Abstract
Background
The phase 2 PACE (Ponatinib Ph+ ALL and CML Evaluation) trial of ponatinib showed robust long-term benefit in relapsed Philadelphia chromosome-positive (Ph+) leukemia; arterial occlusive events (AOEs) occurred in ≥ 25% of patients based on investigator reporting. However, AOE rates vary depending on the definitions and reporting approach used.
Methods
To better understand clinically relevant AOEs with ponatinib, an independent cardiovascular adjudication committee reviewed 5-year AOE data from the PACE trial according to a charter-defined process and standardized event definitions.
Results
A total of 449 patients with chronic myeloid leukemia (CML) or Ph+ acute lymphoblastic leukemia (ALL) received ponatinib (median age 59 y; 47% female; 93% ≥ 2 prior tyrosine kinase inhibitors (TKIs); median follow-up, 37.3 months). The adjudicated AOE rate (17%) was lower than the non-adjudicated rate (i.e., rate before adjudication; 25%). The only adjudicated AOE in > 2% of patients was peripheral arterial occlusive disease (4%). Exposure-adjusted incidence of newly occurring adjudicated AOEs decreased over time. Patients with multiple baseline cardiovascular risk factors had higher adjudicated AOE rates than those without risk factors.
Conclusions
This independent adjudication study identified lower AOE rates than previously reported, suggesting earlier overestimation that may inaccurately reflect AOE risk with ponatinib. This trial was registered under ClinicalTrials.gov identifier NCT01207440 on September 23, 2010 (https://clinicaltrials.gov/ct2/show/NCT01207440).
Keywords: Acute lymphoblastic leukemia, Chronic myeloid leukemia, Safety, Tyrosine kinase inhibitor
Background
Ponatinib, a pan-BCR::ABL1 inhibitor, is an orally active third-generation tyrosine kinase inhibitor (TKI) designed to potently inhibit BCR::ABL1 with or without any point mutation, including BCR::ABL1T315I [1]. In the pivotal phase 2 PACE (Ponatinib Ph+ ALL and CML Evaluation) trial, ponatinib demonstrated robust clinical activity with rapid, deep, and long-term responses, progression-free survival (PFS), and overall survival in patients with chronic-phase chronic myeloid leukemia (CP-CML), ≥ 90% of whom had failed treatment with ≥ 2 TKIs, regardless of the presence or absence of BCR::ABL1 mutations, including T315I [2, 3]. The 5-year results of the PACE trial confirmed the durability of these responses with a 5-year overall survival rate of 73% for CP-CML [3]. However, arterial occlusive events (AOEs) were reported by investigators in 25% in the overall population (serious AOEs, 20%) and 31% in the CP-CML population (serious AOEs, 26%) in the 5-year follow-up [3]. The exposure-adjusted incidence of newly occurring AOEs decreased from year 1 (15.8 patients with events per 100 patient-years in the total population) to year 5 (3.9 per 100 patient-years) [3]. The incidence of AOEs associated with ponatinib use has varied widely in subsequent reports. Two retrospective studies have reported an absence or very low incidence (6%) of AOEs [4, 5]. Other real-world studies have reported AOE rates ranging from 18 to 26% [6, 7]. Multiple factors may contribute to variability in reported AOE rates, including differences in patient populations, as well as differences in the clinical definitions used to identify and categorize vascular occlusive events. One of the most important factors is the lack of a standardized approach for defining and capturing AOEs with BCR::ABL1 TKIs.
The AOE incidence rate reported for PACE was based on a list of approximately 400 Medical Dictionary for Regulatory Activities (MedDRA) preferred terms developed by the sponsor. However, differences in the preferred terms used to define AOEs led to variability in AOE incidence rates. Some preferred terms included in the AOE analysis of PACE are highly sensitive for identification of potential AOEs but may not themselves indicate the occurrence of arterial occlusions, frequently including symptoms or descriptions rather than events; these include chest pain, cold hands, dysarthria, and poor peripheral circulation. This approach to characterize AOEs based on adverse event terms results in broadly capturing non-specific symptoms that may be associated with AOE rather than true AOEs and may thus overestimate the incidence of clinically meaningful events.
A clear understanding of clinically relevant AOE risk is imperative when characterizing the benefit-risk profile of ponatinib. Patients with CP-CML who become resistant to a second-generation BCR::ABL1 TKI, either with or without a BCR::ABL1 gene mutation, generally experience low response rates and poor survival if treated with another second-generation TKI [8, 9]. Importantly, ponatinib is the only currently available TKI effective in patients with the BCR::ABL1T315I mutation [3]. Therefore, the potential for improved survival and duration of response on ponatinib may outweigh the risk of AOEs [8, 9]. However, the lack of clear data regarding clinically meaningful AOEs has led to confusion about how to optimally use ponatinib to treat relapsed/refractory CML and Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) and, in some instances, avoidance in patients who could potentially benefit. To provide a more accurate characterization of AOE incidence with ponatinib, an independent adjudication committee of experts was convened to retrospectively adjudicate all AOE reports in the PACE trial in a standardized, rigorous manner.
Methods
PACE trial design
The phase 2 PACE trial (ClinicalTrials.gov identifier: NCT01207440) enrolled adults with CML or Ph+ ALL whose disease was resistant or intolerant to dasatinib or nilotinib, or who had the BCR::ABL1T315I mutation regardless of prior TKI use [3]. All patients received ponatinib at a starting dose of 45 mg once daily (qd); dose reductions to 30 or 15 mg qd were applied per protocol (Table 1) to manage adverse events (AEs), or implemented proactively following recommendations from the sponsor in October 2013 in response to AOEs emerging as notable AEs. The trial has been completed; detailed methods are published [2, 3].
Table 1.
Dose reduction recommendations (as of 2013)
| Dose reduction recommendations |
| In October 2013, the following specific recommendations were formulated after discussions with the US FDA on evolving observations of arterial occlusive events in patients treated with ponatinib: |
| All chronic phase chronic myeloid leukemia (CP-CML) patients on study who already had achieved major cytogenetic response (MCyR) should have had their dose reduced to 15 mg daily, unless, in the judgment of the investigator, the benefit/risk analysis, taking into account the patient's disease characteristics, BCR::ABL mutation status, and the patient's cardiovascular risk justified treatment with a higher dose |
| All CP-CML patients on study who had not yet achieved MCyR should have had their dose reduced to 30 mg daily, unless, in the judgment of the investigator, the benefit/risk analysis, taking into account the patient's disease characteristics, BCR::ABL mutation status, and the patient's cardiovascular risk justified treatment with a higher dose |
| All acute phase chronic myeloid leukemia (AP-CML), blast phase chronic myeloid leukemia (BP-CML), and Ph+ acute lymphoblastic leukemia (ALL) patients on study should have had their dose reduced to 30 mg daily, unless, in the judgment of the investigator, the benefit/risk analysis, taking into account the patient's disease characteristics, BCR::ABL mutation status, and the patient's cardiovascular risk justified treatment with a higher dose |
| All patients who lost response at a lower dose may have their dose escalated (up to a maximum of 45 mg daily) as long as the dose was not lowered as a result of an adverse event (AE) |
Adjudication methods
All activities related to the adjudication of AOEs were conducted by ACI Clinical (Bala Cynwyd, PA), including the identification of an independent adjudication committee. ACI Clinical is a clinical research organization with expertise in Endpoint Adjudication and Data Monitoring Committees to support safety decisions around clinical development programs. ACI Clinical was contracted by the sponsor; adjudication activities were not part of the PACE trial.
Identification of AEs for adjudication
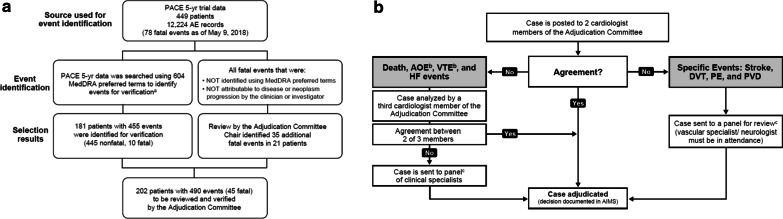
To ensure all relevant potential events were captured, the PACE AE dataset (449 patients with 12,224 AE records; extraction date: May 9, 2018) was searched using a comprehensive set of 604 preferred terms potentially relevant to AOEs that was developed by the sponsor (Table 2). This search strategy, which was more comprehensive than that used in initial analyses of the PACE trial, identified 181 patients and 455 AE records for adjudication (Fig. 1A). In addition, all patient deaths not attributable to disease progression by the clinical investigator were reviewed by the chair of the adjudication committee (described below) for identification of potential fatal AOEs. The adjudication committee identified 45 fatal events for review. In total, 202 patients and 490 events were submitted to the independent adjudication committee for review (Fig. 1A).
Table 2.
List of 604 preferred terms used to identify AEs for adjudication
| Preferred term (MEdDRA 21.0) | ||
|---|---|---|
| Acute aortic syndrome | Diplegia | Pituitary infarction |
| Acute coronary syndrome | Directional Doppler flow tests abnormal | Placental infarction |
| Acute myocardial infarction | Dissecting coronary artery aneurysm | Pneumatic compression therapy |
| Administration site thrombosis | Disseminated intravascular coagulation | Poor peripheral circulation |
| Adrenal thrombosis | Disseminated intravascular coagulation in newborn | Popliteal artery entrapment syndrome |
| Agnosia | Dry gangrene | Portal shunt procedure |
| Amaurosis | Dysarthria | Portal vein cavernous transformation |
| Amaurosis fugax | ECG electrically inactive area | Portal vein occlusion |
| Amputation | ECG signs of myocardial infarction | Portal vein stenosis |
| Angina pectoris | ECG signs of myocardial ischaemia | Portal vein thrombosis |
| Angina unstable | Electrocardiogram Q wave abnormal | Portosplenomesenteric venous thrombosis |
| Anginal equivalent | Electrocardiogram ST segment abnormal | Post angioplasty restenosis |
| Angiogram abnormal | Electrocardiogram ST segment depression | Post cardiac arrest syndrome |
| Angiogram cerebral abnormal | Electrocardiogram ST segment elevation | Post procedural myocardial infarction |
| Angiogram peripheral abnormal | Electrocardiogram ST-T segment abnormal | Post procedural pulmonary embolism |
| Angioplasty | Electrocardiogram ST-T segment depression | Post procedural stroke |
| Angiosclerosis | Electrocardiogram ST-T segment elevation | Post stroke depression |
| Anterior segment ischaemia | Electrocardiogram T wave abnormal | Post thrombotic syndrome |
| Aortic arteriosclerosis | Electrocardiogram T wave inversion | Posthaemorrhagic hydrocephalus |
| Aortic bypass | Electrocardiogram U wave inversion | Postinfarction angina |
| Aortic embolus | Embolia cutis medicamentosa | Postoperative thrombosis |
| Aortic occlusion | Embolic cerebral infarction | Postpartum thrombosis |
| Aortic restenosis | Embolic pneumonia | Postpartum venous thrombosis |
| Aortic stenosis | Embolic stroke | Precerebral arteriosclerosis |
| Aortic surgery | Embolism | Precerebral artery occlusion |
| Aortic thrombosis | Embolism arterial | Precerebral artery thrombosis |
| Aortogram abnormal | Embolism venous | Prinzmetal angina |
| Aphasia | Endarterectomy | Profundaplasty |
| Application site thrombosis | Exercise electrocardiogram abnormal | Prosthetic vessel implantation |
| Arm amputation | Exercise test abnormal | Pulmonary artery occlusion |
| Arterectomy | External counterpulsation | Pulmonary artery stenosis |
| Arterectomy with graft replacement | Extremity necrosis | Pulmonary artery therapeutic procedure |
| Arterial bypass occlusion | Extrinsic iliac vein compression | Pulmonary artery thrombosis |
| Arterial bypass operation | Femoral artery embolism | Pulmonary embolism |
| Arterial bypass stenosis | Finger amputation | Pulmonary endarterectomy |
| Arterial bypass thrombosis | Foetal cerebrovascular disorder | Pulmonary infarction |
| Arterial disorder | Foot amputation | Pulmonary microemboli |
| Arterial graft | Gangrene | Pulmonary thrombosis |
| Arterial insufficiency | Gastrointestinal ischaemia | Pulmonary tumour thrombotic microangiopathy |
| Arterial occlusive disease | Glomerular vascular disorder | Pulmonary vein occlusion |
| Arterial restenosis | Graft ischaemia | Pulmonary vein stenosis |
| Arterial stenosis | Graft thrombosis | Pulmonary veno-occlusive disease |
| Arterial stent insertion | Haemorrhage coronary artery | Pulmonary venous thrombosis |
| Arterial therapeutic procedure | Haemorrhagic adrenal infarction | Quadriparesis |
| Arterial thrombosis | Haemorrhagic cerebral infarction | Quadriplegia |
| Arteriogram abnormal | Haemorrhagic infarction | Raynaud's phenomenon |
| Arteriogram carotid abnormal | Haemorrhagic stroke | Renal arteriosclerosis |
| Arteriogram coronary abnormal | Haemorrhagic transformation stroke | Renal artery angioplasty |
| Arteriogram renal abnormal | Haemorrhagic vasculitis | Renal artery arteriosclerosis |
| Arteriosclerosis | Haemorrhoids thrombosed | Renal artery occlusion |
| Arteriosclerosis coronary artery | Hand amputation | Renal artery stenosis |
| Arteriosclerosis Monckeberg type | Hemianaesthesia | Renal artery thrombosis |
| Arteriosclerotic gangrene | Hemiparesis | Renal embolism |
| Arteriosclerotic retinopathy | Hemiplegia | Renal infarct |
| Arteriospasm coronary | Heparin-induced thrombocytopenia | Renal ischaemia |
| Arteriotomy | Hepatic artery embolism | Renal vascular thrombosis |
| Arteriovenous fistula occlusion | Hepatic artery occlusion | Renal vein embolism |
| Arteriovenous fistula thrombosis | Hepatic artery stenosis | Renal vein occlusion |
| Arteriovenous graft site stenosis | Hepatic artery thrombosis | Renal vein thrombosis |
| Arteriovenous graft thrombosis | Hepatic infarction | Retinal artery embolism |
| Arteritis | Hepatic ischaemia | Retinal artery occlusion |
| Artificial blood vessel occlusion | Hepatic vascular thrombosis | Retinal artery stenosis |
| Atherectomy | Hepatic vein embolism | Retinal artery thrombosis |
| Atherosclerotic plaque rupture | Hepatic vein occlusion | Retinal infarction |
| Atrial appendage closure | Hepatic vein stenosis | Retinal ischaemia |
| Atrial thrombosis | Hepatic vein thrombosis | Retinal vascular disorder |
| Axillary vein thrombosis | Homans' sign positive | Retinal vascular occlusion |
| Balint's syndrome | Hypothenar hammer syndrome | Retinal vascular thrombosis |
| Basal ganglia infarction | Hypoxic–ischaemic encephalopathy | Retinal vein occlusion |
| Basal ganglia stroke | Iliac artery disease | Retinal vein thrombosis |
| Basilar artery occlusion | Iliac artery embolism | Reversible cerebral vasoconstriction syndrome |
| Basilar artery stenosis | Iliac artery occlusion | Reversible ischaemic neurological deficit |
| Basilar artery thrombosis | Iliac vein occlusion | Right hemisphere deficit syndrome |
| Biliary ischaemia | Implant site thrombosis | Scan myocardial perfusion abnormal |
| Blindness transient | Incision site vessel occlusion | Shunt occlusion |
| Blood creatine phosphokinase abnormal | Infarction | Shunt thrombosis |
| Blood creatine phosphokinase increased | Inferior vena cava syndrome | SI QIII TIII pattern |
| Blood creatine phosphokinase MB abnormal | Inferior vena caval occlusion | Silent myocardial infarction |
| Blood creatine phosphokinase MB increased | Infusion site thrombosis | Skin ulcer |
| Bone infarction | Injection site thrombosis | Soft tissue necrosis |
| Bone marrow ischaemia | Inner ear infarction | Spinal artery embolism |
| Brachial artery entrapment syndrome | Instillation site thrombosis | Spinal artery thrombosis |
| Brachiocephalic arteriosclerosis | Intermittent claudication | Spinal cord infarction |
| Brachiocephalic artery occlusion | Interscapulothoracic amputation | Spinal cord ischaemia |
| Brachiocephalic artery stenosis | Intestinal infarction | Spinal vascular disorder |
| Brachiocephalic vein occlusion | Intestinal ischaemia | Splenic artery stenosis |
| Brachiocephalic vein stenosis | Intra-aortic balloon placement | Splenic artery thrombosis |
| Brachiocephalic vein thrombosis | Intracardiac mass | Splenic embolism |
| Brain hypoxia | Intracardiac thrombus | Splenic infarction |
| Brain stem embolism | Intracranial artery dissection | Splenic thrombosis |
| Brain stem infarction | Intracranial venous sinus thrombosis | Splenic vein occlusion |
| Brain stem ischaemia | Intraoperative cerebral artery occlusion | Splenic vein thrombosis |
| Brain stem stroke | Ischaemia | Spontaneous amputation |
| Brain stem thrombosis | Ischaemic cardiomyopathy | Stoma site thrombosis |
| Budd–Chiari syndrome | Ischaemic cerebral infarction | Stress cardiomyopathy |
| Capsular warning syndrome | Ischaemic contracture of the left ventricle | Stress echocardiogram abnormal |
| Cardiac arrest | Ischaemic enteritis | Stroke in evolution |
| Cardiac discomfort | Ischaemic gastritis | Subclavian artery embolism |
| Cardiac stress test abnormal | Ischaemic heart disease prophylaxis | Subclavian artery occlusion |
| Cardiac ventricular scarring | Ischaemic hepatitis | Subclavian artery stenosis |
| Cardiac ventricular thrombosis | Ischaemic limb pain | Subclavian artery thrombosis |
| Cardiopulmonary exercise test abnormal | Ischaemic mitral regurgitation | Subclavian coronary steal syndrome |
| Cardio-respiratory arrest | Ischaemic nephropathy | Subclavian steal syndrome |
| Cardiovascular disorder | Ischaemic neuropathy | Subclavian vein occlusion |
| Cardiovascular insufficiency | Ischaemic pancreatitis | Subclavian vein stenosis |
| Carotid angioplasty | Ischaemic skin ulcer | Subclavian vein thrombosis |
| Carotid arterial embolus | Ischaemic stroke | Subendocardial ischaemia |
| Carotid arteriosclerosis | Jugular vein occlusion | Superior mesenteric artery syndrome |
| Carotid artery bypass | Jugular vein thrombosis | Superior sagittal sinus thrombosis |
| Carotid artery calcification | Kounis syndrome | Superior vena cava occlusion |
| Carotid artery disease | Lacunar infarction | Superior vena cava syndrome |
| Carotid artery insufficiency | Lacunar stroke | Surgical vascular shunt |
| Carotid artery occlusion | Lateral medullary syndrome | Testicular infarction |
| Carotid artery restenosis | Leg amputation | Thalamic infarction |
| Carotid artery stenosis | Leriche syndrome | Thrombectomy |
| Carotid artery stent insertion | Limb amputation | Thromboangiitis obliterans |
| Carotid artery stent removal | Limb traumatic amputation | Thromboembolectomy |
| Carotid artery thrombosis | Macular ischaemia | Thrombolysis |
| Carotid endarterectomy | Mahler sign | Thrombophlebitis |
| Carotid revascularisation | May–Thurner syndrome | Thrombophlebitis migrans |
| Catheter site thrombosis | Medical device site thrombosis | Thrombophlebitis neonatal |
| Catheterisation venous | Mesenteric arterial occlusion | Thrombophlebitis superficial |
| Cavernous sinus thrombosis | Mesenteric arteriosclerosis | Thrombosed varicose vein |
| Central pain syndrome | Mesenteric artery embolism | Thrombosis |
| Central venous catheterisation | Mesenteric artery stenosis | Thrombosis corpora cavernosa |
| Cerebellar artery occlusion | Mesenteric artery stent insertion | Thrombosis in device |
| Cerebellar artery thrombosis | Mesenteric artery thrombosis | Thrombosis mesenteric vessel |
| Cerebellar embolism | Mesenteric phlebosclerosis | Thrombosis prophylaxis |
| Cerebellar infarction | Mesenteric vascular insufficiency | Thrombotic cerebral infarction |
| Cerebellar ischaemia | Mesenteric vascular occlusion | Thrombotic microangiopathy |
| Cerebellar stroke | Mesenteric vein thrombosis | Thrombotic stroke |
| Cerebral arteriosclerosis | Mesenteric venous occlusion | Thrombotic thrombocytopenic purpura |
| Cerebral artery embolism | Microembolism | Thyroid infarction |
| Cerebral artery occlusion | Microvascular coronary artery disease | Toe amputation |
| Cerebral artery restenosis | Migrainous infarction | Tongue infarction |
| Cerebral artery stenosis | Millard–Gubler syndrome | Transient ischaemic attack |
| Cerebral artery thrombosis | Monoparesis | Transverse sinus thrombosis |
| Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy | Monoplegia | Troponin I increased |
| Cerebral congestion | Moyamoya disease | Troponin increased |
| Cerebral gas embolism | Myocardial hypoxia | Troponin T increased |
| Cerebral hypoperfusion | Myocardial infarction | Truncus coeliacus thrombosis |
| Cerebral infarction | Myocardial ischaemia | Tumour embolism |
| Cerebral infarction foetal | Myocardial necrosis | Tumour thrombosis |
| Cerebral ischaemia | Myocardial necrosis marker increased | Ultrasonic angiogram abnormal |
| Cerebral microembolism | Myocardial reperfusion injury | Ultrasound Doppler abnormal |
| Cerebral reperfusion injury | Myocardial stunning | Umbilical cord occlusion |
| Cerebral revascularisation | Necrosis | Umbilical cord thrombosis |
| Cerebral septic infarct | Necrosis ischaemic | Uterine ischaemia |
| Cerebral small vessel ischaemic disease | Nephroangiosclerosis | Vaccination site thrombosis |
| Cerebral thrombosis | NIH stroke scale abnormal | Vascular access site occlusion |
| Cerebral vascular occlusion | NIH stroke scale score decreased | Vascular access site thrombosis |
| Cerebral vasoconstriction | NIH stroke scale score increased | Vascular encephalopathy |
| Cerebral venous thrombosis | Non-cardiac chest pain | Vascular graft |
| Cerebrospinal thrombotic tamponade | Obstetrical pulmonary embolism | Vascular graft occlusion |
| Cerebrovascular accident | Obstructive shock | Vascular graft restenosis |
| Cerebrovascular accident prophylaxis | Ocular ischaemic syndrome | Vascular graft stenosis |
| Cerebrovascular disorder | Ocular vascular disorder | Vascular graft thrombosis |
| Cerebrovascular insufficiency | Omental infarction | Vascular insufficiency |
| Cerebrovascular operation | Ophthalmic vein thrombosis | Vascular occlusion |
| Cerebrovascular stenosis | Optic ischaemic neuropathy | Vascular operation |
| Chest discomfort | Optic nerve infarction | Vascular pseudoaneurysm thrombosis |
| Chest pain | Ovarian vein thrombosis | Vascular shunt |
| Choroidal infarction | Paget–Schroetter syndrome | Vascular skin disorder |
| Choroidal sclerosis | Pancreatic infarction | Vascular stenosis |
| Claudication of jaw muscles | Papillary muscle infarction | Vascular stent insertion |
| Clumsiness | Paradoxical embolism | Vascular stent occlusion |
| Coeliac artery occlusion | Paralysis | Vascular stent restenosis |
| Coeliac artery stenosis | Paraneoplastic thrombosis | Vascular stent stenosis |
| Colitis ischaemic | Paraparesis | Vascular stent thrombosis |
| Collateral circulation | Paraplegia | Vasculitis |
| Compression garment application | Paresis | Vasoconstriction |
| Computerised tomogram coronary artery abnormal | Pelvic venous thrombosis | Vasodilation procedure |
| Coronary angioplasty | Penetrating atherosclerotic ulcer | Vena cava embolism |
| Coronary arterial stent insertion | Penile artery occlusion | Vena cava filter insertion |
| Coronary artery bypass | Penile vein thrombosis | Vena cava filter removal |
| Coronary artery compression | Percutaneous coronary intervention | Vena cava thrombosis |
| Coronary artery disease | Perinatal stroke | Venogram abnormal |
| Coronary artery dissection | Peripheral arterial occlusive disease | Venoocclusive disease |
| Coronary artery embolism | Peripheral arterial reocclusion | Venoocclusive liver disease |
| Coronary artery insufficiency | Peripheral artery angioplasty | Venous angioplasty |
| Coronary artery occlusion | Peripheral artery bypass | Venous occlusion |
| Coronary artery reocclusion | Peripheral artery occlusion | Venous operation |
| Coronary artery restenosis | Peripheral artery restenosis | Venous recanalisation |
| Coronary artery stenosis | Peripheral artery stenosis | Venous repair |
| Coronary artery surgery | Peripheral artery stent insertion | Venous stenosis |
| Coronary artery thrombosis | Peripheral artery thrombosis | Venous stent insertion |
| Coronary brachytherapy | Peripheral coldness | Venous thrombosis |
| Coronary bypass stenosis | Peripheral embolism | Venous thrombosis in pregnancy |
| Coronary bypass thrombosis | Peripheral endarterectomy | Venous thrombosis limb |
| Coronary endarterectomy | Peripheral ischaemia | Venous thrombosis neonatal |
| Coronary no-reflow phenomenon | Peripheral revascularisation | Vertebral artery occlusion |
| Coronary ostial stenosis | Peripheral vascular disorder | Vertebral artery stenosis |
| Coronary revascularisation | Periprocedural myocardial infarction | Vertebral artery thrombosis |
| Coronary vascular graft occlusion | Phlebectomy | Vertebrobasilar insufficiency |
| Coronary vascular graft stenosis | Phlebitis | Vessel puncture site occlusion |
| Coronary vein stenosis | Phlebosclerosis | Vessel puncture site thrombosis |
| Deep vein thrombosis | Vestibular ischaemia | |
| Deep vein thrombosis postoperative | Visceral venous thrombosis | |
| Delayed ischaemic neurological deficit | Visual acuity reduced transiently | |
| Dependent rubor | Visual agnosia | |
| Device embolisation | Visual midline shift syndrome | |
| Device occlusion | Wall motion score index abnormal | |
| Device related thrombosis | ||
| Diabetic macroangiopathy | ||
| Diabetic microangiopathy | ||
| Diabetic vascular disorder | ||
Fig. 1.
CONSORT flow diagram and process for adjudication of arterial occlusive events (AOEs). A CONSORT diagram: Identification of AOEs for review by the adjudication committee. B Adjudication process flow charts. AE adverse event, AC adjudication committee, AIM Applied Clinical Intelligence Information Management System, MedDRA Medical Dictionary for Regulatory Activities, PACE Ponatinib Ph+ ALL and CML Evaluation, PE pulmonary embolism, PVD peripheral vascular disease, VTE venous thromboembolism. aThe Adjudication Committee also reviewed any events included in the Cardiac Failure Standard MedDRA Query (SMQ) to determine whether any heart failure events were AOEs. bAOEs evaluated on the left panel excluded events evaluated in the right panel (stroke, DVT, and PE). cPer the charter, panel meetings were convened to discuss events for which a decision was not reached via independent voting. The quorum for panel meeting attendance was dependent on the type of event(s) to be discussed (i.e., cardiologist, neurologist, or vascular specialist)
An individual case package containing all available clinical information (including medical history) was created for each event and provided to the adjudication committee members for their review. If a patient experienced more than 1 event within 48 h, these events were adjudicated as potentially representing a single clinical event, unless the case evidence suggested they were independent events. Individual events occurring > 48 h apart were adjudicated as independent events. All data were from the clinical trial database that was in SAS format and structured in conformance to CDISC SDTM format; no other source material was available.
Adjudication procedure
An adjudication committee of academic research clinicians who are highly experienced in adjudication activities in cardiovascular trials was appointed by ACI Clinical. The adjudication committee of 5 independent academic experts (3 cardiologists, 1 vascular medicine specialist, and 1 vascular neurologist) retrospectively adjudicated suspected cases of arterial occlusive events in the PACE study. The committee followed a predefined process outlined in the adjudication charter developed by ACI clinical. The charter defined the responsibilities of the adjudication committee and the adjudication endpoints using established definitions developed by the 2014 American College of Cardiology (ACC)/American Heart Association (AHA) guideline [10], and the definitions for cardiovascular and stroke outcomes developed by the Standardized Data Collection for Cardiovascular Trials Initiative (SCTI) and the US Food and Drug Administration [11, 12]. All suspected AOEs identified in the PT search were assessed using the charter definitions (Table 3) for myocardial infarction; heart failure if attributed to an AOE, which may include coronary artery disease, arterial hypertension, cardiomyopathy, or myocardial infarction; hospitalization for unstable angina; stroke and other cerebrovascular events; and peripheral vascular disease. Any events meeting the criteria of these endpoints were considered adjudicated AOEs. Specific criteria were required (e.g., revascularization, change in cardiac biomarkers, diagnostic evidence as shown by computerized tomography scan, magnetic resonance imaging, etc.) to determine the presence of a clinical endpoint. The adjudication committee members were blind to ponatinib dose at the time of the event, whether dose modifications were made, and the investigator’s opinion on AE causality.
Table 3.
Adjudication committee prespecified definitions of events
| Events | Definitions |
|---|---|
| Cardiovascular (CV) death | The cause of death will be determined by the principal condition that caused the death, not the immediate mode of death. Members of the adjudication committee will review all available information and use their clinical expertise to adjudicate the cause of death |
| CV death includes death resulting from an acute myocardial infarction (MI), sudden cardiac death, death due to heart failure (HF), death due to stroke, death due to CV procedures, death due to CV hemorrhage, death due to pulmonary embolism, and death due to other CV causes | |
| Death associated with acute myocardial infarction | Refers to a death by any CV mechanism (e.g., arrhythmia, sudden death, heart failure, stroke, pulmonary embolus, peripheral arterial disease) ≤ 30 days after a MI related to the immediate consequences of the MI, such as progressive heart failure or recalcitrant arrhythmia. Acute MI should be verified to the extent possible by the diagnostic criteria outlined for acute MI (see below) or by autopsy findings showing recent MI or recent coronary thrombosis |
| Death resulting from a procedure to treat a MI (percutaneous coronary intervention (PCI), coronary artery bypass graft surgery (CABG), or to treat a complication resulting from MI, should also be considered death due to acute MI | |
| Death resulting from an elective coronary procedure to treat myocardial ischemia (i.e., chronic stable angina) or death due to a MI that occurs as a direct consequence of a CV investigation/procedure/operation should be considered as a death due to a CV procedure | |
| Sudden cardiac death | Sudden cardiac death refers to death that occurs unexpectedly, not following an acute MI (as defined above) and includes the following deaths: |
| Witnessed and occurring without new or worsening symptoms | |
| Witnessed within 60 min of the onset of new or worsening cardiac symptoms, unless the symptoms suggest acute MI | |
| Witnessed and attributed to an identified arrhythmia (e.g., captured on an electrocardiographic (ECG) recording or witnessed on a monitor, or unwitnessed but found on implantable cardioverter-defibrillator review) | |
| After unsuccessful resuscitation from cardiac arrest (e.g., implantable cardioverter-defibrillator [ICD] unresponsive sudden cardiac death, pulseless electrical activity arrest) | |
| After successful resuscitation from cardiac arrest and without identification of a specific cardiac or non-cardiac etiology | |
| Unwitnessed death in a subject seen alive and clinically stable ≤ 24 h prior to being found dead without any evidence supporting a specific non-CV cause of death (information regarding the patient’s clinical status preceding death should be provided, if available) | |
| Note: Unless additional information suggests an alternate specific cause of death (e.g., Death due to other CV causes), if a patient is seen alive ≤ 24 h of being found dead, sudden cardiac death should be recorded. For patients who were not observed alive within 24 h of death, undetermined cause of death should be recorded (e.g., a subject found dead in bed, but who had not been seen by family for several days) | |
| Note: Successful resuscitation without death should be captured as a resuscitated sudden cardiac death in the non-fatal voting flow | |
| Death due to HF | Refers to death associated with clinically worsening symptoms and/or signs of HF regardless of etiology. Deaths due to HF can have various etiologies, including single or recurrent MIs, ischemic or non-ischemic cardiomyopathy, hypertension, or valvular disease |
| Note: Due to the pro-thrombotic nature of the subject population, a thrombo-embolic option is included during voting. See rules in the non-fatal heart failure definition | |
| Death due to stroke | Refers to death within 30 days that is either a direct consequence of the stroke or a complication of the stroke. Acute stroke should be verified to the extent possible by the diagnostic criteria outlined for stroke |
| Death due to CV procedures | Refers to death caused by the immediate complications of a cardiac procedure not in the context of treatment for acute MI |
| Death due to CV hemorrhage | Refers to death related to hemorrhage such as a non-stroke intracranial hemorrhage, non-procedural or non-traumatic vascular rupture (e.g., aortic aneurysm), or hemorrhage causing cardiac tamponade |
| Death due to other CV causes | Refers to a CV death not included in the above categories but with a specific, known cause (e.g., pulmonary embolism or peripheral vascular disease (venous or arterial disease) |
| Non-CV death | Non-CV death is defined as any death with a specific cause that is not thought to be of CV nature. Adjudication committee members will be asked to indicate the most likely cause of non-cardiovascular death on their voting form |
| Examples of non-CV death are: pulmonary causes, renal causes, gastrointestinal causes, hepatobiliary causes, pancreatic causes, infection (including sepsis), inflammatory (e.g., systemic inflammatory response syndrome (SIRS))/immune (including autoimmune)(may include anaphylaxis from environmental (e.g., food allergies), hemorrhage that is neither cardiovascular bleeding or stroke, non-CV procedure or surgery, trauma, suicide, non-prescription drug reaction or overdose, prescription drug reaction or overdose (many include anaphylaxis), neurological (non-cardiovascular), malignancy (i.e., new malignancy, worsening of prior malignancy) or other (should be specified) | |
| Undetermined cause of death | Undetermined cause of death refers to a death not attributable to one of the above categories. Inability to classify the cause of death may be due to lack of information (e.g., the only available information is “patient died”) or when there is insufficient supporting information or detail to assign the cause of death. In general, most deaths should be classifiable as CV or non-CV, and the use of this category of death, therefore, should be discouraged and should apply to few patients in well-run clinical trials |
| Non-fatal event definitions | |
| Myocardial infarction (non-fatal) | Criteria for acute MI: The term MI should be used when there is evidence of myocardial necrosis in a clinical setting consistent with acute myocardial ischemia. In general MI is defined as a combination of evidence of myocardial necrosis (changes in cardiac biomarkers) and supporting information (derived from the clinical presentation, electrocardiographic changes or the results of a myocardial or coronary artery imaging). Under these conditions, any one of the following criteria A to G meets the diagnosis for MI |
| Spontaneous MI (type 1): To identify a type 1 MI, patients should demonstrate spontaneous symptoms of myocardial ischemia unprovoked by supply/demand inequity, together with at least one of the following criteria: | |
| Cardiac biomarker elevation: Troponin is the preferred marker for use to adjudicate the presence of acute MI. At least one value should show a rise and/or fall above the lowest cut-point providing 10% imprecision (typically the upper reference limit for the troponin run per standard of clinical care). Creatine kinase-MB is a secondary choice to troponin; a rise of CK-MB above the local upper reference limit would be consistent with myocardial injury. Total CK may be used in the absence of CK-MB and troponin | |
| Imaging evidence of new non-viable myocardium or new wall motion abnormality | |
| ECG changes consistent with new ischemic changes | |
| ECG changes indicative of new ischemia [new ST-T changes or new left bundle branch block (LBBB)]* | |
| Development of pathological Q-waves in the ECG** | |
| *ECG manifestations of acute myocardial ischemia (in absence of left ventricular hypertrophy (LVH) and left bundle branch block (LBBB)): | |
| ST elevation: New ST elevation at the J-point in two contiguous leads with the cut-off points: ≥ 0.2 mV in men or ≥ 0.15 mV in women in leads V2–V3 and/or ≥ 0.1 mV in other leads | |
| ST depression and T-wave changes: New horizontal or down- sloping ST depression ≥ 0.05 mV in two contiguous leads; and/or T inversion ≥ 0.1 mV in two contiguous leads with prominent R-wave or R/S ratio > 1 | |
| **Pathological Q-waves: | |
| Any Q-wave in leads V2–V3 ≥ 0.02 s or QS complex in leads V2 and V3 | |
| Q-wave ≥ 0.03 s and ≥ 0.1 mV deep or QS complex in leads I, II, aVL, aVF, or V4-V6 in any two leads of a contiguous lead grouping (I, aVL, V6; V4–V6; II, III, and aVF) | |
| “Demand” related MI (type 2): Patients with type 2 MI should be considered with similar diagnostic criteria as a type 1 MI, however type 2 MI should be considered present when myocardial ischemia and infarction are consequent to supply/demand inequity, rather than a spontaneous plaque rupture and coronary thrombosis | |
| Percutaneous coronary intervention-related MI (type 4a): For percutaneous coronary interventions (PCI) in patients with normal baseline troponin values, elevations of cardiac biomarkers above the 99th percentile URL, within 24 h of the procedure, are indicative of peri-procedural myocardial necrosis. By convention, increases of biomarkers greater than 5 × 99th percentile URL (Troponin or CK-MB > 5 × 99th percentile URL) are consistent with PCI-related MI. If the cardiac biomarker is elevated prior to PCI, a ≥ 20% increase of the value in the second cardiac biomarker sample within 24 h of the PCI and documentation that cardiac biomarker values were decreasing (2 samples at least 6 h apart) prior to the suspected recurrent MI is also consistent with PCI-related MI. In addition to biomarker elevation one of the following must exist: | |
| Symptoms suggestive of myocardial ischemia | |
| New ischemic ECG changes or new LBBB | |
| Angiographic findings consistent with procedural complication (e.g., Loss of patency, persistent slow/non-flow or embolization) | |
| Imaging demonstration of new loss of viable myocardium or new regional wall motion abnormality | |
| MI associated with stent thrombosis or stent restenosis as documented by angiography or at autopsy will also be captured as subtypes 4b and 4c | |
| Stent thrombosis related MI (type 4b): MI associated with stent thrombosis as detected by coronary angiography or at autopsy, where symptoms suggestive of myocardial ischemia are present, and with a rise and/or fall of cardiac biomarker values with at least 1 value > 99th percentile of the URL. If found with autopsy, it will be captured under cardiac death | |
| Definite stent thrombosis is considered to have occurred by either angiographic or pathological confirmation: | |
| Angiographic confirmation of stent thrombosis (Incidental angiographic documentation of stent occlusion in the absence of clinical signs or symptoms is not considered a confirmed stent thrombosis [silent occlusion]). The presence of a thrombus (intracoronary) that originates in the stent or in the segment 5 mm proximal or distal to the stent and presence of at least 1 of the following criteria within a 48-h time window: | |
| Acute onset of ischemic symptoms at rest | |
| New ischemic ECG changes that suggest acute ischemia | |
| Typical rise and fall in cardiac biomarkers (refer to definition of spontaneous MI) | |
| Non-occlusive thrombus | |
| Intracoronary thrombus is defined as a (spheric, ovoid, or irregular) non-calcified filling defect or lucency surrounded by contrast material (on 3 sides or within a coronary stenosis) seen in multiple projections, or persistence of contrast material within the lumen, or a visible embolization of intraluminal material downstream | |
| Occlusive thrombus TIMI 0 or TIMI 1 intrastent or proximal to a stent up to the most adjacent proximal side branch or main branch (if originates from the side branch) | |
| Pathological confirmation of stent thrombosis: Evidence of recent thrombus within the stent determined at autopsy or via examination of tissue retrieved following thrombectomy | |
| Probable stent thrombosis: Clinical definition of probable stent thrombosis is considered to have occurred after intracoronary stenting in the following cases: | |
| Any unexplained death within the first 30 days | |
| Irrespective of the time after the index procedure, any MI that is related to documented acute ischemia in the territory of the implanted stent without angiographic confirmation of stent thrombosis and in the absence of any other obvious cause | |
| Stent restenosis-related MI (type 4c): MI associated with stent restenosis as detected by coronary angiography or at autopsy, occurring > 48 h after index PCI without evidence of stent thrombosis but with symptoms suggestive of myocardial ischemia, and with elevation of cardiac biomarker values to > 99th percentile of the URL. This classification also requires the following: | |
| Does not meet criteria for any other classification of MI | |
| Presence of a ≥ 50% stenosis at the site of previous successful stent PCI or a complex lesion and no other significant obstructive CAD of greater severity following: | |
| Initially successful stent deployment | |
| OR | |
| Dilatation of a coronary artery stenosis with balloon angioplasty to < 50% stenosis | |
| If found with autopsy, it will be captured under cardiac death | |
| Coronary artery bypass grafting-related MI (type 5): MI associated with CABG is arbitrarily defined by elevation of cardiac biomarker values > 10 × 99th percentile URL in patients with normal baseline cardiac biomarker values (≤ 99th percentile URL). In addition to any one of the following: | |
| New pathological Q-waves or new LBBB | |
| Angiographic documented new graft or new native coronary artery occlusion | |
| Imaging evidence of new loss of viable myocardium or new regional wall motion abnormality | |
| Heart failure event | A heart failure event includes hospitalization for heart failure and may include any urgent outpatient visits for heart failure. The date of this event will be the day of hospitalization of the patient (including any overnight stay at the emergency room or chest pain unit) or the day of visit to the urgent outpatient center. Due to the pro-thrombotic nature of the subject population, a thrombo-embolic option is included during voting |
| The following rules may be applied to indicate if heart failure is attributed to an AOE/VTE: | |
| Heart failure may be attributed to an AOE/VTE if related to coronary artery disease, hypertension, cardiomyopathy or myocardial infarction | |
| The relationship of heart failure to an AOE/VTE may be excluded if the underlying cause of heart failure is heart valve disorders, congenital heart disorders or arrhythmias | |
| Heart failure requiring hospitalization | Heart failure hospitalization is defined as an event that meets all the following criteria: |
| Patient is admitted to the hospital with a primary diagnosis of HF | |
| Patient’s length of stay in hospital extends for at least 24 h (or a change in calendar date if the hospital admission and discharge times are unavailable) | |
| Patient exhibits documented new or worsening symptoms due to HF on presentation, including at least ONE of the following: | |
| Dyspnea | |
| Dyspnea with exertion | |
| Orthopnea | |
| Paroxysmal nocturnal dyspnea | |
| Decrease exercise tolerance | |
| Fatigue | |
| Other symptoms of worsened end-organ perfusion or volume overload | |
| Patient has objective evidence of new/worsening HF, consisting of at least TWO physical examination findings OR one physical examination finding and at least one laboratory criterion, including: | |
| Physical examination findings considered to be due to heart failure | |
| Peripheral edema | |
| Increasing abdominal distention or ascites (in the absence of primary hepatic disease) | |
| Pulmonary rales/crackles/crepitations | |
| Increased jugular venous pressure and/or hepatojugular reflux | |
| S3 gallop | |
| Clinically significant or rapid weight gain thought to be related to fluid retention | |
| Laboratory evidence of new or worsening HF, if obtained within 24 h of presentation, including: | |
| Increased b-type natriuretic peptide (BNP)/N-terminal proBNP (NT-proBNP) concentrations consistent with decompensation of heart failure (such as BNP > 500 pg/mL or NT-proBNP > 1800 pg/mL). In patients with chronically elevated natriuretic peptides, a significant increase should be noted above baseline | |
| Radiological evidence of pulmonary congestion | |
| New or worsened bilateral pleural effusions | |
| Noninvasive diagnostic evidence of clinically significant elevated left or right-sided ventricular filling pressure or low cardiac input | |
| Invasive diagnostic evidence with right heart catheterization showing a pulmonary capillary wedge pressure (pulmonary artery occlusion pressure) ≥ 18 mmHg, central venous pressure ≥ 12 mmHg, or a cardiac index < 2.2 L/min/m2 | |
| Patient receives initiation or intensification of treatment specifically for HF (at least one of the following): | |
| Augmentation in oral diuretic therapy or ACE inhibitor | |
| Intravenous diuretic or vasoactive agent (e.g., inotrope, vasopressor, or vasodilator) | |
| Mechanical or surgical intervention: | |
| Mechanical circulatory support (e.g., intra-aortic balloon pump, ventricular assist device, extracorporeal membrane oxygenation, total artificial heart) | |
| Mechanical fluid removal (e.g., dialysis, ultrafiltration, hemofiltration) | |
| Urgent heart failure visit | An urgent heart failure visit is defined as an event that meets all the following criteria: |
| The patient has an urgent, unscheduled office/practice or emergency department visit for a primary diagnosis of heart failure, but not meeting the criteria for a heart failure hospitalization | |
| All signs/symptoms for heart failure hospitalization (i.e., symptoms, physical examination findings/lab evidence of new or worsening HF as indicated under definition for Heart Failure Hospitalization) must be met | |
| The patient receives initiation or intensification of treatment specifically for heart failure, as detailed in the heart failure hospitalization section with the exception of oral diuretic therapy (which will not be sufficient) | |
| Hospitalization for unstable angina | The date of this event will be the day of hospitalization of the patient including any overnight stay at an emergency room or chest pain unit |
| Hospitalization for unstable angina is defined as an event that meets all the following criteria: | |
| Negative cardiac biomarkers and no evidence of acute MI | |
| Ischemic discomfort (angina or other symptoms thought to be equivalent) ≥ 10 min in duration occurring at rest or in an accelerating pattern with frequent episodes associated with progressively decreased exercise capacity | |
| Unscheduled hospitalization within 24 h of the most recent symptoms. Hospitalization is defined as an admission to an inpatient unit or a visit to an emergency department that results in at least a 24 h stay (or a change in calendar date if the hospital admission or discharge times are not available) | |
| At least one of the following: | |
| New or worsening ST or T-wave changes on resting ECG (in absence of confounders such as LBBB or LVH) | |
| ST Elevation: New transient (duration < 20 min) at the J point in two contiguous leads with the cut-points: ≥ 0.1 mV in all leads other than leads V2-V3 where the following cut-points apply: ≥ 0.2 mV in men ≥ 40 years (≥ 0.25 mV in men < 40 years) or ≥ 0.15 mV in women | |
| ST depression and T-wave changes: New horizontal or down-sloping ST depression ≥ 0.05 mV in two contiguous leads and/or a new T inversion ≥ 0.3 mV in two contiguous leads with prominent R -wave or R/S ratio > 1 | |
| Definite evidence of inducible myocardial ischemia as demonstrated by one of the following and believed to be responsible for symptoms: | |
| Early positive stress test (defined as ST elevation or ≥ 2 mm ST depression prior to 5 mets) | |
| Stress echocardiography (reversible wall motion abnormality) | |
| Myocardial scintigraphy (reversible perfusion defect) | |
| MRI (myocardial perfusion deficit under pharmacologic stress) | |
| Angiographic evidence of new or worse ≥ 70% lesion (≥ 50% for left main lesion) and/or thrombus in an epicardial coronary artery that is believed to be responsible for the myocardial ischemic symptoms/signs | |
| Need for coronary revascularization procedure (PCI or CABG) for the presumed culprit lesion(s). This criterion would be fulfilled if revascularization was undertaken during the unscheduled hospitalization, or subsequent to transfer to another institution without interceding home discharge | |
| Stroke | Stroke is defined as an acute episode of focal or global neurological dysfunction caused by brain, spinal cord, or retinal vascular injury as a result of hemorrhage or infarction. Strokes will be classified as ischemic, hemorrhagic, retinal artery occlusion or thrombosis or undetermined |
| General | |
| Stroke is defined as an acute episode of focal or global neurological dysfunction caused by brain, spinal cord, or retinal vascular injury as a result of hemorrhage or infarction, with symptom duration of 24 h or more. Episodes lasting less than 24 h can be considered a stroke if there is an intervention to abort the stroke (e.g., thrombolytic therapy), diagnostic confirmation of the stroke, or patient death prior to reaching the 24 h duration | |
| Subdural and epidural hematomas are intracranial hemorrhagic events and are not strokes | |
| Diagnosis of stroke | |
| For the diagnosis of stroke, the following 4 criteria should be fulfilled: | |
| Acute onset* of a focal/global neurological deficit with at least one of the following: | |
| Change in level of consciousness | |
| Hemiplegia | |
| Hemiparesis | |
| Numbness or sensory loss affecting one side of the body | |
| Dysphasia/Aphasia | |
| Hemianopia (loss of half of the field of vision of one or both eyes) | |
| Other new neurological sign(s)/symptom(s) consistent with stroke | |
| *If the mode of onset is uncertain, a diagnosis of stroke may be made provided that there is no plausible non-stroke cause for the clinical presentation | |
| Duration of a focal/global neurological deficit ≥ 24 h | |
| OR | |
| < 24 h if | |
| This is because of at least one of the following therapeutic interventions: | |
| Pharmacologic (i.e., thrombolytic drug administration) | |
| Non-pharmacologic (i.e., neurointerventional procedure (e.g., intracranial angioplasty)) | |
| or | |
| available brain imaging clearly documents a new hemorrhage or infarct | |
| or | |
| the neurological deficit results in death | |
| No other readily identifiable non-stroke cause for the clinical presentation (e.g., brain tumor, trauma, infection, hypoglycemia, peripheral lesion) | |
| Confirmation of the diagnosis by at least one of the following:** | |
| Neurology or neurosurgical specialist | |
| Brain imaging procedure (at least one of the following): | |
| CT scan | |
| MRI scan | |
| Cerebral vessel angiography | |
| Lumbar puncture (i.e., spinal fluid analysis diagnostic of subarachnoid hemorrhage) | |
| **If a stroke is reported but evidence of confirmation of the diagnosis by the methods outlined above is absent, the event will be discussed at a full EAC meeting. In such cases, the event may be adjudicated as a stroke on the basis of the clinical presentation alone, but full EAC consensus will be mandatory | |
| Classification of stroke | |
| Strokes are sub-classified as follows: | |
| Ischemic (non-hemorrhagic) | |
| Ischemic stroke is defined as an acute episode of focal cerebral, spinal or retinal dysfunction caused by infarction of central nervous system tissue. Hemorrhage may be a consequence of ischemic stroke. In this situation, the stroke is an ischemic stroke with hemorrhagic transformation and not a hemorrhagic stroke | |
| Hemorrhagic | |
| Hemorrhagic stroke is defined as an acute episode of focal or global cerebral or spinal dysfunction caused by intraparenchymal, intraventricular, or subarachnoid hemorrhage | |
| Retinal artery occlusion or thrombosis | |
| Retinal artery occlusion or thrombosis is defined as a blockage in one of the retinal arteries | |
| Occlusions may be caused by a thromboembolism or other risk factors such as atherosclerosis and arrhythmias | |
| Note: Amaurosis fugax is not considered part of this endpoint | |
| Undetermined stroke | |
| Undetermined stroke is defined as an acute episode of focal or global neurological dysfunction caused by presumed brain, spinal cord, as a result of hemorrhage or infarction but with insufficient information to allow categorization as #1 and #2 above | |
| Note: Given the scope of this study, stroke disability will not be measured. TIA definition was intentionally left out for this study; suspected TIA events will be identified for adjudication in order to rule out stroke | |
| Venous thrombosis | Superficial vein thrombosis |
| Superficial vein thrombosis (SVT) refers to a blood clot in one of the superficial veins near the surface of the body. There is usually an inflammatory reaction around the vein and may present with as a painful induration with erythema. An SVT can lead to a serious complication such as a higher risk for pulmonary embolism | |
| Superficial vein thrombosis could be documented by one of the following: | |
| Clinical symptoms (such as warmth, edema, ‘cord-like’ palpable mass, erythema, pain) | |
| Duplex ultrasound | |
| Deep vein thrombosis | Deep vein thrombosis (DVT) refers to a blood clot in one of the deep veins (to include distal and proximal DVT). It may occur anywhere in the body but is most common in the extremities, a clot blocks blood circulation through these veins, which carry blood back to the heart. This commonly causes pain and swelling distal to the thrombus. Severe complications of DVT may occur when a clot embolizes to the lung |
| Deep vein thrombosis could be documented by one of the following: | |
| Venous ultrasonography | |
| Compression ultrasonography (CUS) | |
| Impedance plethysmography (IPG) | |
| Venography | |
| CT scan | |
| MRI | |
| At autopsy | |
| Location | |
| Venous thrombosis (DVT and SVT) will be categorized for location by the EAC | |
| Members as follows: | |
| Lower limb | |
| Upper limb | |
| Retinal vein | |
| Abdominal viscera | |
| Other (e.g., more unusual sites of cerebral venous thrombosis) | |
| Pulmonary embolism | A pulmonary embolism (PE) is a blood clot in the arteries of the lung that typically arise from the veins. The embolus not only prevents the exchange of oxygen and carbon dioxide via the lungs, but it also decreases blood supply to the lung tissue itself, potentially causing infarction. The most common symptoms include pleuritic chest pain, dyspnea, and hemoptysis. A PE may lead to sudden death. Death due to PE refers to death that is either a direct consequence or complication of a PE. Fatal PE is captured in the fatal definition section as death due to other CV causes |
| Pulmonary embolism should be documented by supporting evidence found within any one of the following: | |
| CT scan | |
| Pulmonary angiogram | |
| Ventilation/perfusion lung scan (VPLS) | |
| Inconclusive spiral CT, pulmonary angiography or lung scintigraphy with demonstration of DVT in the lower extremities by CUS or venography with clinical, lab and EKG findings consistent with PE | |
| At autopsy | |
| Other AOE/VTE | Peripheral vascular disease (PVD) |
| Peripheral vascular disease refers to a blood circulation disorder outside of the heart and brain that causes the blood vessels to block, narrow or spasm. PVD can be either in veins or arteries. Physical symptoms may include weak pulses, wounds/ulcers that won’t heal, thin or pale skin | |
| PVD could be documented by one of the following: | |
| Doppler ultrasound | |
| Ankle-brachial index | |
| Angiography | |
| Magnetic resonance angiography | |
| Computerized tomography angiography | |
| Members will be asked to choose if this is a venous or arterial occlusive event | |
| Revascularization procedures | For fatal and non-fatal cardiovascular endpoint events, members must also indicate if the event is associated with a revascularization procedure (PCI, CABG or PVI) |
| Percutaneous coronary intervention (PCI) | |
| Defined as the placement of an angioplasty guidewire, balloon, or other device (e.g., stent, atherectomy, brachytherapy or thrombectomy catheter) into a native coronary artery or CABG for the purpose of mechanical coronary revascularization. The assessment of coronary lesion severity by intravascular ultrasonography, coronary flow reserve, or fractional flow reserve is not considered a PCI procedure | |
| Coronary artery bypass graft (CABG) | |
| Defined as a procedure performed to bypass partially or completely occluded coronary arteries with veins and/or arteries harvested from elsewhere in the body, thereby improving the blood supply to the coronary circulation supplying the myocardium | |
| Peripheral vascular intervention (PVI) | |
| Peripheral vascular intervention is a catheter-based or open surgical procedure designed to improve arterial or venous blood flow or otherwise modify or revise vascular conduits. Procedures may include, but are not limited to percutaneous transluminal balloon angioplasty, stent placement, thrombectomy, embolectomy, atherectomy, dissection repair, aneurysm exclusion, treatment of dialysis conduits, placement of various devices, intravascular thrombolysis or other pharmacotherapies, and open surgical bypass or revision | |
During the adjudication process, the committee reviewed all potential AOEs, as well as any AEs identified in a Cardiac Failure Standard MedDRA Query (SMQ), to determine whether any heart failure events were AOEs. Two members of the adjudication committee independently evaluated whether an individual case met the prespecified event definitions (Fig. 1B). If agreement between 2 members was not reached for cases of AOEs or heart failure, the case was reviewed by a third cardiologist adjudication committee member; if agreement was not reached with 3 votes, the case was reviewed at a panel meeting. If agreement was not reached for cases of stroke, deep vein thrombosis, pulmonary embolism, and peripheral vascular disease, the case was discussed at a panel meeting with the appropriate neurologist and/or vascular specialist member(s). All fatal events were decided by consensus of adjudicators.
Events that met one of the charter-defined endpoint definitions were further categorized depending on the event type (e.g., myocardial infarction, peripheral arterial occlusive disease, deep vein thrombosis, etc.). Non-adjudicated AOEs that were recorded as symptoms (e.g., "non-cardiac chest pain" or "claudication") with a low severity level and no accompanying changes in medication or hospitalization were adjudicated to not be AOEs unless they had an anatomic diagnosis provided (e.g., "severe superficial femoral artery stenosis"). If the term "infarction" was provided for stroke events, the adjudicators categorized the event as ischemic stroke. Revascularization was not always clearly reported by investigators.
Statistics
Exposure-adjusted AOE rates were calculated as: (number of first events in interval)/(total exposure for interval in patient-years) × 100. The relative risk of serious AOEs was analyzed by baseline risk category in patients from the safety population for whom all baseline risk categories were available. Risk categories included commonly recognized cardiovascular risk factors for which data were collected (arterial hypertension, hypercholesterolemia, diabetes mellitus, and obesity), and history of heart disease (non-ischemic or ischemic).
Results
Patient disposition and baseline characteristics
Patient disposition and baseline characteristics in the PACE trial have been published [2, 3]. A total of 449 patients, including 270 CP-CML patients, 85 accelerated-phase (AP) CML patients, 62 blast-phase (BP) CML patients, and 32 Ph+ ALL patients, were enrolled between September 2010 and October 2011. Baseline characteristics are summarized in Table 4. Among all 449 patients, the median age was 59 years and 53% of patients were male. Most (93%) patients had received 2 or more prior TKIs. At baseline, 53% of patients had arterial hypertension, 49% had hypercholesterolemia, and 24% had BMI ≥ 30 kg/m2. Forty-three percent of patients had a baseline history of non-ischemic cardiac disease, and 23% had a history of ischemic cardiovascular disease. Safety data reviewed by the adjudication committee reflect data collected as of February 6, 2017, with median follow-up of 37.3 months for all patients and 56.8 months (range 0.1–73.1 months) for CP-CML patients.
Table 4.
Baseline characteristics and disposition at end-of-study3
| CP-CML n = 270 |
Total N = 449 |
|
|---|---|---|
| Characteristic at baseline | ||
| Median age (range), y | 60 (18–94) | 59 (18–94) |
| Female, n (%) | 126 (47) | 211 (47) |
| Previous use of approved TKIs, n (%)a | ||
| ≥ 2 drugs | 251 (93) | 417 (93) |
| ≥ 3 drugs | 154 (57) | 250 (56) |
| Median duration of previous treatment with approved TKIs (range), ya | 5.4 (0.4–13.3) | 4.6 (0.1–13.3) |
| Resistant or intolerant to dasatinib or nilotinib, n (%) | ||
| Resistant | 215 (80) | 375 (84) |
| Intolerant only | 39 (14) | 49 (11) |
| Both resistant and intolerant | 52 (19) | 81 (18) |
| Mutation status, n (%)b | ||
| No mutation detected | 138 (51) | 198 (44) |
| BCR::ABL1T315I | 64 (24) | 128 (29) |
| Best response of MMR or better to most recent regimen containing dasatinib or nilotinib, n (%)c | 8 (3) | 16 (4) |
| Baseline cardiovascular risk factorsd | ||
| Arterial hypertension | NA | 240 (53) |
| Hypercholesterolemia | NA | 219 (49) |
| Obesity | NA | 109 (24) |
| Diabetes mellitus | NA | 72 (16) |
| Baseline history of cardiovascular disease | ||
| Non-ischemic cardiac disease | NA | 193 (43) |
| Ischemic disease | NA | 102 (23) |
| Patient disposition at end of study | ||
| Median duration of treatment, mo (range) | 32.1 (0.1–73.0) | 16.7 (0.03–73.0) |
| Median follow-up, mo (range) | 56.8 (0.1–73.1) | 37.3 (0.1–73.1) |
| Median dose intensity, mg/d (range) | 27.2 (5–45) | ND |
| Primary reason for discontinuation, n (%) | ||
| Disease progression | 29 (11) | 105 (23) |
| Adverse event | 57 (21) | 79 (18) |
| Patient request | 31 (11) | 42 (9) |
| Lack of efficacy | 15 (6) | 26 (6) |
| Deathe | 9 (3) | 26 (6) |
| Investigator decision | 11 (4) | 17 (4) |
| Lost to follow-up | 0 | 3 (< 1) |
| Non-compliance | 3 (1) | 4 (< 1) |
| Protocol violation | 2 (< 1) | 2 (< 1) |
| Study closuref | 90 (33) | 107 (24) |
| Otherf,g | 14 (5) | 28 (6) |
CML chronic myeloid leukemia, CP chronic phase, MMR major molecular response, ND not determined, TKI tyrosine kinase inhibitor
aApproved TKIs were imatinib, nilotinib, dasatinib, and bosutinib. Previous investigational TKIs received by at least 1% of patients included radotinib (received by 2% of patients), bafetinib (2%), rebastinib (2%), and XL-228 (2%)
bAssessed by conventional Sanger sequencing at baseline
cPercentages were calculated according to the number of patients who received previous dasatinib or nilotinib: 256 patients with CP-CML, 80 patients with AP-CML, 61 patients with BP-CML, and 30 patients with Ph+ ALL
dSmoking and family history were not collected as part of the trial. Patients with significant or active cardiovascular disease, including myocardial infarction, unstable angina or congestive heart failure (in prior 3 months), or history of clinically significant atrial or ventricular arrhythmia were excluded from the trial
eSeven deaths were assessed by investigators as possibly or probably related to ponatinib (CP-CML: pneumonia, acute myocardial infarction; AP-CML: fungal pneumonia, gastrointestinal hemorrhage; BP-CML: hemorrhagic gastritis; Ph+ ALL: cardiac arrest, mesenteric arterial occlusion)
fPatients who continued to derive clinical benefit from their treatment had the option to receive ponatinib through alternative mechanisms
gThis category includes stem cell transplantation (in 11 patients with CP-CML, 5 with AP-CML, 6 with BP-CML, and 1 with Ph+ ALL). The 9 CP-CML patients and 1 AP-CML patient who remained on study at the time of last response assessment are not included in this category.3
Adjudication results
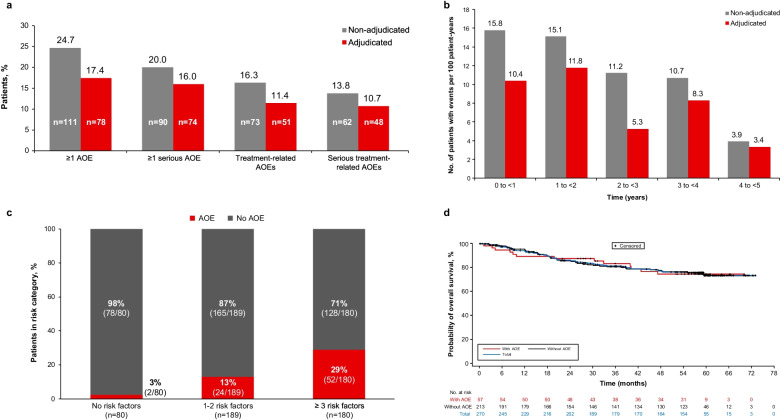
Rates of adjudicated AOEs were lower than rates of non-adjudicated AOEs (Fig. 2A). Overall, 17% (78/449) of patients had adjudicated AOEs compared with 25% (111/449) with non-adjudicated AOEs. Most patients with serious AOEs were adjudicated as having serious AOEs (20% [90/449] non-adjudicated vs. 16% [74/449] adjudicated). Most (95% [74/78]) patients with adjudicated AOEs had serious AOEs. In CP-CML patients, rates of adjudicated AOEs (21% [57/270]) were also lower than rates of non-adjudicated AOEs (31% [84/270]); 95% [54/57] of CP-CML patients with adjudicated AOEs had serious AOEs. The rates of AOEs by AOE type (i.e., cardiovascular, cerebrovascular, and peripheral vascular) are presented for all patients in Table 5 and for CP-CML patients in Table 6.
Fig. 2.
Arterial occlusive event (AOE) rates with ponatinib. A Rates of non-adjudicated and adjudicated AOEs. B Exposure-adjusted incidence of newly occurring arterial occlusive events (AOEs) by year (all patients). Later intervals excluded patients with prior events. Non-adjudicated values were published previously [3]. C Incidence of AOEs (adjudicated) by number of baseline risk factors (all patients). Risk factors included arterial hypertension, hypercholesterolemia, obesity, diabetes mellitus, non-ischemic cardiac disease, and ischemic disease. D Overall survival (OS) in chronic-phase chronic myeloid leukemia (CP-CML) patients with and without AOEs
Table 5.
Rates of non-adjudicated and adjudicated AOEs by type in the total population (n = 449)
| AOE | Non-adjudicated eventsa | Adjudicated eventsb | ||
|---|---|---|---|---|
| Any | Serious | Any | Serious | |
| Any, | 111 (25) | 90 (20) | 78 (17) | 74 (16) |
| Cardiovascularc | 59 (13) | 44 (10) | 38 (8) | 37 (8) |
| Cardiovascular AOEs in ≥ 1% of patients | ||||
| Angina pectoris | 28 (6) | 15 (3) | 0 | 0 |
| Acute MId | 18 (4) | 18 (4) | 8 (2) | 8 (2) |
| MI | d | d | 10 (2) | 10 (2) |
| Coronary artery disease | 14 (3) | 12 (3) | 7 (2) | 7 (2) |
| Acute coronary syndrome | 7 (2) | 7 (2) | 7 (2) | 7 (2) |
| Coronary artery occlusion | 5 (1) | 4 (1) | 0 | 0 |
| Cerebrovascular | 41 (9) | 33 (7) | 28 (6) | 25 (6) |
| Cerebrovascular AOEs in ≥ 1% of patients | ||||
| Cerebrovascular accident | 11 (2) | 11 (2) | 7 (2) | 7 (2) |
| Cerebral infarction | 8 (2) | 8 (2) | 8 (2) | 8 (2) |
| Carotid artery stenosis | 7 (2) | 6 (1) | 7 (2) | 5 (1) |
| Transient ischemic attack | 6 (1) | 4 (1) | 0 | 0 |
| Peripheral vascular | 48 (11) | 38 (8) | 42 (9) | 34 (8) |
| Peripheral vascular AOEs in ≥ 1% of patients | ||||
| Peripheral arterial occlusive disease | 22 (5) | 17 (4) | 19 (4) | 16 (4) |
| Intermittent claudication | 11 (2) | 1 (< 1) | 0 | 0 |
| Peripheral artery stenosis | 10 (2) | 8 (2) | 8 (2) | 7 (2) |
| Peripheral artery occlusion | 7 (2) | 5 (1) | 7 (2) | 5 (1) |
| Peripheral ischemia | 7 (2) | 4 (1) | 5 (1) | 0 |
| Peripheral vascular disorder | 5 (1) | 4 (1) | 0 | 0 |
| Exposure-adjusted newly occurring AOEs, patients with events per 100 patient-years | 13.8 | 10.6 | 8.9 | 8.4 |
Data are no. (%) of patients, unless otherwise specified
AOE arterial occlusive event, CP-CML chronic-phase chronic myeloid leukemia, MedDRA Medical Dictionary for Regulatory Activities, MI myocardial infarction, PT preferred term
aCategorization of AOEs is based on > 400 MedDRA preferred terms related to vascular ischemia or thrombosis
bEvents that were adjudicated as an AOE by the adjudication committee
cDoes not include arterial hypertension AEs
dAcute MI and MI were grouped as a single category in the non-adjudicated analysis
Table 6.
Rates of AOEs non-adjudicated and adjudicated AOEs in CP-CML patients (n = 270)
| AOE | Non-adjudicated eventsa | Adjudicated eventsb | ||
|---|---|---|---|---|
| Any | Serious | Any | Serious | |
| Any, | 84 (31) | 69 (26) | 57 (21) | 54 (20) |
| Cardiovascularc | 42 (16) | 33 (12) | 26 (10) | 25 (9) |
| Cerebrovascular | 35 (13) | 28 (10) | 25 (9) | 22 (8) |
| Peripheral vascular | 38 (14) | 31 (11) | 31 (11) | 26 (10) |
| Exposure-adjusted newly occurring AOEs, patients with events per 100 patient-years | 11.3 | 9.3 | 8.7 | 8.1 |
Data are no. (%) of patients, unless otherwise specified
AOE arterial occlusive event, CP-CML chronic-phase chronic myeloid leukemia, MedDRA Medical Dictionary for Regulatory Activities, MI myocardial infarction, PT preferred term
aCategorization of AOEs is based on > 400 MedDRA preferred terms related to vascular ischemia or thrombosis
bEvents that were adjudicated as an AOE by the adjudication committee
cDoes not include arterial hypertension AEs
The most common non-adjudicated and adjudicated AOEs and serious AOEs are summarized in Table 7. The most common (> 2%) non-adjudicated AOEs were angina pectoris (6%; 28/449), peripheral arterial occlusive disease (5%; 22/449), MI (4%; 18/449), coronary artery disease (3% [14/449]). The only adjudicated AOE reported in > 2% of patients was peripheral arterial occlusive disease (4% [16/449]). Non-adjudicated AOEs that were most commonly adjudicated as not AOEs were angina pectoris, non-cardiac chest pain, and chest pain, as these events were often recorded as symptoms (e.g., "non-cardiac chest pain" or "claudication") or presumptive diagnoses with a low severity level and no accompanying changes in medication or hospitalization.
Table 7.
Arterial occlusive events (AOEs) in ≥ 2.0% of patients (n = 449)
| AOE | Any AOE | Serious AOE | ||
|---|---|---|---|---|
| Non-adjudicateda | Adjudicatedb | Non-adjudicateda | Adjudicatedb | |
| Angina pectoris | 28 (6) | 0 | 15 (3) | 0 |
| Peripheral arterial occlusive disease | 22 (5) | 19 (4) | 17 (4) | 16 (4) |
| Myocardial infarction | 18 (4) | 10 (2) | 18 (4) | 10 (2) |
| Coronary artery disease | 14 (3) | 7 (2) | 12 (3) | 7 (2) |
| Cerebrovascular accident | 11 (2) | 7 (2) | 11 (2) | 7 (2) |
| Intermittent claudication | 11 (2) | 0 | 1 (< 1) | 0 |
| Peripheral artery stenosis | 10 (2) | 8 (2) | 8 (2) | 7 (2) |
| Cerebral infarction | 8 (2) | 8 (2) | 8 (2) | 8 (2) |
| Acute coronary syndrome | 7 (2) | 7 (2) | 7 (2) | 7 (2) |
| Carotid artery stenosis | 7 (2) | 7 (2) | 6 (1) | 5 (1) |
| Peripheral artery occlusion | 7 (2) | 7 (2) | 5 (1) | 5 (1) |
| Peripheral ischemia | 7 (2) | 5 (1) | 4 (1) | 0 |
Data are no. (%) of patients
MedDRA Medical Dictionary for Regulatory Activities
aCategorization of AOEs is based on MedDRA preferred terms related to vascular ischemia or thrombosis
bEvents adjudicated as AOEs by the cardiovascular endpoint Adjudication Committee
The exposure-adjusted incidence of adjudicated AOEs (8.9 patients with events per 100 patient-years) and serious AOEs (8.4 patients with events per 100 patient-years) was lower than the exposure-adjusted incidence of non-adjudicated AOEs (11.3 and 9.2 per 100 patient-years, respectively). The exposure-adjusted incidence of newly occurring AOEs decreased over time (Fig. 2B). The median time to onset of the first adjudicated AOE was 14.1 months (range: 0.1 to 49.5; Table 8).
Table 8.
Time to onset of adjudicated AOEs
| Median time to first AOE (range), months | ||
|---|---|---|
| CP-CML patients | All patients | |
| Any AOE |
(n = 57) 16.3 (0.4, 49.5) |
(n = 78) 14.1 (0.1, 49.5) |
| Cardiovascular AOE |
(n = 26) 14.1 (0.6, 52.9) |
(n = 38) 12.3 (0.3, 52.9) |
| Cerebrovascular AOE |
(n = 25) 23.0 (0.4, 53.5) |
(n = 28) 18.9 (0.4, 53.5) |
| Peripheral vascular AOE |
(n = 31) 24.6 (1.8, 49.5) |
(n = 42) 22.2 (0.1, 49.5) |
Resolution of AOEs, dose modifications, and discontinuations
Among the 78 patients with an adjudicated AOE, events resolved in 51 patients. Among 43 patients with just one AOE, 74% (32/43) had resolution of the event; 35 patients had multiple AOEs recorded, with 54% (19/35) patients having resolution of all the events. Most patients continued ponatinib after the AOE, including 36 patients (46%) who continued ponatinib without dose modification and 27 patients (35%) who had their doses reduced and/or interrupted after the event (Table 9). Seven patients (9%) discontinued ponatinib due to an adjudicated AOE. Rates of dose modifications following AOEs are summarized in Table 9.
Table 9.
Ponatinib dose modifications following non-adjudicated and adjudicated arterial occlusive events (AOEs)a
| Any AOE | Serious AOE | |||
|---|---|---|---|---|
| Non-adjudicatedb (n = 111) |
Adjudicatedc (n = 78) |
Non-adjudicatedb (n = 90) |
Adjudicatedc (n = 74) |
|
| No dose modification | 46 (41) | 36 (46) | 28 (31) | 31 (42) |
| Drug interrupted only | 37 (33) | 25 (32) | 37 (41) | 26 (35) |
| Dose reduced only | 6 (5) | 0 | 5 (6) | 0 |
| Dose reduced + drug interrupted | 5 (5) | 2 (3) | 4 (4) | 2 (3) |
| Drug interrupted + drug withdrawn | 0 | 2 (3) | 0 | 2 (3) |
| Drug withdrawn | 17 (15) | 5 (6) | 16 (18) | 5 (7) |
| Not applicable/unknown | 0 | 8 (10) | 0 | 8 (11) |
Data are no. (%) of patients with an AOE
MedDRA Medical Dictionary for Regulatory Activities
aWhen a patient had multiple events, dose modification was derived as the most severe one across all events with the following severity order (high to low): drug withdrawn, drug reduced plus drug interrupted, drug reduced only, drug interrupted only, no dose modification
bCategorization of AOEs is based on MedDRA preferred terms related to vascular ischemia or thrombosis
cEvents adjudicated as AOEs by the cardiovascular endpoint Adjudication Committee
Risk factor analysis
The most common baseline risk factors in patients who developed an AOE were arterial hypertension and hypercholesterolemia (Table 10). Patients with adjudicated AOEs also had higher rates of concomitant use of antihypertensive medications, platelet aggregation inhibitor medications, and anti-diabetic agents compared with patients who did not have AOEs (Table 11).
Table 10.
Prevalence of baseline risk factors by adjudicated AOE and serious AOE status
| No. (%) of patients | Any AOE | Any serious AOE | ||
|---|---|---|---|---|
| No (n = 371) | Yes (n = 78) | No (n = 375) | Yes (n = 74) | |
| Age, ≥ 65 years | 118 (32) | 37 (47) | 120 (32) | 35 (47) |
| Sex, male | 187 (50) | 51 (65) | 188 (50) | 50 (68) |
| History of ischemic disease | 45 (12) | 22 (28) | 45 (12) | 22 (30) |
| Diabetes mellitus | 45 (12) | 27 (35) | 48 (13) | 24 (32) |
| Baseline glucose grade ≥ 2 | 24 (6) | 14 (18) | 25 (7) | 13 (18) |
| Venous thromboembolism | 30 (8) | 8 (10) | 30 (8) | 8 (11) |
| Arterial hypertension | 181 (49) | 59 (76) | 185 (49) | 55 (74.3) |
| Baseline blood pressure grade ≥ 2 | 32 (9) | 7 (9) | 32 (9) | 7 (9) |
| Hypercholesterolemia | 167 (45) | 52 (67) | 169 (45) | 50 (68) |
| Baseline triglycerides grade ≥ 1 | 112 (30) | 28 (36) | 114 (30) | 26 (35) |
| History of non-ischemic cardiac disease | 120 (32) | 30 (38) | 121 (32) | 29 (39) |
| Obesity | 88 (24) | 21 (27) | 90 (24) | 19 (26) |
| Baseline BMI ≥ 30 kg/m−2 | 86 (23) | 21 (27) | 88 (23) | 19 (26) |
AOE arterial occlusive event, BMI body mass index
Table 11.
Concomitant medication use by adjudicated AOE and serious AOE status
| Total (n = 449) | No AOE (n = 371) | Any AOE (n = 78) | Serious AOE (n = 74) | |
|---|---|---|---|---|
| Baseline concomitant medications | ||||
| Antihypertensives | 86 (19) | 63 (17) | 23 (29) | 22 (30) |
| Acetylsalicylic acid | 39 (9) | 23 (6) | 16 (21) | 15 (20) |
| Platelet aggregation inhibitors | 38 (8) | 22 (6) | 16 (21) | 15 (20) |
| Anti-diabetic agents | 24 (5) | 13 (4) | 11 (14) | 10 (14) |
| Lipid-modifying agents | 22 (5) | 16 (4) | 6 (8) | 6 (8) |
| Anticoagulants | 15 (3) | 13 (4) | 2 (3) | 2 (3) |
| Concomitant medication use at any time | ||||
| Antihypertensives | 233 (52) | 181 (49) | 52 (67) | 50 (68) |
| Acetylsalicylic acid | 125 (28) | 92 (25) | 33 (42) | 33 (45) |
| Platelet aggregation inhibitors | 122 (27) | 85 (23) | 37 (47) | 37 (50) |
| Anticoagulants | 58 (13) | 50 (13) | 8 (10) | 8 (11) |
| Lipid-modifying agents | 51(11) | 39 (11) | 12 (15) | 12 (16) |
| Anti-diabetic agents | 45 (10) | 26 (7) | 19 (24) | 18 (24) |
Data are no. (%) of patients
AOE arterial occlusive event
The incidence of adjudicated AOEs by number of baseline risk factors (including arterial hypertension, hypercholesterolemia, obesity, diabetes mellitus, non-ischemic cardiac disease, and ischemic disease) is shown in Fig. 2C. The rate of adjudicated AOEs was 13% (24/189) among patients with 1–2 risk factors, and 29% (52/180) among patients with 3 or more risk factors. Of the 80 patients without any risk factors at baseline, only 2 (3%) had an AOE.
Fatal AOEs
Separate adjudication of deaths revealed that 11 adjudicated AOEs were associated with death. These included 2 cases of cardiac arrest and 1 each of the following: bradycardic arrest, cardiac failure, intracranial hemorrhage, worsening of congestive heart failure, superior mesenteric artery occlusion, hemorrhagic cerebral infarction, congestive heart failure, ischemic stroke, and acute anterior myocardial infarction. Nine of the 11 patients with AOEs associated with death had a history of cardiovascular events and/or cardiovascular risk factors recorded at baseline (Table 12). The long-term survival of patients with adjudicated AOEs was similar to survival of patients without AOEs (Fig. 2D).
Table 12.
Fatal AOEs and patient baseline characteristics
| Fatal event | Fatal PT | Other AOE PTs reported | CML/ALL status | History of CV events | CV risk factors at baseline |
|---|---|---|---|---|---|
| Bradycardiac arrest | Cardiac arrest |
Cardiac arrest Dry gangrene Peripheral ischemia |
CML |
Congestive heart failure Hypertension Impaired diastolic filling pattern Left atrium enlargement Mild tricuspid regurgitation Mitral valve calcification without significant mitral stenosis Intermittent ventricular tachycardia |
Obesity Diabetes mellitus Arterial hypertension |
| Cardiac failure | Cardiac failure |
Myocardial infarction Coronary artery disease Pulmonary embolism |
CML |
Pericarditis Ischemic heart failure |
|
| Intracranial hemorrhage | Hemorrhage intracranial | CML |
Aortic stenosis Calcified mitral annulus |
||
| Worsening of congestive heart failure | Cardiac failure congestive |
Myocardial infarction Deep vein thrombosis |
QTc prolongation with nilotinib use Stent placement Congestive heart failure Myocardial infarction Coronary artery disease Mitral regurgitation Trace of tricuspid valve regurgitation |
Hyperlipidemia Arterial hypertension |
|
| Superior mesenteric artery occlusion | Mesenteric arterial occlusion | Celiac artery occlusion | ALL |
Paroxysmal atrial fibrillation Thrombophlebitis Bilateral leg deep vein thrombosis Cardiac catheterization |
Hyperlipidemia Arterial hypertension |
| Cardiac arrest | Cardiac arrest | Peripheral vascular disorder | ALL |
Greater saphenous vein thrombosis and cellulitis Aortic valve slightly thickened Left axis deviation Left bundle branch block Hypertension Mild aortic regurgitation Mild pulmonic valve regurgitation Mild to moderate tricuspid regurgitation |
Arterial hypertension |
| Hemorrhagic cerebral infarction | Hemorrhagic cerebral infarction |
Cerebral artery stenosis (2 events) Cerebral infarction (2 events) |
CML |
Diabetes mellitus Arterial hypertension |
|
| Cardiac arrest | Cardiac arrest | CML | |||
| Cardiac arrest | Cardiac arrest | CML |
Ischemic heart disease Angina pectoris |
Coronary artery disease Type 2 diabetes mellitus Hypertension |
|
| Congestive heart failure | Cardiac failure congestive | CML | |||
| Stroke | Cerebrovascular accident | Acute myocardial infarction (2 events) | CML |
Ischemic stroke Ischemic heart disease Coronary artery disease Revascularization and coronary stent placement |
Diabetes mellitus Arterial hypertension Hypercholesterolemia |
ALL acute lymphocytic leukemia, AOE arterial occlusive event, CML chronic myeloid leukemia, CV cardiovascular, PTs preferred terms
Discussion
In this study, adjudication of AOEs by an independent committee of experts allowed for a clinically meaningful description of AOEs associated with ponatinib, which can help to inform health care providers and patients of safety risks in an accurate and objective manner. The search that identified potential AOEs for adjudication was broader (based on 604 MedDRA terms related to vascular ischemia or thrombosis) than that initially used to calculate non-adjudicated AOE rates in the PACE trial (400 MedDRA terms) [3]. Based on 5-year follow-up of the PACE trial, the adjudicated AOE rate (17%) was lower than the non-adjudicated AOE rate (25%) [3]. Although the majority of adjudicated AOEs were serious, 81% of patients with AOEs continued on ponatinib (35% with dose modifications), the benefit of the drug was felt to outweigh the risk of the AOEs. Although vascular occlusive events were rarely reported during the initial development of second-generation BCR::ABL1 TKIs, a meta-analysis found that these events occurred in 5.9% of patients with CML treated with these agents, including bosutinib, dasatinib, nilotinib, and ponatinib [13]. In another review of prospective trials of patients treated with TKIs, including imatinib, nilotinib, dasatinib, and ponatinib, overall incidence of CV events was 45% (range, 41–63%) [14]. Accordingly, a high level of vigilance is indicated to recognize this potential complication of TKI therapy.
Notably, although concern existed around the potential for increasing AOE rates with long-term dosing, as seen with AEs related to other TKIs [15–18] the exposure-adjusted incidence of newly occurring adjudicated AOEs decreased over time on ponatinib, suggesting that the toxicity of ponatinib may not increase with longer treatment duration.
Patients with adjudicated AOEs were more likely to have multiple baseline cardiovascular risk factors (e.g., ischemic cardiac disease, arterial hypertension, hypercholesterolemia, and diabetes mellitus), and only 2 patients had an adjudicated AOE without any cardiovascular risk factors. These observations align with those of previous studies [6, 19]. It is important to identify and manage cardiovascular risk factors before and during therapy with ponatinib or other TKIs [20–22]. In PACE, 80% of CP-CML patients were resistant to dasatinib or nilotinib, and 24% had the BCR::ABL1T315I resistance mutation [3]. Among CP-CML patients, estimated 5-year PFS and OS rates were 53% and 73%, respectively [3]. Data for overall survival in patients with and without adjudicated AOEs suggest that the risk of AOE-related death did not substantially impact survival, with disease-related death being the main driver of the OS curve. This underscores the need for providers to fully understand the therapeutic profile of ponatinib and consider its use when the potential benefits outweigh the risks for a given patient.
This study reinforces the importance of proper assessment of cardiovascular AEs to ensure accurate estimation of cardiovascular risk. The conventional processes of AE reporting and causality assessment may need to be re-assessed to avoid pitfalls associated with over- or under-reporting of AOEs, both of which may adversely affect patient care [23, 24]. Formal adjudication of events is a mainstay for development programs in other therapeutic areas such as diabetes mellitus [25, 26] and cardiology. A better understanding of the AOE risk associated with TKI therapy is a prime example of where formal adjudication is critical because accurate knowledge of risks is crucial before prescribing any TKI. The potential benefits of effective BCR::ABL1 TKI treatment, even with accompanying AEs, may outweigh the potential risks of progression-related mortality in patients with CP-CML and Ph+ ALL receiving second- or third-line therapy. This is particularly true for patients such as those with the BCR::ABL1T315I mutation who may have limited treatment options [27]. Understanding the true incidence of the most significant events is a central element in properly assessing the benefit-risk ratio of an intervention. All later-generation TKIs are associated with risk of cardiovascular AEs [28], and the results of the formal adjudication process suggest the risk of these events with ponatinib may not be dissimilar to the event rates seen with some second-generation BCR::ABL1 TKIs [16–18].
A noteworthy finding in our analysis is that the exposure-adjusted incidence of newly occurring adjudicated AOEs decreased over time on ponatinib. These results are reassuring that the rate of new AOEs may not increase with longer duration of ponatinib treatment. Furthermore, patients with positively adjudicated AOEs were much more likely to have baseline cardiovascular risk factors (e.g., arterial hypertension, hypercholesterolemia, diabetes mellitus) or established cardiovascular disease; of those patients without any cardiovascular risk factors only 2 had a subsequent AOE. These results may provide clinical guidance with respect to the approach to use of ponatinib in patients at risk for an AOE. The ongoing phase 2 OPTIC trial (ClinicalTrials.gov Identifier: NCT02467270) is using a response-based dose reduction protocol approach to evaluate the optimal ponatinib dosing regimen for maximizing efficacy while mitigating toxicity. Results show that higher doses of ponatinib were associated with increased incidence of AOEs, with exposure-adjusted treatment-emergent AOE rates of 5.6%, 3.6%, and 2.1% for the 45-mg, 30-mg, and 15-mg cohorts, respectively [29]. However, the benefit differential was considerably larger with a starting dose of 45 mg, which was associated with a 26.3 percentage-point improvement in the response rate compared with a 15-mg starting dose (51.6% vs. 25.3%) [29]. Overall, the study indicated the best risk/benefit ratio when the 45-mg starting dose was reduced to 15 mg upon achievement of response (BCR::ABL1IS transcript levels ≤ 1%) [29].
This retrospective study has strengths and limitations. The adjudication methodology provided a comprehensive and objective approach for characterizing AOE risk. A limitation is that only data from the clinical trial database were available. Prospective implementation of this strategy, as is being done in 2 ongoing trials, OPTIC and Ponatinib-3001 (NCT03589326), will overcome this challenge and add further value to the methodology and strength to the conclusions. In OPTIC, an independent cardiovascular endpoint adjudication committee is reviewing AOEs as they are reported using source documentation including cardiovascular workup (e.g., echocardiograms, electrocardiograms, biomarkers), hospitalization records, and any cardiovascular examinations performed.
Conclusions
Independent reconsideration of AOEs by an expert adjudication committee showed lower rates of clinically relevant AOEs overall (17% vs. 25%) and serious AOEs (16% vs. 20%) than were originally reported in the PACE trial, suggesting an earlier possible overestimation that may not accurately reflect the AOE risk with ponatinib. The incidence of exposure-adjusted newly occurring AOEs decreased over time during ponatinib treatment. Improved understanding of the AOE profile with ponatinib and risk factors for AOEs can help guide decisions around TKI treatment. Results from the OPTIC study support a novel ponatinib treatment regimen of a 45-mg starting dose reduced to 15 mg upon achievement of response, maximizing response while minimizing toxicity [29].
Acknowledgements
We thank the patients, their families, and their caregivers, and the study investigators and their team members at each site for participation in the PACE trial. Professional medical writing assistance was provided by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, USA, and funded by Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
Presented in part at Januzzi, J.L., Garasic, J., Kasner, S. et al. (2020). "An independent review of arterial occlusive events (AOEs) in the ponatinib (PON) phase II PACE trial (NCT01207440) in patients (pts) with Ph+ leukemia [abstract]." Journal of Clinical Oncology 38(15 suppl): 7550. Januzzi, J., Garasic, J., Kasner, S. et al. (2020). "Retrospective independent review of arterial occlusive events (AOES) in the phase 2 pace trial of ponatinib in Philadelphia chromosome-positive (PH+) leukemia [abstract]." HemaSphere 4(Suppl 1): 338; Januzzi, J., Garasic, J., Kasner, S. et al. (2020). "Retrospective independent review of arterial occlusive events in the phase 2 pace trial of ponatinib in Philadelphia chromosome-positive leukemia [abstract]." Presented at the 8th Annual Meeting (Virtual) of the Society of Hematologic Oncology (SOHO), September 9–12, 2020; Januzzi, J., Garasic, J., Kasner, S. et al. (2020). "Retrospective independent review of arterial occlusive events in the phase 2 pace trial (NCT01207440) of ponatinib in Philadelphia chromosome-positive leukemia [abstract]." Presented at the John Goldman E-Conference on Chronic Myeloid Leukemia: Biology and Therapy (iCMLf) October 1–4, 2020.xxx
Abbreviations
- ACC
American College of Cardiology
- AE
Adverse events
- AHA
American Heart Association
- ALL
Acute lymphoblastic leukemia
- AOE
Arterial occlusive event
- AP
Accelerated-phase
- BP
Blast-phase
- CML
Chronic myeloid leukemia
- CP-CML
Chronic-phase chronic myeloid leukemia
- MeDRA
Medical Dictionary for Regulatory Activities
- PACE
Ponatinib Ph+ ALL and CML Evaluation
- PFS
Progression-free survival
- Ph+
Philadelphia chromosome positive
- qd
Once daily
- SCTI
Standardized Data Collection for Cardiovascular Trials Initiative
- SMQ
Cardiac Failure Standard MedDRA Query
- TKI
Tyrosine kinase inhibitor
Authors' contributions
KC, EB, DN, JX, SS, JLJ, TH, and JC were involved in the conception and design. JC, MM, MD, AH, JP-I, FN, D-WK, DJD, and HK contributed to the provision of study material or patients. JMG, SEK, VM, MCP, JC, MM, MD, AH, JP-I, FN, D-WK, DJD, HK, SS, TH, JX, and DN contributed to the collection and assembly of data. All authors contributed to the data analysis and interpretation. All authors wrote the manuscript. All authors were involved in the final approval of manuscript. All authors are accountable for all aspects of the work. All authors performed data analysis and interpretation, had full access and verified all the data in the study, and had final responsibility for the decision to submit for publication. All authors were involved in drafting and providing critical revision of the article. All authors read and approved the final manuscript.
Funding
The PACE study is sponsored by ARIAD Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
Availability of data and materials
The data sets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results reported in this article, will be made available within three months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection and requirements for consent and anonymization.
Declarations
Ethics approval and consent to participate
PACE was approved by local ethics committees and was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonisation guidelines for good clinical practice. All patients provided written informed consent.
Consent for publication
All authors have critically reviewed the manuscript and consent to publication.
Competing interests
J.L.J.: Consulting/advisory role (Takeda), research funding (Novartis); J.M.G.: Employment and stock/other ownership (family member: Vertex Pharmaceuticals), consulting/advisory role (Clinical Events Committee, ACI; AbbVie; Baim Institute; Parexel), research funding (ReCor Medical); S.E.K.: research funding (Bristol Myers Squibb, Genentech, Medtronic), consulting/advisory role (AbbVie, Abbott, AstraZeneca, BMS, Janssen, Takeda, Medtronic); V.M.: Consulting/advisory role (Takeda, Novartis, Amgen, Bayer), honoraria (Amgen, Novartis), research funding (Novartis, Rigel); M.C.P.: Endpoint adjudication committee (Takeda); J.S.: Employment, leadership role, stock/other ownership (WCG Clinical); M.M.: Consulting/advisory role, travel/accommodations/expenses, and honoraria (Novartis, BMS, Pfizer, Takeda), research funding (all to institution: Sun Pharma, Novartis, BMS); K.C.: Honoraria, consulting/advisory role, travel/accommodations/expenses (Takeda, Abbott, CSI, Philips, Abiomed, Cordis, Boston Scientific), research funding (Abbott, ARIAD, Takeda); E.B.: Honoraria, consulting/advisory role, travel/accommodations/expenses (ARIAD); MD: Consulting/advisory role (Blueprint, Fusion Pharma, Takeda, Humana, Ascentage Pharma, Adelphi, Medscape, Novartis), research funding (Takeda, Pfizer, Novartis, Incyte, SPARC, Blueprint, Leukemia & Lymphoma Society); A.H.: Research funding (Incyte, BMS, Novartis, Pfizer); J.P.I.: Consulting/advisory role (AbbVie, Janssen, AstraZeneca, Novartis, TG Therapeutics, Takeda), speakers bureau (AbbVie, Janssen, AstraZeneca, Takeda), research funding (MEI, Sunesis), patents/royalties/other intellectual property (Sellas); F.N.: Honoraria, speakers bureau, travel/accommodations/expenses (Novartis, Incyte Biosciences), consulting/advisory role (Sun Pharma), research funding (Incyte Biosciences); D.W.K.: Research funding (Novartis, BMS, Pfizer, Takeda, Sun Pharma, Il-Yang Pharm. Co., Ltd.), advisory board (Novartis, BMS); D.J.D.: Consulting/advisory role (Incyte, Pfizer, BMS, Amgen, Novartis, Celgene, Immunogen, Takeda, Blueprint Medicines), research funding (all to institution: Novartis, AbbVie, GlycoMimetics, Blueprint Medicines); H.K.: Honoraria (AbbVie, Amgen, ARIAD, BMS, Immunogen, Orsenix, Pfizer, Agios, Takeda, Actinium Pharmaceuticals), research funding (all to institution: Pfizer, Amgen, BMS, Novartis, ARIAD, Astex Pharmaceuticals, AbbVie, Agios, Cyclacel, Immunogen, Jazz Pharmaceuticals); J.X.: Employment (Takeda); T.H.: Employment (Takeda); S.S.: Employment (Takeda); D.N.: Employment (Takeda); J.C.: Consulting/advisory role (BMS, Novartis, Pfizer, Takeda), research funding (Novartis, Pfizer, Takeda, Sun Pharma).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/23/2022
A Correction to this paper has been published: 10.1186/s13045-022-01239-x
References
- 1.O'Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783–1796. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome–positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132:393–404. doi: 10.1182/blood-2016-09-739086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breccia M, Abruzzese E, Castagnetti F, et al. Ponatinib as second-line treatment in chronic phase chronic myeloid leukemia patients in real-life practice. Ann Hematol. 2018;97:1577–1580. doi: 10.1007/s00277-018-3337-2. [DOI] [PubMed] [Google Scholar]
- 5.Shacham-Abulafia A, Raanani P, Lavie D, et al. Real-life experience with ponatinib in chronic myeloid leukemia: a multicenter observational study. Clin Lymphoma Myeloma Leuk. 2018;18:e295–e301. doi: 10.1016/j.clml.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Caocci G, Mulas O, Abruzzese E, et al. Arterial occlusive events in chronic myeloid leukemia patients treated with ponatinib in the real-life practice are predicted by the Systematic Coronary Risk Evaluation (SCORE) chart. Hematol Oncol. 2019;37:296–302. doi: 10.1002/hon.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heiblig M, Rea D, Chretien ML, et al. Ponatinib evaluation and safety in real-life chronic myelogenous leukemia patients failing more than two tyrosine kinase inhibitors: the PEARL observational study. Exp Hematol. 2018;67:41–48. doi: 10.1016/j.exphem.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Ashaye AO, Thomas C, Dalal M, et al. Treatment of newly diagnosed Philadelphia chromosome positive acute lymphoblastic leukemia using tyrosine kinase inhibitors in combination with chemotherapy: a patient-centered benefit-risk assessment [abstract] Blood. 2020;136(Suppl 1):20–21. doi: 10.1182/blood-2020-137577. [DOI] [Google Scholar]
- 9.Kantarjian HM, Deininger MW, Abruzzese E, et al. Efficacy and safety of ponatinib (PON) in patients with chronic-phase chronic myeloid leukemia (CP-CML) who failed one or more second-generation (2G) tyrosine kinase inhibitors (TKIs): analyses based on PACE and Optic [abstract] Blood. 2020;136(suppl 1):43–44. doi: 10.1182/blood-2020-133922. [DOI] [Google Scholar]
- 10.Hicks KA, Hung HMJ, Mahaffey KW, et al. Standardized definitions for cardiovascular and stroke endpoint events in clinical trials. 2014. Available at https://www.cdisc.org/system/files/all/standard/Draft%20Definitions%20for%20CDISC%20August%2020%2C%202014.pdf. Accessed 14 Nov 2019.
- 11.Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (writing committee to develop cardiovascular endpoints data standards) J Am Coll Cardiol. 2015;66:403–469. doi: 10.1016/j.jacc.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Hicks KA, Mahaffey KW, Mehran R, et al. 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation. 2018;137:961–972. doi: 10.1161/CIRCULATIONAHA.117.033502. [DOI] [PubMed] [Google Scholar]
- 13.Douxfils J, Haguet H, Mullier F, Chatelain C, Graux C, Dogne JM. Association between BCR-ABL tyrosine kinase inhibitors for chronic myeloid leukemia and cardiovascular events, major molecular response, and overall survival: a systematic review and meta-analysis. JAMA Oncol. 2016;2:625–632. doi: 10.1001/jamaoncol.2015.5932. [DOI] [PubMed] [Google Scholar]
- 14.Jain P, Kantarjian H, Boddu PC, et al. Analysis of cardiovascular and arteriothrombotic adverse events in chronic-phase CML patients after frontline TKIs. Blood Adv. 2019;3:851–861. doi: 10.1182/bloodadvances.2018025874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib versus imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–1054. doi: 10.1038/leu.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantarjian HM, Hughes TP, Larson RA, et al. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia. 2021;35:440–453. doi: 10.1038/s41375-020-01111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rea D, Mirault T, Raffoux E, et al. Identification of patients (pts) with chronic myeloid leukemia (CML) at high risk of artery occlusive events (AOE) during treatment with the 2nd generation tyrosine kinase inhibitor (TKI) nilotinib, using risk stratification for cardiovascular diseases (CVD) [abstract] Blood. 2013;122:2726. doi: 10.1182/blood.V122.21.2726.2726. [DOI] [Google Scholar]
- 18.Valent P, Hadzijusufovic E, Schernthaner GH, Wolf D, Rea D, le Coutre P. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood. 2015;125:901–906. doi: 10.1182/blood-2014-09-594432. [DOI] [PubMed] [Google Scholar]
- 19.Dorer DJ, Knickerbocker RK, Baccarani M, et al. Impact of dose intensity of ponatinib on selected adverse events: multivariate analyses from a pooled population of clinical trial patients. Leuk Res. 2016;48:84–91. doi: 10.1016/j.leukres.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Cortes J. How to manage CML patients with comorbidities. Blood. 2020;136:2507–2512. doi: 10.1182/blood.2020006911. [DOI] [PubMed] [Google Scholar]
- 21.Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–984. doi: 10.1038/s41375-020-0776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steegmann JL, Baccarani M, Breccia M, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30:1648–1671. doi: 10.1038/leu.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo Rossi A, Breccia M, Abruzzese E, et al. Outcome of 82 chronic myeloid leukemia patients treated with nilotinib or dasatinib after failure of two prior tyrosine kinase inhibitors. Haematologica. 2013;98:399–403. doi: 10.3324/haematol.2012.064337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ongoren S, Eskazan AE, Suzan V, et al. Third-line treatment with second-generation tyrosine kinase inhibitors (dasatinib or nilotinib) in patients with chronic myeloid leukemia after two prior TKIs: real-life data on a single center experience along with the review of the literature. Hematology. 2018;23:212–220. doi: 10.1080/10245332.2017.1385193. [DOI] [PubMed] [Google Scholar]
- 25.Guidance for Industry Diabetes Mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. 2008. Available at https://www.fda.gov/media/71297/download. Accessed 1 Apr 2021.
- 26.US Food and Drug Administration Type 2 diabetes mellitus: evaluating the safety of new drugs for improving glycemic control; draft guidance for industry; availability. Fed Regist. 2020;85:13903–13905. [Google Scholar]
- 27.Moslehi JJ, Deininger M. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol. 2015;33:4210–4218. doi: 10.1200/JCO.2015.62.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medeiros BC, Possick J, Fradley M. Cardiovascular, pulmonary, and metabolic toxicities complicating tyrosine kinase inhibitor therapy in chronic myeloid leukemia: strategies for monitoring, detecting, and managing. Blood Rev. 2018;32:289–299. doi: 10.1016/j.blre.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Cortes J, Apperley JF, Lomaia E, et al. Ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia: a randomized, open-label phase 2 clinical trial. Blood. 2021;138:2042–2050. doi: 10.1182/blood.2021012082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results reported in this article, will be made available within three months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection and requirements for consent and anonymization.