Abstract
Background
The current coronavirus pandemic (COVID-19) was caused by severe acute respiratory syndrome virus 2 (SARS-CoV-2). COVID-19 is characterized by atypical pneumonia, mild colds, and more severe illnesses, such as severe acute respiratory distress, thrombosis, organ failure, and various secondary bacterial and fungal infections. Notably, the severity of COVID-19 in different age groups is not well known, and the validity of clinical laboratory data remains unclear.
Methods
In this retrospective cross-sectional study, we examined differential regulation of clinical, hematologic, and inflammatory biomarkers in COVID-19 patients. We divided 104 COVID-19 patients into five different groups according to age (0−17, 18−45, 46−65, 66−79, and >80 years). Baseline data (sex, comorbidities, intensive care admission, and medications), hematologic markers, liver, and renal function tests, coagulation, and inflammatory markers were examined in these groups. Receiver operator characteristic (ROC) analysis was used to determine the optimal threshold for predicting COVID-19 biological markers.
Results
We found that the highest percentage (45%) of COVID-19 patients was in the age group of 46−65 years. The hematologic parameters (WBC, HB, and PLT) were normal between the patient groups. The area under the curve in ROC analysis showed significant differences in the levels of creatine, GGT, BUN, CRP, D-dimer, ferritin, AST, and procalcitonin between the patients of age groups 46−65 and 66−79 years. Renal biomarkers were significantly high in most patients, regardless of age. In contrast, the liver biomarkers, did not differ significantly between patient groups.
Conclusion
The main finding of our study is that laboratory parameters such as GGT, creatinine, BUN, CRP, procalcitonin, ferritin and D-dimer were differentially regulated in COVID -19 patients of different age groups. Importantly, these laboratory parameters may help as clinical predictors to assess the severity of the disease in the population. We conclude here that age is an important factor influencing COVID-19 severity.
Keywords: SARS CoV-2, COVID-19, Clinical parameters, Hematological parameters, Inflammatory markers, Clinical predictors
Introduction
Coronaviruses (CoVs) are a large family of viruses that cause infections ranging from the common cold to more severe illnesses, such as severe acute respiratory distress, thrombosis, organ failure, and various secondary bacterial and fungal infections [1]. In December 2019, the World Health Organization (WHO) announced cases of pneumonia of unknown cause. In January 2020, Chinese authorities announced that they had identified a new virus called SARS CoV-2 that caused these cases in Wuhan, Hubei province, called Coronavirus Disease 2019 (COVID-19) [2].
In Saudi Arabia, the first case of COVID-19 was reported in March 2020. Since then, numerous protocols have been developed to minimize the spread of the disease [3]. For example, daily statistics and visualization data have been introduced by the National Health Command and Control (NHCC), COVID-19 Data and Informatics Committee, with data sources from the Ministry of Health, COVID-19 Command and Control Center (CCC), and The National Health Emergency Operation Center (NHEOC) [4,5]. However, clinically, confirmed cases are defined as suspected cases with laboratory confirmation of COVID-19 infection based on the polymerase chain reaction (PCR) testing performed in laboratories approved by the Saudi Disease Control and Prevention (CDC) for COVID-19 testing [6]. Furthermore, recovered cases are defined as either (i) recovery 10 days after onset of symptoms plus at least 3 days without symptoms (without fever and respiratory symptoms) or 3 days without symptoms and a PCR negative test (for symptomatic patients) or (ii) remaining asymptomatic for 10 days after the PCR test was positive (for asymptomatic patients).
The WHO and the European Centre for Disease Prevention and Control (ECDC) define the clinical and epidemiological criteria for COVID-19 based on 1. Clinical criteria when a person presents with at least one of the following symptoms: Cough, fever, shortness of breath, partial loss of anosmia, ageusia or dysgeusia, and less specific symptoms (e.g., headache, muscle ache, fatigue, chills, vomiting, and/or diarrhea), and 2. Epidemiologic criteria that include close contact with a confirmed COVID-19 case in the 14 days prior to the onset of symptoms and/or were a resident or staff member in a facility for vulnerable persons where ongoing COVID-19 transmission has been confirmed [7].
However, numerous reports indicate that patients with chronic diseases, such as cardiovascular disease (CVD), diabetes mellitus (DM), and hypertension (HTN) have important risk factors that may promote poor clinical outcome [8]. In addition, other factors such as sex, smoking, and lifestyle may also play an important role in the severity of COVID-19 [9]. There is evidence that asymptomatic carriers are more common among middle-aged people who are in close contact with infected family members [10], and elderly patients have some clinical features that differ from those of young patients [11,12].
The clinical and laboratory data have been used to identify the risk factors that can predict disease severity. Laboratory parameters may be more objective than clinical parameters in evaluating the patient’s condition. It has been reported that abnormal levels of laboratory markers such as C-reactive protein (CRP), D-dimer, lactate dehydrogenase (LDH), interleukin (IL)-6, and fibrinogen correlate with disease severity [[13], [14], [15]]. To date, the relationship between clinical severity and disease progression in different age groups is unclear. A good understanding of the possible risk factors associated with the severity of COVID-19 will help clinicians identify high-risk COVID-19 patients to prevent disease progression and unfavorable outcomes [16]. Therefore, this study aims to evaluate the hematological, biochemical, and clinical data of COVID-19 patients of different age groups. It also aims to evaluate the role of these parameters with the potential risk factors and clinical findings related to the severity of COVID-19.
Study design
This is a retrospective cross-sectional observational study. It was conducted at King Abdulaziz University Hospital (KAUH), Jeddah, Saudi Arabia. Data from three months, June 2020 to August 2020 were included. This study was approved by the Scientific Research Committee (No 303-21), KAUH, Jeddah, Saudi Arabia.
Patients and data sources
Electronic medical records of individual patients (n = 104) were obtained from the COVID-19 wards at KAUH. The inclusion criteria were as follows: i. COVID-19 PCR-confirmed case and ii. Symptomatic patients who required hospitalization. Indication for hospitalization was classified according to severity as per the Saudi Ministry of Health Protocol dated April 12, 2020 (Supplementary Table 1) [17], and all asymptomatic patients were excluded from this study.
Blood tests
Laboratory parameters were collected during the symptomatic phase of the patients included in this study. Although the duration of the patients' symptoms at initial presentation varied, the hematological and biochemical parameters of all patients were collected immediately at the KAUH emergency (ER) department. Complete blood count (CBC), including white blood cells (WBC), hemoglobin (HB), and platelets (PLT), was performed using the Sysmex XN-9000-2-A automated hematology analyzer (Sysmex America Inc., Lincolnshire, Illinois, USA). The coagulation analyzer ACL TOP 550 CTS (Werfen, Barcelona, Spain) was used to determine D-dimer levels with HemosIL D-dimer HS 500 kit (Cat. No.: 0020500100). C-reactive protein (CRP) was measured with the Siemens BN II reliable nephelometric analyzer using the high-sensitivity C-reactive protein (hsCRP) assay kit (Siemens Healthcare GmbH, Erlangen, Germany). Liver function tests (LFTs), such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), albumin (ALB), gamma-glutamyl transferase (GGT), and bilirubin (BILI), and renal function tests (RFTs) such as creatine and blood urea nitrogen (BUN) were performed using Siemens Atellica Solution Immunoassay and Clinical Chemistry Analyzer (IM1300) instrument with Siemens (ALT, AST, TBil_2, Crea_2) kit. Similarly, lactate dehydrogenase (LDH), ferritin, and procalcitonin levels were measured using Siemens Atellica Solution Immunoassay and Clinical Chemistry Analyzer (IM1300) instrument with Siemens Atellica IM kits (Siemens Healthcare GmBH, Erlangen, Germany).
Statistical analysis
Statistical analysis was performed using GraphPad Prism (version 9) software (GraphPad Inc., USA). Means and standard deviation (SD) were calculated, and data were determined by the non-parametric Mann–Whitney U test, which was used to compare the means of two samples. Area under the curve (AUC) and receiver operator characteristic (ROC) with 95% confidence interval were calculated using the predicted probability of abnormal biological markers of COVID-19. All results are expressed as mean ± SD with 95%, 99%, and 99.9% of accuracy (* = P < 0.05, ** = P < 0.01, *** = P < 0.001).
Results
Baseline data
COVID-19 was diagnosed based on quantitative RT-PCR tests performed at KAUH, according to the protocol established by WHO. PCR data were subsequently entered independently into an electronic portal. Hematologic and clinical analyses were performed on blood samples from 104 SARS-CoV-2 positive patients. The mean age of the COVID-19 patients was 52 ± 16.5 years. We divided the patients into five groups according to their age (0−17, 18−45, 46−65, 66−79, and >80 years). We found that the highest percentage (45%) of COVID-19 patients were in the age group 46−65 years, followed by 18% in the age groups 18−45 and 66−79 years, 4% in those over 80 years, and 2% in the age group 0−17 years (Table 1). However, the 0−17 and >80 age groups were be excluded from further analysis due to small sample size.
Table 1.
Baseline characteristics of the COVID-19 patients.
| Male | Female | Total | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Sex | 72 (69) | 32 (31) | 104 (100) |
| Age groups (years) | |||
| 0−17 | 1 (50) | 1 (50) | 2 (2) |
| 18−45 | 16 (50) | 16 (50) | 32 (30) |
| 46−65 | 39 (83) | 8 (17) | 47 (45) |
| 66−79 | 13 (68) | 6 (31) | 19 (18) |
| >80 | 4 (100) | 0 | 4 (4) |
| Comorbidities: | |||
| Diabetes mellitus | 26 (78) | 7 (22) | 33 (32) |
| Hypertension | 24 (77) | 7 (23) | 31 (30) |
| Heart diseases | 8 (72) | 3 (28) | 11 (11) |
| Asthma | 0 | 3 (100) | 3 (3) |
| Rheumatoid arthritis | 0 | 1 (100) | 1 (1) |
| Cancer | 0 | 1 (100) | 1 (1) |
| Pregnancy | – | 3 (100) | 3 (100) |
| Medications: | |||
| Prednisone | 28 (80) | 7 (20) | 35 (34) |
| Tocilizumab | 6 (85) | 1 (15) | 7 (7) |
| O2 requirement | 35 (66) | 18 (34) | 53 (51) |
| Intensive care admission (ICU) | 13 (72) | 5 (27) | 18 (17) |
| Mortality | 6 (75) | 2 (25) | 8 (8) |
Clinical data analysis
Hematological parameters
Hematological parameters differ between age groups, but all values were within the normal range (reference range). However, non-significant differences were found in WBC (P = 0.36) and PLT (P = 0.56), while a significant difference was found between the patient groups in HB (P = 0.01) (Table 2 ).
Table 2.
The heatmap of the CBC results of COVID-19 patients.
 |
Note: The data are represented as the mean ± SD, and the color differences are based on the age group comparison.
LFT/RFT markers
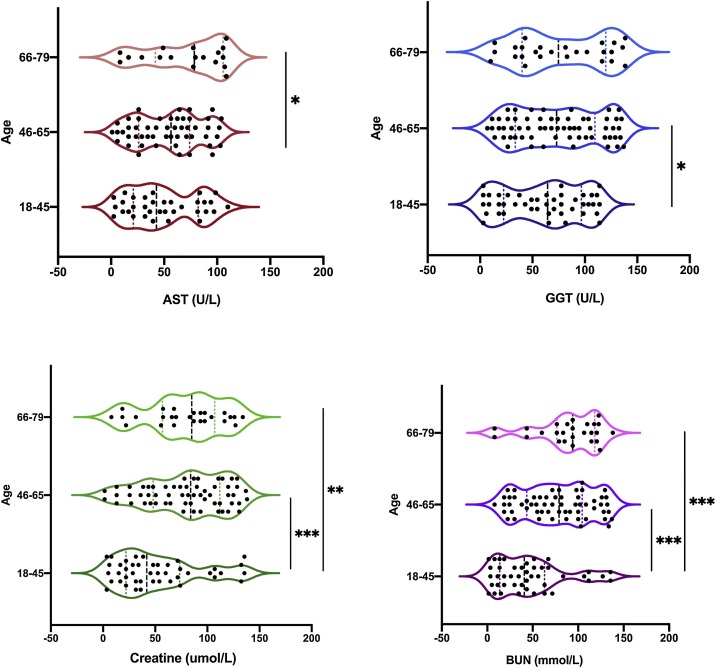
Based on the age groups, the LFTs and RFTs were examined to determine if there was a difference between the groups. In Table 3 , the heat map shows that the values of AST were above the normal range for all age groups of patients. ALT, ALP, ALB, and BILI were all within the normal range with no statistical differences (P = 0.65, 0.26, 0.16, and 0.06 respectively), although patients aged 66−79 years had the highest levels compared to the other groups. GGT had the highest expression in patients in the age groups 46−65 and 66−79 years. Since the other parameters were above the normal range, further analysis was performed within the groups to determine if there were any statistical differences. Fig. 1 shows a significant difference between the patient groups in terms of AST values (P = 0.04) and in GGT levels between patients in the 18−45 and 46−65 age groups (P = 0.05). Creatine levels were significantly higher in patients in the 18−45/46−65 age group than in patients in the 18−45/66−79 age groups (P < 0.001 and P = 0.02, respectively). Blood urea nitrogen levels (BUN) were significantly different between patients in the 18−45/46−65 (P < 0.001), and 18−45/66−79 age groups (P < 0.001). In general, patients in the 46−65 and 66−79 age groups had abnormal values of LFT and RFT markers compared to the other groups.
Table 3.
The heatmap of the LFTs and RFTs levels among COVID-19 patients’ groups.
 |
Note: The data are represented as mean ± SD; * = above the reference range; the color differences are based on the age group comparison.
Fig. 1.
The level of LFTs and RFTs among COVID-19 patients. No statistical difference was detected on the levels of AST and GGT. A significant difference was observed between the patients in the age 18-45 and 46-65 years on the GGT, creatine, and BUN levels, and between the age range 18-45 and 66-79 on the BUN level. Mann–Whitney test, where * = P < 0.05, ** = P < 0.01, *** = P < 0.001, n = 104. AST = aspartate aminotransferase, GGT = gamma-glutamyl transferase, BUN = blood urea nitrogen.
Inflammation and coagulation markers
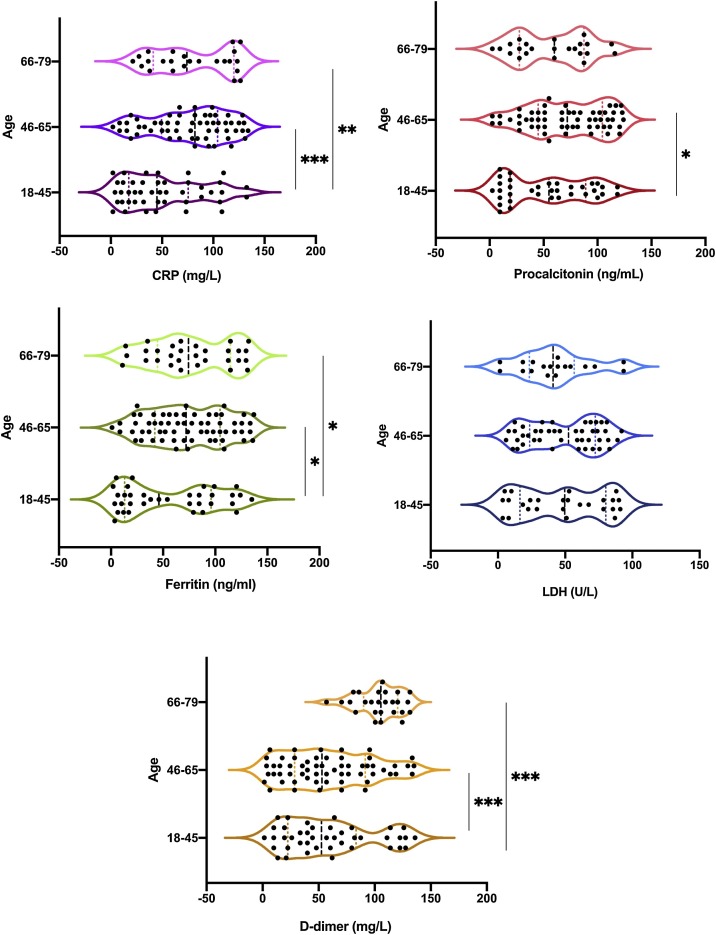
The heat map presented in Table 4 shows that the levels of the inflammatory markers CRP and D-dimer marker (used to indicate blood clot formation) were abnormal in all patients. Ferritin and LDH tests were used to detect signs of damage to body tissues, and these parameters were found to be elevated in all patients aged >18 years. Procalcitonin levels (found in severe bacterial infections compared with viral infections and nonspecific inflammatory diseases) were also elevated in all patients compared with the normal range. Further analysis between patient groups based on age differences was performed to determine the effect of age on disease severity (Fig. 2 ). A significant difference in CRP levels was found between patients in the 18−45/46−65 and 18−45/66−79 age groups (P = 0.001 and P = 0.005, respectively). Procalcitonin levels were higher in patients in the 18−45/46−65 age group (P = 0.009). A significant difference was found in ferritin levels between patients in the 18−45/46−65 and 18−45/66−79 age groups (P = 0.04 and P = 0.05, respectively). No significant difference was found in LDH levels between all patient’s groups (18−45/66−79 P = 0.65, and 46−65/66−79 P = 0.66). D-dimer levels were significantly higher in patients in the 18−45/66−79 (P < 0.001), and 46−65/66−79 (P < 0.001) age groups.
Table 4.
The heatmap of the inflammatory, and coagulation markers among COVID-19 patients’ groups.
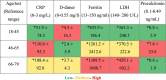 |
Note: The data are represented as mean ± SD; * = above the reference range; the color differences are based on the age group comparison.
Fig. 2.
The comparison between the inflammatory and coagulants markers in different age categories of patients with COVID-19. Most of the significant differences were observed in the CRP, procalcitonin, ferritin and D-dimer levels. No statistical difference was detected in the and LDH level. Mann–Whitney test, where * = P < 0.05, ** = P < 0.01, *** = P < 0.001, n = 104. CRP = C-reactive protein, LDH = lactate dehydrogenase.
ROC analysis
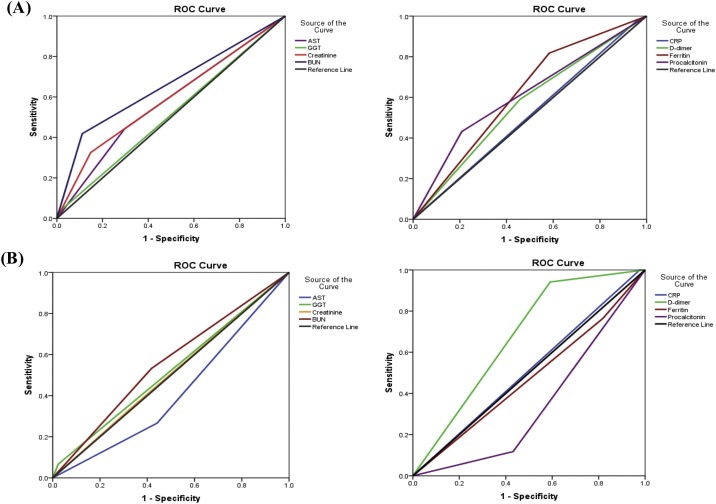
The ROC analysis was used to determine the optimal prediction threshold for COVID -19 biological markers. The AUC of various markers that showed significant expression in different patient groups was used to predict COVID -19 severity. Age-G1 was used as the independent variable (0 = for age group 18−45 years, 1 = for age group 46−65 years) and Age-G2 (0 = 46−65 years, 1 = 66−79 years). As shown in the ROC analysis of Age-G1 (Fig. 3 -A, and Supplementary Table 2), most clinical parameters were above the prediction threshold, and the AUCs of BUN, ferritin, and procalcitonin were the highest among the markers (>0.6). In Age-G2 ROC analysis, GGT, creatinine, BUN, D-dimer, and CRP showed the highest predictive index among markers. In addition, D-dimer and CRP had the highest AUCs (0.587 and 0.791, respectively; Fig. 3-B and Supplementary Table 3).
Fig. 3.
ROC curve comparing the different clinical markers to predict the severity of COVID-19 among different patient groups (A): Age-G1, and (B): Age-G2.
Discussion
A total of 104 COVID-19 patients were enrolled in this study and 16 parameters were used to compare clinical and laboratory markers in different age groups. The age range of our patients was from 18 to 79 years, which may indicate that all age groups are susceptible to COVID-19. In this study, the mean age of the patients was 52 years, which is consistent with a previous study from Saudi Arabia [18], where the median age was 50 years. Other studies from Saudi Arabia showed that the median age of COVID-19 patients was 36 years [19,20]. Another study from Oman showed that the mean age of the 63 patients was 48 years [21]. Most of our patients were men, which is consistent with previous studies [18,19,22]. This suggests that SARS-COV-2 is more likely to infect males, as sex hormones in females may play a protective role in fighting viral infections via innate and adaptive immunity [23]. The most common comorbidities were diabetes mellitus and hypertension in COVID-19 patients, a similar finding to previous studies [22,24].
In general, the groups that showed abnormal laboratory results were the age groups 46−65 and 66−79 years, we excluded two groups from the analysis due to small sample size (0−17 and >80 years). In the present study, about 51% of the patients required O2 supplementation and about 17% were admitted to the ICU due to the severity of their illness. In addition, age often correlated with impaired immune function and the presence of underlying comorbidities which may lead to poorer treatment outcome [25].
Hematologic variables (WBC, HB, and PLT) were normal, despite some insignificant differences between patient groups, except for HB which may vary between patients of different ages. However, these results are consistent with a study by Henry et al. The authors found that the WBC count was increased only in deceased patients (4.5 × 109/L), while the patients with severe disease had a mild increase in WBC count (0.5 × 109/L) and a decrease in PLT count [26]. This is consistent with the results of Gao et al., who found no statistical difference in the number of WBCs in COVID-19 patients [27]. However, Huang et al. reported low numbers of WBCs and lymphocytes in COVID-19 patients [28]. Fan et al. showed that the patients who were in the ICU had a greater decrease in HB values, absolute lymphocyte count, and absolute monocyte count compared with the non-ICU group [15].
COVID-19 is a systemic disease affecting many organs, including the liver and kidneys [29,30]. The incidence of liver dysfunction has been reported to be approximately 14–53% [29]. In this study, liver biomarkers, including ALT, ALP, ALB, and BILI, did not differ significantly between patient groups. However, the AST marker value was higher than the normal range in all patients. Cai et al. showed that after the abnormal liver test results of 417 patients with COVID-19, severe pneumonia occurred and 21.5% of patients suffered liver injury during hospitalization. The authors also reported that taking lopinavir/ritonavir medications increases liver injury, which should be monitored and assessed frequently. [31]. Yoo et al. showed that the hypoalbuminemia and abnormal AST levels were prominent in severe COVID-19 patients, and abnormal albumin levels were strongly correlated with COVID-19 severity with 78% of patients in the severe group having hypoalbuminemia [13].
Renal biomarkers including creatinine and BUN were significantly high in most patients, regardless of age. Hansrivijit et al. showed that creatinine level was a potential predictive factor for acute kidney injury (AKI) in patients with COVID-19 [32].Moreover, peak serum creatinine and blood urea nitrogen levels were associated with intrinsic AKI in men. However, urine analysis may not help to differentiate intrinsic AKI from other causes of AKI [33].
In addition, CRP is a commonly used inflammatory marker that indicates inflammation caused by various conditions, including infections. Besides, several reports have indicated that CRP is a marker for the identification of cytokine storms in COVID-19 patients as well as for disease mortality [34,35]. Here, we found that the CRP and procalcitonin levels were above the normal range in all patient groups. Gao et al. found that CRP levels were higher in the severe group (39 ± 28 mg/L) than in the mild group (19 ± 22 mg/L); however, procalcitonin levels showed no difference between the groups [27]. Wang et al. showed that procalcitonin levels were above normal range in patients admitted to the ICU and in patients not admitted to the ICU [36].
Previous studies have shown that the abnormal coagulation parameters were observed more frequently in the moderate and severe groups than in the mild group [[37], [38], [39]]. Moreover, impaired coagulation is considered an important risk factor for severe disease and death. Recent findings have shown that COVID-19 patients have abnormal coagulation in both clinical and laboratory examinations [38,40]. Ferritin, LDH, and D-dimer are inflammatory markers that are considered predictive factors for severe COVID-19 and disease progression [41]. In our study, no significant differences were found between the patient groups in terms of LDH levels; however, the levels of this marker were above the normal ranges. Serum ferritin level has shown a strong association with the incidence of COVID-19 [42,43], which is consistent with our findings. For this reason, patients with unusual ferritin levels should be carefully monitored [43,44]. Previous reports have also shown that the coagulation marker (D-dimer) was significantly higher in the severe group of patients with COVID-19 and those admitted to the ICU than in the mild group of patients with COVID-19 or those not admitted to the ICU [27,36,43]. Tang et al. showed that the elevated D-dimer and fibrin levels, and the prothrombin time (PT) in admitted patients, were related to poor prognosis [38].
The area under the ROC curve for all selected parameters was largest in patients aged 18−45 and 46−65 years (>0.5), while GGT, creatinine, BUN, d-dimer, and CRP levels in the predictive index were higher in patients aged 46−65 and 66−79 years. A previous study by Goa et al. showed that the AUC of D-dimer (0.750) was high in patients with severe COVID-19 [27]. In addition, the initial CRP measurement based on the ROC curve for severe COVID-19 could be used as a risk model and early predictor [45].
These laboratory parameters may be associated with severity of COVID-19 in different age groups of patients. In addition, abnormal laboratory values of these parameters in elderly patients may be an early sign of severe disease and poor outcomes. A study by Luo et al. showed that elderly patients were more than twice as likely to have severe or critical illness when compared with middle-aged patients [12]. Several studies have shown that elderly patients do not respond well to treatment or do not improve significantly after treatment and are likely to be at higher risk of other diseases, such as acute respiratory distress syndrome and respiratory failure, or even death compared to younger patients [34,[46], [47], [48]]. Our study warrants further investigation of the clinical, hematologic, and inflammatory parameters obtained from a large cohort of COVID-19 patients with mild to severe disease to better understand or decipher the clinical predictors of COVID-19 disease severity in the clinical setting.
Conclusion
The main finding of our study is that laboratory parameters such as GGT, creatinine, BUN, CRP, procalcitonin, ferritin and D-dimer were differentially regulated in COVID-19 patients of different age groups. Importantly, these laboratory parameters may help as clinical predictors to assess the severity of the disease in the population, as age is an important factor influencing COVID -19 severity. However, the main limitation of this study was the small sample size, which may have affected the statistical power. In addition, not all tests were performed in all patients. For this reason, future studies should include more COVID-19 patients and healthy volunteers for further investigation to compare the values of these parameters between COVID-19 positive and negative participants in a typical clinical milieu.
Funding
No funding to declare.
Competing interests
The Author(s) declare(s) that there is no conflict of interest.
Ethical approval
The study was approved by the Ethics Committee of King Abdulaziz University Hospital (KAUH) (Reference No 303-21), Jeddah, Saudi Arabia.
Author contributions
LAD, SB, and PNP proposed this idea. SB, AA, YB, and MM collected the data. LAD, ID, and PNP analyzed the data. LAD, AA, and PNP wrote the manuscript. All authors read and approved the manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jiph.2021.12.013.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Cascella M., Rajnik M., Aleem A., Dulebohn S.C., Di Napoli R. StatPearls [Internet] StatPearls Publishing; Treasure Island (FL): 2021. Features, evaluation, and treatment of coronavirus (COVID-19) PMID: 32150360. [PubMed] [Google Scholar]
- 2.WHO | Pneumonia of unknown cause — China n.d. https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/. [Accessed 5 May 2021].
- 3.Al-Hanawi M.K., Angawi K., Alshareef N., Qattan A.M.N., Helmy H.Z., Abudawood Y., et al. Knowledge, attitude and practice toward COVID-19 among the public in the Kingdom of Saudi Arabia: a cross-sectional study. Front Public Health. 2020;8:217. doi: 10.3389/fpubh.2020.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CCC — Command and Control Center, n.d. https://www.moh.gov.sa/en/CCC/Pages/default.aspx. [Accessed 5 May 2021].
- 5.MOH News — Strenuous Efforts Made by Health Emergency Operations Center in COVID-19 Fight, n.d. https://www.moh.gov.sa/en/Ministry/MediaCenter/News/Pages/News-2020-03-11-001.aspx. [Accessed 5 May 2021].
- 6.CDC Global Health — Saudi Arabia, n.d. https://www.cdc.gov/globalhealth/countries/saudi_arabia/default.htm. [Accessed 5 May 2021].
- 7.Sisó-Almirall A., Brito-Zerón P., Ferrín L.C., Kostov B., Moreno A.M., Mestres J., et al. Long covid-19: proposed primary care clinical guidelines for diagnosis and disease management. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18084350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Almeida-Pititto B., Dualib P.M., Zajdenverg L., Dantas J.R., de Souza F.D., Rodacki M., et al. Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol Metab Syndr. 2020;12:75. doi: 10.1186/s13098-020-00586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Y.-D., Ding M., Dong X., Zhang J.-J., Kursat Azkur A., Azkur D., et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76:428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Liu Y., Liu L., Wang X., Luo N., Li L. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at hospital admission in Shenzhen, China. J Infect Dis. 2020;221:1770–1774. doi: 10.1093/infdis/jiaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020;80:e14–e18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo H., Liu S., Wang Y., Phillips-Howard P.A., Ju S., Yang Y., et al. Age differences in clinical features and outcomes in patients with COVID-19, Jiangsu, China: a retrospective, multicentre cohort study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-039887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo E.H., Chang S.H., Song D.Y., Lee C.H., Cheong G.Y., Park S., et al. Comprehensive laboratory data analysis to predict the clinical severity of coronavirus disease 2019 in 1,952 patients in daegu, korea. Ann Lab Med. 2021;42:24–35. doi: 10.3343/ALM.2022.42.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skevaki C., Fragkou P.C., Cheng C., Xie M., Renz H. Laboratory characteristics of patients infected with the novel SARS-CoV-2 virus. J Infect. 2020;81:205–212. doi: 10.1016/j.jinf.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan B.E., Chong V.C.L., Chan S.S.W., Lim G.H., Lim K.G.E., Tan G.B., et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95:E131–4. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 16.Sokolowska M., Lukasik Z.M., Agache I., Akdis C.A., Akdis D., Akdis M., et al. Immunology of COVID-19: mechanisms, clinical outcome, diagnostics, and perspectives—a report of the European Academy of Allergy and Clinical Immunology (EAACI) Allergy. 2020;75:2445–2476. doi: 10.1111/all.14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saudi Ministry of Health . Saudi Minist Heal; 2021. Saudi MoH Protocol for Adults Patients suspected of / confirmed with COVID-19 Supportive care and antiviral treatment of suspected or confirmed COVID-19 infection; pp. 1–6. [Google Scholar]
- 18.AlJishi J.M., Alhajjaj A.H., Alkhabbaz F.L., AlAbduljabar T.H., Alsaif A., Alsaif H., et al. Clinical characteristics of asymptomatic and symptomatic COVID-19 patients in the Eastern Province of Saudi Arabia. J Infect Public Health. 2021;14:6–11. doi: 10.1016/j.jiph.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Omari A., Alhuqbani W.N., Zaidi A.R.Z., Al-Subaie M.F., AlHindi A.M., Abogosh A.K., et al. Clinical characteristics of non-intensive care unit COVID-19 patients in Saudi Arabia: a descriptive cross-sectional study. J Infect Public Health. 2020;13:1639–1644. doi: 10.1016/j.jiph.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alsofayan Y.M., Althunayyan S.M., Khan A.A., Hakawi A.M., Assiri A.M. Clinical characteristics of COVID-19 in Saudi Arabia: a national retrospective study. J Infect Public Health. 2020;13:920–925. doi: 10.1016/j.jiph.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khamis F., Al-Zakwani I., Al Naamani H., Al Lawati S., Pandak N., Omar M.B., et al. Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID-19: an experience from Oman. J Infect Public Health. 2020;13:906–913. doi: 10.1016/j.jiph.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamel F.O., Magadmi R.M., Alqutub S.T., Badawi M., Al-Sayes F., Badawi M., et al. Clinical and hematologic presentations of adults with COVID-19 patients in Jeddah: a case control study. J Infect Public Health. 2021;14:709–716. doi: 10.1016/j.jiph.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaillon S., Berthenet K., Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol. 2019;56:308–321. doi: 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- 24.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Opal S.M., Girard T.D., Ely E.W. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis. 2005;41(Suppl 7):S504–12. doi: 10.1086/432007. [DOI] [PubMed] [Google Scholar]
- 26.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 27.Gao Y., Li T., Han M., Li X., Wu D., Xu Y., et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92:791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li N., Qiao H., Guo J.-F., Yang H.-Y., Li X.-Y., Li S.-L., et al. Preoperative hypoalbuminemia was associated with acute kidney injury in high-risk patients following non-cardiac surgery: a retrospective cohort study. BMC Anesthesiol. 2019;19:171. doi: 10.1186/s12871-019-0842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai Q., Huang D., Yu H., Zhu Z., Xia Z., Su Y., et al. COVID-19: abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansrivijit P., Qian C., Boonpheng B., Thongprayoon C., Vallabhajosyula S., Cheungpasitporn W., et al. Incidence of acute kidney injury and its association with mortality in patients with COVID-19: a meta-analysis. J Investig Med. 2020;68:1261–1270. doi: 10.1136/jim-2020-001407. [DOI] [PubMed] [Google Scholar]
- 33.Hansrivijit P., Gadhiya K.P., Gangireddy M., Goldman J.D. Risk factors, clinical characteristics, and prognosis of acute kidney injury in hospitalized COVID-19 patients: a retrospective cohort study. Medicines. 2021;8:4. doi: 10.3390/medicines8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang K., Zhang Z., Yu M., Tao Y., Xie M. 15-day mortality and associated risk factors for hospitalized patients with COVID-19 in Wuhan, China: an ambispective observational cohort study. Intensive Care Med. 2020;46:1472–1474. doi: 10.1007/s00134-020-06047-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao D., Zhou F., Luo L., Xu M., Wang H., Xia J., et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 2020;7:e671–8. doi: 10.1016/S2352-3026(20)30217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong M., Liang X., Wei Y.-D. Changes in blood coagulation in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Br J Haematol. 2020;189:1050–1052. doi: 10.1111/bjh.16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu T., Zhang J., Yang Y., Ma H., Li Z., Zhang J., et al. The role of interleukin‐6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med. 2020;12:1–12. doi: 10.15252/emmm.202012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hussein M., Taha Z.B., Gailan Malek A., Akram Rasul K., Qasim Hazim D., Jalal Ahmed R., et al. D-dimer and serum ferritin as an independent risk factor for severity in COVID-19 patients. Mater Today Proc. 2021 doi: 10.1016/j.matpr.2021.04.009. 10.1016/j.matpr.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao P., Zhang Y., Huang Z., Sullivan M.A., He Z., Wang J., et al. The preventative effects of procyanidin on binge ethanol-induced lipid accumulation and ROS overproduction via the promotion of hepatic autophagy. Mol Nutr Food Res. 2019;63 doi: 10.1002/mnfr.201801255. [DOI] [PubMed] [Google Scholar]
- 45.Hu H., Du H., Li J., Wang Y., Wu X., Wang C., et al. Early prediction and identification for severe patients during the pandemic of COVID-19: a severe COVID-19 risk model constructed by multivariate logistic regression analysis. J Glob Health. 2020;10:20510. doi: 10.7189/jogh.10.020510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mo P., Xing Y., Xiao Y., Deng L., Zhao Q., Wang H., et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020;73 doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lai C.-C., Liu Y.H., Wang C.-Y., Wang Y.-H., Hsueh S.-C., Yen M.-Y., et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020;53:404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.