010;13(6):563-572. doi: 10.1177/1087054709344194 [DOI] [PubMed] [Google Scholar]
- 24.Lyall K, Song L, Botteron K, et al. The association between parental age and autism-related outcomes in children at high familial risk for autism. Autism Res. 2020;13(6):998-1010. doi: 10.1002/aur.2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27(5):358-368. doi: 10.1055/s-0029-1237424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broder-Fingert S, Mateo CM, Zuckerman KE. Structural Racism and Autism. Pediatrics. 2020;146(3):e2020015420. doi: 10.1542/peds.2020-015420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Constantino JN, Abbacchi AM, Saulnier C, et al. Timing of the diagnosis of autism in African American children. Pediatrics. 2020;146(3):e20193629. doi: 10.1542/peds.2019-3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rucklidge JJ. Gender differences in attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2010;33(2):357-373. doi: 10.1016/j.psc.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 29.Hasson R, Fine JG. Gender differences among children with ADHD on continuous performance tests: a meta-analytic review. J Atten Disord. 2012;16(3):190-198. doi: 10.1177/1087054711427398 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Definition of Neurodevelopmental Disorders of Interest
eTable 2. Patient Characteristics Among Children With the Specific Neurodevelopmental Disorder of Interest vs Children Without Any Neurodevelopmental Disorders, After 1:4 Matching on State and Follow-up, Separately for Publicly and Privately Insured Children
eTable 3. Patient Characteristics Among Children, Stratified by Length of Continuous Enrollment, Separately for A) Publicly Insured Children and B) Privately Insured Children
eFigure 1. Cumulative Incidence Curves for Each of the Specific Neurodevelopmental Disorders of Interest, Stratified by Insurance Type, Child’s Sex, Year of Birth, Maternal Age, and Race/Ethnicity
eFigure 2. Children’s Mean Age in Years (95% Confidence Interval) at Diagnosis of Each of the Specific Neurodevelopmental Disorders of Interest, Separately for Publicly and Privately Insured Children, Stratified by A) Child’s Sex, B) Maternal Age, and C) Race/Ethnicity (Public Insurance Only)
eFigure 3. Length of Continuous Enrollment Stratified by Birth Year, Among A) Publicly Insured and B) Privately Insured Children
eAppendix 1. Description and Patient Characteristics of the Publicly and Privately Insured Mother-Child Linked Cohorts
eAppendix 2. Calculation of Mean Age at Diagnosis and 95% Confidence Intervals Accounting for Loss to Follow up