Abstract
Purpose of review:
To provide the current state of the development and application of cardiovascular disease (CVD) prediction tools in people living with HIV (PLWH).
Recent findings:
Several risk prediction models developed on the general population are available to predict CVD risk, the most notable being the US-based pooled cohort equations (PCE), the Framingham risk functions, and the Europe-based SCORE (Systematic COronary Risk Evaluation). In validation studies in cohorts of PLWH, these models generally underestimate CVD risk, especially in individuals who are younger, women, Black race or predicted to be at low/intermediate risk. An HIV-specific CVD prediction model, the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) model, is available, but its performance is modest, especially in US-based cohorts. Enhancing CVD prediction with novel biomarkers of inflammation or coronary artery calcification is of interest but has not yet been evaluated in PLWH. Finally, studies on CVD risk prediction are lacking in diverse PLWH globally.
Summary:
While available risk models for CVD prediction in PLWH remain suboptimal, clinicians should remain vigilant of higher CVD risk in this population and should use any of these risk scores for risk-stratification to guide preventive interventions. Focus on established traditional risk factors such as smoking remains critical in PLWH. Risk prediction functions tailored to PLWH in diverse settings will enhance clinicians’ ability to deliver optimal preventive care.
Keywords: HIV, cardiovascular, risk, prediction, Framingham, ASCVD
INTRODUCTION
While people living with HIV (PLWH) with access to antiretroviral therapy (ART) continue to live longer and with improved quality of life, they have a high burden of comorbidities, including cardiovascular disease (CVD).1,2 Compared to the general population, PLWH are at approximately 1.5 to 2 times higher risk of CVD, which they tend to experience at a relatively younger age.2-4 From 1990-2015, the global population-attributable fraction and disability-adjusted life years of HIV-associated CVD have increased nearly 3-fold and are expected to rise with aging of this population.4 The cause of this increased risk is likely multi-factorial: HIV associated immune dysfunction and inflammation, higher rates of traditional risk factors in PLWH (e.g. smoking), adverse effects of ART including metabolic effects, and higher prevalence of socio-economic disadvantage in this population.5-7 Particularly intriguing and a core issue in regards to CVD in PLWH is the prospect of unique drivers of CVD risk in HIV that may not be present or contribute to CVD risk in the general population and that are not included in established CVD risk prediction paradigms.8
In an effort to prevent CVD in the context of unique factors promoting risk in the HIV population, there has been an intense interest in the evaluation and development of CVD risk prediction tools in PLWH over the last decade. Cardiovascular risk prediction tools, first developed through the Framingham Heart Study,9-11 can enhance clinical decision-making beyond clinical judgement, especially in less clinically apparent scenarios, such as primary prevention. When used in clinical practice, risk prediction tools may improve clinicians’ ability to identify high-risk individuals who could be targeted for interventions while also having the potential to inform further insight into risk level. Moreover, by providing a risk estimate of an outcome, risk prediction can assist in patient behavioral counseling, for example when addressing behavioral risk factors such as smoking. Risk prediction can also advance policy decision-making when surveyed over a large population sample, helping guide investment, education and focused interventions. Overall, risk assessment holds promise as an important tool in reducing the incidence of CVD in PLWH. While the utility of risk prediction tools in general has not been evaluated in large, prospective randomized trials, they are widely utilized in clinical care and in the context of CVD prevention and are recommended by multiple guidelines.12,13 This assumption of benefit, however, is predicated on such predictive tools being accurate, which in the HIV population remains in doubt.
The purpose of this review is to provide an overview of the current state of CVD prediction in PLWH. We first discuss the basics of how to appraise a prediction model. We then consider the characteristics, performance and current limitations of common CVD risk prediction tools that have been evaluated in PLWH. We focus on prospective studies from the last 3-4 years that have evaluated clinical endpoints for which models were originally developed. Understanding the performance and limitations of cardiovascular risk prediction functions in HIV is critical because established functions do not include novel, HIV-specific risk factors and thus may underestimate risk and incorrectly discriminate. Tailoring risk prediction functions to include HIV-specific factors may improve their ability to accurately classify and predict risk and enhance cardiovascular disease prevention in this population.
APPRAISING A PREDICTION MODEL
A prediction model is a mathematical equation based on risk factor data that predicts the probability of occurrence of an event of interest in a given time period, as usually defined through studies in epidemiologic and other larger cohort studies. Prediction models enable clinicians to estimate an overall risk of an outcome representing a composite of individual risk factors. While details of appraising a prediction model are beyond the scope of this review and are reviewed elsewhere,14-17 we highlight some of the important features referred to in this review.
Model calibration
Model calibration refers to the ability of a model to predict the actual observed risk of an event over a given time period.16 The closer the observed risk is to the predicted risk, the better the model is said to be calibrated. If a model predicts the risk of an event to be >10% over a year for a group of individuals with a given set of risk factors, but the observed risk is only 5% in that group, then it would be considered as poorly calibrated. A graph of observed risk of an event over categories (e.g. deciles) of predicted risk provides an informative and intuitive assessment of the model performance. Calibration is assessed using a chi square statistic.18 Good model calibration is important when an actual estimate of risk is needed, such as when making decisions on preventative interventions.
Model discrimination
Model discrimination refers to the ability of a model to accurately assess high-risk and low-risk individuals – and perhaps especially intermediate risk individuals – while also distinguishing among these groups. Model discrimination is commonly measured as the area under receiver operating curve (AUC) or Concordance (C)-statistic.17 A C-statistic value of 0.5 indicates the model cannot discriminate more than at random; a value of ≥0.7 is generally considered useful; and a value of ≥0.8 is considered excellent and is rarely achieved.17 For example, the C-statistic of the Pooled Cohort Equations (PCE) score recommended by the American Heart Association (AHA) for predicting CVD ranges between 0.7 to 0.8 in the original cohort depending on race and sex. Arguably, the discrimination of a model is more important than its calibration; clinicians are often interested in identifying intermediate- or high-risk individuals correctly (e.g. 10-year risk of CVD ≥7.5% for using lipid-lowering therapies), but not necessarily in accurately predicting risk (e.g. it may not impact clinical decision making if the actual risk is 8% versus 15% in the above example as long as the model correctly identifies the individual as high-risk).
Validation
Ultimately, the true value of a model is assessed by its performance (e.g. calibration and discrimination) when applied to real-world cohorts outside of the settings in which the model was developed.15,17 It is accepted that models would perform predictably well in populations similar to those in which the models were derived. External validation of prediction models can yield humbling results, often due to different patient characteristics, frequency of risk factors, and other unknown factors in discrete populations. Poor external validation provides an opportunity to further investigate risk factors for a disease and to consider developing more subgroup-specific prediction models (e.g. PCE model provides separate equations by race and sex).19
Other key characteristics of a prediction model
The quality of both original and validation studies is understandably important and is reflected in factors such as a representative study population, prospective nature, robust ascertainment of risk factor and outcome measurements, and low loss to follow-up.17 Finally, a prediction model is only useful if it is simple to use, if risk factors in the model are routinely measured, and if appropriate interventions are available for high-risk individuals (e.g. more intensive behavioral counselling and lipid-lowering therapy in the case of preventing CVD).13
OVERVIEW OF COMMONLY USED CVD PREDICTION MODELS IN PLWH
Over 350 models have been developed for CVD risk prediction in the general population,20 of which very few have been evaluated independently in cohorts of PLWH. The Framingham Heart Study (FHS) risk prediction models, developed on a multi-generational population from Framingham, Massachusetts (US), have now been widely used for several decades.8,9,21,22 The Framingham group developed and evaluated CVD risk prediction functions, addressing the question of whether individual risk factors could be combined into a multifactorial function to assess CVD risk over a given time period and representing a paradigm shift in conceptualizing risk. Framingham risk functions have been developed for multiple endpoints that include hard coronary heart disease, stroke and global cardiovascular disease. The updated FHS model for the composite endpoint of cardiovascular disease (FHS-CVD in Table 1) in 2008 has been adopted by contemporary guidelines.21 More recently, the American College of Cardiology (ACC) and the AHA developed the PCE in 2013, based on several large diverse community-based US cohorts (PCE in Table 1) and validated externally.19 The prediction tools built on the Framingham functions by including stroke in addition to hard coronary events and by generating separate equations by race in addition to by sex. The PCE equations have been endorsed by multiple guidelines, although not without extensive discussion regarding possible over- and under-estimation of risk.23 Similarly, the SCORE (Systematic COronary Risk Evaluation) model was developed by the European union based on multiple European cohorts.24 All of these prediction tools predict hard CVD endpoints: a composite of myocardial infarction, stroke (fatal or non-fatal for PCE; fatal only for SCORE) and in the case of FHS-CVD, other atherosclerotic endpoints (Table 1). The FHS-CVD, PCE, and SCORE models have been most commonly evaluated in HIV cohorts. There are subtle variations in the risk factors incorporated and in the CVD outcomes predicted. Table 1 reviews the key features of these models and population characteristics in the original publication. Of note, the PCE model was disseminated in updated guidelines that incorporated a clinical approach to risk assessment, identifying four patient groups who warranted LDL-C lowering and statin therapy independent of any risk score: known ASCVD, diabetes, LDL-C > 190 mg/dL or primary prevention with calculated risk of 7.5% or greater over 10 years.25 This strategy highlights the complexity of the clinical application of risk prediction scores in the general population, a process likely to be even more complicated in the setting of HIV where risk factors differ and where the role of statins – especially in low to moderate risk individuals – is still being delineated.
Table 1:
Summary of CVD Risk Prediction Models Commonly Used in PLWH
| Model | Source population | Mean age (years) |
Females | Black race | Current or former smoker |
Risk factors | Predicted outcome |
|---|---|---|---|---|---|---|---|
| Framingham Heart Study (FHS)- CVD21 | US adults from the Framingham Heart Study Original and Offspring Cohorts (Framingham, MA, US) | 49 | 50% | <10% | 35% | Age, SBP, BP Rx, smoking, total cholesterol, HDL-C, diabetes (sex-specific estimates) | Composite CHD (coronary death, MI, coronary insufficiency, angina), cerebrovascular events (stroke, TIA), PAD, heart failure |
| ACC/AHA Pooled Cohort equations (PCE)19 | US adults from 4 cohorts | 40-70 (variable by cohort) | 56% | 21% | 24-30% | Age, SBP, BP Rx, smoking, total cholesterol, HDL-C, diabetes (sex- and race-specific estimates) | MI, fatal or nonfatal stroke, CHD death |
| Systematic COronary Risk Evaluation (SCORE) high-risk Equation (SCORE)24 | Adults from 12 European countries | Variable by cohort | 43% | Not reported | 20-40% in women, 40-60% in men | Age, sex, SBP, smoking, total cholesterol | CHD death, fatal stroke |
| Data Collection on Adverse Effects of Antiretroviral Drugs (D: A:D) Study model35 | HIV-infected adults from clinics in the U.S., Europe, Argentina, and Australia | 39 | 26% | Not reported | 69% | Age, sex, SBP, smoking, total cholesterol, diabetes, ever smoke, family history of CVD, CD4 count, years of use of PIs and NRTIs, current abacavir use | Composite CVD (fatal or nonfatal MI including sudden death, stroke, TIA, invasive coronary artery procedures including CABG, angioplasty, death from other CHD) |
Note: Summary statistics provided are approximate and are taken from source publications of the study. CHD death included fatal MI, peripheral vascular disease (PVD), or coronary artery disease (CAD). PVD and CAD diagnoses were confirmed on the basis of aortography, angiography, or arterial Doppler. Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; BP Rx, blood pressure treatment; CABG, coronary artery bypass graft; CHD, coronary heart disease; CVD, cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; PAD, peripheral arterial disease; SBP, systolic blood pressure; TIA, transient ischemic attack; NRTIs, nucleoside reverse transcriptase inhibitors; PIs, protease inhibitors.
Performance of general population CVD prediction models in cohorts of PLWH
While multiple studies have applied CVD risk prediction models in PLWH or assessed concordance among models, relatively few studies have formally assessed model performance in cohorts of PLWH. Table 2 summarizes key prospective studies that have evaluated CVD prediction models in PLWH. The performance of these models in cohorts of PLWH has generally demonstrated suboptimal discrimination and calibration, although performance varies by model and within subgroups. In a large, multi-site, US-based cohort, Centers for AIDS Research Network of Integrated Clinical Systems (CNICS), the PCE showed good discrimination of CVD risk overall (C-statistic 0.75), but only modest calibration in regards to accuracy.26 Overall, in HIV, the PCE underestimated the risk of CVD, particularly in individuals who were female, Black, or predicted to be at low/moderate risk of CVD. In a Boston-based cohort of over 1200 men, FHS-CVD and PCE showed modest discrimination (C-statistic of 0.67 and 0.65 respectively) and both systematically underestimated CVD risk, particularly in low/moderate risk groups (Figure 1).27 Notably, recalibrating the original FHS-CVD or PCE equations did not significantly improve model performance, suggesting that unique HIV-associated risk-factors could be conferring risk. In another US-based study of the HIV Outpatients Study (HOPS) cohort, PCE and the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) model (discussed in detail below) underestimated risk, and FHS-CVD and SCORE showed modest and poor discrimination (Table 2).28 Most recently, in a large population-based Dutch cohort which was predominantly White with high rates of viral suppression, all of the prediction scores discriminated well, with acceptable C-statistics (e.g. 0.76 for PCE).29 All models demonstrated statistically significant chi-square P values indicating inadequate calibration, but model fit was poorest for SCORE. PCE, D:A:D, and SCORE underestimated risk in low/moderate predicted risk groups, whereas FHS-CVD overestimated risk, especially in groups with high predicted risk.29
Table 2:
Summary of published validation studies for CVD risk prediction models in PLWH
| Study | Year | Setting | N | Age (mean) |
Female (%) |
Black race (%) |
Current or former smoker (%) |
Virologically suppressed (%) or viral load (median, IQR) |
Mean follow- up (years) |
Events (N) |
C-statistic (discrimination) Chi square P value (calibration) |
Summary findings | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FHS-CVD | PCE | SCORE | D:A:D | ||||||||||||
| Thompson-Paul et al.28 | 2016 | HOPS; multiple US sites | 2283 | 42 | 24 | 34 | 42 | 490 (25–21600) | 6.5 | 195 | 0.66 0.89 |
0.71 <0.001 |
0.59 0.48 |
0.72 <0.001 |
-PCE and D:A:D models underestimated risk by nearly 20% -Only the FRS accurately estimated risk |
| Feinstein et al.26 | 2017 | CNICS; 5 US sites | 11288 | 40-43 | 18 | 38 | 25-32 | 64-9 | 4 | 247 | - - |
0.75 0.16 |
- - |
- - |
-Only evaluated myocardial infarction endpoint -PCE model underestimated risk, especially in female, Black, and low/intermediate risk |
| Triant et al.27 | 2018 | Partners; Boston healthcare system | 1272 | 51 | 0 | 20 | 45 | 77 | 4 | 78 | 0.67 0.0004 |
0.65 0.001 |
- - |
- - |
-Both FHS-CVD and PCE models underestimated risk (Figure 1) -Recalibration did not improve model fit |
| van Zoest et al.29 | 2019 | ATHENA; 26 Netherlands clinics | 16070 | 43 | 18 | 19 | 59 | 89 | 5.5 | ~550 | 0.75 <0.0001 |
0.76 0.0035 |
0.73 <0.0001 |
0.77 0.0004 |
-Large cohort accounting for ~63% of population of the Netherlands -All models discriminated well -PCE, SCORE, D:A:D underestimated risk in low/intermediate |
Note: Summary statistics are best approximate from available published data. Abbreviations: FHS-CVD= Framingham heart study (FHS) model for composite cardiovascular disease (CVD) outcome (2008); PCE=American College of Cardiology (ACC) and American Heart Association (AHA) pooled cohort equations (PCE) (2013); SCORE=Systematic COronary Risk Evaluation; D:A:D=Data Collection on Adverse Events of Anti-HIV Drugs. See text for details on these models.
Figure-1: Observed vs predicted CVD risk over deciles of predicted risk by pooled cohort equation (PCE) in a US cohort.
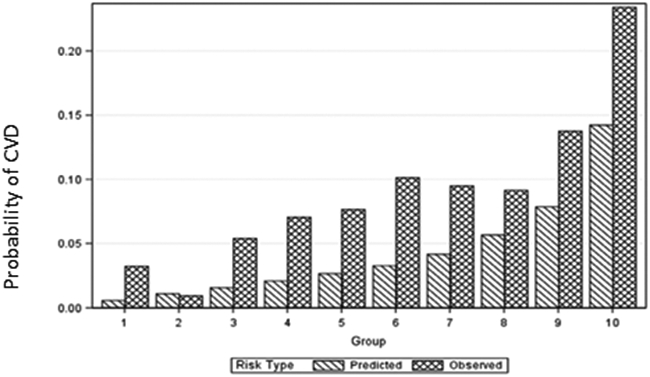
Taken with permission from Triant et al.27 Figure illustrates underestimation of CVD risk by Pooled cohort equation (PCE) model in People living with HIV (PLWH). See text for details.
Suboptimal performance of established risk prediction models in PLWH is likely due to multiple factors. The demographic and comorbidity profiles of HIV populations is likely to differ from those of the populations on which the prediction models were developed. PLWH tend to be younger with a higher proportion of non-White individuals, have higher smoking rates, and have a higher comorbidity burden.2,30 The role of HIV infection itself cannot be overstated. Importantly, inflammation and immune dysfunction are likely play integral roles in increasing CVD risk in the setting of HIV and are not reflected in widely-used prediction functions.31,32 Embedded in this issue is the related possibility that HIV additionally confers unique drivers of risk that may not be present, or as present, in the general population, including for example other concomitant viral infections or other aspects of immune dysregulation.33 Additional limitations of these studies should be acknowledged. All of the general population models discussed above had their enrollment periods in the 1970s to 1980s, when smoking rates were higher, and preventive care has improved since that time period. Most validation studies have been relatively small with shorter follow-up periods of approximately 4-5 years and relatively small numbers of events (Table 2). Risk prediction models on contemporary cohorts with longer follow-up periods are therefore needed, both in the general population and in PLWH. Finally, CVD risk prediction models, including non-laboratory-based risk functions, are yet to be properly evaluated in prospective studies in PLWH living in low- and middle-income countries, especially sub-Saharan Africa, where a larger burden of HIV-associated CVD lies.4
HIV specific risk-prediction models
The D:A:D model is the main HIV-specific risk prediction model, initially developed in 201034 and updated in 2016.35 The D:A:D study developed a model for predicting 5-year CVD risk based on a large cohort of predominantly European PLWH (N>30,000) with prospective collection of CVD- and HIV-related risk factors and adjudication of CVD outcomes. In addition to traditional CVD risk factors, the full D:A:D model incorporates HIV-related factors including CD4 count, recent use of abacavir, and cumulative use of nucleoside reverse transcriptase inhibitors (NRTIs) and protease inhibitors (PIs).35 Its performance in validation studies has been similar to the FHS-CVD, PCE and SCORE models (Table 2): Performance was generally modest in US cohorts,26,28 while the prediction score performed better in a Dutch cohort with a similar population to the D:A:D derivation cohort.29 Evaluation of the D:A:D model has been limited in part by inclusion of family history of CVD, a variable which is not available in many cohorts of PLWH. Several de novo HIV-specific risk scores were also developed from the CNICS cohort that included variables from the ASCVD risk score in addition to HIV-specific variables and utilized a derivation and holdout cohort for internal validation.26 The two scores that were developed discriminated adequately but demonstrated worse calibration than PCE.26
Role of novel risk factors to improve CVD prediction
Over the last two decades, there have been intensive efforts in identifying novel risk factors and biomarkers to improve CVD risk prediction. The main interest in evaluating novel biomarkers has been to more accurately identify individuals predicted to have low/intermediate risk by traditional risk prediction models but who go on to develop clinical CVD in the future. Some biomarkers or indicators of interest have included: coronary artery calcium (CAC) scoring (quantification of calcification of coronary vessels measured on a CT scan), high-sensitivity C reactive protein (hsCRP), ankle-brachial index (ABI), advanced lipoprotein testing, and lipoprotein-a (LPa). Of these, CAC has demonstrated strong data on the negative predictive value for CVD risk with a CAC value of zero over subsequent windows of time; of relevance to HIV, CAC testing may be limited in regards to its use in younger individuals.13 While these additional measures are not currently endorsed for routine use in primary prevention of CVD by the AHA, United States Preventive Services Taskforce or European guidelines,12,13,36 use of these biomarkers and additional measures are now discussed, especially in those with intermediate predicted risk of CVD by traditional models where therapeutic intervention is uncertain and in clinical scenarios such as strong family history of premature CAD where concern is heightened.12,13
In the context of HIV infection, novel biomarkers or disease indicators have not been formally evaluated with regards to their capacity to improve discrimination or calibration in prospective studies employing hard CVD endpoints. Biomarkers of inflammation and coagulation activation, such as hsCRP, interleukin-6 and D-dimer, have been linked to CVD and subclinical atherosclerosis in PLWH but have not been formally evaluated as components of risk prediction models, given that are not routinely obtained clinically.37,38 The CAC score has also been of interest, and in one recent cross-sectional study in PLWH aged >50 years, use of CAC reclassified 20% of individuals in low-risk groups identified by the PCE model to a higher-risk group.39 However, in the absence of prospective data on clinical endpoints, it was unclear whether this reclassification was correct. Future studies on CAC, biomarkers of inflammation and immune activation, and HIV-specific factors will be important to improve our understanding of the role of novel risk factors in CVD risk prediction in PLWH.
Clinical implications of data on CVD risk prediction in HIV
Underestimation of CVD risk in PLWH by established risk prediction models is an important problem which can defer appropriate implementation of preventive measures, in particular in Black individuals, women, and in those predicted to be at low/moderate risk. Even with current suboptimal risk stratification strategies, implementation of CVD prevention is limited in HIV clinics globally; in a large international survey of PLWH, only 19% ever had CVD discussed by their physician, and 42% of smokers reported never having discussed smoking.40 In recognition of potential underestimation of CVD risk in the HIV population, the AHA released a scientific statement on this topic in 2019, providing guidance on CVD prevention in PLWH41 based on the 2018 cholesterol guidelines13 and the 2013 AHA guidelines.19 Notably, the guidance indicated that a clear best risk estimation model for HIV has not been established. For those who do not qualify for lipid-lowering therapy from other indications, the guidance suggests using the FHS-CVD, PCE or D:A:D model and considering HIV as a “risk enhancing factor” by 1.5 to 2 times, particularly in those PLWH with history of delayed ART initiation/prolonged viremia, low CD4 count or hepatitis C co-infection. The guidance cautions against routinely using risk enhancers such as CAC or hsCRP in PLWH as they would be used in the general population given the relative scarcity of data in HIV. Moreover, risk enhancers which might aid in calibration or discrimination in the general population may not similarly enhance models in HIV; for example, CAC alone may not reflect underlying coronary artery disease among PLWH, who tend to have more noncalcified plaque compared with uninfected individuals. Ultimately, it remains to be seen whether PLWH predicted to be at low/intermediate CVD risk by established prediction models will merit aggressive prevention therapies due to elevated CVD risk from HIV infection itself. An ongoing, large randomized trial, REPRIEVE (Randomized Trial to Prevent Vascular Events in HIV), is investigating this question and has enrolled a diverse group including participants from sites in Asia and sub-Saharan Africa.42 While awaiting REPRIEVE data, as outlined in the 2019 AHA Scientific Statement, primary CVD prevention in PLWH should focus on identifying high-risk individuals, implementing interventions on dietary and lifestyle factors – particularly smoking which occurs at elevated rates in PLWH – and using lipid-lowering therapies when indicated by current guidance.25,41
CONCLUSIONS AND FUTURE DIRECTIONS
In summary, accurate prediction of CVD risk is one of the important tools in mitigating morbidity and mortality from CVD in PLWH, yet established risk prediction models have been shown to be inaccurate in this group. Risk prediction models used in the general population, first developed and refined by the Framingham Heart Study group9-11 and including FHS-CVD, PCE and SCORE, are based on traditional CVD risk-factors combined into a multifactorial function to estimate risk. While they serve as a cornerstone of CVD preventive care for the general population, current established functions may misrepresent the relative contributions of traditional CVD risk factors in PLWH and do not incorporate novel risk factors related to HIV-associated inflammation and immune dysregulation which may drive excess CVD risk in this group. While established prediction models do a fair to acceptable job of discriminating between high- and low-risk individuals, they have been shown to underestimate CVD risk in PLWH, particularly in individuals with low/intermediate predicted risk in whom accurate risk estimation to guide clinical decision-making is arguably most important. Future studies should focus on the inclusion of novel and HIV-specific risk factors to refine risk prediction models and should include diverse populations, including women, Black individuals and PLWH in low- and middle-income countries. Optimizing the performance of risk prediction models in PLWH will be critical to reduce disparities in the receipt of appropriate CVD preventative care and to enhance the long-term health of this group.
References
- 1.Lerner AM, Eisinger RW, Fauci AS. Comorbidities in Persons With HIV: The Lingering Challenge. JAMA. 2019. [DOI] [PubMed] [Google Scholar]
- 2.Schouten J, Wit FW, Stolte IG, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV Cohort Study. Clin Infect Dis. 2014. [DOI] [PubMed] [Google Scholar]
- 3.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. JCEM. 2007;92(7):2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah ASV, Stelzle D, Lee KK, et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV. Circulation. 2018;138(11):1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Achhra AC, Mocroft A, Reiss P, et al. Short-term weight gain after antiretroviral therapy initiation and subsequent risk of cardiovascular disease and diabetes: the D:A:D study. HIV Med. 2016;17(4):255–268. [DOI] [PubMed] [Google Scholar]
- 6.Ladapo JA, Richards AK, DeWitt CM, et al. Disparities in the Quality of Cardiovascular Care Between HIV-Infected Versus HIV-Uninfected Adults in the United States: A Cross-Sectional Study. J Am Heart Assoc. 2017;6(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.So-Armah K, Benjamin LA, Bloomfield GS, et al. HIV and cardiovascular disease. The lancet HIV. 2020;7(4):e279–e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Agostino RB Sr. Cardiovascular Risk Estimation in 2012: Lessons Learned and Applicability to the HIV Population. J Infect Dis. 2012;205 Suppl 3:S362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Agostino RB Sr., Grundy S, Sullivan LM, Wilson P, Group CHDRP. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation.[see comment]. JAMA. 2001;286(2):180–187. [DOI] [PubMed] [Google Scholar]
- 10.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23(13):2109–2123. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan LM, Massaro JM, D'Agostino RB Sr. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23(10):1631–1660. [DOI] [PubMed] [Google Scholar]
- 12.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundy SM, Stone NJ, Bailey AL, et al. 2018. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2018:CIR0000000000000625. [Google Scholar]
- 14.D'Agostino RB Sr., Pencina MJ, Massaro JM, Coady S. Cardiovascular Disease Risk Assessment: Insights from Framingham. Glob Heart. 2013;8(1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moons KG, Kengne AP, Grobbee DE, et al. Risk prediction models: II. External validation, model updating, and impact assessment. Heart. 2012;98(9):691–698. [DOI] [PubMed] [Google Scholar]
- 16.Moons KG, Kengne AP, Woodward M, et al. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart. 2012;98(9):683–690. [DOI] [PubMed] [Google Scholar]
- 17.Grant SW, Collins GS, Nashef SAM. Statistical Primer: developing and validating a risk prediction model. Eur J Cardiothorac Surg. 2018;54(2):203–208. [DOI] [PubMed] [Google Scholar]
- 18.D'Agostino RB, Nam BH. Evaluation of the performance of survival analysis models: discrimination and calibration measures. Vol 23: Elsevier Science B.V.; 2004. [Google Scholar]
- 19.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. [DOI] [PubMed] [Google Scholar]
- 20.Damen JA, Hooft L, Schuit E, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Agostino RB Sr., Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. [DOI] [PubMed] [Google Scholar]
- 22.Pencina MJ, D'Agostino RB Sr., Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the framingham heart study. Circulation. 2009;119(24):3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amin NP, Martin SS, Blaha MJ, Nasir K, Blumenthal RS, Michos ED. Headed in the Right Direction But at Risk for Miscalculation: A Critical Appraisal of the 2013 ACC/AHA Risk Assessment Guidelines. J Am Coll Cardiol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003. [DOI] [PubMed] [Google Scholar]
- 25.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–45. [DOI] [PubMed] [Google Scholar]
- 26.Feinstein MJ, Nance RM, Drozd DR, et al. Assessing and Refining Myocardial Infarction Risk Estimation Among Patients With Human Immunodeficiency Virus: A Study by the Centers for AIDS Research Network of Integrated Clinical Systems. JAMA cardiology. 2017;2(2):155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Triant VA, Perez J, Regan S, et al. Cardiovascular Risk Prediction Functions Underestimate Risk in HIV Infection. Circulation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson-Paul AM, Lichtenstein KA, Armon C, et al. Cardiovascular Disease Risk Prediction in the HIV Outpatient Study. Clin Infect Dis. 2016;63(11):1508–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Zoest RA, Law M, Sabin CA, et al. Predictive Performance of Cardiovascular Disease Risk Prediction Algorithms in People Living With HIV. J Acquir Immune Defic Syndr. 2019;81(5):562–571. [DOI] [PubMed] [Google Scholar]
- 30.Giles ML, Gartner C, Boyd MA. Smoking and HIV: what are the risks and what harm reduction strategies do we have at our disposal? AIDS Res Ther. 2018;15(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Titanji B, Gavegnano C, Hsue P, Schinazi R, Marconi VC. Targeting Inflammation to Reduce Atherosclerotic Cardiovascular Risk in People With HIV Infection. J Am Heart Assoc. 2020;9(3):e014873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54(23):2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsue PY, Deeks SG, Hunt PW. Immunologic Basis of Cardiovascular Disease in HIV-Infected Adults. J Infect Dis. 2012;205 Suppl 3:S375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friis-Moller N, Thiebaut R, Reiss P, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17(5):491–501. [DOI] [PubMed] [Google Scholar]
- 35.Friis-Moller N, Ryom L, Smith C, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: The Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol. 2016;23(2):214–223. [DOI] [PubMed] [Google Scholar]
- 36.Risk Assessment for Cardiovascular Disease with Nontraditional Risk Factors: Recommendation Statement. Am Fam Physician. 2019;99(2):Online. [PubMed] [Google Scholar]
- 37.Baker JV, Duprez D. Biomarkers and HIV-associated cardiovascular disease. Curr Opin HIV AIDS. 2010;5(6):511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51(3):268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereira B, Mazzitelli M, Milinkovic A, et al. Use of Coronary Artery Calcium Scoring to Improve Cardiovascular Risk Stratification and Guide Decisions to Start Statin Therapy in People Living With HIV. J Acquir Immune Defic Syndr. 2020;85(1):98–105. [DOI] [PubMed] [Google Scholar]
- 40.Sherer R, Solomon S, Schechter M, Nachega JB, Rockstroh J, Zuniga JM. HIV provider-patient communication regarding cardiovascular risk: results from the AIDS Treatment for Life International Survey. J Int Assoc Provid AIDS Care. 2014;13(4):342–345. [DOI] [PubMed] [Google Scholar]
- 41.Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation. 2019;140(2):e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grinspoon SK, Douglas PS, Hoffmann U, Ribaudo HJ. Leveraging a Landmark Trial of Primary Cardiovascular Disease Prevention in Human Immunodeficiency Virus: Introduction From the REPRIEVE Coprincipal Investigators. J Infect Dis. 2020;222(Suppl 1):S1–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]


