Abstract
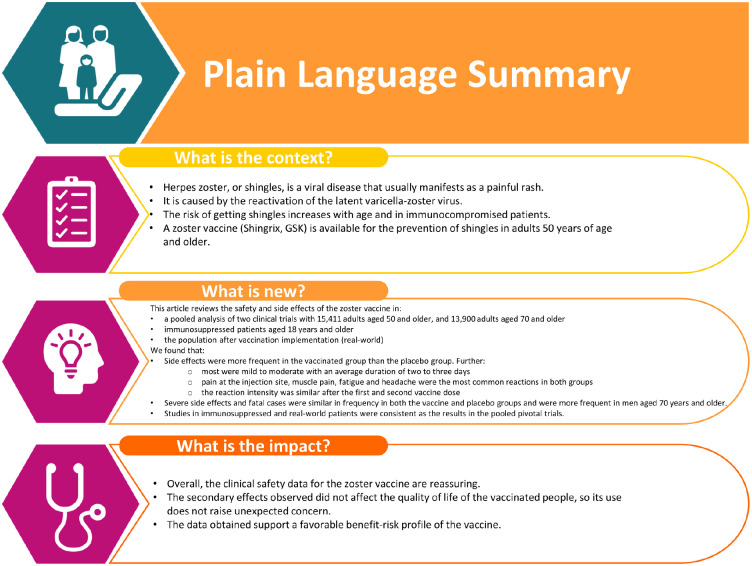
An adjuvanted recombinant zoster vaccine (RZV) is licensed for the prevention of herpes zoster. This paper reviews its safety and reactogenicity. A pooled analysis of two pivotal randomized Phase-3 trials (NCT01165177, NCT01165229) in adults ⩾50 years found that more solicited adverse events (AEs) were reported with RZV than placebo. Injection site pain was the most common solicited AE (RZV: 78.0% participants; placebo: 10.9%). Grade-3 pain occurred in 6.4% of RZV and 0.3% of placebo recipients. Myalgia, fatigue, and headache were the most commonly reported general solicited AEs (RZV: 44.7%, 44.5%, and 37.7%, respectively; placebo: 11.7%, 16.5%, and 15.5%, respectively). Most symptoms were mild to moderate in intensity with a median duration of 2–3 days. The intensity of reactogenicity symptoms did not differ substantially after the first and second vaccine doses. The pooled analysis of the pivotal Phase-3 trials did not identify any clinically relevant differences in the overall incidence of serious adverse events (SAEs), fatal AEs or potential immune-mediated diseases (pIMDs) between RZV and placebo. Reactogenicity in five studies of immunocompromised patients ⩾18 years (autologous stem cell transplant, human immunodeficiency virus, solid tumors, hematological malignancies, and renal transplant; NCT01610414, NCT01165203, NCT01798056, NCT01767467, and NCT02058589) was consistent with that observed in the pivotal Phase-3 trials. There were no clinically relevant differences between RZV and placebo in the immunocompromised populations with regard to overall incidence of SAEs, fatal AEs, pIMDs, or AEs related to patients’ underlying condition. Post-marketing surveillance found that the most commonly reported AEs were consistent with the reactogenicity profile of the vaccine in clinical trials. Overall, the clinical safety data for RZV are reassuring.
Keywords: clinical trial, reactogenicity, real-world, safety, zoster vaccine
Movie 1.
Introduction
After primary infection with Varicella Zoster Virus (VZV) which manifests as chickenpox, the virus becomes latent in the cranial, dorsal root, and autonomic nerve ganglia. 1 Typically, years after the primary infection, reactivation of the latent virus occurs and presents as herpes zoster (HZ) which usually manifests as a painful blistering dermatomal rash. 2 HZ is common, with approximately 1 million cases occurring annually in the United States (US). 3 Its incidence rises with age, increasing from five cases per 1,000 persons per year in individuals 50–59 years of age to 11 cases per 1,000 persons per year in individuals ⩾80 years of age. 3 Lifetime risk for HZ in the US is one in three, increasing to one in two for persons ⩾85 years of age. 4 The risk of HZ is also higher in populations who are immunocompromised due to underlying disease or immunosuppressive therapy.3,5,6
The most common complication of HZ is refractory, long-term neuropathic pain known as post-herpetic neuralgia, commonly defined as persistent pain for at least 90 days following the resolution of the HZ rash. 7 Post herpetic neuralgia has been shown to occur in 5%, 10−17% and 20% of HZ cases in persons <60, 60−79, and ⩾80 years of age, respectively. 8 Other complications of HZ include secondary bacterial infection, ocular involvement with uveitis, neurological complications including encephalitis, vasculopathy leading to stroke or myocardial infarction, and disseminated infections. 9
An adjuvanted recombinant zoster vaccine (RZV; Shingrix, GSK) is licensed for the prevention of HZ in many countries worldwide for adults ⩾50 years of age. In the US, it is also approved for adults aged 18 years and older who are or will be at increased risk of HZ due to immunodeficiency or immunosuppression caused by known disease or therapy. In addition, in the European Union, the vaccine is also licensed for the prevention of post-herpetic neuralgia and for use in adults ⩾18 years of age who are at increased risk of HZ. RZV consists of a recombinant subunit VZV glycoprotein E antigen and AS01B. It is administered intramuscularly as a two-dose series with the second dose administered 2−6 months after the first (the timing of the second dose varies by indication and the recommendations of regulatory authorities). Two pivotal Phase-3 trials have shown that RZV reduces the risk of developing HZ by over 90% in immunocompetent adults ⩾50 years of age.10,11 In addition, the efficacy and/or immunogenicity and safety of RZV have been evaluated in adults ⩾18 years of age with varying immunocompromising conditions or receiving immunosuppressive therapies.12–19 Although the vaccine demonstrates transient local and systemic reactogenicity, it has a favorable benefit–risk profile in the age and risk groups studied.10–19 Nevertheless, continued monitoring of safety data following introduction of the vaccine is important to detect any new safety signals and potential shift in the risk–benefit ratio.
In this paper, we summarize the safety and reactogenicity data of RZV in immunocompetent adults ⩾50 years of age, as well as in immunocompromised adults ⩾18 years of age. We have focused on the marketed dose and formulation of RZV; although some studies of different dosing regimens and formulations have been done, they are not discussed in the present paper. In addition, published post-marketing surveillance conducted since first launch is summarized.
Pooled analysis of two pivotal Phase-3 trials in older adults
The two pivotal Phase-3 trials of RZV (ZOE-50, NCT01165177; ZOE-70, NCT01165229) were conducted in 18 countries in Europe, North America, South America, Asia, and Australia.10,11 Adults ⩾50 years of age (ZOE-50, N = 15,411) or ⩾70 years of age (ZOE-70, N = 13,900) were randomized 1:1 to receive either RZV or placebo in a two-dose series given 2 months apart. In both studies, solicited local and general adverse events (AEs) were recorded by a sub-cohort of study participants on diary cards for 7 days after each vaccination. All local AEs were considered related to vaccination. Events were graded on a scale from 1 (mild; not interfering with everyday activities) to 3 (severe; significant at rest and prevent normal, everyday activities) (Supplemental Table S1). In addition, all study participants recorded unsolicited AEs (comprising both non-serious AEs and serious AEs [SAEs]) for 30 days after each vaccination; data on non-serious unsolicited AEs are not reviewed in this paper. SAEs were recorded for 1 year after vaccination, while fatal AEs, SAEs considered related to study vaccines and potential immune-mediated diseases (pIMDs) were recorded for the entire study duration. pIMDs were pre-defined in the protocol and included autoimmune diseases and other inflammatory or neurological disorders that might or might not have an autoimmune etiology. Specific information on pIMDs was collected because of the theoretical concern that the adjuvant component of the vaccine might precipitate the development of autoimmune syndromes. 20
A pooled analysis of safety from ZOE-50 and ZOE-70 has been reported previously and is summarized here.21,22 The analysis included 14,645 RZV recipients and 14,660 placebo recipients, with a median follow-up of 4.4 years. Most participants were White, 58% were female and the mean age was 68.6 years.
Solicited AEs (reactogenicity)
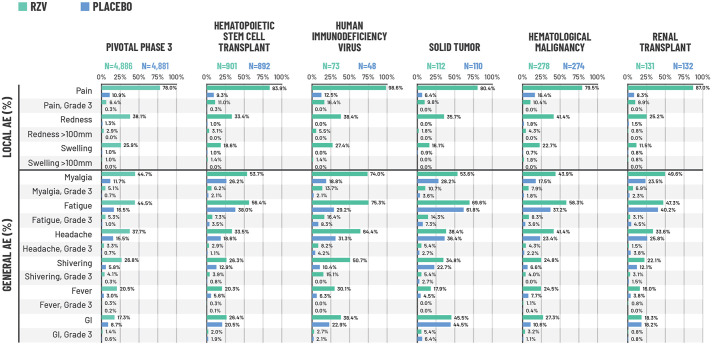
For the RZV group, the reactogenicity sub-cohort consisted of 4886 participants in the RZV group and 4881 participants in the placebo group. 22 More solicited AEs were reported with RZV than placebo. However, most symptoms were of mild to moderate intensity, with a median duration of 2−3 days. Pain at the injection site was the most common solicited AE, reported by 78.0% of participants in the RZV group and 10.9% of those receiving placebo (Figure 1). 22 Grade-3 pain (i.e. prevented normal, everyday activity) occurred in 6.4% of RZV and 0.3% of placebo recipients. Redness and swelling were also reported by more participants receiving RZV compared with placebo, although the incidence of Grade-3 redness and swelling was low in both study groups (Figure 1). 22 Myalgia, fatigue and headache were the most commonly reported general solicited AEs, occurring respectively in 44.7%, 44.5%, and 37.7% of RZV recipients and 11.7%, 16.5%, and 15.5% of placebo recipients (Figure 1). 22 Grade-3 myalgia, fatigue and headache were uncommon but occurred in more RZV recipients than with placebo (Figure 1). 22
Figure 1.
Percentage of participants reporting solicited local and general AEs during the 7-day post-vaccination period in the combined analysis of the two pivotal phase-3 trials (ZOE-50 and ZOE-70)21,22 and studies of immunocompromised populations.
*Each population was evaluated in a different study. Pivotal phase-3 trials: data from the pooled safety analysis of the ZOE-50 and ZOE-70 trials in adults ⩾50 years of age. For the pivotal phase-3 ZOE trials, N corresponds to a sub-cohort of participants who were asked to complete a 7-day diary card recording solicited AEs and who received ⩾1 vaccine dose. This sub-cohort consisted of 4886 participants in the RZV group and 4881 participants in the placebo group; 4884 participants in the RZV group and 4880 participants in the placebo group returned their diary cards reporting local events, while 4876 participants in the RZV group and 4881 participants in the placebo group returned their diary cards reporting general events. For the studies in immunocompromised populations, all participants in the TVC were asked to complete a diary card; the N values shown correspond to the number who returned a diary card and received ⩾1 vaccine dose. AEs were recorded for 7 days (on the day of vaccination and 6 days thereafter). Grade-3 pain and general solicited AEs: prevented normal, everyday activities. Grade-3 redness and swelling: >100 mm. Fever: temperature ⩾37.5°C. Grade-3 fever: temperature >39.0°C.
AE, adverse event; GI, gastrointestinal; HSCT, hematopoietic stem cell transplant; RZV, recombinant zoster vaccine; TVC, total vaccinated cohort.
A post hoc analysis showed that the incidence of local and general solicited AEs (all grades and Grade 3) was higher in women than in men (Table 1). 23 Local AEs (all grades and Grade 3) and Grade-3 general AEs were higher in participants 50−69 years of age than in those ⩾70 years of age. When analyzed according to race and ethnicity, there was a trend toward higher reactogenicity in the RZV group among participants of Asian heritage and those not of American–Hispanic or Latino ethnicity (Table 1). 23 However, the ZOE studies were not powered to evaluate differences between population subgroups and the numbers were very small in some subgroups; the data should therefore be interpreted with caution. Reactogenicity might be higher in women than men because of anatomical differences in skin thickness, blood flow, and nervous system structure. 24 Furthermore, immune responses and cytokine levels are affected by sex hormones, with androgens and high doses of oestrogens shown to be immunosuppressive. 24 The lower incidence of solicited AEs in older study participants is unsurprising, as it has been previously observed that reporting rates reduce during adult life, possibly as a result of higher tolerance to pain and symptoms of illness gained with age and/or the waning of innate immune defense mechanisms. 24 Older people exhibit lower systemic levels of interleukin (IL)-6, IL-10, and C-reactive protein after vaccination, which might explain the trend of reporting fewer systemic AEs, in particular fever. 24
Table 1.
Reactogenicity (solicited AEs) reported during the 7-day post-vaccination period according to gender, race, and ethnicity in the combined analysis of the two pivotal Phase-3 trials (ZOE-50 and ZOE-70, reactogenicity sub-cohort). 23 .
| Local AEs | General AEs | |||
|---|---|---|---|---|
| RZV, n (%) | Placebo, n (%) | RZV, n (%) | Placebo, n (%) | |
| Age | ||||
| 50−69 years | 2,287 (87.1) | 354 (13.5) | 1,907 (72.7) | 866 (33.1) |
| Grade 3 | 297 (11.3) | 13 (0.5) | 375 (14.3) | 74 (2.8) |
| ⩾70 years | 1,657 (73.4) | 218 (9.6) | 1,252 (55.6) | 553 (24.4) |
| Grade 3 | 162 (7.2) | 4 (0.2) | 153 (6.8) | 42 (1.9) |
| Gender | ||||
| Male | 1,465 (75.5) | 201 (9.9) | 1,126 (58.1) | 483 (23.8) |
| Grade 3 | 137 (7.1) | 5 (0.2) | 127 (6.6) | 37 (1.8) |
| Female | 2,479 (84.2) | 371 (13.0) | 2,033 (69.2) | 936 (32.8) |
| Grade 3 | 322 (10.9) | 12 (0.4) | 401 (13.6) | 79 (2.8) |
| Race | ||||
| African/African–American | 52 (72.2) | 9 (13.8) | 44 (61.1) | 22 (33.8) |
| Grade 3 | 7 (9.7) | 0 | 5 (6.9) | 1 (1.5) |
| Asian | 785 (84.6) | 142 (15.3) | 666 (71.8) | 263 (28.3) |
| Grade 3 | 99 (10.7) | 3 (0.3) | 89 (9.6) | 15 (1.6) |
| White | 2,861 (79.8) | 360 (10.0) | 2,255 (63.0) | 1,054 (29.4) |
| Grade 3 | 304 (8.5) | 9 (0.3) | 373 (10.4) | 87 (2.4) |
| Other | 246 (82.0) | 61 (20.3) | 194 (65.1) | 80 (26.7) |
| Grade 3 | 49 (16.3) | 5 (1.7) | 61 (20.5) | 13 (4.3) |
| Ethnicity | ||||
| American–Hispanic or Latino | 376 (76.4) | 85 (17.3) | 295 (60.2) | 121 (24.6) |
| Grade 3 | 71 (14.4) | 5 (1.0) | 79 (16.1) | 17 (3.5) |
| Other | 3,568 (81.2) | 487 (11.1) | 2,864 (65.3) | 1,298 (29.6) |
| Grade 3 | 388 (8.8) | 12 (0.3) | 449 (10.2) | 99 (2.3) |
AE, adverse event; n (%), number and percentage of patients experiencing an AE; RZV, recombinant zoster vaccine.
The reactogenicity sub-cohort comprised participants who completed a diary card recording AEs after vaccination (during the 7-day post-vaccination period). Grade-3 pain and general solicited AEs: prevented normal, everyday activities; grade-3 redness and swelling: >100 mm.
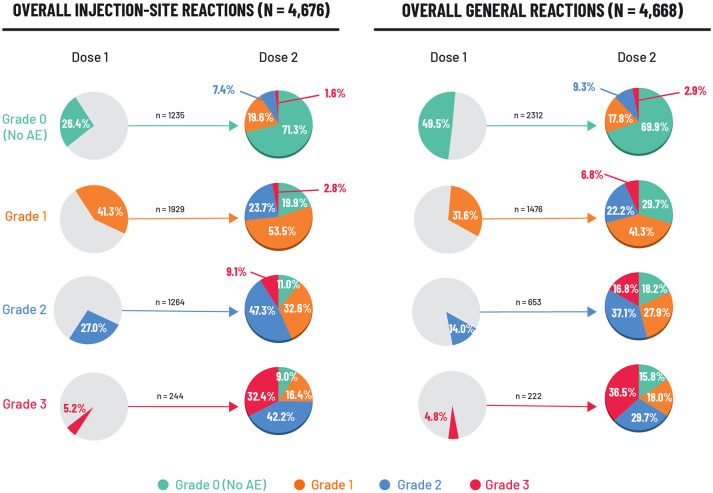
The intensity of solicited AEs in the RZV group after the second vaccine dose in relation to the first dose was explored in a post hoc analysis. 25 Local AEs were evaluated in 4676 vaccinees. A total of 244 (5.2%) individuals reported a Grade-3 local AE after the first dose, of whom 165 (67.6%) experienced the same event at a lower intensity (grade ⩽ 2) after the second dose (Figure 2). A total of 1235 (26.4%) vaccinees reported no local AE after the first dose; of these 1235 individuals, 71.3% again reported no local AE following the second dose (Figure 2).
Figure 2.
Intensity (grade) of solicited local and general AEs reported after the second RZV dose in relation to the first dose (ZOE-50 and ZOE-70). 25
Data are reported for the reactogenicity sub-cohort which comprised participants who completed a diary card recording AEs after vaccination. AEs were recorded for 7 days (on the day of vaccination and 6 days thereafter). N: number of RZV vaccinees with both doses administered and corresponding event intensity after Dose 1 and/or after Dose 2. n%: number and percentage of RZV vaccinees with events at a specific grade. There were nine events (four injection site and five general events) with missing grading after Dose 1 and 6 events (three injection site and three general events) with missing grading after Dose 2. Injection site events included: pain at the injection site, redness at the injection site, and swelling at the injection site. General events included: fatigue, gastrointestinal symptoms (nausea, vomiting, diarrhea and/or abdominal pain), headache, myalgia, shiver, and fever.
AE, adverse event; RZV, recombinant zoster vaccine.
In the corresponding analysis of general solicited AEs in 4668 vaccinees, 222 (4.8%) reported a Grade-3 general AE after the first dose; of these participants, 141 (63.5%) experienced the same event at lower intensity (grade ⩽ 2) following the second dose (Figure 2). 25 A total of 2312 (49.5%) vaccinees experienced no general AE after the first dose, of whom 1617 (69.9%) also experienced no event after the second dose (Figure 2). A similar pattern was seen when each specific local and general solicited AE was analyzed individually.
SAEs, fatal AEs and pIMDs
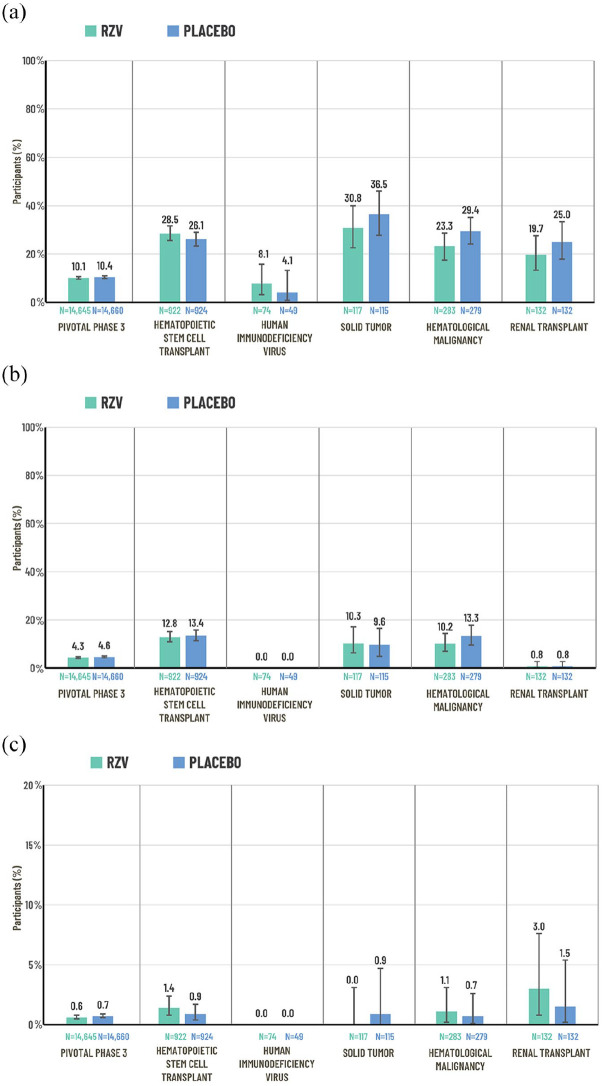
SAEs were reported by 1482 (10.1%) participants receiving RZV and 1525 (10.4%) receiving placebo within 1 year after the last vaccine dose (Figure 3(a)).21,22 The relative risk (RR) of a SAE for RZV versus placebo was 0.97 (95% confidence interval (CI) 0.91−1.05; p = 0.46).21,22
Figure 3.
Percentage of participants reporting SAEs, fatal AEs and pIMDs in the combined analysis of the two pivotal Phase-3 trials (ZOE-50 and ZOE-70)21,22 and studies of immunocompromised populations:12–14,16–19 (a) SAEsa, (b) fatal AEs,a and (c) pIMDsa.
Pivotal Phase-3 trials: data from the pooled safety analysis of the ZOE-50 and ZOE-70 trials in adults ⩾50 years of age. Data are reported for the total vaccinated cohort which comprised participants who received at least one vaccine dose. SAEs and pIMDs were recorded over the following study periods: ZOE and HSCT: from first vaccination up to 1 year after last vaccination; other studies: from first vaccination until study end (approximately 12 months after the last scheduled dose). Fatal AEs were recorded from first vaccination until study end (approximately 12 months after the last scheduled dose for all studies except HSCT and ZOE; the median duration of the safety follow-up was 29 months for the HSCT trial and 4.4 years for the pooled safety analysis of the ZOE trials). Error bars represent 95% CIs.
AE, adverse event; CI, confidence interval; N, number of patients in the total vaccinated cohort per group; pIMD, potential immune-mediated disease; RZV, recombinant zoster vaccine; SAE, serious adverse event; TVC, total vaccinated cohort.
aEach population was evaluated in a different study.
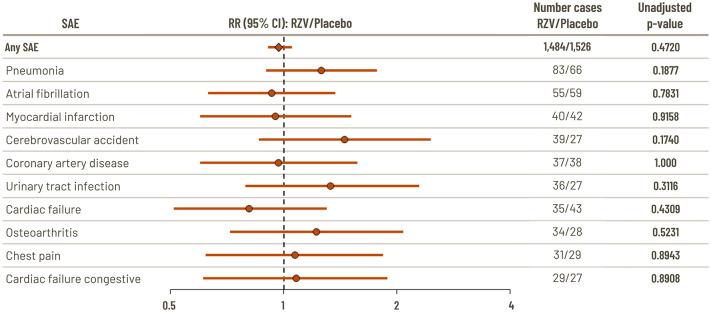
The nature of the events was as expected in a study population of this age (⩾50 years), and the most frequently reported SAEs reported within 1 year post-vaccination were pneumonia (0.57% RZV and 0.45% placebo) and atrial fibrillation (0.38% RZV and 0.40% placebo).21,22 There were no significant differences between RZV and placebo in RR for the 10 most common SAEs with an incidence ⩾0.2% in the RZV group (Figure 4). 22 Only one SAE, supraventricular tachycardia, was reported significantly more often in RZV vaccinees than placebo vaccinees (six participants versus none; p = 0.03).21,22 To explore this further, a follow-up analysis was conducted of a group of SAEs that are pathologically related to supraventricular tachycardia (arrhythmia supraventricular, atrial fibrillation, atrial flutter, atrial tachycardia, cardiac flutter, supraventricular tachycardia, tachyarrhythmia, and tachycardia paroxysmal). The analysis found no significant difference between RZV and placebo (incidence 0.47% with RZV and 0.45% with placebo; RR 1.1 (95% CI: 0.7, 1.5); p = 0.86). 21 Three SAEs were reported significantly more frequently with placebo versus RZV: aortic stenosis, cardiorespiratory arrest, and retinal detachment.
Figure 4.
Relative risk versus placebo of SAEs (10 most frequently reported with incidence ⩾0.2% in the RZV group) occurring within 1 year of last vaccination in the combined analysis of the two pivotal Phase-3 trials (ZOE-50 and ZOE-70). 22
Data are reported for the total vaccinated cohort which comprised participants who received at least one vaccine dose.
CI, confidence interval; RR, relative risk; RZV, recombinant zoster vaccine; SAE, serious adverse event.
A descriptive analysis did not identify any difference between RZV and placebo in the incidence of SAEs within 1 year after the last vaccine dose in different racial subgroups: 10.3% RZV versus 10.6% placebo for White participants; 10.5% RZV versus 13.3% placebo for African/African–American participants; 10.4% for RZV versus 10.7% placebo for Asian participants; and 7.0% RZV versus 6.9% placebo for participants of other racial subgroups. 21 SAEs were experienced more frequently in men (11.9% RZV versus 12.5% placebo) than in women (8.8% RZV versus 8.9% placebo) and in participants ⩾70 years of age (12.7% RZV versus 13.3% placebo) than in those 50−69 years of age (6.2% RZV versus 6.1% placebo).
During the entire study period (median follow-up 4.4 years), SAEs considered by investigators to be related to vaccination occurred in 15 participants (0.1%) in both the RZV and placebo groups; only two events occurred in more than one participant (rheumatoid arthritis in two participants and syncope in two participants, all of whom had received placebo). 21
Fatal AEs occurred in 634 (4.4%) participants in the RZV group and 680 (4.6%) participants in the placebo group during the entire study period (Figure 3(b)). The most frequently reported fatal AEs were cardiac failure (0.3% RZV versus 0.4% placebo), pneumonia (0.3% RZV versus 0.3% placebo), myocardial infarction (0.3% RZV versus 0.3% placebo), death with no specified cause (0.2% RZV versus 0.3% placebo) and cardiac arrest (0.2% RZV versus 0.2% placebo). 21 One fatal AE was considered possibly related to RZV by the investigator.21,22 The study participant was male, 90 years of age and had a medical history of stable immune-mediated thrombocytopenia, as well as several cardiac-related conditions. He was diagnosed with acute myeloid leukemia 75 days after the first vaccine dose and was hospitalized and withdrawn from the study. He was readmitted to hospital 96 days after vaccination with febrile neutropenia and died a day later due to neutropenic sepsis.
A new pIMD or a possible exacerbation of an existing pIMD was experienced by 0.6% and 0.7% of participants in the RZV and placebo groups, respectively, in the year post last vaccination and by 1.2% and 1.4%, respectively, during the entire study period (Figure 3(c)). 21 Overall, in different racial subgroups, men and women, and participants 50−69 and ⩾70 years of age, the incidence of pIMD was similar between RZV and placebo recipients. Over the entire study period, the pIMDs reported in ⩾0.1% of RZV recipients were polymyalgia rheumatica (0.2% RZV versus 0.2% placebo), rheumatoid arthritis (0.1% RZV versus 0.2% placebo), psoriasis (0.1% RZV versus 0.1% placebo), autoimmune thyroiditis (0.1% RZV versus 0.1% placebo) and VIIth nerve paralysis (0.1% RZV versus 0% placebo). 21 An analysis of participants with a pre-existing pIMD showed that 95.6% of RZV recipients and 95.0% of placebo recipients did not experience either an exacerbation of their existing condition nor a new pIMD. 21
Overall, except for the expected local and systemic symptoms, the safety results were comparable between the RZV and placebo groups irrespective of participant race (White, Black, Asian, Other). 21 In Asian populations ⩾50 years of age, RZV has an acceptable safety profile, similar to what was observed in the general ZOE-50/70 populations. 26
Studies in immunocompromised populations
The burden of HZ is higher in immunocompromised populations than in the general population. A study of a US health care claims database showed that the incidence of HZ was more than three times higher in patients who had received recent care for transplantation, HIV infection or cancer (10.3 per 1,000 persons per year) than individuals without such care (3.0 per 1,000 persons per year). 3 Another study of a US health care plan reported that patients with hematologic malignancies and those with solid tumors had an age- and sex-standardized rate of HZ that was 4.8 times higher and 1.9 times higher, respectively, than the rate in the general population. 5 A study of patients who had received an autologous hematopoietic stem cell transplant (HSCT) reported an overall HZ incidence of 62 per 1,000 persons per year (31 per 1,000 in patients who received antiviral prophylaxis and 152 per 1,000 in those who did not), 27 while another study in solid organ transplant recipients reported an incidence of 22 per 1,000 persons per year. 28 Patients living with HIV are also at greater risk of HZ, even in the age of highly active antiretroviral treatment, with rates approximately three times higher than the general population.29,30 Complication rates are also higher in HIV-infected individuals. 29
This review focuses on five randomized, placebo-controlled studies of RZV in immunocompromised adults ⩾18 years of age: autologous HSCT (NCT01610414);12,13 HIV (NCT01165203);14,15 solid tumors (NCT01798056); 16 hematologic malignancies (NCT01767467);17,18 and renal transplant (NCT02058589). 19 An overview of the study design, patient population, and vaccination schedule is shown in Table 2. The mean age at first vaccination ranged from 52 to 59 years, with exception of the HIV trial in which the mean age was approximately 45 years. Excluding the HIV trial, 25−37% of vaccinees were 18−49 years of age; in the HIV trial, approximately 65% were 18−49 years of age (Table 2).
Table 2.
Study design and patient population in clinical trials of RZV in immunocompromised populations.
| Autologous HSCT12,13 | HIV 14 | Solid tumor 16 | Hematological malignancy17,18 | Renal transplant 19 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study characteristics | ||||||||||
| Study design and trial registration number | Randomized, phase-3,
placebo-controlled NCT01610414 |
Randomized, phase-1/2,
placebo-controlled NCT01165203 |
Randomized, phase-2/3,
placebo-controlled NCT01798056 |
Randomized, phase-3,
placebo-controlled NCT01767467 |
Randomized, phase-3,
placebo-controlled NCT02058589 |
|||||
| Study location | 28 countries | Germany, USA, UK | Canada, Czech Republic, France, Republic of Korea, Spain, UK | 77 centers worldwide | Belgium, Canada, Czech Republic, Finland, Italy, Panama, Republic of Korea, Spain, Taiwan | |||||
| Age eligibility criterion | ⩾18 years | ⩾18 years | ⩾18 years | ⩾18 years | ⩾18 years | |||||
| Vaccination schedule | Two-dose schedule Dose 1: 50−70 days after transplant Dose 2: 1−2 months after Dose 1 |
Three-dose schedule at Months 0, 2, and 6 | Two-dose schedule Doses administered 1−2 months apart. First dose was given either prior to or at the start of the chemotherapy cycle. Second dose was given with a subsequent chemotherapy cycle. a |
Two-dose schedule Doses administered 1−2 months apart during or after full cancer therapy course b |
Two-dose schedule Dose 1: 4−18 months post-transplant Dose 2: 1−2 months after Dose 1 |
|||||
| Patient characteristics | RZV | Placebo | RZV | Placebo | RZV | Placebo | RZV | Placebo | RZV | Placebo |
| N | 922 | 924 | 74 | 49 | 117 | 115 | 283 | 279 | 132 | 132 |
| Mean age (years) | 54.8 | 55.1 | 46.6 | 45.1 | 57.1 | 58.5 | 56.8 | 57.8 | 52.3 | 52.4 |
| Percentage ⩾50 years | 75.1% | 75.2% | 37.8% | 30.6% | 73.5% | 73.9% | 73.9% | 73.8% | 63.6% | 62.9% |
| Percentage men | 62.9% | 62.6% | 93.2% | 95.9% | 40.2% | 40.0% | 59.7% | 59.1% | 71.2% | 68.9% |
HIV, human immunodeficiency virus; HSCT, hematopoietic stem cell transplant; N, number of participants in the total vaccinated cohort; RZV, recombinant zoster vaccine.
The total vaccinated cohort for safety included all participants with at least one documented dose.
Participants were stratified (4:1) according to the timing of the first RZV or placebo dose with respect to the start of the first (or occasionally second) cycle of a chemotherapy course: first vaccination 8−30 days before the start of a cycle (pre-chemotherapy groups) or first vaccination within 1 day of the start of a cycle (on-chemotherapy groups). Paticipants received their second vaccination with a subsequent chemotherapy cycle.
Participants were vaccinated during a cancer therapy course (each dose at least 10 days before and after any cancer therapy) or after the full cancer therapy course (first dose between 10 days and 6 months after therapy).
In line with the pivotal phase-3 trials in older adults (⩾50 years), injection site pain, fatigue, myalgia and headache were the most common solicited AEs, and occurred more often in the RZV group than in the placebo group (Figure 1). All five studies found that there were no clinically relevant differences in the incidence of SAEs, fatal SAEs or pIMDs between the RZV and placebo groups (Figure 3). SAEs were reported by 28.5% (RZV) and 26.1% (placebo) of participants in the autologous HSCT study, 8.1% (RZV) and 4.1% (placebo) in the HIV study, 30.8% (RZV) and 36.5% (placebo) in the solid tumor study, 23.3% (RZV) and 29.4% (placebo) in the hematologic malignancies study and 19.7% (RZV) and 25.0% (placebo) in the renal transplant study (Figure 3(a)). The most frequent SAEs by system organ class (SOC) were neoplasms in the autologous HSCT trial, 12 and infections and infestations in the solid tumors, hematologic malignancies, and renal transplant trials.16,18,19 In the HIV trial, only six and two participants in the RZV and placebo groups, respectively, reported a SAE; no event occurred more than once in a SOC. The percentage of participants who experienced fatal AEs or pIMDs in the studies is shown in Figure 3(b) and (c). In the hematologic malignancy trial, one neonatal death occurred in an offspring born at 36 weeks’ gestation to a mother who received RZV before pregnancy (approximately 34 days before her last menstrual period). The investigator assessed this fatal SAE as possibly related to study vaccine. The mother was treated with chemotherapy before pregnancy for her underlying malignancy. The neonate was born with no apparent congenital anomalies and died 30 minutes after birth because of breathing difficulties. The Company considered that the neonatal death was possibly due to perinatal causes. 17
The studies in immunocompromised populations also assessed specific AEs of interest related to patients’ underlying conditions. In the autologous HSCT trial, 26% of RZV recipients and 27% of placebo recipients had a malignancy relapse during the entire study period. 12 In the HIV trial, 12% of participants in the RZV group and 10% in the placebo group reported worsening of HIV disease through to Month 7 of the study. 14 Overall, RZV had no sustained impact on CD4+ cell counts or HIV RNA loads. 14 In the hematologic malignancies trial, relapse or progression of the original malignancy was reported in 16% of RZV recipients and 21% of placebo recipients during the entire study period. 17 In the study of renal transplant patients, biopsy-proven rejection occurred in 3% and 5% of RZV and placebo recipients, respectively, during the whole study period; of these, one of four rejections in the RZV group and seven of seven rejections in the placebo group occurred in patients at low risk of rejection. 19 In the solid tumor trial, relapse or worsening of patients’ condition was not a pre-defined safety endpoint. 16
Post-marketing surveillance
Monitoring of vaccine safety in the real-world setting after licensure is essential. Post-marketing safety surveillance of spontaneously reported AEs following vaccine administration allows data to be collected rapidly following real-world use of the product in the general population, including people who would not normally be included in clinical trials (such as high-risk individuals or those receiving concomitant medication). In addition, real-world surveillance allows identification of less frequent events that would not be detected in the smaller clinical trial populations.
Post-marketing safety surveillance data, comprising spontaneous reports of AEs following RZV vaccination, have been reported for the period 13 October 2017 to 10 February 2019. 31 Spontaneous report data were either reported voluntarily to GSK directly or were collated by GSK from the scientific literature or interactive digital media. Follow-up was conducted if required to obtain information needed for scientific evaluation of the event. Spontaneous report data from GSK sources were analyzed using the Signal Mining and Management (SMM) tool which flagged signals if there was disproportionate reporting or an unexpected time-to-onset distribution. Data from external sources were reviewed separately for the purpose of signal detection, including spontaneous report data from public safety databases: the US Vaccine Adverse Event Reporting System (VAERS), the Canada Vigilance Adverse Reaction Online Database, and the European Medicines Agency EudraVigilance system. In addition, observed-to-expected analyses were performed for all-cause mortality and the most frequently reported pIMDs.
During the reporting period, 9,323,118 vaccine doses were distributed, of which approximately 8.4 million were distributed in the US. There were 15,638 spontaneous reports of individuals experiencing 37,697 AEs following RZV administration, of which 95.3% were considered non-serious. 31 The most commonly reported AEs were consistent with the reactogenicity profile of the vaccine observed in clinical trials, for example, pain, redness, and swelling at the injection site, fatigue, headache, and myalgia (Table 3). A specific analysis of symptoms potentially related to reactogenicity identified 4639 reports, corresponding to a rate of 49.8 reports per 100,000 doses distributed. Injection site reactions comprised 61.4% of these reports, most commonly pain. Most events were non-serious (95.9%), occurred within the first few days after vaccination, and generally lasted for 3−4 days. 31 Of the 15,638 total reports, 805 (5.1%) described symptoms potentially linked to severe reactogenicity. Of these, the most commonly reported AEs were decreased mobility of the injected arm (1.8 reports per 100,000 doses distributed) and extensive swelling of the injected arm (1.4 reports per 100,000 doses distributed). These events occurred within the first few days after vaccination and generally lasted for 3–4 days, although symptoms persisted for a week or more on rare occasions. 31
Table 3.
Common AEs with RZV reported in post-marketing surveillance (occurring at a reporting rate of ⩾5 per 100,000 doses distributed). 31
| Symptom (MedDRA preferred term) | Number (%) of reports a | Reporting rate per 100,000 doses distributed |
|---|---|---|
| Injection site pain | 1,699 (10.9) | 18.2 |
| Pyrexia | 1,658 (10.6) | 17.8 |
| Pain in extremity | 1,466 (9.4) | 15.7 |
| Pain | 1,326 (8.5) | 14.2 |
| Chills | 1,240 (7.9) | 13.3 |
| Injection site erythema | 1,221 (7.8) | 13.1 |
| Fatigue | 1,085 (6.9) | 11.6 |
| Headache | 1,076 (6.9) | 11.5 |
| Influenza-like illness | 866 (5.5) | 9.3 |
| Herpes zoster | 837 (5.4) | 9.0 |
| Myalgia | 802 (5.1) | 8.6 |
| Injection site swelling | 787 (5.0) | 8.4 |
| Erythema | 649 (4.2) | 7.0 |
| Malaise | 647 (4.1) | 6.9 |
| Nausea | 556 (3.6) | 6.0 |
| Rash | 540 (3.5) | 5.8 |
AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities; RZV, recombinant zoster vaccine.
Percentage calculated from the total of 15,638 reports.
A total of 741 (4.7%) reports were classified as serious, defined as any untoward medical occurrence that results in death, is life-threatening, requires hospitalization or prolongs existing hospitalization, results in disability or incapacity, is a congenital anomaly or birth defect in the offspring, or is a medically important event. 31 The most commonly reported events were HZ (27.6%), pyrexia (9.6%), pain in extremity (9.2%), and pain (8.4%). Nine deaths were reported, for which five reports did not contain sufficient information for further evaluation. Of the other four reports, one individual died at an unspecified time after vaccination, possibly due to sepsis; this person was possibly immunosuppressed and was undergoing treatment with rituximab for primary membranous nephropathy. The deaths of two individuals with cardiac risk factors, who died on the same day and 3 days after vaccination, were associated with cardiovascular disease. The fourth report was of Guillain–Barré Syndrome (GBS) which occurred at an unspecified time after the second dose of RZV and an unknown quadrivalent influenza vaccine; the individual died possibly due to GBS complications 1 week after diagnosis. The observed-to-expected analysis found that all-cause mortality in the 7-day period following vaccination was below the expected range, possibly indicating a high level of under-reporting. 31
A total of 114 pIMDs were reported in 104 individuals, corresponding to a reporting rate of 1.1 per 100,000 doses distributed. 31 The pIMDs reported were diverse and fell into a range of disease categories. Events occurring in five or more vaccinees were Bell’s palsy (25 events), GBS (17 events), polymyalgia rheumatica (6 events), and five events each of uveitis, rheumatoid arthritis and vasculitis. All reports with a known time-to-onset occurred within 60 days post-vaccination and more than half occurred within 1 week post-vaccination. The reports of Bell’s palsy and GBS during the reporting period (13 October 2017 to 10 February 2019) corresponded to 0.27 and 0.18 cases per 100,000 doses, respectively. Observed-to-expected analysis of Bell’s palsy considering risk periods of 7 or 30 days after vaccination found a below than expected number of events. A lower than expected number of GBS cases was also found considering a risk period of 42 days after vaccination.
There were 865 spontaneous reports of HZ, comprising 837 HZ cases and 50 HZ complications. 31 A suspected vaccination failure was defined as the occurrence of HZ clinical symptoms suggestive of VZV infection occurring ⩾30 days after completion of a full RZV vaccination schedule. A confirmed vaccination failure was defined as the occurrence of HZ clinical symptoms and laboratory confirmation of VZV infection occurring ⩾30 days after completion of a full RZV vaccination schedule. A total of 176 reports met the criteria for a suspected vaccination failure (1.9 reports per 100,000 doses distributed) and two reports met the criteria for a confirmed vaccination failure (0.02 reports per 100,000 doses distributed). Most reports came from Canada (68.5%) from a non-medically confirmed source, possibly as a result of the RZV Facebook page in Canada turning on the capability to comment on the page. The reports of HZ complications comprised 25 reports of HZ ophthalmicus, 21 reports of PHN, two reports of HZ with neurologic infection and two reports of HZ oticus.
A substantial proportion of the spontaneous reports (3579/15,638, 22.9%) was linked to vaccination error. 31 The most common vaccination errors were product preparation errors (n = 1062, 29.7%), inappropriate or incomplete course of administration (n = 956, 26.7%), incorrect route of administration (n = 585, 16.3%), and storage errors (n = 463, 12.9%). A total of 17.3% of vaccination error reports were associated with symptoms, mainly injection site reactions following subcutaneous instead of intramuscular injection. Most of the errors during vaccine preparation were the administration of the liquid AS01B only or mixing the RZV lyophilized antigen with a diluent other than the supplied adjuvant system. Most errors occurred in the US (where most doses were distributed) and might be explained by unfamiliarity of health care professionals with RZV in the early days of its use. 31 Some confusion might have arisen because the live attenuated virus zoster vaccine, available in the US for a decade, is reconstituted prior to subcutaneous administration by injecting diluent into a vial containing the lyophilized component. Early VAERS data on RZV administration errors during the first 4 months following licensure in the US drew similar conclusions. 32 Errors decreased substantially following a program of education and training of health care professionals involved in administering the vaccine. 31
During routine medical review of post-marketing data from the reporting period October 2017 to February 2019, a possible causal relationship between RZV and certain hypersensitivity reactions (mainly different types of rash, urticaria, and angioedema) could not be excluded and the prescribing information has been updated accordingly. 33 The RZV vaccine is contraindicated in persons with a history of severe allergic reactions (e.g. anaphylaxis) to any component of the vaccine or after a previous dose of the vaccine. 33 In the US, the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) have conducted a safety analysis of RZV in VAERS. 34 During the first 8 months of use, VAERS received 4381 AE reports. It was concluded that no unexpected patterns were detected in reports of AEs or SAEs, and the findings were consistent with the safety profile of RZV in prelicensure clinical trials. 34
In 2018, a statistical signal for GBS following RZV receipt was detected in the Vaccine Safety Datalink (VSD) Rapid Cycle Analysis (RCA). The US CDC consulted with the FDA on additional analyses in other databases. 35 Subsequently, the FDA in collaboration with the CDC and Center for Medicare & Medicaid Services (CMS) initiated an assessment of the risk of GBS following RZV receipt in the US Medicare population aged 65 years or older. In 2020, new information on GBS emerged from the post-marketing observational study in individuals aged 65 years or older which showed an increased risk of GBS (estimated 3 excess cases per million doses administered) during the 42 days following vaccination.33,35,36 Based on this evaluation, the FDA has determined that the results of this observational study show an association of GBS with RZV, but that available evidence is insufficient to establish a causal relationship. Moreover, the FDA determined that the benefits of vaccination with RZV continue to outweigh its risks. 36 Having considered the findings of the additional CDC and FDA analyses in February 2021, the Herpes Zoster Work Group of the United States Advisory Committee on Immunization Practices (ACIP) also concluded that clinical trials, observational studies, and benefit-risk analysis confirm the considerable benefits of RZV vaccination in preventing HZ, severe disease and complications.
Spontaneously reported AEs are analyzed continuously. To date, reports received are generally consistent with what was observed in the clinical trials and with the reactogenicity profile of the vaccine, in line with published safety surveillance data 31 and as reflected in approved prescribing information for RZV.
Conclusion
In clinical trials, the incidence and nature of SAEs and fatal AEs were similar in the RZV and placebo groups. Reactogenicity symptoms occurred more frequently with RZV than with placebo.10–12,14,16,17,19,21 This is likely a secondary consequence of the enhanced innate immune responses elicited at the site of injection and therefore is not unexpected. It is consistently observed in studies evaluating AS01B and other adjuvanted vaccines, in which higher frequencies of mostly mild and transient local and systemic reactions are observed with adjuvanted versus non-adjuvanted vaccines.24,37 These data do not raise concern. Following the first year of post-marketing surveillance, the safety profile of RZV is consistent with that observed in clinical trials. 31
Importantly, reactogenicity following RZV administration did not affect quality of life, although participants who experienced Grade-3 reactogenicity had a transient decrease in physical functioning on the Short Form (36) Health Survey and in the Euroqol-5 dimensions score.38,39 The reported quality-adjusted life-year losses resulting from reactogenicity were 300−3000 times lower than those associated with HZ.38–41
Several studies are currently ongoing and planned to further assess the safety of RZV in real-world settings. Overall, the clinical safety data for RZV are reassuring. Along with high vaccine efficacy shown in the clinical trials, the available data support a favorable benefit–risk profile for RZV.
Supplemental Material
Supplemental material, sj-docx-1-tav-10.1177_25151355211057479 for Safety and reactogenicity of the adjuvanted recombinant zoster vaccine: experience from clinical trials and post-marketing surveillance by Joseph Fiore, Maribel Miranda Co-van der Mee, Andrés Maldonado, Lisa Glasser and Phil Watson in Therapeutic Advances in Vaccines and Immunotherapy
Acknowledgments
The authors would like to thank the Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Maxime Bessières coordinated publication development and editorial support. Mary Greenacre (An Sgriobhadair Ltd) provided writing support.
Footnotes
Author contributions: All authors comply with the ICMJE criteria for authorship. All authors were involved in the conception and/or the design of the study. AM, JF, and MC participated in the data collection or generation of the study data and analysis and conducted the study. All authors were involved in the interpretation of the data. All authors reviewed and approved the final manuscript.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AM, JF, MC, and PW are employed by the GSK group of companies. AM, JF, and PW hold shares in the GSK group of companies. LG was employed by the GSK group of companies at the moment of the study and is now employed by AstraZeneca. The authors declare no other financial and non-financial relationships and activities.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: GlaxoSmithKline Biologicals SA funded this study/research and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals S.A. also took in charge all costs associated with the development and publication of this manuscript.
Trademark: Shingrix is a trademark owned by or licensed to the GSK group of companies
ORCID iD: Joseph Fiore  https://orcid.org/0000-0003-0591-1877
https://orcid.org/0000-0003-0591-1877
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Joseph Fiore, GSK, Philadelphia, PA 19112, USA.
Maribel Miranda Co-van der Mee, GSK, Wavre, Belgium.
Andrés Maldonado, ViiV Healthcare, Wavre, Belgium.
Lisa Glasser, Former affiliation: GSK, Philadelphia, PA, USA; Current affiliation: AstraZeneca, Washington, DC, USA.
Phil Watson, GSK, Brentford, UK.
References
- 1. Gilden D, Cohrs RJ, Mahalingam R, et al. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol 2009; 8: 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lecrenier N, Beukelaers P, Colindres R, et al. Development of adjuvanted recombinant zoster vaccine and its implications for shingles prevention. Expert Rev Vaccines 2018; 17: 619–634. [DOI] [PubMed] [Google Scholar]
- 3. Insinga RP, Itzler RF, Pellissier JM, et al. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med 2005; 20: 748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57: 1–30; quiz CE32–CE34. [PubMed] [Google Scholar]
- 5. Habel LA, Ray GT, Silverberg MJ, et al. The epidemiology of herpes zoster in patients with newly diagnosed cancer. Cancer Epidemiol Biomarkers Prev 2013; 22: 82–90. [DOI] [PubMed] [Google Scholar]
- 6. Chanan-Khan A, Sonneveld P, Schuster MW, et al. Analysis of herpes zoster events among bortezomib-treated patients in the phase III APEX study. J Clin Oncol 2008; 26: 4784–4790. [DOI] [PubMed] [Google Scholar]
- 7. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization practices for use of herpes zoster vaccines. Morb Mortal Wkly Rep 2018; 67: 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yawn BP, Saddier P, Wollan PC, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007; 82: 1341–1349. [DOI] [PubMed] [Google Scholar]
- 9. Johnson RW, Alvarez-Pasquin MJ, Bijl M, et al. Herpes zoster epidemiology, management, and disease and economic burden in Europe: a multidisciplinary perspective. Ther Adv Vaccines 2015; 3: 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016; 375: 1019–1032. [DOI] [PubMed] [Google Scholar]
- 11. Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372: 2087–2096. [DOI] [PubMed] [Google Scholar]
- 12. Bastidas A, de la Serna J, El Idrissi M, et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA 2019; 322: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. GlaxoSmithKline. Clinical study report for study 115523 (ZOSTER-002), https://www.gsk-studyregister.com/en/trial-details/?id=115523 (2018, accessed 23 June 2020).
- 14. Berkowitz EM, Moyle G, Stellbrink HJ, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis 2015; 211: 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. GlaxoSmithKline. Clinical study report for study 112673 (ZOSTER-015), https://www.gsk-studyregister.com/en/trial-details/?id=112673 (2013, accessed 5 November 2020).
- 16. Vink P, Delgado Mingorance I, Maximiano Alonso C, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: a randomized trial. Cancer 2019; 125: 1301–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis 2019; 19: 988–1000. [DOI] [PubMed] [Google Scholar]
- 18. GlaxoSmithKline. Clinical study report for study: 116428 (ZOSTER-039), https://www.gsk-studyregister.com/en/trial-details/?id=116428 (2018, accessed 23 June 2020).
- 19. Vink P, Ramon Torrell JM, Sanchez Fructuoso A, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in chronically immunosuppressed adults following renal transplant: a phase 3, randomized clinical trial. Clin Infect Dis 2020; 70: 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tavares Da Silva F, De Keyser F, Lambert PH, et al. Optimal approaches to data collection and analysis of potential immune mediated disorders in clinical trials of new vaccines. Vaccine 2013; 31: 1870–1876. [DOI] [PubMed] [Google Scholar]
- 21. López-Fauqued M, Campora L, Delannois F, et al. Safety profile of the adjuvanted recombinant zoster vaccine: pooled analysis of two large randomised phase 3 trials. Vaccine 2019; 37: 2482–2493. [DOI] [PubMed] [Google Scholar]
- 22. GlaxoSmithKline Biologicals. Briefing document. SHINGRIX (zoster vaccine recombinant, adjuvanted). Vaccines and Related Biological Products Advisory Committee, https://www.fda.gov/media/107553/download (2017, accessed 5 July 2020).
- 23. GlaxoSmithKline. Solicited adverse events of herpes zoster subunit vaccine in adults > 50 years of age by age, gender, race and ethnicity. Data on File 2017N342761_00, 2019. [Google Scholar]
- 24. Hervé C, Laupèze B, Del Giudice G, et al. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines 2019; 4: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Colindres R, Wascotte V, Brecx A, et al. Post hoc analysis of reactogenicity trends between dose 1 and dose 2 of the adjuvanted recombinant zoster vaccine in two parallel randomized trials. Hum Vaccin Immunother 2020; 16: 2628–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim JH, Diaz-Decaro J, Jiang N, et al. The adjuvanted recombinant zoster vaccine is efficacious and safe in Asian adults ⩾ 50 years of age: a sub-cohort analysis of the ZOE-50 and ZOE-70 randomized trials. Hum Vaccin Immunother 2021; 17: 2050–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mao J, McPheeters JT, Zhang D, et al. Herpes zoster incidence and cost in patients receiving autologous hematopoietic stem-cell transplant. Curr Med Res Opin 2018; 34: 741–749. [DOI] [PubMed] [Google Scholar]
- 28. Pergam SA, Forsberg CW, Boeckh MJ, et al. Herpes zoster incidence in a multicenter cohort of solid organ transplant recipients. Transpl Infect Dis 2011; 13: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blank LJ, Polydefkis MJ, Moore RD, et al. Herpes zoster among persons living with HIV in the current antiretroviral therapy era. J Acquir Immune Defic Syndr 2012; 61: 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grabar S, Tattevin P, Selinger-Leneman H, et al. Incidence of herpes zoster in HIV-infected adults in the combined antiretroviral therapy era: results from the FHDH-ANRS CO4 cohort. Clin Infect Dis 2015; 60: 1269–1277. [DOI] [PubMed] [Google Scholar]
- 31. Tavares-Da-Silva F, Co MM, Dessart C, et al. Review of the initial post-marketing safety surveillance for the recombinant zoster vaccine. Vaccine 2020; 38: 3489–3500. [DOI] [PubMed] [Google Scholar]
- 32. Shimabukuro TT, Miller ER, Strikas RA, et al. Notes from the field: vaccine administration errors involving recombinant zoster vaccine – United States, 2017-2018. Morb Mortal Wkly Rep 2018; 67: 585–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Food and Drug Administration. SHINGRIX (zoster vaccine recombinant, adjuvanted), suspension for intramuscular injection. Prescribing information, https://www.fda.gov/media/108597/download (2021, accessed 20 July 2020).
- 34. Hesse EM, Shimabukuro TT, Su JR, et al. Postlicensure safety surveillance of recombinant zoster vaccine (Shingrix) – United States, October 2017-June 2018. Morb Mortal Wkly Rep 2019; 68: 91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Food and Drug Administration. Risk of Guillain-Barre syndrome (GBS) following recombinant Zoster Vaccine (RZV). In: Advisory Committee on Immunization Practices meeting, 24–25 February 2021, https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/24-25/02-Zoster-Vaccines-Forshee.pdf (accessed April 2021).
- 36. Food and Drug Administration. FDA requires a warning about Guillain-Barré Syndrome (GBS) be included in the prescribing information for Shingrix. FDA Safety Communication – March 24, 2021, https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-requires-warning-about-guillain-barre-syndrome-gbs-be-included-prescribing-information-shingrix (2021, accessed April 2021).
- 37. Stassijns J, Bollaerts K, Baay M, et al. A systematic review and meta-analysis on the safety of newly adjuvanted vaccines among children. Vaccine 2016; 34: 714–722. [DOI] [PubMed] [Google Scholar]
- 38. Schmader KE, Levin MJ, Chen M, et al. Impact of reactogenicity after two doses of recombinant zoster vaccine upon physical functioning and quality of life: an open phase III trial in older adults. J Gerontol A Biol Sci Med Sci 2021; 76: 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schmader KE, Levin MJ, Grupping K, et al. The impact of reactogenicity after the first dose of recombinant zoster vaccine on the physical functioning and quality of life of older adults: an open-label, phase III trial. J Gerontol A Biol Sci Med Sci 2019; 74: 1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pellissier JM, Brisson M, Levin MJ. Evaluation of the cost-effectiveness in the United States of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Vaccine 2007; 25: 8326–8337. [DOI] [PubMed] [Google Scholar]
- 41. Moore L, Remy V, Martin M, et al. A health economic model for evaluating a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in the UK. Cost Eff Resour Alloc 2010; 8: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tav-10.1177_25151355211057479 for Safety and reactogenicity of the adjuvanted recombinant zoster vaccine: experience from clinical trials and post-marketing surveillance by Joseph Fiore, Maribel Miranda Co-van der Mee, Andrés Maldonado, Lisa Glasser and Phil Watson in Therapeutic Advances in Vaccines and Immunotherapy