Abstract
The ubiquitously expressed c-Abl tyrosine kinase localizes to the nucleus and cytoplasm. Using confocal microscopy, we demonstrated that c-Abl colocalizes with the endoplasmic reticulum (ER)-associated protein grp78. Expression of c-Abl in the ER was confirmed by immunoelectron microscopy. Subcellular fractionation studies further indicate that over 20% of cellular c-Abl is detectable in the ER. The results also demonstrate that induction of ER stress with calcium ionophore A23187, brefeldin A, or tunicamycin is associated with translocation of ER-associated c-Abl to mitochondria. In concert with targeting of c-Abl to mitochondria, cytochrome c is released in the response to ER stress by a c-Abl-dependent mechanism, and ER stress-induced apoptosis is attenuated in c-Abl-deficient cells. These findings indicate that c-Abl is involved in signaling from the ER to mitochondria and thereby the apoptotic response to ER stress.
The c-Abl protein tyrosine kinase localizes to the nucleus and cytoplasm. Nuclear c-Abl is activated in the response to DNA damage (16) by the DNA-dependent protein kinase (10, 13) and the product of the gene mutated in ataxia telangiectasia (2, 28). Activation of nuclear c-Abl by genotoxic stress contributes to induction of the proapoptotic c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) and p38 mitogen-activated protein kinase pathways (14–16, 25). Nuclear c-Abl also contributes to DNA damage-induced apoptosis by mechanisms in part dependent on the p53 tumor suppressor and its homolog p73 (1, 7, 38, 40, 41). Other studies have demonstrated that the cytoplasmic form of c-Abl is activated in the cellular response to oxidative stress (30). Reactive oxygen species induce cytoplasmic c-Abl activity by a mechanism dependent on protein kinase Cδ (PKCδ) (31). Moreover, c-Abl is required for reactive oxygen species-induced release of mitochondrial cytochrome c, caspase-3 activation, and apoptosis (30). These findings have provided support for the involvement of c-Abl in the responses to genotoxic and oxidative stress.
The endoplasmic reticulum (ER) functions as an oxidizing compartment for the folding of membrane and secretory proteins (11). Accumulation of unfolded intermediates in the ER activates stress signals referred to as the unfolded protein response (5). The IRE1α and IRE1β ER transmembrane protein kinases sense ER stress and activate transcription of genes that encode protein chaperones and other ER-resident proteins (33, 36). ER stress also induces activity of the PRK-like ER kinase (PERK), phosphorylation of the eukaryotic initiation factor 2 α subunit and inhibition of mRNA translation (8). Treatment of cells with inducers of ER stress, such as the calcium ionophore A23187, is associated with the induction of CHOP/GADD153 expression and apoptosis (3, 21, 26, 27). Other studies have demonstrated that caspase-12 is activated by ER stress and that caspase-12 contributes to ER stress-induced apoptosis (24).
The present studies show that the c-Abl kinase localizes to the ER and is targeted to mitochondria by ER stress. The results also demonstrate that ER stress induces mitochondrial cytochrome c release and apoptosis by a c-Abl-dependent mechanism.
MATERIALS AND METHODS
Cell culture.
Rat1 cells and wild-type, Abl−/−, and Abl+ (Abl−/− cells reconstituted to stably express c-Abl) mouse embryo fibroblasts (MEFs) (16, 19, 34) were cultured in Dulbecco's modified Eagle's medium containing 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. Cells were treated with A23187, brefeldin A, or tunicamycin (all from Sigma).
Digital confocal immunofluorescence microscopy.
Cells grown on poly-d-lysine-coated glass coverslips were fixed (3.7% formaldehyde in phosphate-buffered saline [PBS], pH 7.4; 10 min), permeabilized (0.2% Triton X-100; 10 min), and blocked for 30 min in medium containing serum. After rinsing with PBS, immunostaining was performed by incubating the cells with 50 ng of anti-c-Abl (K-12 rabbit polyclonal; Santa Cruz) and anti-grp-78 (C-20 goat polyclonal; Santa Cruz) per slide. After being washed with PBS, cells were incubated with a 1:250 dilution of CY-3 or fluorescein isothiocyanate-conjugated anti-rabbit or anti-goat immunoglobulin G (IgG) secondary antibodies (Jackson ImmunoResearch) for 1 h. Mitochondria were stained with 0.006 ng of Mitotracker Green FM (Molecular Probes) per slide. Nuclei were stained with 4, 6-diamino-2-phenylindole (DAPI; 1 μg/ml in PBS). Coverslips were mounted onto slides with 0.1 M Tris (pH 7.0) in 50% glycerol. Cells were visualized by digital confocal immunofluorescence, and images were captured with a cooled charge-coupled device camera mounted on a Zeiss Axioplan 2 microscope. Images were deconvolved using Slidebook software (Intelligent Imaging Innovations, Inc., Denver, Colo.).
Immunoelectron microscopic analysis.
Cells were fixed with 2% paraformaldehyde in 0.1 M sodium cacodylate buffer for 10 min, washed with three changes of cacodylate buffer, postfixed with 1% osmium tetroxide for 5 min, dehydrated in graded ethanol, and infiltrated and polymerized with Poly/bed 812 overnight. Ultrathin sections were cut with an ultramicrotome (Nova; Leica). After etching with sodium periodate for 10 min, the sections were rinsed with buffer and incubated with anti-c-Abl at a dilution of 1:10 overnight at 4°C. The sections were rinsed with buffer, incubated with protein A-gold (15 nm) for 1 h, rinsed again, and then fixed with 2% glutaradehyde in PBS for 2 min. After air drying, the sections were stained with 25 aqueous uranyl acetate and with 0.5% lead citrate. The sections were examined and photographed using a Hitachi H-600 electron microscope (Nessei Sagnyo) at 75 kV.
Isolation of the ER fraction.
Cells were washed with PBS, lysed in homogenization buffer (50 mM Tris-HC1 [pH 8.0], 1 mM β-mercaptoethanol, 1 mM EDTA, 0.32 M sucrose, and 0.1 mM phenylmethylsulfonyl fluoride), and then centrifuged at 5,000 × g for 10 min. The supernatant was collected and centrifuged at 105,000 × g for 1 h. The pellet was disrupted in lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% NP-40, 1 mM dithiothreitol, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 mM sodium fluoride, 10 μg of leuptin and aprotinin/ml) at 4°C and then centrifuged at 15,000 × g for 20 min. The resulting supernatant was used as the ER fraction.
Isolation of cytoplasmic and nuclear fractions.
The cytoplasmic and nuclear fractions were isolated as described previously (30).
Isolation of mitochondria.
Cells were washed twice with PBS, homogenized in buffer A (210 mM mannitol, 70 mM sucrose, 1 mM EGTA, 5 mM HEPES [pH 7.4]) with 110 μg of digitonin per ml in a glass homogenizer (Pyrex no. 7727-07) and then centrifuged at 5,000 × g for 5 min. Pellets were resuspended in buffer A, homogenized in a glass homogenizer, and centrifuged at 1,500 × g for 5 min. The supernatant was collected and centrifuged at 10,000 × g for 10 min. Mitochondrial pellets were disrupted in lysis buffer at 4°C and then centrifuged at 15,000 × g for 20 min. Protein concentration was determined by the Bio-Rad protein estimation kit.
Isolation of ER and plasma membranes.
Cellular membranes were prepared as described previously (4). The membranes were applied to a discontinuous sucrose gradient and centrifuged at 100,000 × g for 2.5 h at 4°C. Plasma membranes were isolated from the interface between 0.25 and 1.2 M sucrose. ER membranes were isolated from the interface between 1.2 and 2.0 M sucrose (4).
Immunoblot analysis.
Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed with anti-c-Abl (Calbiochem), anti-grp78 (Santa Cruz), anticalreticulin (StressGen), anti-HSP60 (StressGen), anti-β-actin (Sigma), anti-PCNA (Calbiochem), anti-cytochrome c (18), or anti-platelet-derived growth factor receptor (anti-PDGF-R; Oncogene). Antigen-antibody complexes were visualized by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech).
Analysis of c-Abl activity.
Cell lysates were prepared as described previously (30) and subjected to immunoprecipitation with anti-c-Abl (K-12; Santa Cruz). The immunoprecipitates were resuspended in kinase buffer (30) containing 2.5 μCi of [γ-32P]ATP and glutathione S-transferase (GST)–Crk(120–225) or GST-Crk(120–212) for 15 min at 30°C. The reaction products were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography.
Apoptosis assays.
DNA content was assessed by staining ethanol-fixed cells with propidium iodide and monitoring by FACScan (Becton Dickinson).
RESULTS
Localization of c-Abl to the ER.
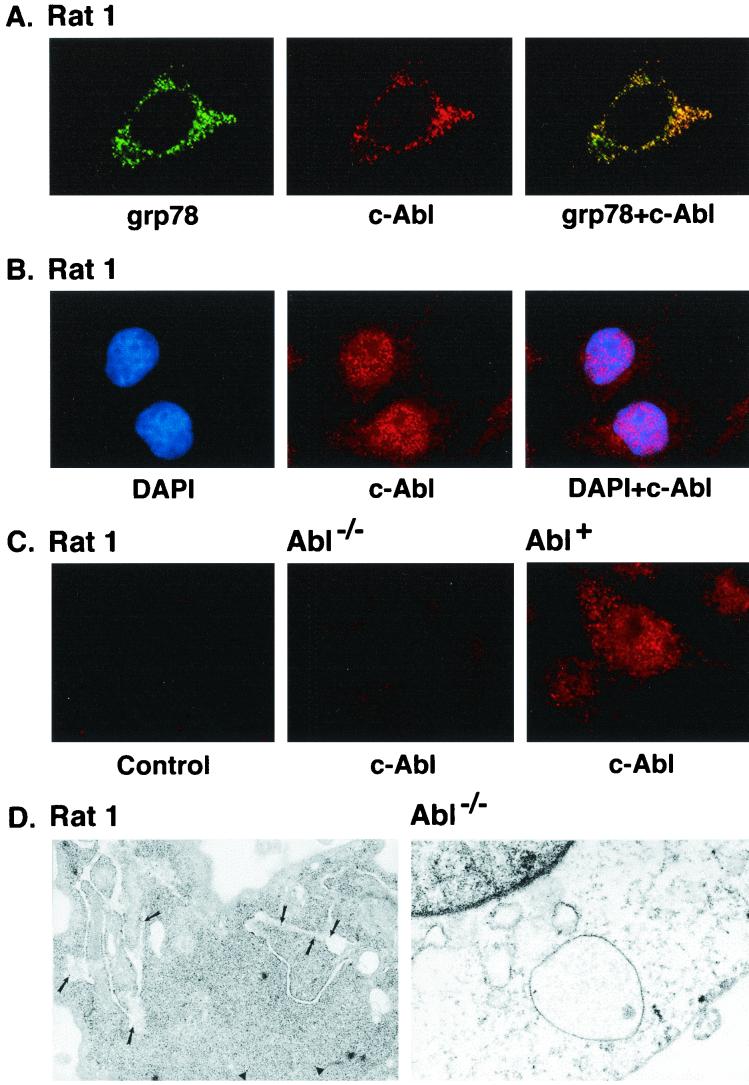
To assess the subcellular distribution of c-Abl, confocal microscopy was performed to detect colocalization of c-Abl with proteins that are selectively expressed in different organelles. Using an antibody against the ER protein grp78 and a digital confocal image set for the ER, the distribution of immunofluorescence was compared to that obtained with anti-c-Abl (Fig. 1A). Colocalization of grp78 (green) and c-Abl (red) was supported by overlay of the signals (overlay of red and green yields a yellow-orange signal). (Fig. 1A). These findings provided support for localization of c-Abl to the ER. As c-Abl is also expressed in the nucleus, digital confocal images set at a different depth confirmed nuclear localization of the c-Abl protein (Fig. 1B). As a control, similar studies were performed on Abl−/− and Abl+ cells. The finding that cytoplasmic and nuclear staining is detectable in Abl+ but not Abl−/− cells confirmed specificity of the anti-c-Abl antibody (Fig. 1C). To extend the analysis, cells were subjected to immunogold labeling with anti-c-Abl. The results demonstrate expression of c-Abl in the cytoplasm, mitochondria, and rough ER (Fig. 1D, left). By contrast, there were no detectable signals when similar studies were performed on Abl−/− cells (Fig. 1D, right). These findings indicate that c-Abl localizes to the ER.
FIG. 1.
(A) Colocalization of c-Abl and ER-associated proteins. Rat1 cells grown on poly-d-lysine-coated coverslips were fixed, permeabilized, and blocked in medium containing serum. Rat1 cells were subjected to immunofluorescence staining with goat anti-grp78 antibody and rabbit anti-c-Abl. The green signals for grp78 were obtained with fluorescein isothiocyanate-conjugated donkey anti-goat IgG (left). The red signal (c-Abl) was obtained with CY-3-conjugated donkey anti-rabbit IgG secondary antibody (middle). Overlay resulted in yellow signals indicative of colocalization (right). The digital confocal image was set for the ER. (B) Rat1 cells were incubated with DAPI (left, blue signal) and rabbit anti-c-Abl. The red signal for c-Abl was obtained with the CY-3-conjugated donkey anti-rabbit IgG (middle). The overlay demonstrates localization of c-Abl in the nucleus (right). The confocal image was set for the nucleus. (C) Rat1 cells were incubated with CY-3-conjugated donkey anti-rabbit IgG (no anti-c-Abl; left). Abl−/− (middle) and Abl+ (right) cells were incubated with anti-c-Abl and CY-3-conjugated donkey anti-rabbit IgG. The confocal image was set for the nucleus and cytoplasm. (D) Rat1 (left) and Abl−/− (right) cells were subjected to immunogold labeling with anti-c-Abl. Gold particles were counted in nine Rat1 cells. The average number of gold particles per cell was 29 ± 14 (mean ± standard deviation). The percentages of total particles in the following subcellular fractions were 57% ± 14% (nucleus), 12% ± 8% (ER), 2% ± 4% (mitochondria), and 29% ± 9% (cytoplasm). Magnification, ×30,000.
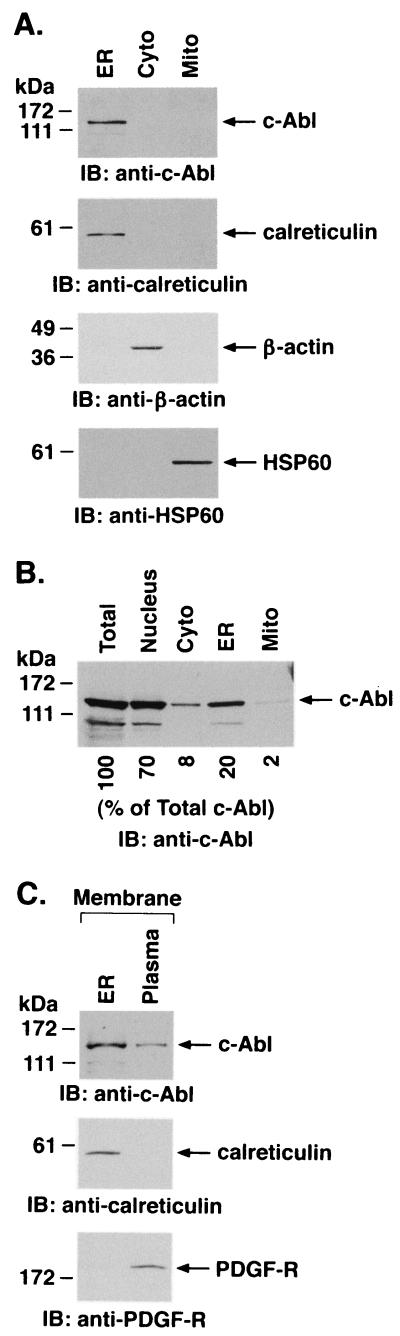
Subcellular fractionation studies were performed to define the fraction of c-Abl that associates with the ER. To assess intracellular distribution, ER, cytosolic, and mitochondrial fractions were subjected to immunoblotting with anti-c-Abl. Analysis of equal amounts of proteins from the fractions indicated that the concentration of c-Abl in the ER is higher than that found in the cytosol or mitochondria (Fig. 2A). The purity of the ER fraction was confirmed by immunoblotting with antibodies against calreticulin, β-actin, and HSP60. Thus, the ER fraction included calreticulin and little if any cytosolic β-actin or mitochondrial HSP60 (Fig. 2A). Whereas these studies used equal amounts of proteins from the fractions, additional experiments were performed by immunoblot analysis of fractions obtained from equal numbers of cells. Analysis of c-Abl protein in the different fractions, including the nucleus, indicated that c-Abl localized to the ER comprises about 20% of c-Abl protein in the total cell lysate (Fig. 2B). To determine whether c-Abl associates with ER membrane, cell membrane preparations were fractionated by sucrose density centrifugation. Immunoblot analysis of ER membranes demonstrated levels of c-Abl expression that were higher than that found in equal amounts of protein from plasma membranes (Fig. 2C). Purity of the membrane preparations was confirmed by immunoblotting with antibodies against grp78 and PDGF-R (Fig. 2C). These findings collectively demonstrate that c-Abl associates with ER membranes.
FIG. 2.
Subcellular distribution of c-Abl. (A) ER, cytoplasmic (Cyto), and mitochondrial (Mito) fractions were isolated from Rat1 cells. Equal amounts of protein (5 μg) from each fraction were subjected to immunoblotting (IB) with anti-c-Abl, anticalreticulin, anti-β-actin, or anti-HSP60. (B) Rat1 cells (2 × 107) were divided into five aliquots for preparation of total cell, nuclear, cytoplasmic, ER, and mitochondrial lysates. The lysates were adjusted to 500 μl with PBS, and aliquots (20 μl) were subjected to immunoblotting with anti-c-Abl. Signal intensities were analyzed by densitometric scanning. The results are presented as the percentage of c-Abl in each subcellular fraction compared to that in the total cell lysate. (C) ER and plasma membrane preparations were isolated from Rat1 cells. Equal amounts of protein (5 μg) were subjected to immunoblot analysis with anti-c-Abl, anticalreticulin, and anti-PDGF-R.
ER stress decreases ER-associated c-Abl.
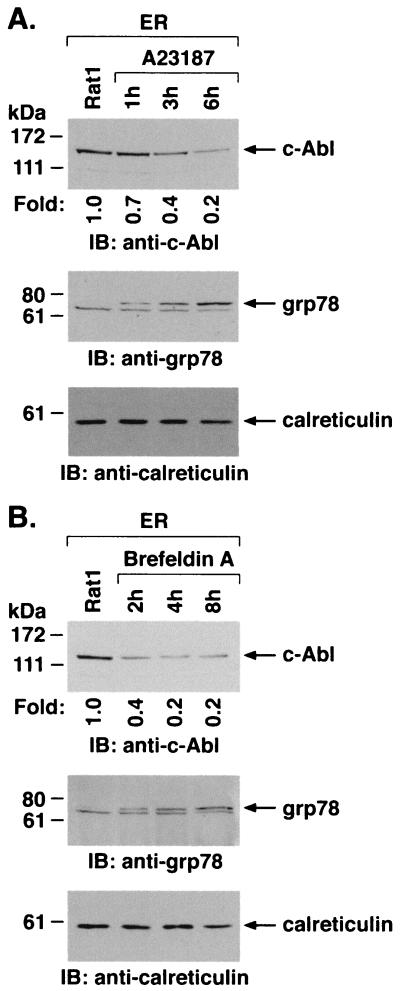
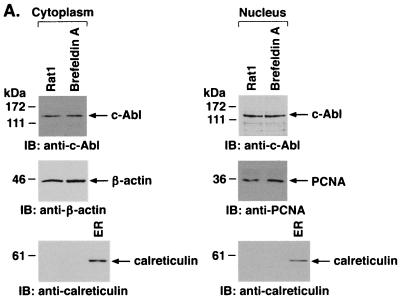
To assess whether ER stress affects the subcellular localization of c-Abl, ER fractions were isolated from cells treated with A23187. Immunoblot analysis demonstrated that A23187 treatment is associated with a time-dependent decrease in c-Abl levels (Fig. 3A). As shown previously (20), ER stress induced by A23187 was associated with increases in expression of grp78 (Fig. 3A). Equal loading of the lanes was confirmed by immunoblotting with anticalreticulin (Fig. 3A). ER fractions isolated from cells treated with brefeldin A to inhibit transport of protein from the ER to the Golgi were also subjected to immunoblotting with anti-c-Abl. The results demonstrate that brefeldin A, like A23187, decreases levels of c-Abl associated with the ER (Fig. 3B). Brefeldin A treatment was also associated with increases in grp78 and had little if any effect on levels of calreticulin (Fig. 3B). These findings demonstrate that ER stress downregulates localization of c-Abl to the ER.
FIG. 3.
ER stress decreases ER-associated c-Abl. Rat1 cells were treated with 10 μM A23187 (A) or 10 μg of brefeldin A per ml (B) and harvested at the indicated times. ER fractions were isolated and subjected to immunoblotting with anti-c-Abl (upper panels), anti-grp78 (middle panels), or anticalreticulin (lower panels). The signal intensities of c-Abl protein were compared to that of the control.
ER stress targets c-Abl to mitochondria.
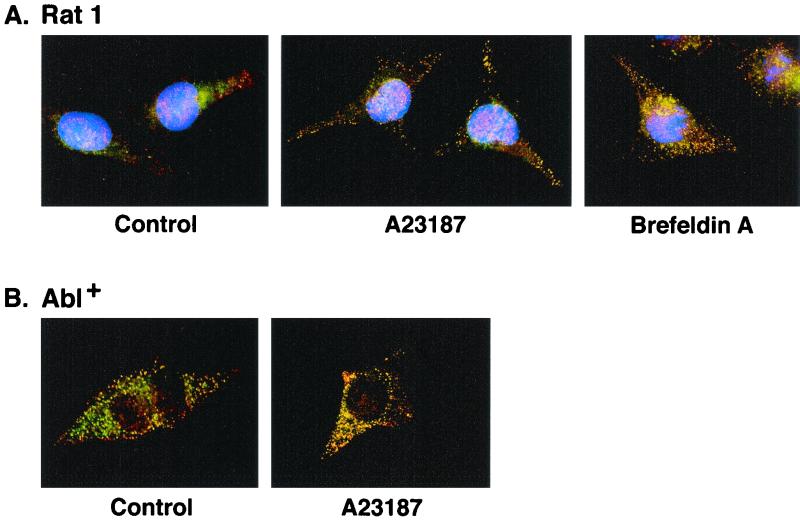
The subcellular relocalization of c-Abl in response to ER stress was investigated by measuring intracellular fluorescence. Examination of the distribution of fluorescence markers in control Rat1 cells showed distinct patterns for anti-c-Abl (red signal) and a mitochondrion-selective dye (Mitotracker; green signal) (Fig. 4A). By contrast, treatment with A23187 was associated with a change in fluorescence signals (red and green yield yellow-orange) supporting translocation of c-Abl to mitochondria (Fig. 4A). Similar results were obtained with brefeldin A-treated Rat1 cells (Fig. 4A) and with A23187-treated Abl+ cells (Fig. 4B). By contrast, there was little if any change in expression of c-Abl in the cytoplasm or nucleus (data not shown). These results indicate that ER stress-induced downregulation of c-Abl in the ER is associated with targeting of c-Abl to mitochondria.
FIG. 4.
ER stress targets c-Abl to mitochondria. (A) Rat1 cells (left) were treated with 10 μM A23187 for 6 h (middle) or 10 μg of brefeldin A per ml for 8 h (right). (B) Abl+ cells (left) were treated with 10 μM A23187 for 6 h (right). After being washed, the cells were immobilized on slides, fixed, and incubated with anti-c-Abl antibody followed by Texas red-conjugated goat anti-rabbit IgG. Rat1 cells were also stained with DAPI, while no DAPI was used for staining of the Abl+ cells. Mitochondria were stained with the mitochondrion-selective dye Mitotracker green. The slides were visualized using a fluorescence microscope coupled to a high-sensitivity charge-coupled device camera and image analyzer. Red signal, c-Abl; green signal, Mitotracker; yellow-orange signals, colocalization of c-Abl and Mitotracker.
ER stress activates the c-Abl kinase.
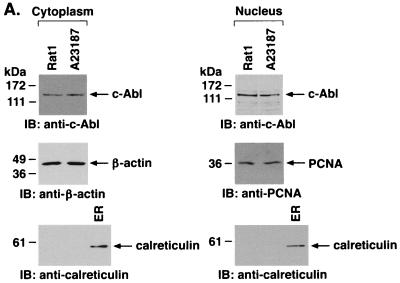
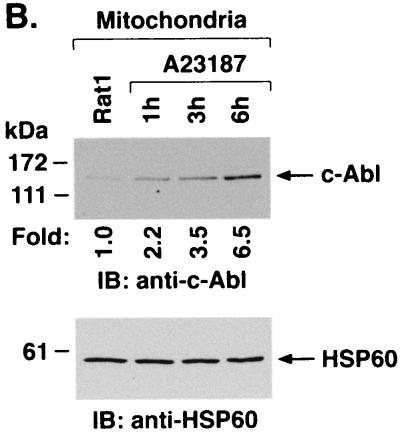
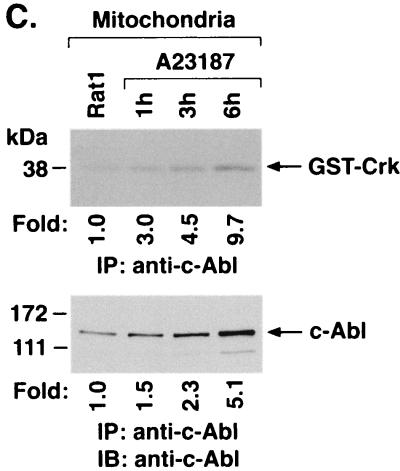
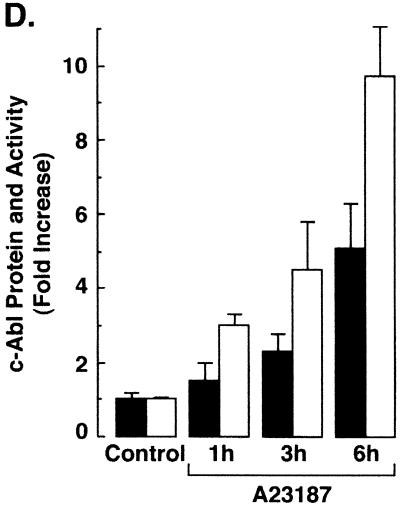
To further define the distribution of c-Abl in response to ER stress, cytoplasmic and nuclear fractions from A23187-treated cells were assessed by immunoblot analysis with anti-c-Abl. The results demonstrate that A23187 has little if any effect on c-Abl levels in the cytoplasm or nucleus (Fig. 5A). Purity of the fractions was confirmed by immunoblotting with anti-β-actin, anti-PCNA, and anticalreticulin (Fig. 5A). In contrast to the cytoplasm and nucleus, immunoblot analysis of the mitochondrial fraction from A23187-treated cells demonstrated a time-dependent increase in c-Abl protein (Fig. 5B). The mitochondrial fraction was also subjected to immunoprecipitation with anti-c-Abl. Analysis of the immunoprecipitates for phosphorylation of GST-Crk (120–225) demonstrated that A23187 treatment is associated with increases in mitochondrial c-Abl activity (Fig. 5C). As a control, there was no detectable phosphorylation of GST-Crk (120–212) that lacks the c-Abl Y-221 phosphorylation site (data not shown). Densitometric scanning of the signals obtained for phosphorylation of GST-Crk (120–225) compared to those obtained for immunoprecipitated c-Abl protein indicated that A23187 induces c-Abl activity (Fig. 5C). The average increase in mitochondrial c-Abl activity compared to that for c-Abl protein for three separate experiments is shown (Fig. 5D). The results support activation of the c-Abl protein that localizes to mitochondria.
FIG. 5.
A23187 induces mitochondrial translocation of c-Abl. (A) Rat1 cells were treated with 10 μM A23187 and harvested at 6 h. Cytoplasmic and nuclear fractions were isolated and subjected to immunoblotting (IB) with anti-c-Abl, anti-β-actin, anti-PCNA, or anticalreticulin. (B) Rat1 cells were treated with 10 μM A23187 and harvested at the indicated times. Mitochondrial fractions were isolated and subjected to immunoblotting with anti-c-Abl or anti-HSP60. The signal intensities of c-Abl protein were compared to that of the control. (C) Rat1 cells were treated with 10 μM A23187 and harvested at the indicated times. Mitochondrial fractions were subjected to immunoprecipitation (IP) with anti-c-Abl. The precipitates were analyzed in a c-Abl kinase assay using GST-Crk(120–225) as the substrate or subjected to immunoblotting with anti-c-Abl. The signal intensities of c-Abl activity and protein were compared to that of the controls. (D) The increases in mitochondrial c-Abl protein (solid bars) and activity (open bars) are expressed as the means plus standard deviations obtained from three separate experiments.
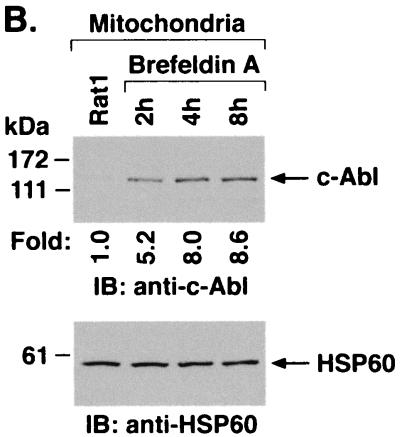
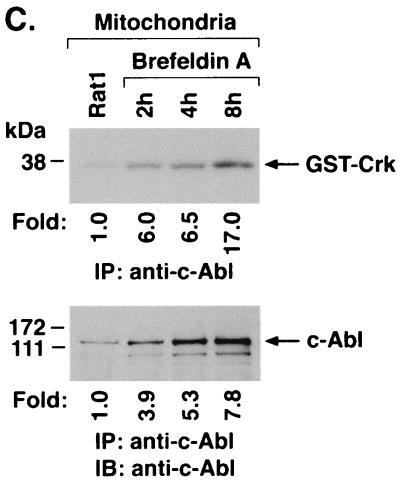
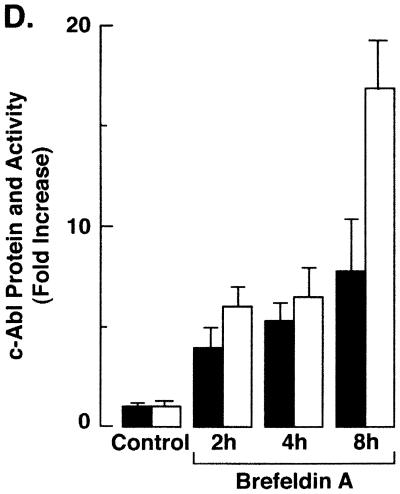
Targeting of c-Abl to mitochondria was similarly assessed in cells treated with brefeldin A. Immunoblot analysis of the cytoplasmic and nuclear fractions showed no detectable effect of brefeldin A on c-Abl levels (Fig. 6A). As found with A23187, analysis of the mitochondrial fraction demonstrated brefeldin A-induced increases in c-Abl protein (Fig. 6B). In addition, brefeldin A treatment was associated with increases in mitochondrial c-Abl activity (Fig. 6C). Comparison of the signals found for GST-Crk (120–225) phosphorylation and c-Abl protein indicated that brefeldin A induces activation of the c-Abl kinase (Fig. 6C and D). These findings and those obtained with A23187 demonstrate that ER stress is associated with targeting of c-Abl to mitochondria and stimulation of c-Abl activity.
FIG. 6.
Brefeldin A induces mitochondrial translocation of c-Abl. (A) Rat1 cells were treated with 10 μg of brefeldin A per ml for 8 h. Cytoplasmic and nuclear fractions were subjected to immunoblotting (IB) with anti-c-Abl, anti-β-actin, anti-PCNA, or anticalreticulin. (B) Rat1 cells were treated with 10 μg of brefeldin A per ml for the indicated times. Mitochondrial fractions were subjected to immunoblotting with anti-c-Abl or anti-HSP60. The signal intensities of c-Abl protein were compared to that of the control. (C) Rat1 cells were treated with 10 μg of brefeldin A per ml and harvested at the indicated times. Mitochondrial fractions were subjected to immunoprecipitation (IP) with anti-c-Abl. The precipitates were analyzed in a c-Abl kinase assay using GST-Crk(120–225) as the substrate or subjected to immunoblotting with anti-c-Abl. The signal intensities of c-Abl activity and protein were compared to that of the control. (D) The increases in mitochondrial c-Abl protein (solid bars) and activity (open bars) are expressed as the means plus standard deviations obtained from three separate experiments.
ER stress induces cytochrome c release and apoptosis by a c-Abl-dependent mechanism.
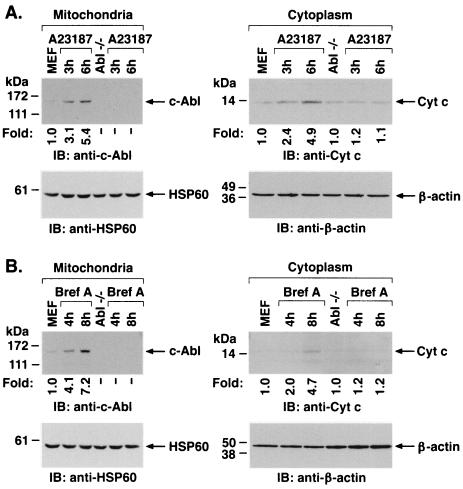
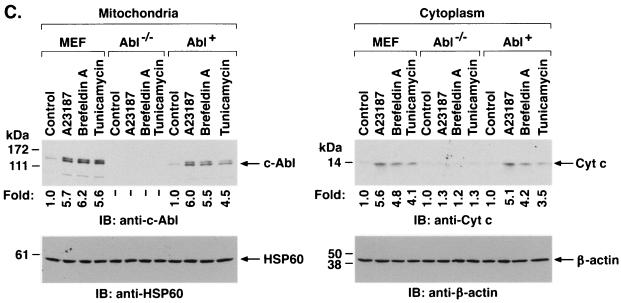
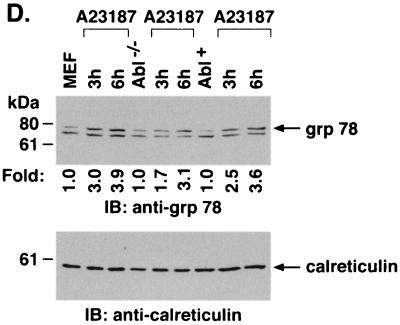
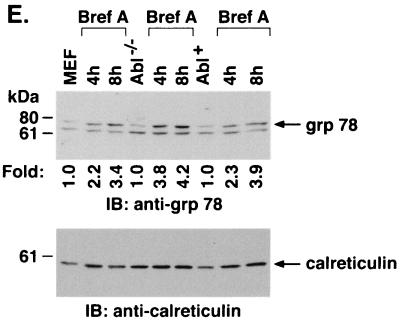
To assess the functional significance of ER stress-induced targeting of c-Abl to mitochondria, wild-type and Abl−/− MEFs were treated with A23187. Immunoblot analysis of the mitochondrial fraction demonstrated A23187-induced increases in mitochondrial c-Abl levels in wild-type but not Abl−/− cells (Fig. 7A). Cytoplasmic fractions were also subjected to immunoblot analysis to assess release of mitochondrial cytochrome c. The results demonstrate that A23187 induces the release of cytochrome c in wild-type but not Abl−/− MEFs (Fig. 7A). Similar results were obtained in wild-type and Abl−/− cells treated with brefeldin A (Fig. 7B). To confirm dependence on c-Abl, Abl+ cells were treated with inducers of the ER stress. The results demonstrate that A23187 treatment is associated with targeting of c-Abl to mitochondria and cytochrome c release (Fig. 7C). Similar results were obtained with brefeldin A and tunicamycin (Fig. 7C). As additional controls, wild-type, Abl−/−, and Abl+ cells were treated with A23187 and analyzed for activation of grp78. The results demonstrate that grp78 is activated in the different cell types (Fig. 7D). Similar findings were obtained with brefeldin A (Fig. 7E). These results demonstrate that c-Abl is not involved in initiating ER stress but is required for transducing ER stress signals to mitochondria.
FIG. 7.
ER stress induces cytochrome c release and apoptosis by a c-Abl-dependent mechanism. (A) MEF (c-Abl+/+) and c-Abl−/− cells were treated with 10 μM A23187 and harvested at the indicated times. Mitochondrial fractions were isolated and subjected to immunoblotting (IB) with anti-c-Abl or anti-HSP60. Cytoplasmic fractions were subjected to immunoblotting with anti-cytochrome c (Cyt c) or anti-β-actin. The signal intensities of c-Abl and cytochrome c were compared to that of the control. (B) MEF (c-Abl+/+) and c-Abl−/− cells were treated with 10 μg of brefeldin A per ml (Bref A) and harvested at the indicated times. Mitochondrial fractions were subjected to immunoblotting with anti-c-Abl or anti-HSP60. Cytoplasmic fractions were subjected to immunoblotting with anti-cytochrome c or anti-β-actin. (C) MEF (c-Abl+/+), Abl−/−, and Abl+ cells were treated with 10 μM A23187 for 6 h, 10 μg of brefeldin A per ml for 8 h, or 10 μg of tunicamycin per ml for 8 h. Mitochondrial fractions were analyzed by immunoblotting with anti-c-Abl and anti-HSP60. Cytoplasmic fractions were analyzed by immunoblotting with anti-cytochrome c and anti-β-actin. (D and E) MEF (c-Abl+/+), Abl−/−, and Abl+ cells were treated with 10 μM A23187 (D) or 10 μM of brefeldin A per ml (E) for the indicated times. ER fractions were analyzed by immunoblotting with anti-grp78 and anticalreticulin.
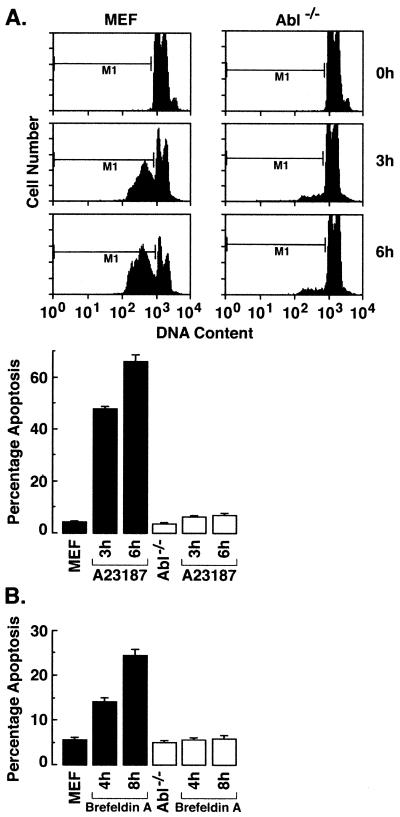
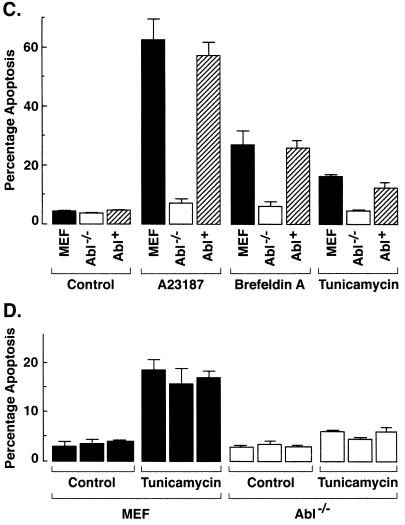
In concert with these findings, A23187 treatment was associated with the induction of sub-G1 DNA in wild-type cells but had little effect on the induction of apoptosis in c-Abl−/− cells (Fig. 8A). The finding that ER stress-induced apoptosis is also abolished in Abl−/− MEFs treated with brefeldin A provided further support for involvement of c-Abl in this response (Fig. 8B). To extend these studies, Abl+ cells were analyzed for ER stress-induced apoptosis. The results demonstrate that Abl+ cells respond to A23187 with induction of sub-G1 DNA (Fig. 8C). Similar results were obtained with brefeldin A and tunicamycin (Fig. 8C). As additional controls, independently derived wild-type, Abl−/− MEFs (19) were treated with tunicamycin. Wild-type but not Abl−/− cells responded to tunicamycin with the induction of apoptosis (Fig. 8D). These results demonstrate that ER stress induces cytochrome c release and apoptosis by a c-Abl-dependent mechanism.
FIG. 8.
ER stress induces apoptosis by a c-Abl-dependent mechanism. (A) MEF (c-Abl+/+) and c-Abl−/− cells were treated with 10 μM A23187 and harvested at the indicated times. After being fixed, cells were stained with propidium iodide, and sub-G1 DNA content was measured using FACScan. The percentage of apoptotic cells with sub-G1 DNA content is expressed as the means plus standard deviations from three independent experiments, each performed in duplicate. (B) MEF (c-Abl+/+) and Abl−/− cells were treated with 10 μg of brefeldin A per ml and harvested at the indicated times. (C) MEF (c-Abl+/+), Abl−/−, and Abl+ cells were treated with 10 μM A23187 for 6 h, 10 μg of brefeldin A per ml for 8 h, or 10 μg of tunicamycin per ml for 8 h. (D) Independently derived MEFs and Abl−/− cells were treated with 10 μg of tunicamycin per ml for 8 h. The percentage of apoptotic cells with sub-G1 DNA content is expressed as the means plus standard deviations from three independent experiments, each performed in duplicate.
DISCUSSION
Stress signaling from the ER to mitochondria.
The ER responds to alterations in homeostasis with the transduction of signals to the nucleus and cytoplasm. In this context, eukaryotic cells respond to the accumulation of unfolded or excess proteins in the ER with (i) transcriptional activation of genes encoding ER-resident proteins and (ii) repression of protein synthesis (23). The ER-resident transmembrane kinases, IRE1α and IRE1β, are activated by the presence of incorrectly folded proteins within the ER lumen and transduce signals that induce JNK/SAPK activity and gene transcription (29, 33, 35). Inhibition of protein synthesis in the response to unfolded proteins is signaled by the PERK transmembrane ER-resident kinase (8). PERK has a luminal domain similar to that of IRE1 and a cytoplasmic kinase domain that phosphorylates eIF2α (8). ER stress responses are also activated by disruption of ER calcium homeostasis. The calcium ionophore A23187 induces ER stress by increasing intracellular calcium pools (11). Brefeldin A, by contrast, induces ER stress by blocking transport of proteins from the ER to the Golgi. Under conditions of excessive ER stress, cells activate signaling pathways that induce apoptosis (37). However, the mechanisms responsible for ER stress-induced apoptosis have been largely unknown. The results of the present studies demonstrate that the ER responds to diverse types of stress with the transduction of signals to mitochondria and thereby the induction of apoptosis.
c-Abl confers ER stress signals to mitochondria.
The available evidence has shown that c-Abl is expressed in the nucleus and cytoplasm. The present results demonstrate that c-Abl also localizes to the ER. Confocal microscopy studies demonstrate that c-Abl colocalizes with the ER-associated grp78 protein. Localization of c-Abl to the ER was confirmed by immunoelectron microscopy and subcellular fractionation studies. Nuclear c-Abl is activated in the cellular response to genotoxic stress by mechanisms dependent on DNA-dependent protein kinase and the product of the gene mutated in ataxia telangiectasia (2, 10, 13, 28). Cytoplasmic c-Abl is activated in the response to oxidative stress by a PKCδ-dependent mechanism (30, 31). Other studies have supported a role for c-Abl in the apoptotic response to both genotoxic and oxidative stress (9, 30, 39). The finding that c-Abl is required for the release of cytochrome c in the oxidative stress response has further supported a role for c-Abl in targeting proapoptotic signals to mitochondria (30). The present studies extend the link between c-Abl and cellular stress by demonstrating that ER stress is associated with mitochondrial targeting of c-Abl. The results support a model in which ER stress induces translocation of the ER-associated c-Abl to mitochondria. The results also support a functional role for c-Abl in transducing proapoptotic signals that are activated by ER stress.
ER stress induces cytochrome c release and apoptosis by targeting c-Abl to mitochondria.
The cellular response to genotoxic stress includes c-Abl-dependent signaling that mediates the release of mitochondrial cytochrome c and induction of apoptosis (16, 17). Activation of c-Abl in the response to oxidative stress has also been associated with release of cytochrome c and the induction of apoptosis by a c-Abl-dependent mechanism (30). In the cytosol, cytochrome c associates with a complex of Apaf-1 and caspase-9 and thereby induces the activation of caspase-3 (22, 42). The induction of apoptosis is associated with caspase-3-mediated cleavage of poly (ADP-ribose) polymerase, PKCδ, and other proteins (6, 12, 32). While ER stress can induce apoptosis (37), the involvement of cytochrome c release in this response has been unknown. In the present studies, the finding that ER stress induces the release of mitochondrial cytochrome c provided further support for signaling from the ER to mitochondria. Importantly, the induction of cytochrome c release by ER stress was attenuated in Abl−/− cells. Moreover, Abl−/− cells were defective in the apoptotic response to ER stress. These findings indicate that ER stress-induced cytochrome c release and apoptosis are mediated by targeting c-Abl from the ER to mitochondria.
ACKNOWLEDGMENTS
This work was supported by grant CA42802 awarded by the National Cancer Institute, DHHS, and by the office of Health and Biological Research, U.S. Department of Energy, cooperative agreement DE-FC04-96AL76406.
We appreciate the technical assistance of Kamal Chauhan.
Y. Ito, P. Pandey, and N. Mishra contributed equally to this work.
REFERENCES
- 1.Agami R, Blandino G, Oren M, Shaul Y. Interaction of c-Abl and p73α and their collaboration to induce apoptosis. Nature. 1999;399:809–813. doi: 10.1038/21697. [DOI] [PubMed] [Google Scholar]
- 2.Baskaran R, Wood L D, Whitaker L L, Xu Y, Barlow C, Canman C E, Morgan S E, Baltimore D, Wynshaw-Boris A, Kastan M B, Wang J Y J. Ataxia telangiectasia mutant protein activates c-abl tyrosine kinase in response to ionizing radiation. Nature. 1997;387:516–519. doi: 10.1038/387516a0. [DOI] [PubMed] [Google Scholar]
- 3.Dricu A, Carlberg M, Wang M, Larsson O. Inhibition of N-linked glycosylation using tunicamycin causes cell death in malignant cells: role of down-regulation of the insulin-like growth factor 1 receptor in induction of apoptosis. Cancer Res. 1997;57:543–548. [PubMed] [Google Scholar]
- 4.Frangioni J V, Beahm P H, Shifrin V, Jost C A, Neel B G. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell. 1992;68:545–560. doi: 10.1016/0092-8674(92)90190-n. [DOI] [PubMed] [Google Scholar]
- 5.Gething M, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 6.Ghayur T, Hugunin M, Talanian R V, Ratnofsky S, Quinlan C, Emoto Y, Pandey P, Datta R, Kharbanda S, Allen H, Kamen R, Wong W, Kufe D. Proteolytic activation of protein kinase C δ by an ICE/CED 3-like protease induces characteristics of apoptosis. J Exp Med. 1996;184:2399–2404. doi: 10.1084/jem.184.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong J, Costanzo A, Yang H, Melino G, Kaelin W, Jr, Levrero M, Wang J Y J. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- 8.Harding H P, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Yuan Z M, Ishiko T, Nakada S, Utsugisawa T, Kato T, Kharbanda S, Kufe D W. Pro-apoptotic effect of the c-Abl tyrosine kinase in the cellular response to 1-β-d-arabinofuranosylcytosine. Oncogene. 1997;15:1947–1952. doi: 10.1038/sj.onc.1201376. [DOI] [PubMed] [Google Scholar]
- 10.Jin S, Kharbanda S, Mayer B, Kufe D, Weaver D T. Binding of Ku and c-Abl at the kinase homology region of DNA-dependent protein kinase catalytic subunit. J Biol Chem. 1997;272:24763–24766. doi: 10.1074/jbc.272.40.24763. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman R J. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann S H, Desnoyers S, Ottaviano Y, Davidson N E, Poirier G G. Specific proteolytic cleavage of poly (ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976–3985. [PubMed] [Google Scholar]
- 13.Kharbanda S, Pandey P, Jin S, Inoue S, Bharti A, Yuan Z-M, Weichselbaum R, Weaver D, Kufe D. Functional interaction of DNA-PK and c-Abl in response to DNA damage. Nature. 1997;386:732–735. doi: 10.1038/386732a0. [DOI] [PubMed] [Google Scholar]
- 14.Kharbanda S, Pandey P, Ren R, Feller S, Mayer B, Zon L, Kufe D. c-Abl activation regulates induction of the SEK1/stress activated protein kinase pathway in the cellular response to 1-β-d-arabinofuranosylcytosine. J Biol Chem. 1995;270:30278–30281. doi: 10.1074/jbc.270.51.30278. [DOI] [PubMed] [Google Scholar]
- 15.Kharbanda S, Pandey P, Yamauchi T, Kumar S, Kaneki M, Kumar V, Bharti A, Yuan Z, Ghanem L, Rana A, Weichselbaum R, Johnson G, Kufe D. Activation of MEK kinase-1 by the c-Abl protein tyrosine kinase in response to DNA-damage. Mol Cell Biol. 2000;20:4979–4989. doi: 10.1128/mcb.20.14.4979-4989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kharbanda S, Ren R, Pandey P, Shafman T D, Feller S M, Weichselbaum R R, Kufe D W. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature. 1995;376:785–788. doi: 10.1038/376785a0. [DOI] [PubMed] [Google Scholar]
- 17.Kharbanda S, Saxena S, Yoshida K, Pandey P, Kaneki M, Wang Q, Cheng K, Chen Y, Campbell A, Thangrila S, Yuan Z, Narula J, Weichselbaum R, Nalin C, Kufe D. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-xL in response to DNA damage. J Biol Chem. 2000;275:322–327. doi: 10.1074/jbc.275.1.322. [DOI] [PubMed] [Google Scholar]
- 18.Kirken R, Lincoln A, Fink P, Prochaska L. High yield purification of a four subunit caa3-type cytochrome oxidase from the thermophilic bacterium Bacillus PS3 using fast protein liquid chromatography. Protein Expr Purif. 1995;6:707–715. doi: 10.1006/prep.1995.1093. [DOI] [PubMed] [Google Scholar]
- 19.Koleske A J, Gifford A M, Scott M L, Nee M, Bronson R T, Miczek K A, Baltimore D. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21:1259–1272. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- 20.Kozutsumi Y, Segal M, Normington K, Gething M J, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 21.Larsson O, Carlberg M, Zetterberg A. Selective killing induced by an inhibitor of N-linked glycosylation. J Cell Sci. 1993;106:299–307. doi: 10.1242/jcs.106.1.299. [DOI] [PubMed] [Google Scholar]
- 22.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 23.Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–454. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner B A, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 25.Pandey P, Raingeaud J, Kaneki M, Weichselbaum R, Davis R, Kufe D, Kharbanda S. Activation of p38 MAP kinase by c-Abl-dependent and -independent mechanisms. J Biol Chem. 1996;271:23775–23779. doi: 10.1074/jbc.271.39.23775. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Sala D, Mollinedo F. Inhibition of N-linked glycosylation induces early apoptosis in human promyelocytic HL-60 cells. J Cell Physiol. 1995;163:523–531. doi: 10.1002/jcp.1041630312. [DOI] [PubMed] [Google Scholar]
- 27.Price B D, Calderwood S K. Gadd45 and Gadd153 messenger RNA levels are increased during hypoxia and after exposure of cells to agents which elevate the levels of the glucose-regulated proteins. Cancer Res. 1992;52:3814–3817. [PubMed] [Google Scholar]
- 28.Shafman T, Khanna K K, Kedar P, Spring K, Kozlov S, Yen T, Hobson K, Gatei M, Zhang N, Watters D, Egerton M, Shiloh Y, Kharbanda S, Kufe D, Lavin M F. Interaction between ATM protein and c-Abl in response to DNA damage. Nature. 1997;387:520–523. doi: 10.1038/387520a0. [DOI] [PubMed] [Google Scholar]
- 29.Shamu C E, Walter P. Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 1996;15:3028–3039. [PMC free article] [PubMed] [Google Scholar]
- 30.Sun X, Majumder P, Shioya H, Wu F, Kumar S, Weichselbaum R, Kharbanda S, Kufe D. Activation of the cytoplasmic c-Abl tyrosine kinase by reactive oxygen species. J Biol Chem. 2000;275:17237–17240. doi: 10.1074/jbc.C000099200. [DOI] [PubMed] [Google Scholar]
- 31.Sun X, Wu F, Datta R, Kharbanda S, Kufe D. Interaction between protein kinase C δ and the c-Abl tyrosine kinase in the cellular response to oxidative stress. J Biol Chem. 2000;275:7470–7473. doi: 10.1074/jbc.275.11.7470. [DOI] [PubMed] [Google Scholar]
- 32.Tewari M, Quan L T, O'Rourke K, Desnoyers S, Zeng Z, Beidler D R, Poirier G G, Salvesen G S, Dixit V M. Yama/CPP32β, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly (ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 33.Tirasophon W, Welihinda A A, Kaufman R J. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998;12:1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tybulewicz V L J, Crawford C E, Jackson P K, Bronson R T, Mulligan R C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 35.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding H P, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase. IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 36.Wang X Z, Harding H P, Zhang Y, Jolicoeur E M, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998;17:5708–5717. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welihinda A A, Tirasophon W, Kaufman R J. The cellular response to protein misfolding in the endoplasmic reticulum. Gene Expr. 1999;7:293–300. [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan Z, Huang Y, Fan M-M, Sawers C, Kharbanda S, Kufe D. Genotoxic drugs induce interaction of the c-Abl tyrosine kinase and the tumor suppressor protein p53. J Biol Chem. 1996;271:26457–26460. doi: 10.1074/jbc.271.43.26457. [DOI] [PubMed] [Google Scholar]
- 39.Yuan Z, Huang Y, Ishiko T, Kharbanda S, Weichselbaum R, Kufe D. Regulation of DNA damage-induced apoptosis by the c-Abl tyrosine kinase. Proc Natl Acad Sci USA. 1997;94:1437–1440. doi: 10.1073/pnas.94.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan Z M, Huang Y, Whang Y, Sawyers C, Weichselbaum R, Kharbanda S, Kufe D. Role for the c-Abl tyrosine kinase in the growth arrest response to DNA damage. Nature. 1996;382:272–274. doi: 10.1038/382272a0. [DOI] [PubMed] [Google Scholar]
- 41.Yuan Z M, Shioya H, Ishiko T, Sun X, Huang Y, Lu H, Kharbanda S, Weichselbaum R, Kufe D. p73 is regulated by the c-Abl tyrosine kinase in the apoptotic response to DNA damage. Nature. 1999;399:814–817. doi: 10.1038/21704. [DOI] [PubMed] [Google Scholar]
- 42.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]