Abstract
An open prospective clinical, microbiological, and molecular analysis of a national molecular diagnostic service for tuberculous meningitis (TBM) using an in-house IS6110-targeted PCR for molecular “Fastrack” diagnosis was carried out. Between April 1997 and June 1998. Consecutive cerebrospinal fluid (CSF) samples from 131 patients were assessed. Against a culture on the same sample, PCR had a sensitivity of 75% and a specificity of 94%. Of samples from patients classified as definite or probable TBM cases based on clinical criteria, 81% had raised CSF protein levels and 73% had a lymphocytosis, although 57% of all submitted samples showed a raised lymphocyte count. While only 46% had a CSF glucose level below the normal range, the CSF glucose level was significantly lower (P = 0.0281) than in cases of meningitis of other etiologies. Levels of tumor necrosis factor alpha were also found to be significantly raised in definite or probable TBM cases (P = 0.028), while adenosine deaminase levels were not. The study showed IS6110-targeted PCR to be a rapid, sensitive, and specific test in routine use for the diagnosis of TBM.
Due to inconsistent clinical presentations, rarity (106 notifications in England and Wales, 1996 [17]), and the lack of a rapid, sensitive, and specific test, tuberculous meningitis (TBM) is particularly difficult to diagnose (27). While conventional microscopy and culture are widely used, smear microscopy is insensitive (usually 10 to 20%, although sometimes higher, especially when multiple samples or large volumes are processed) (5), and culture takes up to 4 to 6 weeks to provide a result, limiting the value of these methods in aiding diagnosis and immediate decisions on treatment. Any delays in initiating the correct drug regime lead to increased neurological sequelae and mortality rates, and so diagnosis is made on assessment of clinical presentation, cerebrospinal fluid (CSF) biochemistry, microscopy, and evidence of current or prior tuberculosis (TB) (7). However, presentations are diverse and rarely fit a “classical picture” of TBM symptoms. At present, the culture method provides a retrospective “gold standard” diagnosis and rapid, sensitive, and specific tests are urgently needed to aid the clinician. Many tests have been advocated for the diagnosis of TBM, including the bromide partition test (11, 26, 29), the adenosine deaminase assay (14, 18, 31) and, more recently, latex particle agglutination (9), high-pressure liquid chromatography (3), and various PCR (2, 12, 16, 20, 25, 30)- and enzyme-linked immunosorbent assay (ELISA) (1, 13, 15)-based tests. While immunodiagnostic techniques may show promise, at present they lack sufficient sensitivity and often the necessary specificity. PCR is the most widely applied alternative rapid diagnostic technique for TBM. The present study was an open prospective analysis of samples referred to the Public Health Laboratory Service Mycobacterium Reference Unit (PHLS MRU) for England and Wales for testing under a national “Fastrack” PCR diagnostic service for TBM between April 1997 and June 1998. A nested-PCR assay was chosen to maximize sensitivity since volumes of CSF available for TBM PCR are often lower than requested. Stringent precautions were taken against contamination, namely, there were three separate dedicated rooms for preparation of master mixes (clean area), extraction of DNA and addition of sample (gray area), and amplification and detection of sample (dirty area). Negative controls and inhibition controls were included with each run. Assessments of sensitivity and specificity were made using both culture as a gold standard and assessment of clinical data and treatment. While PCR has been shown to be more sensitive than culture in several studies, problems with contamination and therefore specificity make it unsuitable as a gold standard until methods and especially controls are standardized across laboratories. A total of 131 samples from 131 patients were assessed during the study period using an in-house PCR assay based on detection of the IS6110 insertion sequence, which was a modification of the method of Wilson et al. (30).
MATERIALS AND METHODS
A national Fastrack PCR diagnostic service was offered for CSF samples referred to the PHLS MRU for TBM testing. Guidance on CSF volumes was provided, with a recommended volume of 1 ml or greater and a minimum volume of 0.5 ml. Where this service was requested, samples were processed as outlined below. Clinical data were sought prospectively in all cases and retrospectively by follow-up questionnaire. The following parameters were requested: name, age, gender, presence of fever, meningism, photophobia, immunocompromised status, computed tomography and magnetic resonance imaging (MRI) scan results, TB contact and history, CSF biochemistry (protein, glucose, white cell count, and lymphocyte count), blood glucose level, microscopy, and the results of other investigations for viral-bacterial infections. Data were also sought retrospectively on the final diagnosis, treatment given, and outcome. Where there was sufficient volume, sample supernatants were also analyzed for adenosine deaminase levels, tumor necrosis factor alpha (TNF-α) levels and, using PCR, for herpes simplex virus (HSV) types 1 and 2 and varicella-zoster virus (VZV) according to established methods (6, 19).
For further analysis patients were classified as definite TBM cases when a positive culture was obtained and as probable TBM cases through assessment of CSF biochemistry, clinical presentation, radiological findings, medical history, treatment, progression, and the clinician's final diagnosis. Between April 1997 and June 1998 131 samples were tested.
Sample processing.
When a >1-ml CSF sample was received, the excess CSF was inoculated directly into an MB BacT culture vial (Organon Teknika, Cambridge, United Kingdom) and onto a Lowenstein-Jensen slope. In cases where a <1-ml CSF sample was received, all of the sample was removed for PCR, and in all cases the sample container was rinsed with Kirchner medium and cultured.
DNA extraction.
DNA was extracted according to the chloroform extraction method of Wilson et al. (30). One milliliter of CSF (or the available volume, with a minimum of 0.5 ml) was centrifuged at 12,000 × g for 15 min. The supernatant was removed and stored at −20°C. The pellet was washed with 1 ml distilled water. Then, 25 μl of distilled water and an equal volume of chloroform was added to the tube. The pellet was resuspended and dispersed by vigorous mixing, followed by incubation in a water bath at 80°C for 20 min. Samples were removed from the water bath and cooled in a freezer for several minutes before being brought back to room temperature and briefly centrifuged to ensure clear separation of the aqueous phase.
IS6110 PCR.
Ten microliters of the aqueous phase was added to the PCR reaction to give a final reaction volume of 40 μl. A 9-μl portion of each extract was also added to a second identical reaction, along with 1 μl of a 500-fg/μl mixture of DNA extracted from BCG (ca. 100 genome equivalents) to serve as an inhibition control. One milliliter of distilled water was processed in parallel with each CSF sample as a negative control, with a minimum of four negative controls per run. A high (500 fg/reaction, 100 genome equivalents) and a low (50 fg/reaction, 10 genome equivalents) positive control of BCG DNA extract were included with each PCR run.
A nested PCR was performed using 0.2-ml thinwall Apex tubes (Alpha, Eastleigh, United Kingdom). Reactions included final concentrations of 16 mM (NH4)SO4 reaction buffer (Bioline, London, United Kingdom); 200 μM concentrations of dATP, dCTP, and dGTP; 100 μM concentrations of dUTP and dTTP (Pharmacia Biotech); 1.5 mM MgCl2; 0.23 mg of bovine serum albumin per ml; 5% (vol/vol) dimethyl sulfoxide; and 20 pmol of primers and 1 U of Taq (Bioline) per reaction. Volumes were made up with distilled water. A 1-μl first-round product was added to 20 μl of the inner reaction mix for the nested reaction. The outer reaction cycle consisted of 2 min at 93°C, followed by 30 cycles of 20 s at 93°C, 30 s at 65°C, and 1 min at 72°C, with a final hold step of 10 min at 72°C. The inner reaction cycle was identical except for an annealing temperature of 48°C, a reaction volume of 20 μl, and 0.5 U of Taq polymerase per reaction. The outer primers were TB294 (5′-GGACAACGCCGAATTGCGAAGGGC-3′) and TB850 (5′-TAGGCGTCGGTGACAAAGGCCACG-3′). The inner primers were TB505 (5′-ACGACCACATCAACC-3′) and TB670 (5′-AGTTTGGTCATCAGCC-3′). PCR was performed in a Perkin-Elmer (Langen, Germany) 9600 Thermocycler. After amplification, the product was visualized by electrophoresis on 2% agarose gel by ethidium bromide staining.
Viral PCR.
PCR for HSV types 1 and 2 and VZV was carried out on 73 samples for which sufficient CSF supernatant remained according to the method of Read et al. (19) and Jeffery et al. (6). Reagent sources were the same as for the IS6110 PCR.
Adenosine deaminase assay.
Adenosine deaminase levels were determined by the colorimetric assay of Giusti (4). An adenosine deaminase-positive control (50 U/liter) was incubated in parallel with the samples in each run. All solutions were prepared using AnalaR water (Merck, Lutterworth, United Kingdom) to minimize background values from ammonium in the water. The formation of blue indophenol was measured at 628 nm with a Genesys 5 spectrophotometer (Life Sciences International, Runcorn, United Kingdom) against a distilled-water blank. All other reagents were from Sigma-Aldrich, Dorset, United Kingdom.
TNF-α assay.
TNF-α assays were performed using sandwich human TNF-α ELISA kits from Genzyme (Cambridge, United Kingdom) according to the manufacturer's instructions. Data were analyzed, and unknowns were determined from standard curves using Graphpad PRISM software (Intuitive Software for Science, San Diego, Calif.).
CSF spiking experiments.
Assays were performed in which CSF samples from patients not suspected of TBM were spiked with Mycobacterium tuberculosis or BCG to demonstrate the sensitivity of the IS6110 PCR test in CSF. Pooled CSF was spiked with IS6110 high-copy-number (17 copies) and low-copy-number (1 copy) M. tuberculosis strains. CSF was also spiked with BCG (one IS6110 copy). Unspiked CSF, distilled water, inhibition controls, and high (500 fg/reaction, 100 genome equivalents) and low (50 fg/reaction, 10 genome equivalents) positive controls (as previously) were included with each run. To minimize clumping of bacteria, cultures were vortexed for 10 s, sonicated for 10 s, revortexed for a further 10 s, and left to stand for 5 min to allow any remaining large clumps to settle. A total of 900 μl of CSF sample was spiked with 100 μl of each culture dilution in triplicate. Colony counts were established from the same dilution series to enumerate the bacteria present. CSF was then processed as for the clinical samples, as outlined above, except that the extractions were made into 50 μl of distilled water plus 50 μl of chloroform in cases where a 25 μl–25 μl volume would be used for clinical samples. Since there was insufficient CSF available, experiments were repeated, spiking distilled water, in place of CSF, to allow 20 replicates to be performed at the minimum CFU count.
RESULTS
IS6110 sensitivity.
The IS6110 PCR detected M. tuberculosis in CSF with a high sensitivity (Table 1). IS6110-targeted PCR showed an increase in sensitivity, detecting the M. tuberculosis strain with 17 IS6110 target copies compared to both BCG (one IS6110 copy) and the M. tuberculosis strain with 1 IS6110 copy using spiked preparations in distilled water. (P = 0.0248, Fisher's exact test) (Table 2).
TABLE 1.
Spiking of CSF with viable M. tuberculosis complex isolate of known CFU number
| M. tuberculosis complex isolate (IS6110 copy no.) | CFU in 10 μl of extract | No. of IS6110 copies | No. positive (%) by IS6110 PCR (n = 3)a |
|---|---|---|---|
| M. tuberculosis (17) | 400 | 6,800 | 3 (100) |
| 40 | 680 | 3 (100) | |
| 4 | 68 | 3 (100) | |
| M. tuberculosis (1) | 50 | 50 | 3 (100) |
| 5 | 5 | 3 (100) | |
| 0.5 | 0.5 | 1 (33.3) | |
| BCG (1) | 1000 | 1000 | 3 (100) |
| 100 | 100 | 2 (66.6) | |
| 10 | 10 | 0 (0) |
That is, the number of three replicates found to be positive by IS6110 PCR.
TABLE 2.
Spiking of distilled water with viable M. tuberculosis complex isolate of known CFU number
| M. tuberculosis complex isolate (IS6110 copy no.) | CFU in 10 μl of extract | No. of IS6110 copies | No. positive (%) by IS6110 PCR (n = 20)a |
|---|---|---|---|
| M. tuberculosis (17) | 2 | 34 | 15 (75) |
| M. tuberculosis (1) | 12 | 12 | 7 (35) |
| BCG (1) | 2 | 2 | 1 (5) |
That is, the number of 20 replicates found to be positive by IS6110 PCR.
CSF analysis.
A total of 131 samples from different patients were assessed by IS6110 PCR during the study period (Table 3). Of these, 23 (17.5%) were found to be positive by culture or PCR or were classified as probable TBM cases through assessment of CSF biochemistry, clinical presentation, radiological findings, medical history, treatment, progression, and the clinician's final diagnosis. Data for these cases are presented in Table 4, along with the results for the sample which tested positive by PCR but which was determined to be a false positive. However, only four samples processed for PCR were culture positive, although in five cases a positive culture was obtained within 3 months from subsequent samples not processed by PCR in cases where the original sample was both PCR and culture negative, one of which was a brain biopsy. In 15 cases no culture result was available.
TABLE 3.
PCR and culture results on Fastrack CSF samples
| PCR category | No. of samples culture:
|
Total no. of samples | ||
|---|---|---|---|---|
| Positive | Negative | Indeterminatea | ||
| PCR positive | 3 | 6 | 0 | 9 |
| PCR negative | 1 (5)b | 99 | 15 | 115 |
| PCR inhibited | 0 | 7 | 0 | 7 |
| Total | 4 | 112 | 15 | 131 |
Indeterminate, culture result not available.
The number of samples culture positive with subsequent samples, not processed by PCR, including one brain biopsy, is shown in parentheses.
TABLE 4.
Principal findings in TBM cases and false-PCR-positive casea
| Patient no. | Culture result | PCR result | Culture on subsequent CSF samples | Clinician diagnosis | Conclusion | Protein level (g/liter) | Glucose level (mmol/liter) | No. of lymphocytes (106/ml) | Notes |
|---|---|---|---|---|---|---|---|---|---|
| 2 | Pos | Pos | NA | Pos | Def | 2.39 | 1.6 | No details available | |
| 4 | Neg | Neg | NA | Pos | Prob | 2.22 | 2.6 | 1.04 | Headache, photophobia abnormal MRI, treated, recovered |
| 5 | Neg | Neg | Pos (BB) | Pos | Def | 309 | HIV, basal ganglion lesions | ||
| 6 | Neg | Neg | NA | Pos | Prob | 1.46 | 1.7 | 3.09 | Died, no PM |
| 16 | Neg | Pos | NA | Neg | Neg | False PCR positive | |||
| 17 | Neg | Neg | NA | Pos | Prob | 1.02 | 3.1 | Nil | Brain lesion, lymph TB, treated |
| 34 | Neg | Neg | NA | Pos | Prob | 1.25 | 2.6 | 0.95 | Full treatment, excellent response |
| 48 | Neg | Neg | Pos | Pos | Def | Raised | No details | ||
| 55 | Neg | Neg | Pos | Pos | Def | 5.98 | 3.7 | 432 | No details |
| 56 | Neg | Neg | NA | Pos | Prob | Full treatment, excellent response | |||
| 57 | Pos | Pos | NA | Pos | Def | 9.47 | 1.4 | 102 | Died, no PM |
| 58 | Neg | Neg | Pos | Pos | Def | 1.53 | 3.4 | Treated, responded | |
| 65 | Neg | Neg | Pos | Pos | Def | 0.03 | Treated | ||
| 67 | Neg | Pos | NA | Pos | Prob | 2.33 | 2.9 | Lesions on CT, treated, improved | |
| 77 | Neg | Pos | NA | Pos | Prob | Raised | Low | 190 | Previous TB adenitis, HIV, treated, recovered |
| 88 | Neg | Neg | NA | Pos | Def | 1.10 | 1.8 | 15 | Lymph TB culture positive |
| 91 | Neg | Pos | NA | Pos | Prob | 0.21 | 6.1 | 285 | Abnormal MRI, previous TBM |
| 97 | Pos | Pos | NA | Pos | Def | 0.72 | 1.9 | Presented with cough, multiple small lesions on MRI, treated | |
| 99 | Neg | Neg | NA | Pos | Prob | Low | 44 | Abnormal MRI | |
| 106 | Neg | Pos | NA | Unknown | Prob | 0.35 | 5.1 | Raised | No details available |
| 112 | Neg | Neg | NA | Pos | Prob | 0.84 | 2.2 | 0.33 | Abnormal MRI, miliary pulmonary TB, treated, recovered |
| 115 | Neg | Neg | NA | Pos | Prob | 7.8 | 1.6 | Previous TB, TB spine? | |
| 123 | Pos | Neg | NA | Pos | Def | Untraceable to follow-up | |||
| 127 | Neg | Pos | NA | Pos | Prob | 0.33 | 3.3 | Abnormal MRI, HIV, treated, recovered |
Abbreviations: BB, brain biopsy; Pos, positive; Neg, negative; Def, definite TBM; Prob, probable TBM; NA, not applicable; PM, post mortem.
Using culture on the same sample as the gold standard of diagnosis, the IS6110 PCR test had a sensitivity of 75% and a specificity of 94%. Of 112 culture-negative patients, 13 were classified as probable cases of TBM (see Table 4 for principal findings and notes), and 5 of these were detected by PCR. One sample which was PCR positive yet culture negative was determined to be a false PCR positive by an examination of the clinical history, treatment, and progression. Attempts were made to follow up the specimen which was culture positive and PCR negative as outlined previously. No sample was available for repeat testing, no response was obtained to questionnaires, and no subsequent specimens were received at the reference center. A summary of the principal clinical findings in definite and/or probable TBM cases is given in Table 4.
The gender distribution of submitted samples was equal, with 56% (n = 73) and 44% (n = 58) from male and female patients, respectively. Of those classified as definite or probable cases of TBM 69.5% (n = 16) were male, while 30% (n = 7) were female. The ages (in years) of 116 (96%) patients were known: 22 (18%) patients were under age 20, 54 (45%) patients were between age 21 and age 50, and 40 (33%) patients were age 50 or older; the age of 5 (4%) patients was not known. The age distribution was similar among positive patients: 24%, (5 patients), 0 to 20 years 43% (10 patients), 21 to 50 years; and 35% (8 patients), 50 years or older. Nine percent of patients were known to be human immunodeficiency virus (HIV) positive; three (13%) of the patients with definite or probable TBM were HIV positive.
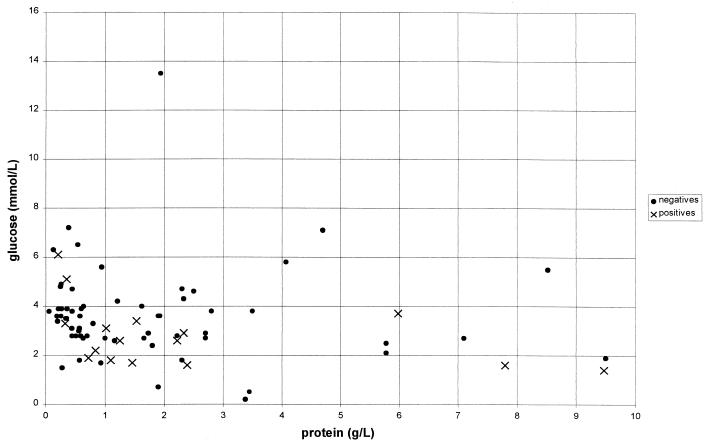
Only 20% (15 of 75) of submitted samples with CSF biochemistry data available fit the classic “raised-protein, low-glucose” profile (Fig. 1), although 61% (46 of 75) had raised protein levels with normal or raised glucose. Therefore, only 19% (14 of 75) had normal or low protein levels. Of patients classified as definite or probable TBM cases, CSF biochemistry results were available for 17. Interestingly, only 8 of the 17 (47%) fit the classic raised-protein, low-glucose picture (see Fig. 1), while 6 (35%) had a raised protein level but normal glucose and 2 (12%) had normal values for both protein and glucose. Of 61 samples, 35 (57%) had raised lymphocyte levels with 8 of 11 (73%) definite or probable TBM cases having raised lymphocyte levels. This was expected, since lymphocytosis would be a key factor in referral for TBM testing; however, a raised lymphocyte count could not be used to differentiate between TBM and meningitis of other etiologies in this study (P = 0.494, Table 3). Of those 23 finally considered definite (culture positive or PCR positive with full treatment and final clinical diagnosis of TBM) or probable TBM cases, culture on a single sample had a sensitivity of only 17%, which rose to 39% when multiple samples were processed, while PCR showed a sensitivity of 35%.
FIG. 1.
CSF protein versus glucose for all samples. Normal values were as follows: glucose, 2.5 to 5.5 mmol/liter; protein, 0.15 to 0.4 g/liter. Symbols: ×, definite and probable TBM cases; ●, all other samples.
Where additional information was available, the numbers of diagnoses of negative patients were as follows: viral, 10; bacterial, 6; trauma, 3; Wegener's granulomatosis, 1; cerebral vasculitis, 2; cancer, 4; Still's disease, 2; multiple sclerosis, 1; idiopathic chronic parchymeningitis, 1; polyradiculopathy, 1; pituitary mass, 1; thyroid storm, 1; retinitis, 1; infective endocarditis, 1; spastic paraplegia, 1; spontaneous recovery, 1; uncertain final diagnosis, 13; lost to follow-up, 3; no response to questionnaire, 57. Of the viral cases, three were HSV (two were independently confirmed in this study), one was VZV (also independently confirmed), one was cytomegalovirus, one was JC virus (JCV), and four were unspecified. Twenty-nine cases for whom there was no response to the questionnaire had negative results for both HSV and VZV by PCR.
Viral PCR.
Sufficient CSF was available for HSV and VZV testing by PCR for 73 of the samples, including 29 of the samples for which no response to the retrospective questionnaire was obtained: 70 samples were negative for both viruses, 2 samples were positive for HSV, and one sample was positive for VZV. In all cases, positive and negative PCR controls performed correctly.
Mann-Whitney analysis.
Glucose was found to be significantly lower in TBM cases than in meningitis cases of other etiologies (P = 0.0317). TNF-α levels were significantly higher (P = 0.037). No value is given for the adenosine deaminase and TNF-α values of culture-positive TBM cases versus negative or viral cases since the number of culture-positive cases where sufficient CSF remained for these tests was too small (Table 5).
TABLE 5.
Mann-Whitney analysis of CSF biochemistrya
| Parameter | Datum group A
|
Datum group B
|
Pb | ||||
|---|---|---|---|---|---|---|---|
| Type | n | Mean | Type | n | Mean | ||
| Adenosine deaminase (U/liter) | Negatives | 52 | 3.1 | TBM | 10 | 9.2 | 0.133 |
| TBM | 10 | 9.2 | Viral | 5 | 2.0 | 0.309 | |
| Viral | 5 | 2.0 | Other negatives | 47 | 3.2 | 0.986 | |
| Viral | 5 | 2.0 | Other negatives+TBM | 57 | 4.2 | 0.825 | |
| Glucose (mmol/liter) | Other TBM | 10 | 1.7 | Culture positive | 5 | 2.0 | 0.992 |
| Negatives | 63 | 3.6 | Prob TBM | 10 | 3.1 | 0.192 | |
| Negatives | 63 | 3.6 | TBM | 16 | 2.8 | 0.028∗ | |
| Negatives | 63 | 3.6 | Culture positive | 6 | 2.3 | 0.033∗ | |
| Viral | 5 | 4.0 | TBM | 15 | 2.8 | 0.091 | |
| Viral | 5 | 4.0 | Culture positive | 6 | 2.3 | 0.082 | |
| Viral | 5 | 4.0 | Prob TBM | 10 | 3.1 | 0.207 | |
| Viral | 5 | 4.0 | Other negatives | 59 | 3.6 | 0.515 | |
| Viral | 5 | 4.0 | Other negatives | 15 | 3.2 | 0.457 | |
| Negatives | 64 | 6.6 | PCR culture positive | 9 | 3.0 | 0.234 | |
| PCR culture positive | 9 | 3.0 | Clinician | 7 | 2.2 | 0.470 | |
| Viral | 5 | 4.0 | All other | 74 | 3.4 | 0.339 | |
| Lymphocytes (106/ml) | Viral | 5 | 24.9 | Other negatives | 14 | 89.9 | 0.431 |
| PCR culture positive | 5 | 136.2 | Clinician | 6 | 48.4 | 0.052 | |
| Viral | 5 | 24.9 | All other | 56 | 106.5 | 0.379 | |
| Negatives | 50 | 96.2 | Prob TBM | 7 | 117.9 | 0.653 | |
| Negatives | 50 | 96.2 | Culture positive | 4 | 113.8 | 0.563 | |
| Prob TBM | 7 | 117.9 | Culture positive | 4 | 113.8 | 1.0 | |
| Negatives | 50 | 96.2 | TBM | 12 | 116.4 | 0.494 | |
| Viral | 5 | 24.9 | Culture positive | 4 | 113.8 | 0.556 | |
| Viral | 5 | 24.9 | TBM | 12 | 116.4 | 0.308 | |
| Viral | 5 | 24.9 | Prob TBM | 7 | 117.9 | 0.343 | |
| Viral | 5 | 24.9 | Other negatives | 45 | 104.1 | 0.431 | |
| Protein (g/liter) | Prob TBM | 11 | 1.7 | Culture positive | 5 | 4.0 | 0.113 |
| Negatives | 67 | 1.8 | Prob TBM | 11 | 1.7 | 0.971 | |
| Negatives | 67 | 1.8 | TBM | 16 | 2.4 | 0.326 | |
| Negatives | 67 | 1.8 | Culture positive | 5 | 4.0 | 0.064 | |
| Viral | 7 | 1.4 | Culture positive | 5 | 4.0 | 0.106 | |
| Viral | 7 | 1.4 | Other negatives | 60 | 1.9 | 0.467 | |
| Viral | 7 | 1.4 | Other dx negatives | 16 | 2.2 | 0.647 | |
| Negatives | 67 | 1.8 | PCR culture positive | 8 | 2.2 | 0.918 | |
| PCR culture positive | 8 | 2.2 | Clinician | 7 | 2.2 | 0.694 | |
| Viral | 7 | 1.4 | All other | 75 | 1.9 | 0.445 | |
| TNF-α (pg/ml) | Negatives | 25 | 45.9 | TBM | 5 | 128.4 | 0.028∗ |
| Viral | 4 | 26.8 | Other negatives | 21 | 49.5 | 0.195 | |
| Viral | 4 | 26.8 | Other negatives+TBM | 26 | 64.7 | 0.106 | |
| Viral | 4 | 26.8 | TBM | 5 | 128.4 | 0.032∗ | |
DISCUSSION
The CSF PCR for TBM, while less sensitive than the culture method, when culture is used as the gold standard, is rapid and therefore of value when the clinical suspicion is high and the results are reviewed in parallel with clinical and other laboratory findings. It is more sensitive than conventional smear microscopy, which detects 10 to 20% of cases, and other available rapid techniques. During follow-up, it became evident that many samples were inappropriately referred to the MRU for TBM testing as part of general screening where there was little or no clinical suspicion of TBM. Due to the rarity of the disease, the fact that a negative result cannot exclude a diagnosis of TBM, the high cost of testing, and the comparatively large volume of fluid required, CSF PCR should not be used as a “screening” procedure, particularly where suspicion of an alternative viral or bacterial diagnosis is high. It is difficult to judge the sensitivity of any test for TBM in the absence of a reliable gold standard, and so a negative result cannot be used to exclude a diagnosis of TBM. This test should only be performed where the index of suspicion for TBM is high, and treatment should not be withheld on the basis of a PCR result alone. In addition, many samples referred to the MRU during the test period were below the requested volume of 1 ml, which, while unavoidable in neonates and young children, compromises the value of negative test results. PCR methodology can add to the overall decision-making process when considered in tandem with the results of microscopy, evidence of TB contact, clinical evidence of TB elsewhere, CSF biochemistry, and radiology. It is useful when combined antibacterial, TB, and antiviral therapy is initiated in cases of severe meningitis with subsequent clinical improvement, but the reason for the therapeutic improvement remains unclear. In these cases the decision to discontinue TB therapy if no other infectious cause is identified can be supported by negative PCR results and continuing negative culture.
A recent paper by Kumar et al. (10) found five clinical and/or laboratory features to be independently associated with the diagnosis of TBM: a prodomal stage of >7 days, fundal optic atrophy, focal deficit, extrapyramidal movements, and a CSF leukocyte level of <50% polymorphs. The presence of three or more of these features had a specificity of 98.4% but a sensitivity of only 54.5%. In this study the CSF glucose was lower in TBM cases than in patients with meningitis of other etiologies (P = 0.0281) although, contrary to expectations, not usually outside a normal range of 2.5 to 5.5 mmol/liter (mean for TBM cases, 2.813 mmol/liter). Undue emphasis should not be placed on the expectation of low glucose values in TBM when assessing the biochemical findings, while a raised protein concentration is a useful indicator but cannot exclude meningitis of other etiologies (P = 0.5608). The CSF glucose level should always be assessed in parallel with the blood glucose level, and apparently this was rarely done, or not recorded, which reduces the utility of a CSF glucose value in differential diagnosis.
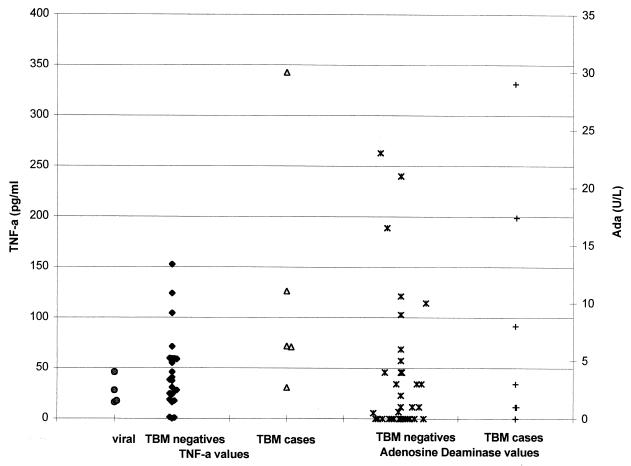
TNF-α was also significantly raised in TBM cases (P = 0.028, Table 5). However, since there is overlap between TBM cases and negative CSF values (Fig. 2), this test is of limited use in isolation. No significant difference was found in adenosine deaminase levels, although other studies have found a cutoff value of 9 to 10 U/liter giving sensitivities of 100%, with specificities of 87.6 to 99% (21, 22, 24).
FIG. 2.
TNF-α and adenosine deaminase CSF values in patients.
An earlier study by Scarpellini et al. (23) had indicated that the detection limits for an IS6110-based PCR assay using spiked bacterial suspensions was 50 CFU/ml. The sensitivity of the assay in this study was comparable to those described earlier (8, 28).
It is clear that PCR is currently the most rapid diagnostic test for TBM that provides an acceptable sensitivity in comparison to the culture method. However, it requires a relatively large volume of CSF (a particular problem in young children, who are more susceptible to TBM), several dedicated but separate laboratory areas, and rigorous quality control to guard against contamination and to maintain sensitivity and specificity. Thus, results should always be reviewed in parallel with clinical findings.
ACKNOWLEDGMENTS
We thank Malcolm Yates and the staff of the MRU for their work on this study, Richard Hooper for his help with the statistical analysis, and all of the clinicians, microbiologists, and laboratory staff at participating centers.
This work was supported by a grant from JRC, KCSMD, and the PHLS.
REFERENCES
- 1.Baig S M. Anti-purified protein derivative cell-enzyme-linked immunosorbent assay, a sensitive method for early diagnosis of tuberculosis meningitis. J Clin Microbiol. 1995;33:304–341. doi: 10.1128/jcm.33.11.3040-3041.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonington A, George-Strang J I, Klapper P E, Hood S V, Rubombora W, Penny M, Willers R, Wilkins E G A. Use of Roche amplicor in early diagnosis of tuberculosis meningitis. J Clin Microbiol. 1998;36:1251–1254. doi: 10.1128/jcm.36.5.1251-1254.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks J B, Syriopoulou V, Butler W R, Saroglow G, Karydis K, Almenoff P L. Development of a quantitative chemical ionization gas chromatography mass spectrometry method to detect tuberculostearic acid in body fluids. J Chromatogr B. 1998;712:1–10. doi: 10.1016/s0378-4347(98)00158-3. [DOI] [PubMed] [Google Scholar]
- 4.Giusti G. Methods in enzymatic analysis: adenosine deaminase. 2nd ed. Wiechen, Germany: Verlag-Chemie; 1974. pp. 1092–1099. [Google Scholar]
- 5.Hopewell P C. Overview of clinical tuberculosis: tuberculosis, pathogenesis, protection, and control. Washington, D.C.: ASM Press; 1994. [Google Scholar]
- 6.Jeffery K J M, Reda S J, Peto T E A, Mayon-White R T, Bangham C R M. Diagnosis of viral infections of the central nervous system: clinical interpretation of PCR results. Lancet. 1997;349:313–317. doi: 10.1016/S0140-6736(96)08107-X. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy D H, Fallon R J. Tuberculosis meningitis. JAMA. 1979;241:264–268. [PubMed] [Google Scholar]
- 8.Kolk A H J, Schuitema A R J, Kuijper S, van Leeuwen J, Hermans P W M, van Embden J D A, Hartskeer R A. Detection of Mycobacterium tuberculosis in clinical samples by using polymerase chain reaction and a nonradioactive detection system. J Clin Microbiol. 1992;30:2567–2575. doi: 10.1128/jcm.30.10.2567-2575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krambovitis E, McIllmurray M B, Lock P E, Hendrickse W, Hovel H. Rapid diagnosis of tuberculosis meningitis by latex particle agglutination. Lancet. 1984;ii:1229–1231. doi: 10.1016/s0140-6736(84)92792-2. [DOI] [PubMed] [Google Scholar]
- 10.Kumar R, Singh S N, Kohli N. A diagnostic rule for tuberculosis meningitis. Arch Dis Child. 1999;81:221–224. doi: 10.1136/adc.81.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandal B K, Evans D I K, Ironside A G, Pullan B R. Radioactive bromide partition test in differential diagnosis of tuberculosis meningitis. Br Med J. 1972;4:413–415. doi: 10.1136/bmj.4.5837.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazurek G H, Reddy V, Murphy D, Ansari T. Detection of mycobacterium tuberculosis in CSF following immunomagnetic enrichment. J Clin Microbiol. 1996;34:450–453. doi: 10.1128/jcm.34.2.450-453.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miörner H, Sjöbring U, Nayak P, Chandramuki A. Diagnosis of tuberculosis meningitis: comparison of three immunoassays, an immune complex assay and the PCR. Tubercle Lung Dis. 1995;76:381–386. doi: 10.1016/0962-8479(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 14.Mishra O P, Nath G, Loiwal V, Ali Z, Chandra L, Das B K. CSF Adenosine deaminase activity and C-reactive protein in tuberculosis and partially treated bacterial meningitis. Indian Pediatr. 1995;32:886–889. [PubMed] [Google Scholar]
- 15.Park S C, Lee B L, Cho S N, Kim W J, Lee B C. Diagnosis of tuberculsis meningitis by detection of IgG antibodies to purified protein derivative and LAM Antigen in CSF. Tubercle Lung Dis. 1993;74:317–322. doi: 10.1016/0962-8479(93)90106-8. [DOI] [PubMed] [Google Scholar]
- 16.Pfyffer G E, Kissling P, Jahn E M I, Welscher H, Salfinger M, Weber R. Diagnostic performance of amplified MTB direct test with CSF and other respiratory and nonrespiratory specimens. J Clin Microbiol. 1996;34:834–841. doi: 10.1128/jcm.34.4.834-841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Public Health Laboratory Service, Communicable Disease Surveillance Center. Communicable disease statistics, 1998, England and Wales. London, United Kingdom: Public Health Laboratory Service, CDSC; 1998. [Google Scholar]
- 18.Prasad R, Kumar A, Khanna B K, Mukerji P K, Agarwai S K, Kumar A, Srivastava V M L. Adenosine deaminase activity in CSF for diagnosis of tuberculosis meningitis. Indian J Tuberculosis. 1991;38:99–102. [Google Scholar]
- 19.Read S J, Jeffery K J M, Bangham C R M. Aseptic meningitis and encephalitis: the role of PCR in the diagnostic laboratory. J Clin Microbiol. 1997;35:691–696. doi: 10.1128/jcm.35.3.691-696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reischl U, Lehn N, Wolf H, Nauman L. Clinical evaluation of the automated COBAS AMPLICOR MTB assay for testing respiratory and nonrespiratory specimens. J Clin Microbiol. 1998;36:2853–2860. doi: 10.1128/jcm.36.10.2853-2860.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ribera E, Martinez-Vazquez J M, Ocaña I, Segura R M, Pascual C. Activity of adenosine deaminase in CSF for diagnosis and follow-up of tuberculosis meningitis in adults. J Infect Dis. 1987;155:603–607. doi: 10.1093/infdis/155.4.603. [DOI] [PubMed] [Google Scholar]
- 22.Rohani M Y, Cheong Y M, Rani J M. The use of adenosine deaminase activity as a biochemical marker for the diagnosis of tuberculosis meningitis. Malays J Pathol. 1995;17:67–71. [PubMed] [Google Scholar]
- 23.Scarpellini P, Braglia S, Brambilla A M, Dalessandro M, Chichero P, Gori A, Lazzarin A. Detection of rifampicin resistance by single-strand conformation polymorphism analysis of cerebrospinal fluid of patients with tuberculosis of the central nervous system. J Clin Microbiol. 1997;35:2802–2806. doi: 10.1128/jcm.35.11.2802-2806.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segura R M, Pascual C, Ocaña I, Martinez-Vazquez J M, Ribera E, Ruiz I, Pelegri M D. Adenosine deaminase in body fluids: a useful diagnostic tool in tuberculosis. Clin Biochem. 1989;22:141–148. doi: 10.1016/s0009-9120(89)80013-x. [DOI] [PubMed] [Google Scholar]
- 25.Seth P, Ahuja G K, Vijaya Bhanu N, Behari M, Bhowmik S, Broor S, Dar L, Chakraborty M. Evaluation of PCR for rapid diagnosis of clinically suspected tuberculosis meningitis. Tubercle Lung Dis. 1996;77:293–388. doi: 10.1016/s0962-8479(96)90101-x. [DOI] [PubMed] [Google Scholar]
- 26.Taylor L M, Smith H V, Hunter G. The blood-CSF barrier to bromide in diagnosis of tuberculosis meningitis. Lancet. 1954;i:700–702. doi: 10.1016/s0140-6736(54)92108-x. [DOI] [PubMed] [Google Scholar]
- 27.Weatherall D J, Leddingham J G G, Warrell D A. Oxford textbook of medicine. 3rd ed. Oxford, United Kingdom: Oxford University Press; 1996. [Google Scholar]
- 28.Whelen A C, Felmlee T A, Hunt J M, Williams D L, Roberts G L, Stockman L, Persing D H. Direct genotypic detection of Mycobacterium tuberculosis rifampicin resistance in clinical specimens by using single-tube heminested PCR. J Clin Microbiol. 1995;33:556–561. doi: 10.1128/jcm.33.3.556-561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiggelinkhuizer J, Mann M. The radioactive bromide partition test in the diagnosis of tuberculosis meningitis in children. J Pediatr. 1980;97:843–847. doi: 10.1016/s0022-3476(80)80286-1. [DOI] [PubMed] [Google Scholar]
- 30.Wilson S M, McNerney R, Nye P M, Godfrey-Faussett G, Stoker N G, Voller A. Progress towards a simplified polymerase chain reaction and its application to diagnosis of tuberculosis. J Clin Microbiol. 1993;31:776–782. doi: 10.1128/jcm.31.4.776-782.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu S Z, Zhao S X, Dai Y. The activities of three enzymes in serum and cerebrospinal fluid for diagnosis of tuberculosis meningitis. Chin J Tuberc Respir Med. 1993;16:39–40. [PubMed] [Google Scholar]