Abstract
A new method was developed to identify and differentiate varicella-zoster virus (VZV) wild-type strains from the attenuated varicella Oka vaccine strain. The PCR technique was used to amplify a VZV open reading frame (ORF) 62 region. A single specific amplicon of 268 bp was obtained from 71 VZV clinical isolates and several laboratory strains. Subsequent digestion of the VZV ORF 62 amplicons with SmaI enabled accurate strain differentiation (three SmaI sites were present in amplicons of vaccine strain VZV, compared with two enzyme cleavage sites for all other VZV strains tested). This method accurately differentiated the Oka vaccine strain from wild-type VZV strains circulating in countries representing all six populated continents. Moreover, the assay more reliably distinguished wild-type Japanese strains from the vaccine strain than did previously described methods.
Varicella-zoster virus (VZV) is the etiologic agent of varicella (chicken pox), which usually occurs in children, and zoster (shingles), which results from the reactivation of a latent VZV infection. While VZV infections are usually mild, they sometimes result in severe disease, particularly in immunocompromised patients (5, 6, 11, 22). A live attenuated varicella vaccine (Oka strain), which confers protection in a high percentage of recipients (2, 7, 11, 23, 29), was licensed and recommended for use in the United States in 1995 (27).
Breakthrough varicella infections after exposure to wild-type VZV have occasionally been noted among vaccinees (3, 9, 24, 28, 29), and Oka vaccine may cause zoster in as many as 6% of immunocompromised vaccinees (9, 12). To monitor potential VZV vaccine-related complications, a technique that discriminates vaccine strain from wild-type VZV is required.
In the past, identification of VZV strains was based on laborious restriction fragment length polymorphism (RFLP) analysis of preparations of viral DNA (15, 25), a method that also required successful culturing of VZV from lesions. Newer PCR methods have eliminated the need to propagate virus for VZV detection (18, 19, 21, 30). In the United States and Australia, wild-type and vaccine strains have been effectively distinguished on the basis of the presence or absence of BglI or PstI sites in amplicons from VZV open reading frames (ORFs) 54 and 38, respectively (18, 19), although this technique fails to distinguish some Japanese wild-type strains (16).
More extensive genotyping, such as amplification analysis of polymorphic repeat regions R5 and R2, was required to distinguish Oka vaccine VZV from Japanese strains (20, 26), a technique that also fails to identify some strains in Japan and the United Kingdom (13, 14, 26).
Argaw et al. identified a sequence variation in VZV ORF 62 between the Oka vaccine strain and the Oka parental strain and used these data as the basis for a PCR-RFLP test (1). In this study we examined various clinical samples and confirmed the ability of this PCR-RFLP assay to differentiate vaccine strain from isolates obtained from patients.
MATERIALS AND METHODS
Viruses, DNA preparation, sequencing.
VZV isolates (excluding those provided by authors of this report) were kindly provided by John Zaia (City of Hope Hospital, Los Angeles, Calif.), Barbara Watson (Philadelphia Department of Public Health), Ann Arvin (Stanford University, Palo Alto, Calif.), Dominic Dwyer (Westmead Hospital, Sydney, Australia), and John Stewart and Joseph J. Esposito (Centers for Disease Control and Prevention, Atlanta, Ga.). Material from 71 specimens was available for testing. Isolates from various geographic locations, including Japan (25 specimens), the United States (26 specimens), Australia (9 specimens), Chad (5 specimens), Congo (5 specimens), Chile (2 specimens), Czech Republic (1 specimen), and France (1 specimen) were collected between 1976 and 1999. VZV DNA samples obtained from cells infected with the Oka vaccine strain and three laboratory VZV strains (Webster, vzv11, and ROD) were also examined. Thirty-nine of the clinical specimens came through general practitioners and infectious disease physicians. Fifty-six preparations of the viruses were isolates, and the remaining 15 were primary virus typed directly from vesicular fluid air dried onto glass slides, cotton swabs, or skin scab lesions. DNA was prepared from vesicular fluid, varicella scabs, and lysates of VZV-infected cells using NucleoSpin Tissue Kits (CLONTECH Laboratories Inc., Palo Alto, Calif.).
Sequencing.
The nucleotide sequences of selected amplicons were sequenced with an ABI Prism dye terminator cycle sequencing kit (Applied Biosystems, Foster City, Calif.) according to the manufacturer's instructions to verify their identity as VZV sequence. Sequences were compared with the VZV ORF 62 sequences of the VZV Dumas strain (GenBank accession number X04370), which were used to design the PCR primers. The Genetics Computer Group (Madison, Wis.) package, DNASIS 2.1 (Hitachi Software, San Bruno, Calif.), and the OLIGO 5 program (National Biosciences, Inc., Plymouth, Minn.) were used for computer analysis of nucleotide sequences.
Evaluation of ORF 62 primers.
The experimental primer sequences used for these studies are described in Table 1. Initial testing of the amplification conditions for each primer set was performed using a standard protocol. Template DNA was prepared from HLF cells infected with VZV strain Webster or from uninfected cells (negative control). PCR assays were completed in a volume of 100 μl of a solution that contained 500 ng of template DNA; 50 mM KCl; 10 mM Tris hydrochloride, pH 8.3; 5 mM MgCl2; a 200 μM concentration (each) of dATP, dCTP, dGTP, and dTTP; a 250 μM concentration of each primer; and 2.5 U of Taq DNA polymerase (PCR Core kit [Boehringer Mannheim Biochemicals, Indianapolis, Ind.] or GeneAmp PCR reagent kit with AmpliTaq or AmpliTaq Gold DNA polymerase [Perkin-Elmer Cetus, Norwalk, Conn.]). Reaction mixtures were passed through 25 cycles of denaturation at 94°C for 1 min, a 1-min annealing step at a gradient of temperatures (55 to 72°C), and a polymerization at 72°C for 1 min, followed by final extension at 72°C for 5 min (Mastercycler gradient; Eppendorf Scientific Inc., Westbury, Conn.). Reaction mixtures for hot-start PCR using AmpliTaq Gold polymerase were incubated for 15 min at 96°C before the start of cycling. To control for contamination, each primer pair in PCR cocktails was run using the above cycling protocol in the absence of DNA template, with an annealing temperature of 60°C.
TABLE 1.
ORF 62 VZV PCR primers
| Primer | Primer sequence (5′ to 3′) | GC content (%) | Tm (°C)a |
|---|---|---|---|
| PKVL6U | TTC CCA CCG CGG CAC AAA CA | 60.0 | 64.0 |
| PKVL7U | AAC TCG CTG GCC CAA AGG TG | 60.0 | 64.0 |
| PKVL1L | GGT TGC TGG TGT TGG ACG CG | 65.0 | 66.0 |
| PKVL2L | GTG TCC GCT TTG AAC GCC CG | 65.0 | 66.0 |
| PKVL3L | TGG TCC TGG CAG CCC TGA GT | 65.0 | 66.0 |
| PKVL4L | GTC CTG GCA GCC CTG AGT AA | 60.0 | 64.0 |
| PKVL5L | GTG GTC GTG GCA GCC CTG AG | 70.0 | 68.0 |
| PHKR1b | AGG TTG GCA AAC GCA GTC | 55.6 | 56.0 |
| PHKR2b | ATT ACT GTC GAC CCG AGA CC | 55.0 | 62.0 |
| PKVL6L | TGG TCC TGG CAG CCC TGA GTA ACC GG | 65.4 | 86.0 |
Tm, annealing temperature of the primers calculated by the nearest-neighbor method using the OLIGO 5 primer analysis software (Molecular Biology Insights, Inc., Plymouth, Minn.).
Previously published by Argaw et al. (1).
PCR assays.
Detection of VZV genome variations in ORFs 38 and 54 was performed using the method described by LaRussa et al. (18). Detection of VZV genome variations in ORF 62 was performed as follows. Reaction mixtures included a 0.1 μM concentration of each oligonucleotide of upper (PKVL_6U [VZV genome position 106036]) and lower (PKVL_1L [VZV genome position 106284]) primers, which are complementary to a variable region of VZV ORF 62, in 100 μl of reaction mixture containing PCR Gold buffer (50 mM KCl; 15 mM Tris-hydrochloride, pH 8.0); 2.5 mM MgCl2; a 200 μM concentration (each) of dATP, dCTP, dGTP, and dTTP; and 2.5 U of AmpliTaq Gold DNA polymerase (PE Biosystems, Foster City, Calif.; Roche Molecular Biochemicals, Indianapolis, Ind.). For amplification, 500 ng of total DNA, prepared from VZV-infected cells using Nucleospin tissue kits, was used as a template. For clinical samples, PCR assays used a 1/100 aliquot of the DNA purified from a single lesion (scab or swab). An initial PCR hot-start step of 96°C for 15 min was followed by 30 cycles of amplification (1 min at 94°C, 1 min at 72°C) and a final extension step at 72°C for 3 min (Mastercycler gradient, Eppendorf Scientific Inc.).
For detection, 10 μl of PCR product was loaded onto precasted 4-to-25% gradient polyacrylamide gels in Tris-borate-EDTA (TBE) buffer (Novex, San Diego, Calif.) and run at 150 V for 1 h. Gels were stained with ethidium bromide to visualize DNA (0.5 μg/ml in TBE buffer, 15 min). Restriction reactions were performed using 5 to 10 μl of the PCR product adjusted to recommended endonuclease buffer and 10 U of SmaI, BglI, or PstI (New England Biolabs, Inc., Beverly, Mass.). Endonuclease-cleaved DNA products were separated by gel electrophoresis as described above. The 50- and 100-bp DNA ladders (GIBCO BRL, Gaithersburg, Md.) were used as DNA size markers.
RESULTS
ORF 62 region primer design and evaluation.
A substitution of C for T in position 106262 (correspondent reference Dumas strain genome position denoted [4]) of the Oka vaccine genome compared with Oka parent strain DNA was recently identified (1). This substitution established an additional SmaI site in the Oka vaccine DNA and provided the basis for developing a new RFLP-PCR test for differentiating the VZV vaccine strain from wild-type strains. Oligonucleotide primers were designed to amplify a region of the VZV genome that codes for the C-terminal portion of the putative ORF 62 protein, approximately 200 nucleotides upstream and downstream of the mutation in position 106262.
Based on our experience with PCR, the most effective amplicon molecular size should be limited to between 250 and 350 bp in length. Amplicons within that size range usually provide optimal sensitivity for an assay, particularly for DNA amplification from clinical samples that may contain limited quantities of template.
As such, we designed several primer sets and assessed their performance on both clinical specimens and laboratory stocks of VZV (Table 1). The G+C content, melting temperature, and length of the primers were chosen and analyzed using Oligo 5 primer design software to ensure they met the essential criteria for optimal PCR primers. In addition to the primer pair described previously (PHKR1 and PHKR2 [Table 1]), eight additional primers were designed (three upstream and five downstream of the mutation); in all, eight 20-mers, one 18-mer, and one 26-mer were assessed; the G+C content of the primers was between 55 and 70%. All of the primers were also analyzed by using OLIGO 5 software for the formation of dimers either within or between pairs; no significant theoretical misprinting was identified on any template.
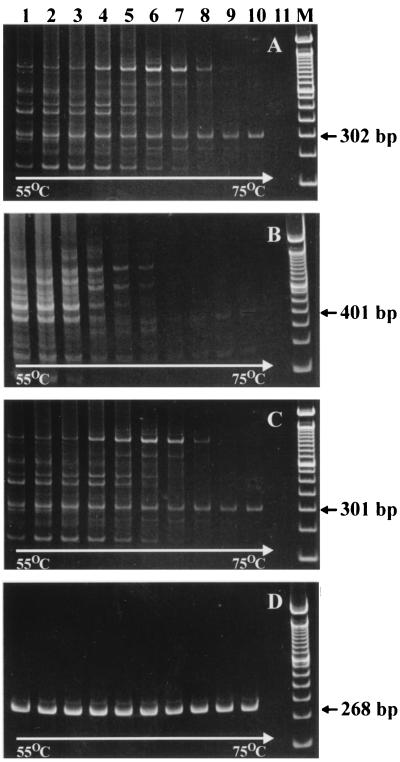
Twenty-one primer combinations were tested altogether, representing each of the three upper primers with each of seven lower primers. Representative results from temperature gradient PCR (55 to 75°C) for four of these experimental primer sets are presented in Fig. 1. Most of the primer pairs amplified a significant number of nonspecific reaction products (e.g., Fig. 1A to C). This was true regardless of whether a high concentration of DNA template (as with laboratory strains and the VZV vaccine strain) isolated from tissue culture or a low concentration from clinical samples was used (data not shown). On this basis, eight of the experimental primers were excluded from further analysis. The primer combination of PKVL6U-PKVL1L provided the best yield of specific product (on the basis of gel band intensity), produced the least amount of nonspecific amplification product, and performed well over a broad range of annealing temperatures (Fig. 1D). The last attribute makes this primer pair more versatile and will permit considerable flexibility in the selection of annealing temperature for VZV-specific PCR if a protocol demands it. For example, we were able to modify the original protocol, eliminating a 55°C annealing step, since at 72°C primer annealing and polymerase enzyme reaction take place with this primer set. Furthermore, the reaction products resulting from PCR using the primer pair PKVL6U-PKVL1L during SmaI RFLP analysis could be easily differentiated by gel electrophoresis. The 268-bp amplicon generated with this primer pair was predicted to produce 153-, 79-, and 36-bp (Oka parent and wild-type strains) or 112-, 79-, 41-, and 36-bp (Oka vaccine strain) SmaI fragment sets. As shown in Fig. 2, SmaI fragments of Oka vaccine strain amplicon can be clearly differentiated from DNA patterns obtained after SmaI cleavage of wild-type amplicons.
FIG. 1.
Representative results for experimental ORF 62 primer pairs. Amplification products were produced with gradient annealing temperature cycling (ranging from 55 to 75°C) with the following primer pairs: PHKR1-PHKR2 (A), PHKR1-PKVL4L (B), PKVL7U-PKVL2L (C), and PKVL6U-PKVL1L (D). Apart from the controlled variation in annealing temperature, all reactions were carried out under identical conditions. Lane 11 is the negative control for all four gels (template DNA prepared from uninfected HLF cells). Lanes M contain a molecular size marker set (100 to 1,500 bp in 100-bp multiples).
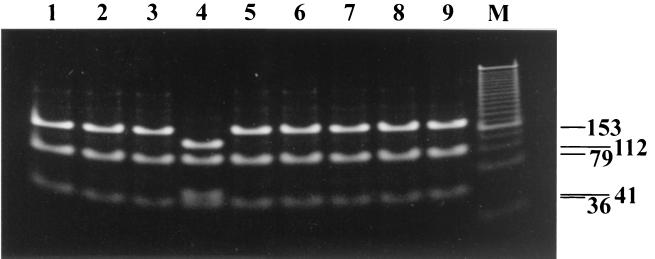
FIG. 2.
Comparative RFLP test results for wild-type and Oka vaccine strain VZV using amplicons generated with the PKVL6U-PKVL1L primer pair. Shown are results for the SmaI RFLP assay for VZV ORF 62 amplicons obtained with wild-type viruses (lanes 1 to 3 and 5 to 9 correspond to samples 44 to 46 and 48 to 52 in Table 1) and Oka vaccine strain (lane 4). Lane M, molecular size marker set (100 to 1,500 bp in 100-bp multiples).
Biochemical optimization of the amplification conditions for this primer set was performed, and final concentrations of 0.1 μM for primers were found to be optimal for the specific amplicon generation (data not shown). Additional modifications of the PCR protocol included independently varying the concentrations of Taq polymerase and MgCl2 in the reaction mixture. Increases in the Taq enzyme activity did not significantly affect the yield of specific product (data not shown). Adjustment of the reaction mixture pH to below 8.0 substantially decreased the sensitivity of detection (data not shown).
We also examined hot-start PCR methodology, including the use of Taq-start antibodies (CLONTECH), Ampli-Taq Gold (Roche), or Platinum Taq (Life Technology). Significant improvement in sensitivity and specificity was seen with all of these hot-start methods, and any of three chemical hot starts were incorporated into the VZV vaccine strain differentiation method described here. Mechanical methods of hot start were deemed impractical using this method, since the assay was designed for use with large numbers of clinical samples.
Sensitivity and specificity of the ORF 62 PCR method.
The primer set PKVL6U-PKVL1L was tested on a panel of VZV-positive and VZV-negative specimens. All VZV-positive samples generated a single specific amplicon 268 bp in size (Fig. 2). The product specificity of the 10 selected amplicons obtained from the PCR was also confirmed by sequence analysis (data not shown). There was no detectable PCR product after nucleic acid extraction and PCR amplification from tissue culture material containing the following human herpesviruses (HHVs): Epstein-Barr virus, cytomegalovirus, herpes simplex virus type 1 (HSV-1) and HSV-2, and human herpesvirus (HHV) 6a, 6b, and 8. In addition, 20 human clinical (swabs and scabs) specimens that were negative both by virological tests and by independent PCR assays for VZV DNA were tested to assess specificity (data not shown). No amplicons were detected in PCR assays using these specimens. On further examination of the primers, we observed no product after PCR amplification with DNA of herpesvirus genome samples as well as DNA isolated from human, monkey, rabbit, mouse, rat, and Escherichia coli (data not shown). These results indicate that the PKVL6U-PKVL1L assay primers are highly specific for VZV.
The lower limit of detection by this method was defined as the smallest amount of DNA in a sample that produced detectable amplicon product (ethidium bromide staining in agarose or polyacrylamide gels) following 30 cycles of PCR. Working from serial dilutions of a preparation of VZV DNA of known concentration, we determined that the ORF 62 primer pair PKVL6U-PKVL1L is able to detect approximately 100 pg of DNA in a specimen. We determined that these primers detected VZV DNA in every specimen from a panel of scab and vesicle fluid clinical samples (12 specimens), even when as little as 1/50 of the DNA preparation was used for the PCR.
PCR analysis of collected VZV DNA specimens.
Seventy-one DNA preparations from cases of chickenpox and zoster were typed by the LaRussa et al. method (ORF 54-ORF 38) (18) and by the ORF 62 method described here. For the ORF 54-ORF 38 method, 222-bp and 350-bp amplicons were produced and digested with PstI and BglI restriction enzymes, respectively (results shown in Table 2). Three of four possible genotypes were detected: 32 specimens were identified as wild-type PstI+ BglI− (i.e., possessing and lacking a PstI and a BglI restriction site, respectively, and 34 specimens were identified as wild-type BglI+ PstI+. Additionally, nine DNAs were typed as Oka vaccine strain (BglI+ PstI−), among which only two specimens, our Oka vaccine virus control specimen and one U.S. case isolate obtained from a child after vaccination, are considered genuine Oka vaccine specimens. The other seven viruses detected as Oka vaccine strain by this method were wild-type viruses isolated from lesions of varicella and zoster patients in Japan. The fourth possible genotype (BglI− PstI−) was not identified in this study.
TABLE 2.
Differential genotyping of Oka vaccine strain and wild-type VZV strains
| Strain(s) and/or isolate(s) | Lesion type | Origin | Restriction enzyme site in:
|
||
|---|---|---|---|---|---|
| ORF 38 | ORF 54 | ORF 62 | |||
| Oka vaccine | Laboratory strain | Merck (VARIVAX)b | PstI− | BglI+ | SmaI+ |
| Webster, vzv 11 | Laboratory strain | CDCc | PstI+ | BglI− | SmaI− |
| MT9, MT202, MT273, MT430, MT813 | Zoster | Japan | PstI+ | BglI+ | SmaI− |
| MT10, MT132, MT160, MT302, MT378, MT435 | Varicella | Japana | PstI− | BglI+ | SmaI− |
| MT202 | Zoster | Japana | PstI− | BglI+ | SmaI− |
| MT124, MT135, MT227, MT257, MT362, MT363, MT365, MT437, MT439, MT810, MT817, MT821, MT858, MT868 | Varicella | Japan | PstI+ | BglI+ | SmaI− |
| N1, N2, N3, N5, N6, N7, N8, N9, N11, N12, N13, N15, N16, N17, 98-sw-01, 99-I-6, 64N, 123J, 509N, 864N, 868N, NICKOLAY, ROD | Varicella | United States | PstI+ | BglI− | SmaI− |
| N10, 454L, 98-scr-3 | Varicella | United States | PstI+ | BglI+ | SmaI− |
| N4 | Varicella | United Statesb | PstI− | BglI+ | SmaI+ |
| 98-v-02 (DR) | Zoster | France | PstI+ | BglI+ | SmaI− |
| 00-I-023 | Varicella | Czech Republic | PstI+ | BglI− | SmaI− |
| 00-I-6 | Varicella | Chile | PstI+ | BglI− | SmaI− |
| 00-I-17 | Varicella | Chile | PstI+ | BglI+ | SmaI− |
| 98-I-013, 98-I-014, 98-I-016, 98-I-025, 98-I-026 | Varicella | Congo | PstI+ | BglI+ | SmaI− |
| Chad1, Chad2, Chad3, Chad4, Chad5 | Varicella | Chad | PstI+ | BglI+ | SmaI− |
| A2 | Zoster | Australia | PstI+ | BglI− | SmaI− |
| A4, A5, A6, A7, A8, A9, A11, A16 | Varicella | Australia | PstI+ | BglI− | SmaI− |
Determined as vaccine strain by PstI/BglI digestion.
Oka vaccine genotype by both methods.
CDC, Centers for Disease Control and Prevention.
Analysis of the specimen set by using the ORF 62 method produced identical results to the ORF 54-ORF 38 method with one exception: the seven Japanese clinical isolates that were identified as the Oka vaccine strain by the ORF 54-ORF 38 method were identified as wild-type isolates by the ORF 62 method. These data are shown in Table 2. All of the amplifications produced the expected 268-bp amplicon, which was digested into 112-, 79-, 41-, and 36-bp SmaI fragments for Oka vaccine control strain DNA and for the U.S. isolate from a vaccinated child or into 153-, 79-, and 36-bp SmaI fragments for the 73 remaining DNA samples tested. As such, this method efficiently detects VZV. More importantly, the ORF 62 method was better able to differentiate the Oka vaccine strain from Japanese wild-type strains.
DISCUSSION
Several PCR methods that can detect and differentiate Oka-vaccine strain from wild-type strains have been described previously (18, 20, 26). This approach has proven to be rapid and is particularly useful as a diagnostic tool for the confirmation of atypical cases of varicella and zoster. It is also useful for the detection of VZV in archival clinical specimens, from which viable VZV is unlikely to be isolated. The most widely used clinical PCR method for discriminating VZV Oka vaccine DNA from wild-type virus is based on PCR-RFLP analysis targeting BglI and PstI sites in amplicons from VZV ORFs 54 and 38, respectively (18). In these studies, we confirmed that most of the non-Japanese VZV wild-type strains can be distinguished from the Oka vaccine strain by using the PstI marker in ORF 38. However, as noted previously (13, 14, 26) the application of this method for Japanese strains and probably some other Asian regions has been limited due to the circulation of strains related to Oka that cannot be distinguished from the Oka strain by using the ORF 38 marker. In the present study, seven wild-type Japanese strains with Oka-like genotypes were found.
The development of PCR methods for VZV strain differentiation has been hampered by the fact that the VZV genome is highly conserved and due to our limited information about the primary DNA sequence of the Oka vaccine strain. Argaw et al. (1) identified a mutation in ORF 62 of the Oka vaccine strain that is absent in the parental isolate from which it was derived, and this site has proven valuable for diagnostic purposes. This mutation, which introduces a new SmaI restriction site into the Oka vaccine strain, formed the basis for the development of the diagnostic test described here.
An additional advantage to the ORF 62 method is that strain discrimination can be accomplished using a single DNA amplification produced from one primer pair and a single restriction enzyme digestion. Thus, the method also requires half the cost, labor, and time of the ORF 54-ORF 38 method. Amplification with the PKVL6U-PKVL1L primer pair results in a PCR product that unambiguously indicates the presence of VZV DNA in test specimens. Subsequent digestion of this 268-bp amplicon with SmaI provides reliable differentiation of VZV Oka vaccine strain and wild-type strains.
Most importantly, the results of this study indicate that the ORF 62-based PCR method distinguishes even closely related wild-type clinical isolates of VZV from the Oka vaccine strain. One valuable benefit of the ORF 62 RFLP assay is that several SmaI sites are present in the targeted amplicon, which helps to monitor restriction enzyme activity during the assay.
The original ORF 62 primers we selected to perform this assay quite effectively amplified VZV DNA from specimens, but they also tended to produce a number of nonspecific reaction products. This was also true of most of the ORF 62 experimental primer pairs we examined in this study. While some of the primers described here may prove useful for alternative diagnostic applications, such as automated DNA or RNA hybridization techniques, the PKVL6U-PKVL1L primer combination clearly outperformed all others tested for RFLP analysis. This primer set generated no detectable nonspecific PCR product in VZV-positive specimens across a broad range of annealing temperatures and produced no detectable PCR product from DNA samples from closely related viruses, including HSV1, HSV2, HHV6a, HHV6b, HHV8, CMV, and EBV. Furthermore, a search of GenBank and EMBL nucleotide sequence databases, querying with the primer sequences described here, identified significant matches only with VZV ORF 62 DNA sequences.
The ORF 62-based PCR method described here successfully verified the presence of VZV both in purified virus DNA from laboratory strains and in a large number of clinical specimens isolated from countries encompassing six continents. Admittedly, we have thus far examined only small numbers of clinical isolates from countries that may or may not reflect VZV strains that are circulating throughout the continent. Nonetheless, testing of additional clinical specimens should help to strengthen the validity of this approach, particularly in specimens from countries where Oka-like strains may still be circulating. Protocols using this approach for diagnosing suspected chickenpox and zoster in clinical samples should be coupled with PCR, using primers specific for beta-globin gene DNA or other cellular markers to confirm that amplification conditions are optimal, thus minimizing false-negative results (8).
The ORF 62-based PCR-RFLP protocol described here should be readily adaptable for use in a variety of laboratories, including hospital facilities with PCR and gel electrophoresis capabilities. The present study extends the usefulness of PCR techniques as a diagnostic method for the detection and differentiation of VZV DNA in clinical specimens.
ACKNOWLEDGMENTS
We thank the following individuals for providing VZV specimens for this study: Ann Arvin, John Zaia, Barbara Watson, Dominic Dwyer, John Stewart, and Joseph J. Esposito. We also thank William C. Reeves, Philip E. Pellett, and Naoki Inoue for valuable intellectual discussions during the completion of this study. Finally, we thank John O'Connor for assistance in editing the manuscript.
REFERENCES
- 1.Argaw T, Cohen J I, Klutch M, Lekstrom K, Yoshikawa T, Asano Y, Krause P R. Nucleotide sequences that distinguish Oka vaccine from parental Oka and other varicella-zoster virus isolates. J Infect Dis. 2000;181:1153–1157. doi: 10.1086/315335. [DOI] [PubMed] [Google Scholar]
- 2.Arvin A M, Gershon A A. Live attenuated varicella vaccine. Annu Rev Microbiol. 1996;50:59–100. doi: 10.1146/annurev.micro.50.1.59. [DOI] [PubMed] [Google Scholar]
- 3.Asano Y, Suga S, Yoshikawa T, Kobayashi I, Yazaki T, Shibata M, Tsuzuki K, Ito S. Experience and reason: twenty-year follow-up of protective immunity of the Oka strain live varicella vaccine. Pediatrics. 1994;94:524–526. [PubMed] [Google Scholar]
- 4.Davison A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 5.Devine S M, Wingard J R. Viral infections in severely immunocompromised cancer patients. Support Care Cancer. 1994;2:355–368. doi: 10.1007/BF00344048. [DOI] [PubMed] [Google Scholar]
- 6.Gatnash A A, Connolly C K. Fatal chickenpox pneumonia in an asthmatic patient on oral steroids and methotrexate. Thorax. 1995;50:422–423. doi: 10.1136/thx.50.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gershon A A. Varicella-zoster virus: prospects for control. Adv Pediatr Infect Dis. 1995;10:93–124. [PubMed] [Google Scholar]
- 8.Gershon A A, Forghani B. Varicella-zoster virus. In: Lennette E H, Lennette D A, Lennette E T, editors. Diagnostic procedures for viral, rickettsial, and chlamydial infections. 7th ed. New York, N.Y: Marcel Dekker; 1995. pp. 601–613. [Google Scholar]
- 9.Gershon A A, LaRussa P, Hardy I, Steinberg S, Silverstein S. Varicella vaccine: the American experience. J Infect Dis. 1992;166(Suppl. 1):S63–S68. doi: 10.1093/infdis/166.supplement_1.s63. [DOI] [PubMed] [Google Scholar]
- 10.Gershon A A, Steinberg S, Gelb L, Galasso G, Borkowsky W, LaRussa P, Ferrara A. A multicentre trial of live attenuated varicella vaccine in children with leukaemia in remission. Postgrad Med J. 1985;61(Suppl. 4):73–78. [PubMed] [Google Scholar]
- 11.Gershon A A, Steinberg S P, LaRussa P, Ferrara A, Hammerschlag M, Gelb L. Immunization of healthy adults with live attenuated varicella vaccine. J Infect Dis. 1988;158:132–137. doi: 10.1093/infdis/158.1.132. [DOI] [PubMed] [Google Scholar]
- 12.Hardy I B, Gershon A, Steinberg S, LaRussa P. The incidence of zoster after immunization with live attenuated varicella vaccine: a study in children with leukemia. N Engl J Med. 1991;325:1545–1550. doi: 10.1056/NEJM199111283252204. [DOI] [PubMed] [Google Scholar]
- 13.Hawrami K, Breuer J. Analysis of United Kingdom wild-type strains of varicella-zoster virus: differentiation from the Oka vaccine strain. J Med Virol. 1997;53:60–62. doi: 10.1002/(sici)1096-9071(199709)53:1<60::aid-jmv10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Hawrami K, Hart L J, Pereira F, Argent S, Bannister B, Bovill B, Carrington D, Ogilvie M, Rawstorne S, Tryhorn Y, Breuer J. Molecular epidemiology of varicella-zoster virus in East London, England, between 1971 and 1995. J Clin Microbiol. 1997;35:2807–2809. doi: 10.1128/jcm.35.11.2807-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayakawa Y, Yamamoto T, Yamanishi K, Takahashi M. Analysis of varicella-zoster virus DNAs of clinical isolates by endonuclease HpaI. J Gen Virol. 1986;67:1817–1829. doi: 10.1099/0022-1317-67-9-1817. [DOI] [PubMed] [Google Scholar]
- 16.Hondo R, Yogo Y, Yoshida M, Fujima A, Itoh S. Distribution of varicella-zoster virus strains carrying a Pst-site-less mutation in Japan and DNA change responsible for the mutation. Jpn J Exp Med. 1989;59:233–237. [PubMed] [Google Scholar]
- 17.Kuter B J, Weibel R E, Guess H A, Matthews H, Morton D H, Neff B J, Provost P J, Watson B A, Starr S E, Plotkin S A. Oka/Merck varicella vaccine in healthy children: final report of a 2-year efficacy study and 7-year follow-up studies. Vaccine. 1991;9:643–647. doi: 10.1016/0264-410x(91)90189-d. [DOI] [PubMed] [Google Scholar]
- 18.LaRussa P, Lungu O, Hardy I, Gershon A, Steinberg S P, Silverstein S. Restriction fragment length polymorphism of polymerase chain reaction products from vaccine and wild-type varicella-zoster virus isolates. J Virol. 1992;66:1016–1020. doi: 10.1128/jvi.66.2.1016-1020.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaRussa P, Steinberg S, Arvin A, Dwyer D, Burgess M, Menegus M, Rekrut K, Yamanishi K, Gershon A. Polymerase chain reaction and restriction fragment length polymorphism analysis of varicella-zoster virus isolates from the United States and other parts of the world. J Infect Dis. 1998;178:S64–S66. doi: 10.1086/514267. [DOI] [PubMed] [Google Scholar]
- 20.Mori C, Takahara R, Toriyama T, Nagai T, Takahashi M, Yamanishi K. Identification of the Oka strain of the live attenuated varicella vaccine from other clinical isolates by molecular epidemiologic analysis. J Infect Dis. 1998;178:35–38. doi: 10.1086/515598. [DOI] [PubMed] [Google Scholar]
- 21.Nahass G T, Mandel M J, Cook S, Fan W, Leonardi C L. Detection of herpes simplex and varicella-zoster infection from cutaneous lesions in different clinical stages with the polymerase chain reaction. J Am Acad Dermatol. 1995;32:730–733. doi: 10.1016/0190-9622(95)91450-1. [DOI] [PubMed] [Google Scholar]
- 22.Parnham A P, Flexman J P, Saker B M, Thatcher G N. Primary varicella in adult renal transplant recipients: a report of three cases plus a review of the literature. Clin Transplant. 1995;9:115–118. [PubMed] [Google Scholar]
- 23.Plotkin S A. Varicella vaccine. Pediatrics. 1996;97:251–253. [PubMed] [Google Scholar]
- 24.Shiraki K, Horiuchi K, Asano Y, Yamanishi K, Takahashi M. Differentiation of oka varicella vaccine strain from wild varicella-zoster virus strains isolated from vaccinees and household contact. J Med Virol. 1991;33:128–132. doi: 10.1002/jmv.1890330212. [DOI] [PubMed] [Google Scholar]
- 25.Straus S E, Hay J, Smith H, Owens J. Genome differences among varicella-zoster virus isolates. J Gen Virol. 1983;64:1031–1041. doi: 10.1099/0022-1317-64-5-1031. [DOI] [PubMed] [Google Scholar]
- 26.Takada M, Suzutani T, Yoshida I, Matoba M, Azuma M. Identification of varicella-zoster virus strains by PCR analysis of three repeat elements and a PstI-site-less region. J Clin Microbiol. 1995;33:658–660. doi: 10.1128/jcm.33.3.658-660.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi M. Clinical overview of varicella vaccine: development and early studies. Pediatrics. 1986;78:736–741. [PubMed] [Google Scholar]
- 28.Takayama N, Minamitani M, Takayama M. High incidence of breakthrough varicella observed in healthy Japanese children immunized with live attenuated varicella vaccine (Oka strain) Acta Paediatr Jpn. 1997;39:663–668. doi: 10.1111/j.1442-200x.1997.tb03664.x. [DOI] [PubMed] [Google Scholar]
- 29.Watson B M, Piercy S A, Plotkin S A, Starr S E. Modified chickenpox in children immunized with the Oka/Merck varicella vaccine. Pediatrics. 1993;91:17–22. [PubMed] [Google Scholar]
- 30.Yoshida M, Tamura T, Hiruma M. Analysis of strain variation of R1 repeated structure in varicella-zoster virus DNA by polymerase chain reaction. J Med Virol. 1999;58:76–78. [PubMed] [Google Scholar]