Abstract
The tumor microenvironment (TME) is characterized by the activation of immune checkpoints, which limit the ability of immune cells to attack the growing cancer. To overcome immune suppression in the clinic, antigen-expressing viruses and bacteria have been developed to induce antitumor immunity. However, the safety and targeting specificity are the main concerns of using bacteria in clinical practice as antitumor agents. In our previous studies, we have developed an attenuated bacterial strain (Brucella melitensis 16M ∆vjbR, henceforth Bm∆vjbR) for clinical use, which is safe in all tested animal models and has been removed from the select agent list by the Centers for Disease Control and Prevention. In this study, we demonstrated that Bm∆vjbR homed to tumor tissue and improved the TME in a murine model of solid cancer. In addition, live Bm∆vjbR promoted proinflammatory M1 polarization of tumor macrophages and increased the number and activity of CD8+ T cells in the tumor. In a murine colon adenocarcinoma model, when combined with adoptive transfer of tumor-specific carcinoembryonic antigen chimeric antigen receptor CD8+ T cells, tumor cell growth and proliferation was almost completely abrogated, and host survival was 100%. Taken together, these findings demonstrate that the live attenuated bacterial treatment can defeat cancer resistance to chimeric antigen receptor T-cell therapy by remodeling the TME to promote macrophage and T cell-mediated antitumor immunity.
Keywords: tumor microenvironment, immunotherapy, adoptive
Introduction
In the tumor microenvironment (TME), cancer cells express factors to suppress immune surveillance, thereby creating a permissive environment for their uncontrolled proliferation.1 2 The immunosuppressive TME is a key factor limiting the efficacy of chimeric antigen receptor T-cell (CAR-T) therapies, especially for solid tumors.3 Several strategies are being developed to overcome TME-associated immunosuppression, including the activation of antitumor immunity by antigen-expressing viruses and bacteria.4–6 However, improvements in the safety, targeting specificity, and efficacy of these agents are required for widespread adoption.7 Here, we demonstrate that a safe, live attenuated bacterium (Brucella melitensis 16M ∆vjbR, henceforth Bm∆vjbR) homed to tumor tissue and improved the TME in a murine model of cancer. Moreover, we show that Bm∆vjbR, when paired with CAR-T therapy, displayed remarkable anticancer efficacy in this model.
Bm∆vjbR has been developed by our groups for clinical applications.8 9 This strain is genetically and functionally defective in LuxR-type regulatory protein VjbR, which is required for expression of the bacterial type IV secretion system, an essential component of bacterial virulence.10 A series of safety studies in immune-compromised mice and non-human primates showed that Bm∆vjbR does not induce disease-associated symptoms and resulted in removal of BmΔvjbR from the select agent list by the Centers for Disease Control and Prevention.8 11 12 Here, we show that Bm∆vjbR can remodel the TME to a proinflammatory state. Moreover, when Bm∆vjbR treatment was combined with the adoptive transfer of carcinoembryonic antigen CEA-Ag-specific CD8+ T cells, tumor growth and proliferation were dramatically impaired.
Materials and methods
Bacterial culture and inoculation
Freshly cultured Bm∆vjbR in tryptone soya broth was collected by centrifugation and washed and resuspended in 1× phosphate-buffered saline (PBS, pH 7.4). For in vitro inoculation, bacteria were added in each well of a 24-well plate with macrophage monolayer at a multiplicity of infection (MOI) of 20 in Dulbecco’s Modified Eagle’s Medium (DMEM) (Thermo Fisher Scientific), and the plate was centrifuged at 500× g for 5 min to enhance bacterial interaction with the macrophages. After incubation at 37℃ for 30 min to allow the macrophages to uptake the bacteria, the non-internalized bacteria were removed by washing the cell monolayer twice with warm PBS, and then fresh DMEM medium containing 50 µg/mL of gentamicin was added into each well for cell growth until assay. For in vivo animal experiment, at 9-day postinoculation of tumor cells in mice, 5×107 colony forming units (CFUs) of Bm∆vjbR in 100 µL of 1× PBS was intravenously injected into each mouse.
Macrophage cultures
For murine bone marrow-derived macrophage (BMDM) generation, bone marrow cells were harvested from the tibia and femur of C57BL/6 mice of 6–8 weeks and cultured as described previously.13 Murine RAW264.7 (ATCC TIB-71) and J774A.1 (ATCC TIB-67) macrophage cell lines were both cultured in DMEM media containing 10% FBS and penicillin–streptomycin (100 IU/mL and 100 µg/mL).
Cytokine responses
BMDMs were seeded in 24-well plates at a concentration of 2.0×105 cells/well in DMEM without antibiotics. After overnight culture, the cells were inoculated with heat-killed (HK) or live Bm∆vjbR bacteria at a MOI of 20. At 24 hours post-treatment, cell culture supernatant was collected and analyzed for the presence of cytokines/chemokines by using a Multiplex Mouse Cytokine/Chemokine Array 31-Plex technology (MD31, Eve Technologies).
Flow cytometric analysis
CD8+ T cells, isolated by using mouse CD8+ T-cell isolation kit (BioLegend), were cocultured in vitro with Bm∆vjbR-treated macrophages. The CD8+ T cells were then analyzed by flow cytometry following exclusion of dead cells by using Aqua Zombie NIR staining dye (BioLegend) and specific gating CD8+ marker. The CD8+ T-cell markers of programmed cell death protein 1 (PD-1), CD69, 4-1BB, CD27, CD62L, OX40, granzyme B (GrB), and perforin (Prf) were assessed either immediately after coculture with infected BMDMs or 3 days after re-stimulation with anti-CD3/CD28 antibodies. Intracellular cytokine staining was performed by using monensin and brefeldin (BioLegend) and the production of interleukin 2 (IL-2), tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ) was assessed. Similarly, the BMDMs were separately analyzed for the expression of CD38 on M1 macrophages. All flow cytometry data were acquired on a Fortessa X 20 (BD Biosciences, CA) and analyzed by using FlowJo (Treestar, OR).
CAR-T cell preparation
The MSGV1 γ retroviral vector backbone was modified to express CEA specific scFv, as described in our previous study.14 Briefly, CD8+ T cells isolated from B6 Thy 1.2 mice were transduced with the viral supernatants containing CEA in the presence of 5 µg/mL Polybrene (Sigma Aldrich, USA), following a protocol as described previously.15 The transduced cells were positively identified by expression of c-Myc.
Animal experimentation
The wild-type C57BL/6 (B6) Thy 1.1 mice (Jackson Laboratories) 6–8 weeks old were subcutaneously injected with 1×106 MC32 CEA cancer cells in the right lateral flank on day 0. Subsequently, the mice were divided into three different groups (n=5) with each group receiving either 1× PBS control (Ctrl), HK bacteria or live attenuated bacteria (Live) on day 9 postinoculation of tumor cells. On day 12 postinduction of the tumor, all the groups of mice received the CEA CAR-Ts isolated and prepared from Thy 1.2 mice 6–8 weeks old. Mice were housed in Texas A&M University, Laboratory Animal Resources and Research Facility, and checked daily. Tumor growth was monitored every other day and tumor volumes were calculated using the formula: Tumor Volume (mm3)=0.5 × length × width2. Mice were humanely sacrificed if tumor size reached above 4000 mm3.
Fluorescence imaging of Bm∆vjbR
Formaldehyde fixed tissue or macrophage monolayer were used for Bm∆vjbR staining. For staining bacteria in tumor tissue, formalin fixed, paraffin-embedded sections of MC32 tumor tissue were deparaffinized in xylene and rehydrated through graded alcohols, and then antigen was retrieved in a pressure cooker using a citrate buffer. The cells were stained with rabbit anti-Brucella antibodies (Bioss Inc.) for 1 hour followed by appropriate secondary antibody for 1 hour. Cells were mounted with ProLong Glass Antifade Mountant with NucBlue Stain (Thermo Fisher Scientific). All the images were acquired using a Nikon Eclipse Ti2 fluorescence microscope.
Bacterial quantification
For detecting Bm∆vjbR survival in BMDMs, J774A.1 or RAW 264.7 cell lines, cells were seeded in a 24-well plate in 1 mL of DMEM without antibiotics at 2.0×105 cells/well. The CFU of bacteria at different postinoculation times was assayed by spotting serial dilution on tryptone soya agar (TSA) plates. For CFU assay of Bm∆vjbR in different organs of cancer bearing mice, the organ-homogenates were obtained 19 days postinoculation and spotted on TSA plates for enumeration of bacteria.
Comparative metabolic analysis
The differences in the glycolytic states of CD8+ T cells were analyzed using extracellular flux (XF) analyzers (Agilent) using a protocol described previously.16 Briefly, after coculture with Bm∆vjbR infected BMDMs for 16 hours, T cells in suspension were removed from the cocultured medium and seeded on 96-well seahorse plates. Their XF and compensatory glycolysis were assessed by using glycolytic activators and inhibitors as described in the Seahorse XF protocol.
Imaging and immunohistochemistry of tumor sections
Paraffin-embedded solid tumor samples were sliced into 5 µm sections with microtome. The slides that were prepared from these sections were processed for fluorescence microscopy, H&E staining, and mass cytometry analysis. The H&E stained slides were scored for inhibition of tumor by assessing the necrotic areas and infiltration of immune cells on a scale of 1–5. The score was represented as tumor inhibition score in the comparative bar–graph analysis.
Imaging mass cytometry (IMC) analysis
IMC analysis of tumor samples derived from Bm∆vjbR treated mice or PBS controls were processed for the quantification, imaging, and analysis of DNA, Ki67 antigen, CD8+ T cells, B220 (B cells), CD11c (dendritic cells), and F4/80 (macrophages) respectively. A dimensionality reduction technique was adopted to construct t-distributed stochastic neighbor embedding (t-SNE) plots from the heatmaps of treated or untreated groups of mice. The neighborhood analysis was constructed to find the probability of enriched cell-to-cell interactions using basic statistical methods as described previously.17
Statistical analysis
All analyses were performed using Graphpad Prism V.9. Unpaired t-test was performed to compare the difference between the groups. A p value of <0.05 was considered statistically significant.
Results
Live Bm∆vjbR induces anticancer phenotypes in BMDMs and CD8+ T cells
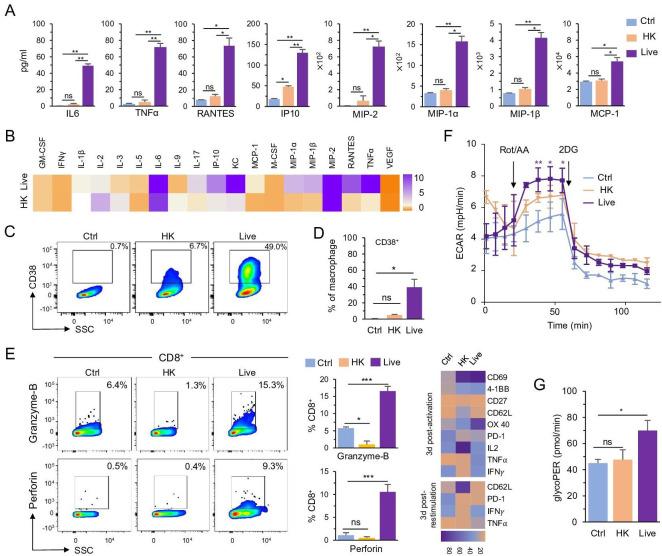
To test the hypothesis that Bm∆vjbR elicits anti-cancer proinflammatory phenotypes from immune cells, we incubated the live attenuated strain with murine BMDMs for 24 hours, and then measured cytokine secretion and macrophage polarization. We found that, in contrast to HK or no-treatment Ctrl, live BmΔvjbR (Live) enhanced the secretion of proinflammatory cytokines and chemokines (figure 1A, B). Most of these BMDMs were polarized to M1 macrophages, which express CD38, an M1 exclusive marker, on their surface (figure 1C, D). Collectively, these data suggested that live BmΔvjbR activates macrophages and induces the production of proinflammatory cytokines and T cell-mediated chemo-attractants.18 19
Figure 1.
Live BmΔvjbR treatment activates CD8+ T cells to produce proinflammatory cytokines by polarizing macrophages. (A, B) Cytokine array analysis of the culture medium of BMDMs after treatment with 1× PBS (Ctrl), HK or live) BmΔvjbR for 24 hours shown live BmΔvjbR promotes BMDMs to secret proinflammatory cytokines and chemokines. (C, D) Flowcytometric analysis of CD38 expression on BMDMs after treatment with HK or live BmΔvjbR for 24 hours. (E) Coculturing with live BmΔvjbR-infected BMDMs activates CD8+ T cells to produce granzyme B and perforin (left) and express activation markers and cytokines (right heatmap). (F, G) Cocultivation with live BmΔvjbR-infected BMDMs increases the glycolysis of CD8+ T cells. Data represent means±SD from three independent experiments. *, **, ***Significance at p<0.05, 0.01, and 0.001, respectively. BMDM, bone marrow-derived macrophage; Ctrl, control; ECAR, extracellular acidification rate; GM-CSF, granulocyte-macrophage colony-stimulating factor;HK, heat-killed; IFN-γ, interferon gamma; IL, interleukin; IP-10, interferon gamma-induced protein 10; KC, keratinocytes-derived chemokine; MCP-1, monocyte chemoattractant protein 1; M-CSF, macrophage colony-stimulating factor; MIP, macrophage inflammatory protein; ns, not significant; RANTES, chemokine (C-C motif) ligand 5; VEGF, vascular endothelial growth factor; PBS, phosphate-buffered saline; SSC, side scatter; TNF-α, tumor necrosis factor alpha.
After coculturing CD8+ T cells with BMDMs pre-treated with either live or HK bacteria, we found that BMDMs exposed to live BmΔvjbR activated CD8+ T cells more efficiently compared with HK controls through upregulating the expression of GrB and Prf (figure 1E, left). The live BmΔvjbR-treated BMDMs also induced significantly higher production of TNF-α, IFN-γ, and IL-2 from CD8+ T cells (figure 1E, right top). Moreover, costimulatory marker expression, including OX40 and 4-1BB, was higher in CD8+ T cells cocultured with BmΔvjbR-treated BMDMs (figure 1E, right top). To test the hypothesis that the activated CD8+ T cells retained functional recall ability, a feature critical for antitumor efficacy,20 we used anti-CD3/anti-CD28 antibodies to restimulate CD8+ T cells at 3-day postactivation. We found that the CD8+ T-cell recall responses were enhanced postrestimulation, exhibiting lower PD-1 expression and higher expression of proinflammatory cytokines (figure 1E, right bottom). CD8+ T cells also had a significantly higher extracellular acidification rate and showed higher glycolytic activity when activated with BMDMs treated with live Bm∆vjbR (figure 1F, G).
Bm∆vjbR induces diverse cellular responses
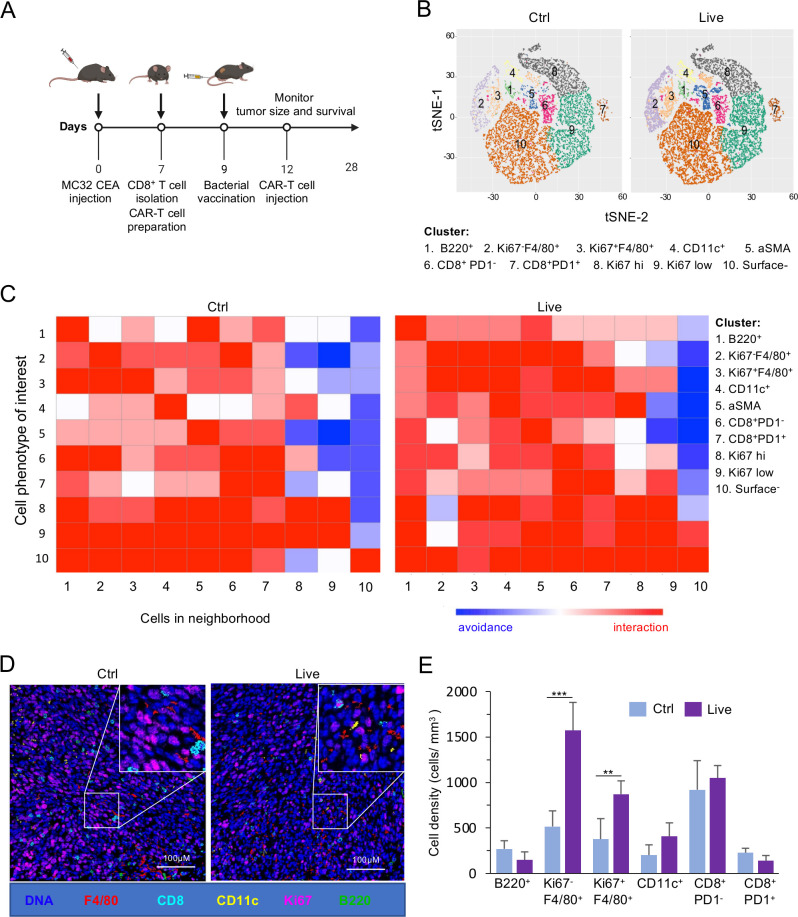
We hypothesized that Bm∆vjbR treatment may alter the TME in an in vivo murine solid-tumor system. To test this hypothesis, we performed an IMC analysis to quantify the abundance of B cells as well as proliferating and non-proliferating immune cells from explanted solid tumors. A well-established MC32 colon cancer murine model was used for the experiment, following the protocol shown in figure 2A. We found that live Bm∆vjbR-treated mice had a higher complexity of immune cells in the TME (figure 2B, C) compared with controls. To determine the identities of enriched interactions between or within the cell phenotypes in the TME, we constructed neighborhood joining plots from the IMC data. The t-distributed stochastic neighbor embedding (t-SNE) plots (figure 2B) and neighborhood joining analysis (figure 2C) showed that innate immune cells were activated and quantitatively higher in the TME of mice receiving the treatment. The reconstructed image from the mass cytometry analysis showed more immune cells, especially F4/80+ macrophages, in the TME of Bm∆vjbR treated mice receiving adoptive transfer of CAR-Ts (figure 2D). Therefore, we quantified the specific innate immune cells from the TME and found that the numbers of Ki67-F4/80+ (non-proliferating macrophages) and Ki67+F4/80+ (proliferating macrophages) were significantly increased in Bm∆vjbR-treated mice receiving adoptive transfer of CAR-Ts (figure 2E). Overall, our results indicated that the numbers of macrophages and dendritic cells were significantly increased in the TME of treated mice receiving adoptive transfer of CAR-Ts, consistent with the hypothesis that these immune cells promote CAR-T tumor infiltration and drive tumor regression.
Figure 2.
Live BmΔvjbR-treated mice show a significant increase in innate immune cells. Mass cytometry analysis of MC32 CEA derived tumor samples (three samples/group) from Thy 1.1 mice either intravenously injected with live BmΔvjbR or 1× PBS (Ctrl) prior to intravenous administration of CEA CAR-Ts. (A) Schematic diagram showing adoptive T-cell therapy and BmΔvjbR treatment protocol. (B) Visualization of t-distributed stochastic neighbor embedding (viSNE) plots of comparative immune cell populations in the Ctrl and live BmΔvjbR treated tumor samples. (C) Neighborhood joining plots of different immune cell populations in tumor tissues with highly interacting neighbored cells shown in red, whereas the avoided interactions are shown in blue. (D) Reconstructed image of immune cell infiltration into tumor samples. (E) Quantification of macrophages, dendritic cells, and B cells in tumor samples (three fields/sample were analyzed). **, ***Significance at p< 0.01 and 0.001, respectively. The markers representing the different immune cell populations are B220 (B cells), F4/80 (macrophages), CD11c (dendritic cells), Ki67 (proliferating cells), CD8+ (CD8+ T cells) and surface− (cells are negative to all tested makers). CAR-T, chimeric antigen receptor T cell; CEA, carcinoembryonic antigen; Ctrl, control; PBS, phosphate-buffered saline.
Bm∆vjbR treatment enhances antitumor efficacy and selectively colonizes tumor tissue
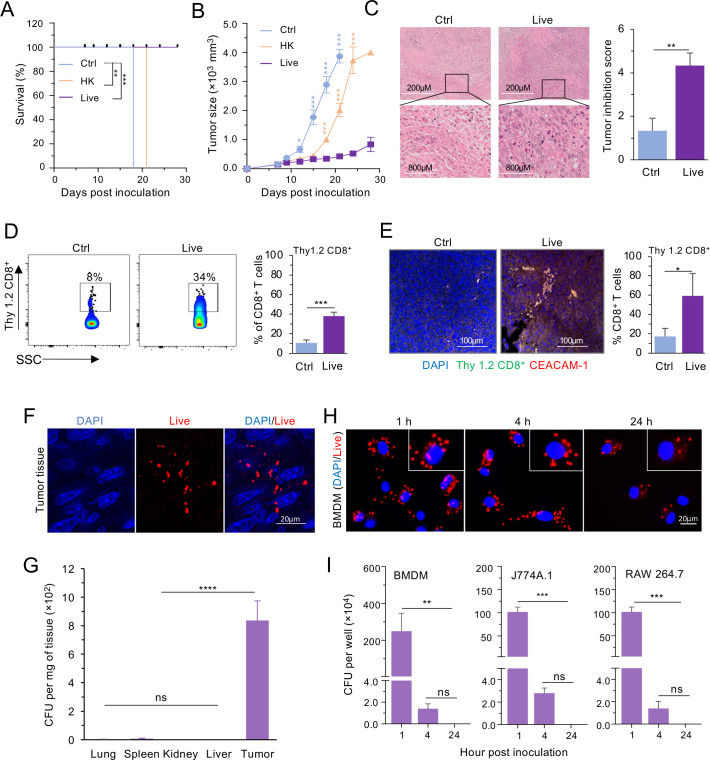
Encouraged by our findings, we tested the hypothesis that Bm∆vjbR treatment enhances the antitumor efficacy of CAR-T therapy. We found that Bm∆vjbR-treated mice displayed significantly greater survival (figure 3A) and had drastically lower tumor burden than controls (figure 3B, C). We found that there were significantly increased numbers of CD8+ T cells infiltrating into the solid tumor of mice that were treated with live Bm∆vjbR, in comparison to control (figure 3D, E).
Figure 3.
BmΔvjbR accumulates in tumor tissue and suppresses tumor growth. Three groups of MC32 tumor-bearing C57BL/6 (B6) mice (five mice/group) were intravenously injected with either live, HK BmΔvjbR, or 1× PBS (Ctrl). On day 3 postbacteria treatment, all three groups of mice were intravenously injected with CEA CAR-Ts. (A) Survival of mice is significantly improved in the group receiving BmΔvjbR from 18 days onwards compared with the control untreated group (n=5 mice/group). (B) Live BmΔvjbR immunization followed by adoptive T-cell transfer significantly suppresses the tumor growth from 18 days postinitiation of the experiment compared with both non-bacterial (Ctrl) and HK BmΔvjbR (HK) treatment groups (n=5 mice/group). (C) H&E staining shows significant improvement in tumor in the group of mice receiving BmΔvjbR compared with the Ctrl group. (D, E) Flow cytometry (D) and confocal microscopy (E) followed by graphical representation of infiltrating lymphocytes (Thy 1.2 CD8+ T cells) confirm significantly higher infiltration of adoptively transferred CEA CD8+ T cells. (F) Representative immunofluorescence microscopy images show BmΔvjbR survival in tumor tissue 19 days postinjection. (G) BmΔvjbR mainly colonizes in tumor (n=3). (H) BmΔvjbR can be observed in BMDMs with immunofluorescence microscopy after 1, 4, and 24 hpi. (I) The BmΔvjbR can be recovered from BMDMs, J774A.1, and RAW 264.7 macrophages at 1 hpi and four hpi, but no bacteria survived in these macrophages at 24 hpi. Three independent experiments were performed for H and I. Data represent means±SD. *, **, ***, ****Significance at p<0.05, 0.01, 0.001, and 0.0001, respectively. BMDM, bone marrow derived macrophage; CFU, colony forming unit; CAR-T, chimeric antigen receptor T cell; Ctrl, control; DAPI, 4′,6-diamidino-2-phenylindole; HK, heat-killed; hpi, hours postinoculation; ns, not significant; PBS, phosphate-buffered saline.
We also measured Bm∆vjbR clearance from treated mice. Nineteen days after intravenous injection, we found Bm∆vjbR in tumor tissue (figure 3F) but not in other organs (figure 3G). We also monitored the survival of Bm∆vjbR in macrophages in vitro using immunofluorescence staining and CFU enumeration. We found numerous bacterial cells in BMDMs at 1 and 4 hours postinoculation (hpi). However, fewer were observed at 24 hpi (figure 3H). Importantly, live bacteria were only recovered from BMDMs at 1 and 4 hpi, and no bacteria survived longer than 24 hpi in BMDMs, J774A.1, and RAW 264.7 (figure 3I). These results indicate the Bm∆vjbR strain selectively targeted the tumor, survived for only short times in macrophages and were rapidly cleared from non-tumor tissue after treatment.
Discussion
Cancer cells suppress immune surveillance, thereby creating a permissive environment for cancer cell proliferation. In this work, we show that a novel and safe live attenuated bacterial strain Bm∆vjbR can remodel the TME to a proinflammatory status and thereby limit cancer progression and tumorigenesis. Moreover, we have shown that Bm∆vjbR treatment, when combined with the adoptive transfer of antigen-specific CD8+ T cells, results in dramatically impaired tumor growth and proliferation. Therefore, this live attenuated bacterial strain potentiates immune surveillance and control of cancer.
Previous studies have demonstrated that treatment with live attenuated bacteria can limit tumorigenesis by a variety of mechanisms, such as activating T cells and expressing tumor antigens.6 21 22 Even though some of these bacterial approaches have entered clinical trials,23 24 most previously used bacterial vectors have intrinsic deleterious or toxic features, and suboptimal safety profiles or routes of delivery that may significantly limit their broad utility in cancer therapy/treatment. Among the negative features observed are intraperitoneal route of delivery,25 significant endotoxin activity, pathogenic reversion potential and limitations due to pre-existing host immunity.26 27 So far, we have no evidence to suggest that Bm∆vjbR possesses the common deleterious properties shared by many of the previously studied bacterial vectors.28 Moreover, this work provides the first description of combining live attenuated bacterium treatment with CAR-T therapy and thereby demonstrates the synergy that can be achieved with these approaches.
Acknowledgments
We thank Robbie Moore from the College of Medicine Cell Analysis Facility, Malea Murphy from the Integrated Microscopy and Imaging Laboratory, Sankar P Chaki from the College of Veterinary Medicine, and Elizabeth Bustamante and Julia Plocica from Health Science Center at Texas A&M University, and the Immunomonitoring Core facility at Houston Methodist Hospital-Texas Medical Center for their technical support.
Footnotes
FG and JKD contributed equally.
Contributors: FG, JKD, PDF, JS, RCA, and TAF conceived and designed the experiments. FG and JKD performed the experiments and analyzed the data. PDF, JS, FG, JKD, RCA, TAF, Q-MQ, and KSK wrote the manuscript and provided critical feedback. PDF and JS supervised the research. All the authors read and approved the final manuscript.
Funding: This work was supported by funding from the National Institutes of Health (R01AI121180 and R01CA221867 to JS, R01AI110642 to RCA, R01HD084339 to TAF, and R01AI141607-01A1 to PDF, NSF DBI1532188, and NSF0854684 to PDF).
Competing interests: No, there are no competing interests.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. The data presented in this report are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study does not involve human subjects. All experiments were approved and performed in compliance with the regulations of The Texas A&M University Animal Care Committee (Institutional Animal Care & Use Committee, #2018–0065) and in accordance with the guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care.
References
- 1.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013;19:1423–37. 10.1038/nm.3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fridman WH, Pagès F, Sautès-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298–306. 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 3.Giraldo NA, Sanchez-Salas R, Peske JD, et al. The clinical role of the Tme in solid cancer. Br J Cancer 2019;120:45–53. 10.1038/s41416-018-0327-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishikawa H, Sato E, Briones G, et al. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J Clin Invest 2006;116:1946–54. 10.1172/JCI28045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quispe-Tintaya W, Chandra D, Jahangir A, et al. Nontoxic radioactive Listeria(at) is a highly effective therapy against metastatic pancreatic cancer. Proc Natl Acad Sci U S A 2013;110:8668–73. 10.1073/pnas.1211287110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvanesan BC, Chandra D, Quispe-Tintaya W. Tumor-targeted delivery of childhood vaccine recall antigens by attenuated Listeria reduces pancreatic cancer. bioRxiv 2021. [Google Scholar]
- 7.Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer 2010;10:785–94. 10.1038/nrc2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arenas-Gamboa AM, Rice-Ficht AC, Fan Y, et al. Extended safety and efficacy studies of the attenuated Brucella vaccine candidates 16 M(Delta)vjbR and S19(Delta)vjbR in the immunocompromised IRF-1-/- mouse model. Clin Vaccine Immunol 2012;19:249–60. 10.1128/CVI.05321-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Figueiredo P, Ficht TA, Rice-Ficht A, et al. Pathogenesis and immunobiology of brucellosis: review of Brucella-host interactions. Am J Pathol 2015;185:1505–17. 10.1016/j.ajpath.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delrue R-M, Deschamps C, Léonard S, et al. A quorum-sensing regulator controls expression of both the type IV secretion system and the flagellar apparatus of Brucella melitensis. Cell Microbiol 2005;7:1151–61. 10.1111/j.1462-5822.2005.00543.x [DOI] [PubMed] [Google Scholar]
- 11.Lee KM, Chiu KB, Sansing HA, et al. Aerosol-induced brucellosis increases TLR-2 expression and increased complexity in the microanatomy of astroglia in rhesus macaques. Front Cell Infect Microbiol 2013;3:86. 10.3389/fcimb.2013.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC . 2014 attenuated strains of overlap select agents excluded from the requirements of 9 cfr part 121 and 42 cfr part 73, 2014. Available: https://www.cdc.gov/selectagent/exclusions-overlap.html
- 13.Eshghjoo S, Kim DM, Jayaraman A, et al. A comprehensive high-efficiency protocol for isolation, culture, polarization, and glycolytic characterization of bone marrow-derived macrophages. JoVE 2021. 10.3791/61959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan J, Das JK, Xiong X, et al. Development of CAR-T cell persistence in adoptive immunotherapy of solid tumors. Front Oncol 2020;10:574860. 10.3389/fonc.2020.574860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song J, So T, Cheng M, et al. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity 2005;22:621–31. 10.1016/j.immuni.2005.03.012 [DOI] [PubMed] [Google Scholar]
- 16.Eshghjoo S, Kim DM, Jayaraman A, et al. A comprehensive high-efficiency protocol for isolation, culture, polarization, and glycolytic characterization of bone marrow-derived macrophages. J Vis Exp 2021:e61959. 10.3791/61959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schapiro D, Jackson HW, Raghuraman S, et al. histoCAT: analysis of cell phenotypes and interactions in multiplex image cytometry data. Nat Methods 2017;14:873–6. 10.1038/nmeth.4391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pathria P, Louis TL, Varner JA. Targeting tumor-associated macrophages in cancer. Trends Immunol 2019;40:310–27. 10.1016/j.it.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Li F, Ping Y, et al. Local production of the chemokines CCL5 and CXCL10 attracts CD8+ T lymphocytes into esophageal squamous cell carcinoma. Oncotarget 2015;6:24978–89. 10.18632/oncotarget.4617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liikanen I, Lauhan C, Quon S, et al. Hypoxia-Inducible factor activity promotes antitumor effector function and tissue residency by CD8+ T cells. J Clin Invest 2021;131. 10.1172/JCI143729. [Epub ahead of print: 01 04 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SH, Castro F, Paterson Y, et al. High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res 2009;69:5860–6. 10.1158/0008-5472.CAN-08-4855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandra D, Quispe-Tintaya W, Jahangir A, et al. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol Res 2014;2:901–10. 10.1158/2326-6066.CIR-13-0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toso JF, Gill VJ, Hwu P, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol 2002;20:142–52. 10.1200/JCO.2002.20.1.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassan R, Alley E, Kindler H, et al. Clinical Response of Live-Attenuated, Listeria monocytogenes Expressing Mesothelin (CRS-207) with Chemotherapy in Patients with Malignant Pleural Mesothelioma. Clin Cancer Res 2019;25:5787–98. 10.1158/1078-0432.CCR-19-0070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Tome Y, Suetsugu A, et al. Determination of the optimal route of administration of Salmonella typhimurium A1-R to target breast cancer in nude mice. Anticancer Res 2012;32:2501–8. [PubMed] [Google Scholar]
- 26.Goldszmid RS, Dzutsev A, Trinchieri G. Host immune response to infection and cancer: unexpected commonalities. Cell Host Microbe 2014;15:295–305. 10.1016/j.chom.2014.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Tang H, Chen P, et al. Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome. Signal Transduct Target Ther 2019;4:41. 10.1038/s41392-019-0074-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forbes NS, Munn LL, Fukumura D, et al. Sparse initial entrapment of systemically injected Salmonella typhimurium leads to heterogeneous accumulation within tumors. Cancer Res 2003;63:5188–93. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. The data presented in this report are available from the corresponding author on reasonable request.