Abstract
Female hypocretin knockout (Hcrt KO) mice have increased body weight despite decreased food intake compared to wild type (WT) mice. In order to understand the nature of the increased body weight, we carried out a detailed study of Hcrt KO and WT, male, and female mice. Female KO mice showed consistently higher body weight than WT mice, from 4 to 20 months (20–60%). Fat, muscle, and free fluid levels were all significantly higher in adult (7–9 months) as well as old (18–20 months) female KO mice compared to age-matched WT mice. Old male KO mice showed significantly higher fat content (150%) compared to age-matched WT mice, but no significant change in body weight. Respiratory quotient (−19%) and metabolic rates (−14%) were significantly lower in KO mice compared to WT mice, regardless of gender or age. Female KO mice had significantly higher serum leptin levels (191%) than WT mice at 18–20 months, but no difference between male mice were observed. Conversely, insulin resistance was significantly higher in both male (73%) and female (93%) KO mice compared to age- and sex-matched WT mice. We conclude that absence of the Hcrt peptide has gender-specific effects. In contrast, Hcrt-ataxin mice and human narcoleptics, with loss of the whole Hcrt cell, show weight gain in both sexes.
Keywords: adiposity, body weight, gender, hypocretin/orexin, insulin, leptin
The hypothalamic neuropeptide, hypocretin (Hcrt), also known as orexin, has been implicated in energy metabolism, body weight control, sleep–wake regulation, reward-motivated behaviors and addiction (Hara et al. 2001; Mochizuki et al. 2004; Zhang et al. 2007; Tsuneki et al. 2008; McGregor et al. 2011; Mahler et al. 2012). Several studies have shown that loss of Hcrt neurons causes narcolepsy with cataplexy in humans (Peyron et al. 2000; Thannickal et al. 2000). Similar symptoms are observed in hypocretin knockout (KO) and Hcrt-ataxin mice (Chemelli et al. 1999; Hara et al. 2001).
In humans, increased body mass index (BMI) has been noted in both men and women narcoleptics compared to the general population. Poli et al. (2009) reported that patients with narcolepsy with cataplexy (all HLA DQBI*0602 positive, with CSF levels of orexin A < 110 pg/mL) had a higher BMI and BMI-independent metabolic alterations, including increased waist circumference, high density lipo-protein-cholesterol levels, and glucose/insulin ratio (insulin resistance index), compared to patients with idiopathic hypersomnia (with CSF levels of orexin A > 300 pg/mL). This suggests that loss of Hcrt neurons may lead to altered metabolic control, resulting in obesity, insulin resistance, and increased risk of type 2 diabetes. Despite increased BMI, normal or decreased leptin levels were seen in both men and women narcoleptics, compared to the general population. Donjacour et al. (2013) reported no changes in total ghrelin or leptin levels in narcoleptic patients on or off sodium oxybate, compared to the general population. On the other hand, Kok et al. (2003) reported that male narcoleptics had a large reduction in leptin levels compared to control humans, suggesting that lowered leptin levels may predispose narcoleptic humans to weight gain. Beitinger et al. (2012) reported that narcoleptic, nonobese patients, who have low levels of Hcrt, showed a tendency to decreased insulin sensitivity. Honda et al. (1986) showed that noninsulin-dependent diabetes mellitus was significantly increased in narcoleptics, compared to the general Japanese population, irrespective of their obesity index.
In animals, several authors have reported increased body weight in female but not male Hcrt KO mice compared to wild type (WT) littermate controls (Fujiki et al. 2006; Tsuneki et al. 2008). This is despite the fact that both sexes showed decreased food intake (Tsuneki et al. 2008) and decreased locomotor activity (Kayaba et al. 2003; Mochizuki et al. 2006; Tsuneki et al. 2008). Tsuneki et al. (2008) further reported that impaired glucose tolerance, decreased insulin sensitivity, and increased serum insulin levels were observed in both male and female Hcrt KO mice, compared to gender-matched WT mice. However, increased serum leptin levels were only observed in female mice. Fujiki et al. (2006) reported that obesity was more prominent in female, than male Hcrt KO and Hcrt-ataxin transgenic (TG) mice compared to WT controls, and was associated with higher serum leptin levels, suggesting a gender-specific alteration of leptin-hypocretin signaling. Hara et al. (2001) reported that male Hcrt-ataxin TG mice exhibited increased body weight, decreased food intake and decreased locomotor activity compared to WT mice. Zhang et al. (2007) similarly reported that male Hcrt-ataxin TG mice exhibited decreased feeding and drinking behavior, as well as decreased energy expenditure and decreased locomotor activity compared to their WT littermates. This may account for the increased body weight observed in these male Hcrt-ataxin TG mice.
Female Hcrt KO mice have increased body weight despite decreased food and water intake compared to female WT mice. In order to understand the nature of the increased body weight, we carried out a detailed study of Hcrt KO and WT, male and female mice. The mice were followed from 4 to 20 months, a longer period than has been used in prior studies. We also analyzed, for the first time, changes in the amount of fat, muscle, free fluid content and white adipose tissue (WAT), and brown adipose tissue (BAT), as well as respiratory quotient (RQ), and metabolic rate (MR). The mice were then sacrificed and blood glucose, serum insulin, and leptin levels were measured.
Methods
Ethical approval
All procedures were approved by the Institutional Animal Care and Use Committee of the University of California at Los Angeles (UCLA) and the Veterans Administration Greater Los Angeles Health Care System (VAGLAHS). Every measure was taken to minimize pain or discomfort in the animals.
Animals
Hcrt KO mice, with a mixed C57BL/6J-129/SvEv background, and their WT littermates were obtained from our breeding colony at the Division of Laboratory Animal Medicine at UCLA. All mice used in this study were derived from heterozygote parents. Between 4 and 5 littermates or age-matched mice, of each genotype and gender, were used (four male WT, five male KO, five female WT and five female KO). The animals were housed under standard light (12 h light/dark cycle, lights on at 7:00 hours) and temperature conditions (24°C ± 1°C), and were given free access to normal laboratory diet (PicoLab Rodent Diet 20 irradiated 5053 from Lab Diet/PMI Nutrition International, St Louis, MO, USA).
Body weight
The mice were weighed every month from 9 to 18 months. Between 18 and 20 months, the mice were transferred to the core Mouse Metabolic Syndrome Phenotype Facility at UCLA, for body composition and metabolic studies. Since female Hcrt KO mice started showing significant increases in body weight at 9 months (the first time point in the initial phase of our study), we monitored another group of female mice (five KO and five WT) starting at 3 months, and subjected them to body composition and metabolic analyses at 7–9 months.
Body composition
The amount of total fat, muscle, and free fluid content of each mouse, was determined by NMR measurement, using the minispec mq series from Bruker (Bruker Optics Inc. Billerica, MA, USA). Each mouse was placed in the chamber for approximately 1–2 min. One reading was taken for each mouse, during the light phase.
Metabolic studies
Indirect calorimetry was recorded using the Comprehensive Laboratory Animal Monitoring System (Columbus Instruments, Columbus, OH, USA). The mice were housed individually in calorimeter chambers. After 3 days of acclimatization, one reading was taken per mouse, during the light phase. Metabolic parameters were calculated as described below (Funato et al. 2009):
Blood and serum analysis
After completing the studies on body composition and metabolic changes, the mice now aged about 20 months, were fasted overnight, and then sacrificed by carbon dioxide inhalation. Blood was collected by cardiac puncture, into red capiject tubes with a serum separator, left at 24°C for 20 min, and then centrifuged for 20 min at 142 g. Blood glucose levels were assayed with a glucometer (ReliOn Ultima), and serum leptin and insulin levels with the Mouse Leptin (Cat # 90030) and the Ultra Sensitive Mouse Insulin (Cat # 90080) ELISA kits respectively (Crystal Chem Inc., Downers Grove, IL, USA), according to manufacturer’s instructions (Funato et al. 2009). The homeostasis model assessment of insulin resistance (HOMA-IR) and beta-cell activity (HOMA-%β) was calculated using fasting glucose and insulin concentrations, with the following formula (Matthews et al. 1985):
Statistical analysis
Three-way repeated measures anova with post hoc Tukey test, was used to determine the interaction between gender, genotype, and age, for body weight analyses, among old (18–20 months) male and female mice, while two-way anova with post hoc Newman–Keuls, was used to determine genotype and gender-specific differences, in the analyses of body composition, metabolic parameters and serum components. Unpaired Student’s t-test was used to determine the differences in body weight, body composition, and metabolic parameters between adult (7–9 months) KO and WT female mice. The significance level was set at p < 0.05.
Results
Body weight
A significant effect of genotype (F = 13.78, p = 0.003), age (F = 31.35, p < 0.0001), interaction between gender and genotype (F = 15.07, p = 0.002), gender and age (F = 5.64, p < 0.0001), and genotype and age (F = 2.93, p = 0.004) on body weight, was observed among old male and female mice. Post hoc Tukey comparisons indicated that female KO mice had significantly higher body weight (ranging from 50 to 60%, p < 0.01) than female WT mice, starting as early as 9 months and persisting until 18 months (Fig. 1a). Male KO mice did not show any significant changes in body weight compared to male WT mice, throughout this period. On the other hand, female WT mice had significantly lower body weight compared to male WT mice between 9 and 17 months (ranging from 37 to 14%, p < 0.01), while female KO mice had significantly higher body weight compared to male KO mice between 16 and 18 months (averaging around 18%, p < 0.01). Since female KO mice started showing significant increases in body weight at 9 months (the first time point in the initial phase of our study), we monitored another group of female mice (five KO and five WT) starting at 3 months. We found that the difference in body weight between female KO and WT mice actually started to become significant as early as 4 months (20%, p < 0.05, Fig. 1b), and continued to increase with age.
Fig. 1.
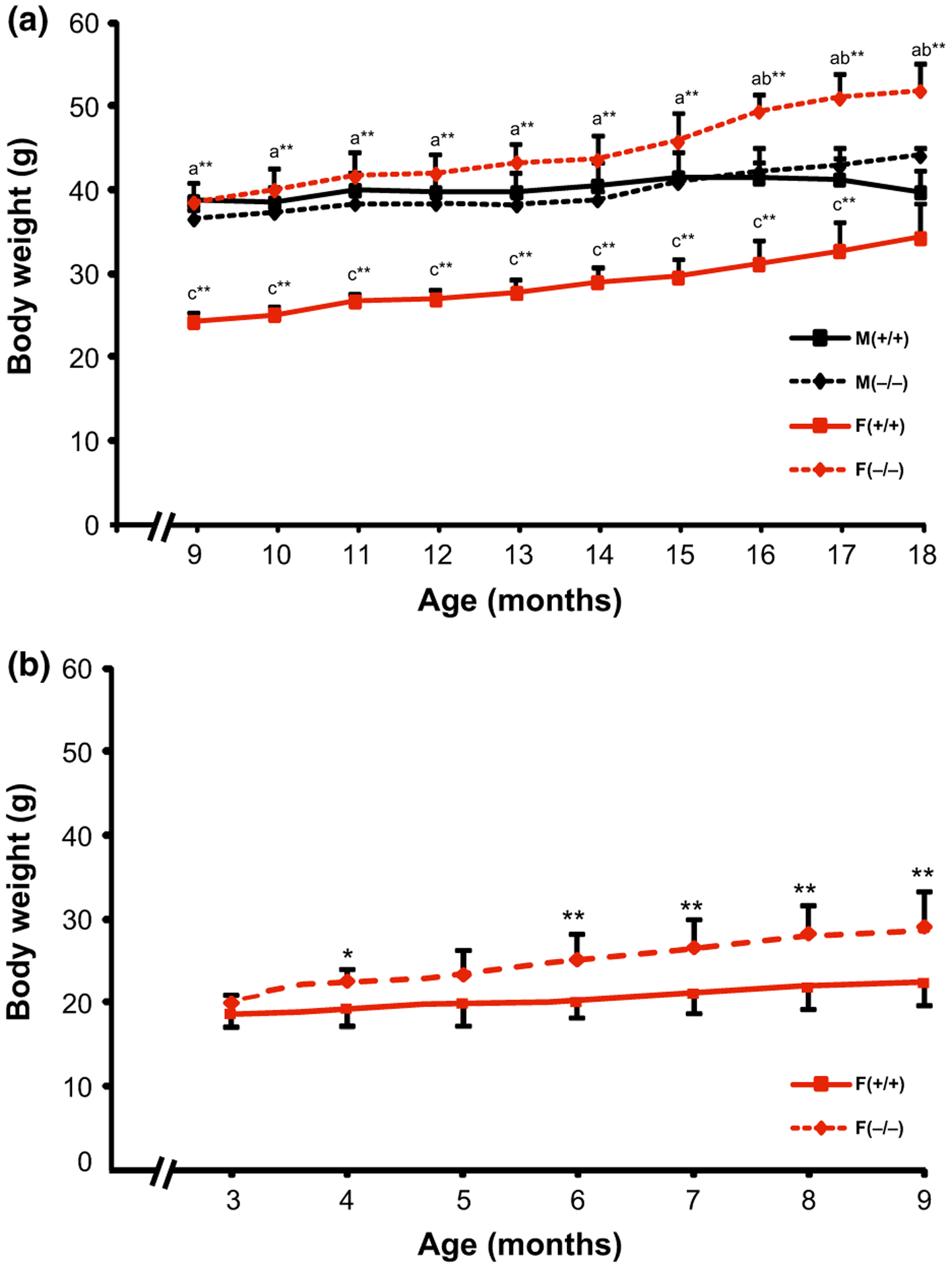
Changes in body weight across the ages in old (9–18 months) male and female (a) and adult (3–9 months) female (b) Hcrt KO and WT mice. Each bar represents the mean value + SEM. a = Significant difference between female WT (+/+) and female KO (−/−) mice, b = significant difference between female and male KO mice, c = significant difference between female and male WT mice, at the level of p < 0.0* and p < 0.01**.
Body composition
Two-way anova showed significant effects of gender (F = 17.68, p = 0.0008) and genotype (F = 19.76, p = 0.0005) in the fat, muscle, and free fluid content. Post hoc Newman–Keuls revealed that old female KO mice had significantly more fat, muscle, and free fluid (90%, 27%, both p < 0.01, and 86%, p < 0.05, respectively) levels compared to female WT mice (Fig. 2a and c). In contrast, the only significant difference between old male mice was the fat content, with male KO mice having significantly more fat (150%, p < 0.01, Fig. 2a) than male WT mice. Muscle and free fluid levels were not significantly different between old male KO and WT mice. Furthermore, female KO mice had significantly more fat (82%, p < 0.01) and free fluid (60%, p < 0.05) content than male KO mice, while female WT mice had significantly more fat (141%, p < 0.05), but less muscle (−25%, p < 0.01) mass than male WT mice. The unpaired Student’s t-test indicated that adult (7–9 months) female KO mice also had significantly higher body fat, muscle, and free fluid (272%, 19%, both p < 0.01, and 42%, p < 0.05, respectively) levels than adult female WT mice (Fig. 2d–f).
Fig. 2.
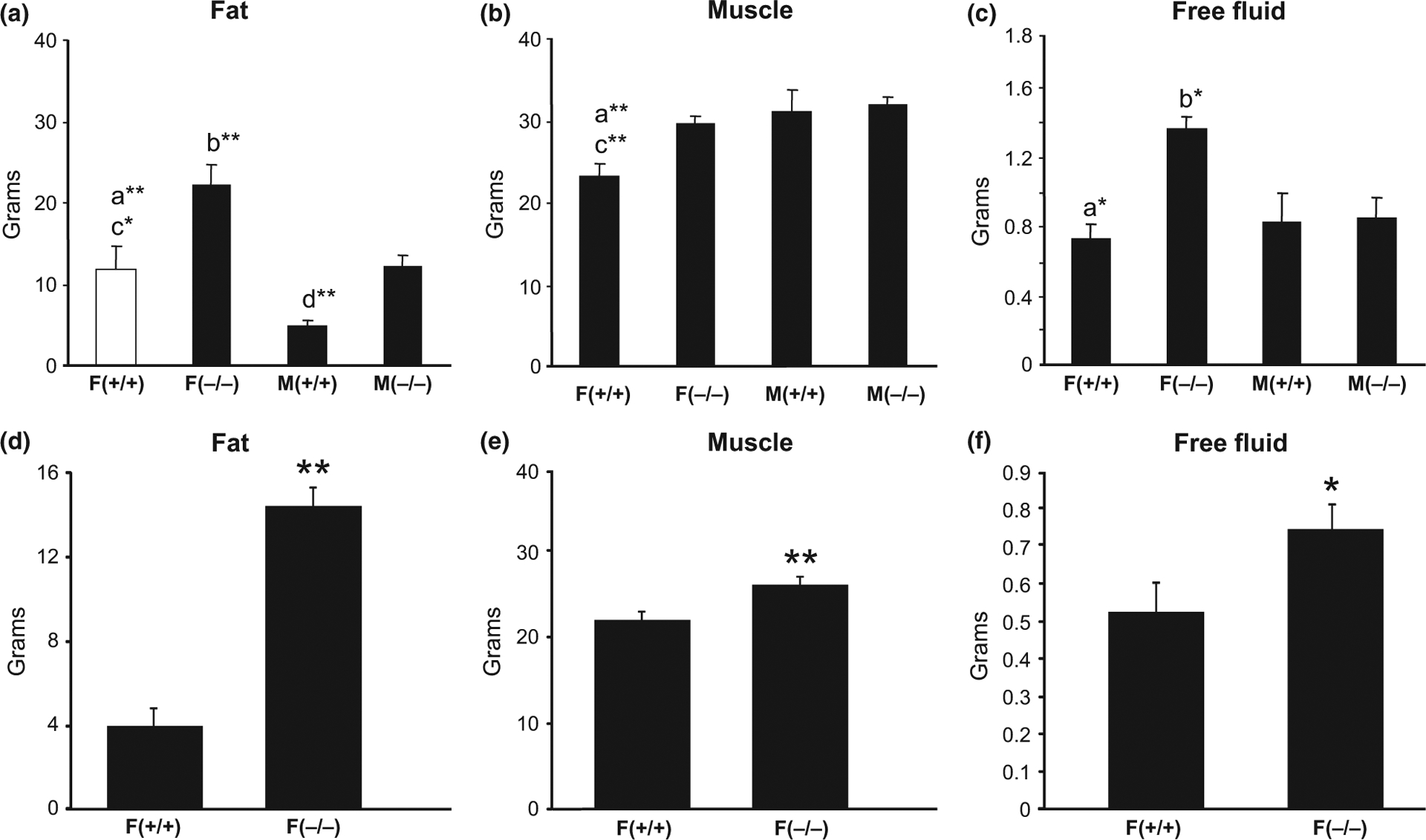
Differences in the major components of the body (fat, muscle and free fluid levels) between old (18–20 months) male and female (a–c) and adult (7–9 months) female (d–f) Hcrt KO and WT mice. Each bar represents the mean value + SEM. a = Significant difference between female WT (+/+) and female KO (−/−) mice, b = significant difference between female and male KO mice, c = significant difference between female and male WT mice, d = significant difference between male WT and KO mice, at the level of p < 0.05*, p < 0.01**.
Metabolic parameters
After completing the analysis for body composition, metabolic parameters, including RQ and MR were measured. Two-way anova indicated a significant effect of genotype, on both RQ (F = 4.97, p < 0.05) and MR (F = 4.85, p < 0.05), with KO mice showing lower RQ (−19%) and lower MR (−14%) than WT mice, irrespective of gender (Fig. 3a and b). Adult female KO mice also had significantly lower (−23%, p = 0.003) MR compared to age-matched female WT mice, but RQ was not significantly different (Fig. 3c and d).
Fig. 3.
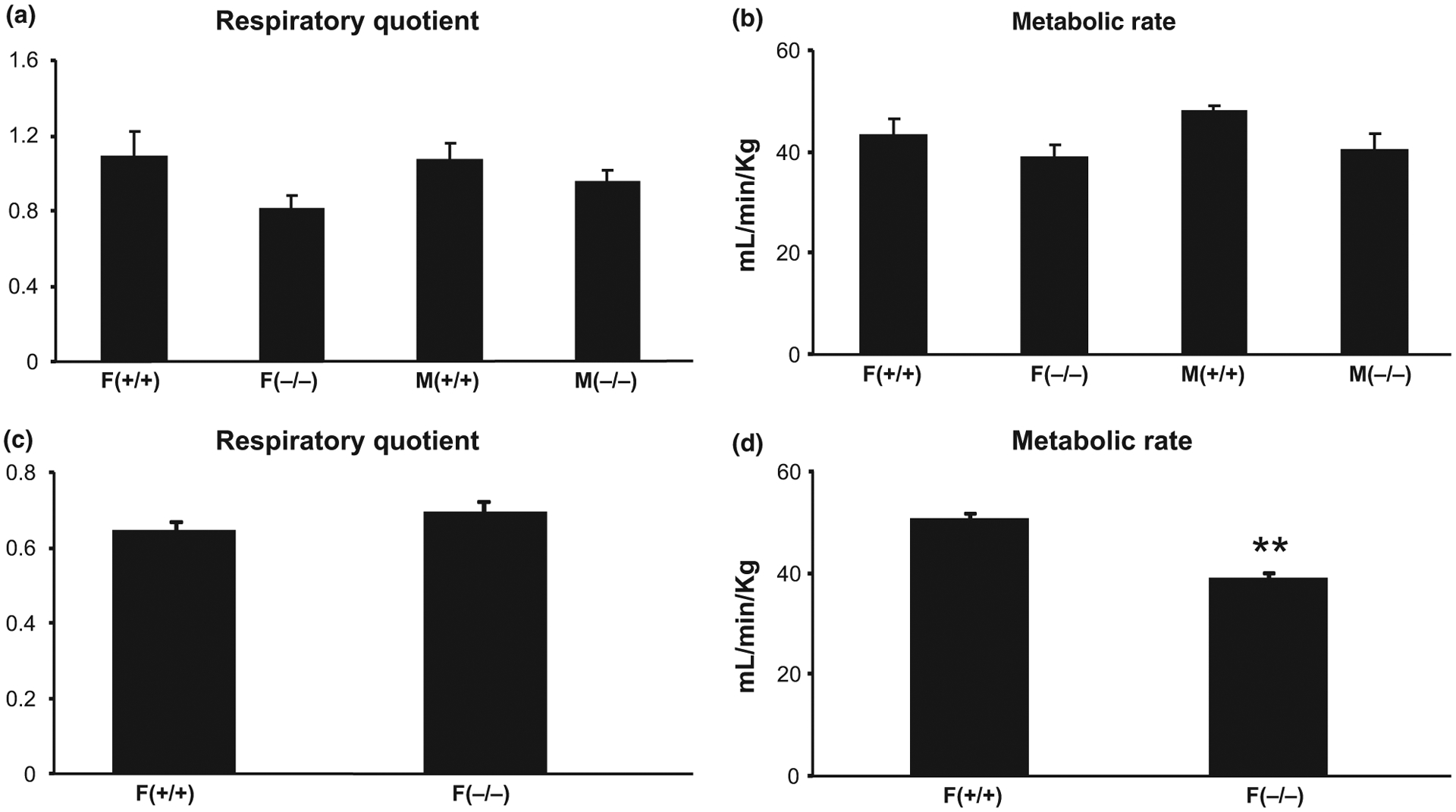
Differences in metabolic markers [respiratory quotient and metabolic rate (MR)] between old (18–20 months) male and female (a and b) and adult (7–9 months) female (c and d) Hcrt KO and WT mice. Each bar represents the mean value + SEM. **Significant difference between Hcrt KO and WT mice at the levels of p < 0.01.
Serum leptin levels
The mice were between 18 and 20 months old when they were sacrificed. Two-way anova indicated a significant effect of genotype (F = 9.88, p = 0.007), and the interaction between gender and genotype (F = 5.87, p = 0.03), on serum leptin levels. Post hoc Newman–Keuls comparisons showed that female KO mice had the highest serum leptin levels, compared to all the other groups (female KO vs. female WT= 191%, p < 0.01and female KO vs. male KO = 111%, p < 0.01, Fig. 4a). No significant differences in serum leptin levels between male KO and male WT mice or between male and female WT mice were observed. The mice at 20 months were of drastically different body weight, with female KO mice still being the heaviest (Fig. 4b). After adjusting for body weight, female KO mice still showed the highest serum leptin/body weight values compared to all the other groups (female KO vs. female WT = 93%, p < 0.05 and female KO vs. male KO = 95%, p < 0.05, Fig. 4c). Serum leptin levels paralleled the amount of body fat and body weight, with female KO mice having the highest levels of all three parameters.
Fig. 4.
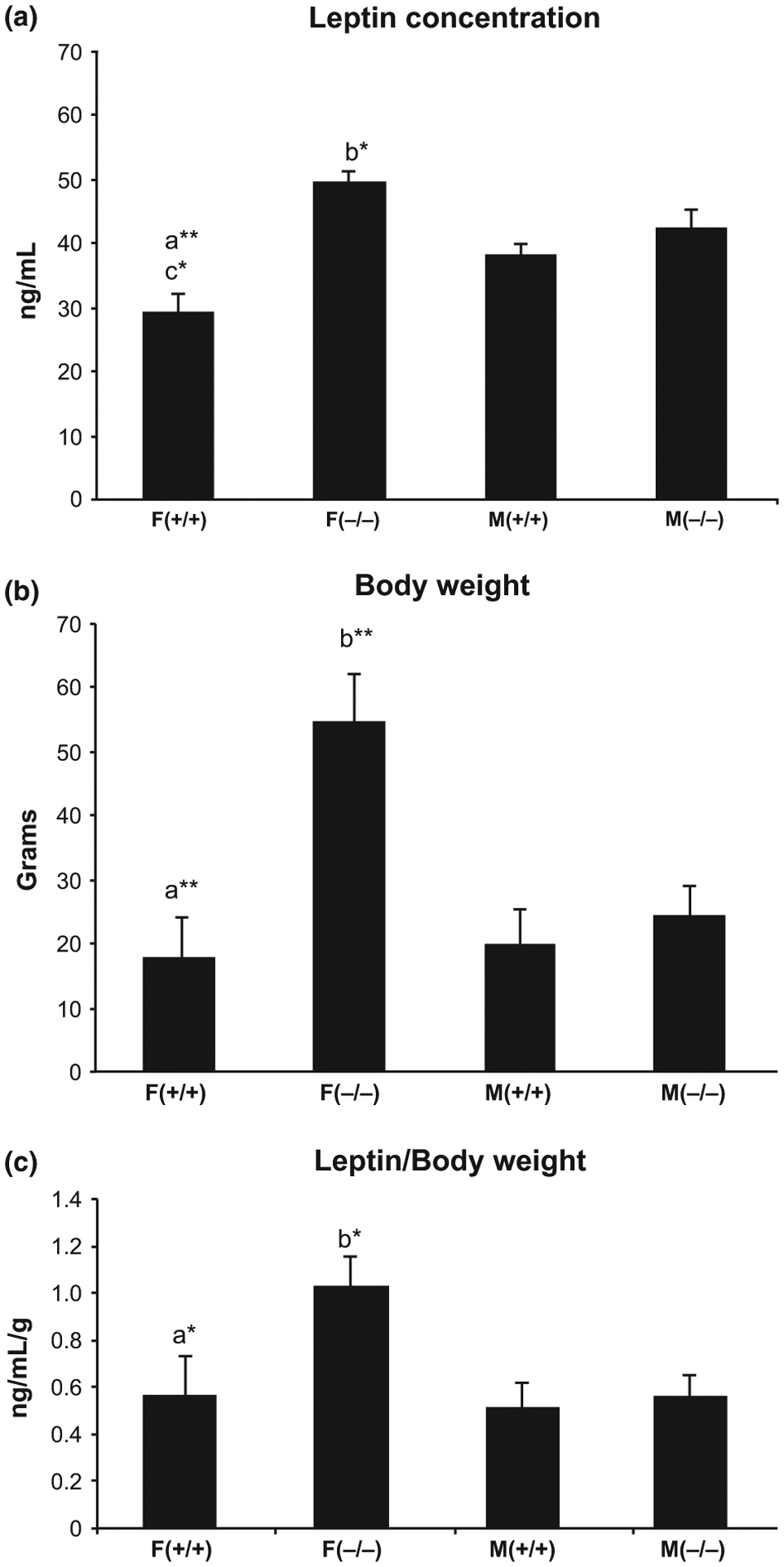
Differences in serum leptin concentration (a), body weight (b) and leptin concentration/body weight (c) between old (20 months) male and female Hcrt KO and WT mice. Each bar represents the mean value + SEM. a = Significant difference between female WT (+/+) and female KO (−/−) mice, b = significant difference between female and male KO mice, c = significant difference between female and male WT mice, at the level of p < 0.05*, p < 0.01**.
Insulin resistance and insulin release
The HOMA-IR was calculated using fasting blood glucose and serum insulin concentrations (Fig. 5a). Two-way anova revealed a significant effect of genotype on insulin resistance (F = 23.35, p = 0.0002), with both male and female Hcrt KO mice showing higher insulin resistance compared to sex-matched WT mice (male KO vs. male WT = 73%, p < 0.05 and female KO vs. female WT = 93%, p < 0.01). Although not significant, male KO mice showed a trend toward higher insulin release from beta cells (HOMA-%β) compared to male WT mice (90%, p = 0.09, Fig. 5b).
Fig. 5.
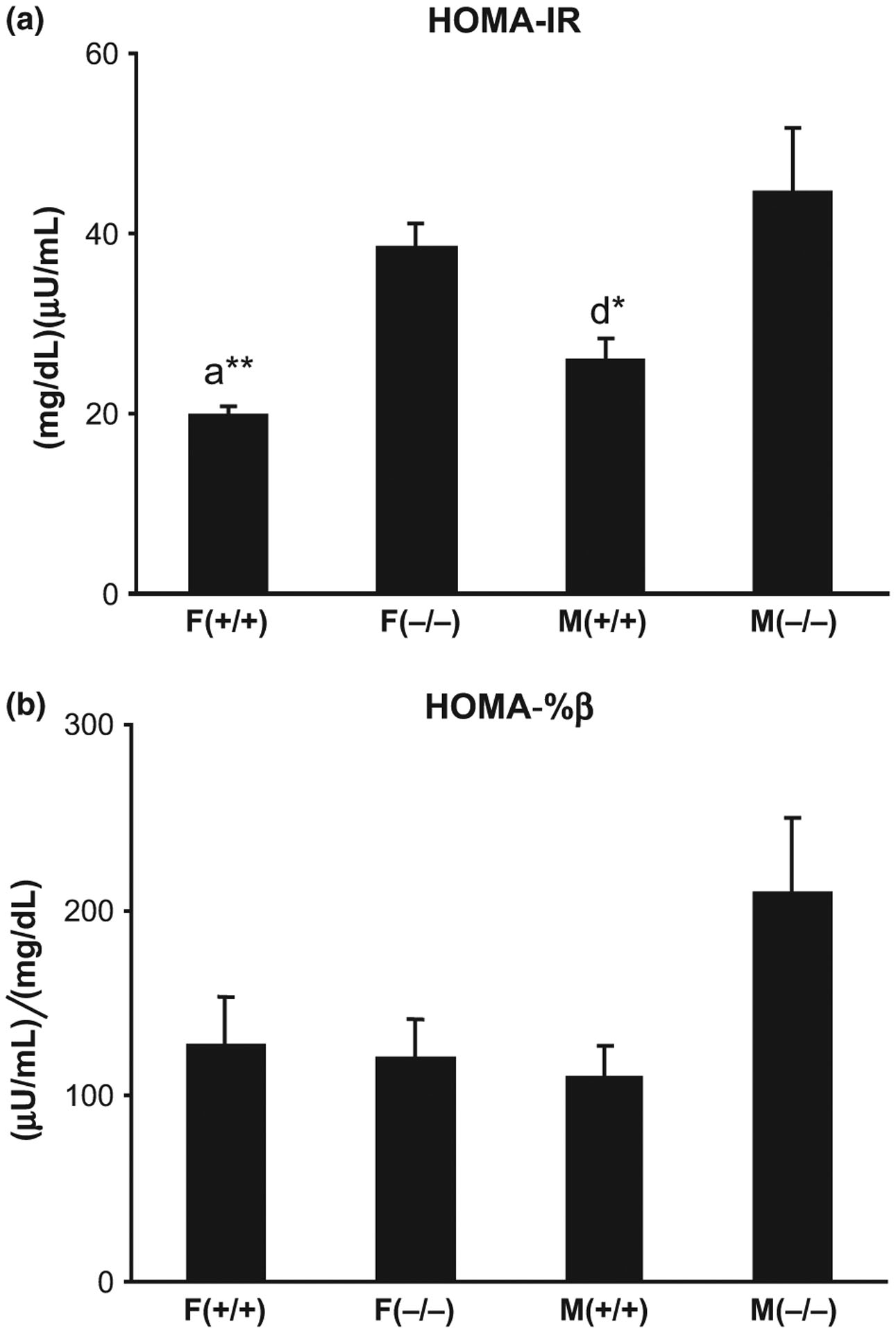
Differences in insulin resistance (a) and insulin release (b) between old (20 months) male and female Hcrt KO and WT mice. Each bar represents the mean value + SEM. a = Significant difference between female WT (+/+) and female KO (−/−) mice and d = significant difference between male WT and KO mice, at the level of p < 0.05*, p < 0.01**.
Weight of adipose tissue
Since both adult and old female Hcrt KO mice showed significantly larger body weight, compared to age-matched female WT mice, we also analyzed the weight of BAT and WAT (abdominal) in these mice (Fig. 6). Two way anova revealed a significant effect of genotype (F = 21.67, p = 0.0003) on BAT, as well as age (F = 18.13, p = 0.0006) and genotype (F = 47.21, p < 0.0001) on WAT. Both old (20 months) and adult (10 months) female KO mice had significantly more BAT (69% and 84%, p < 0.05, respectively, Fig. 6a) than age-matched WT females. Similarly, both old and adult female KO mice had more WAT (290% and 452%, p < 0.01, respectively, Fig. 6b) compared to age-matched female WT mice. Furthermore, old KO females exhibited more WAT compared to adult KO females (61%, p < 0.01), but no significant difference was noted between old and adult WT female mice.
Fig. 6.
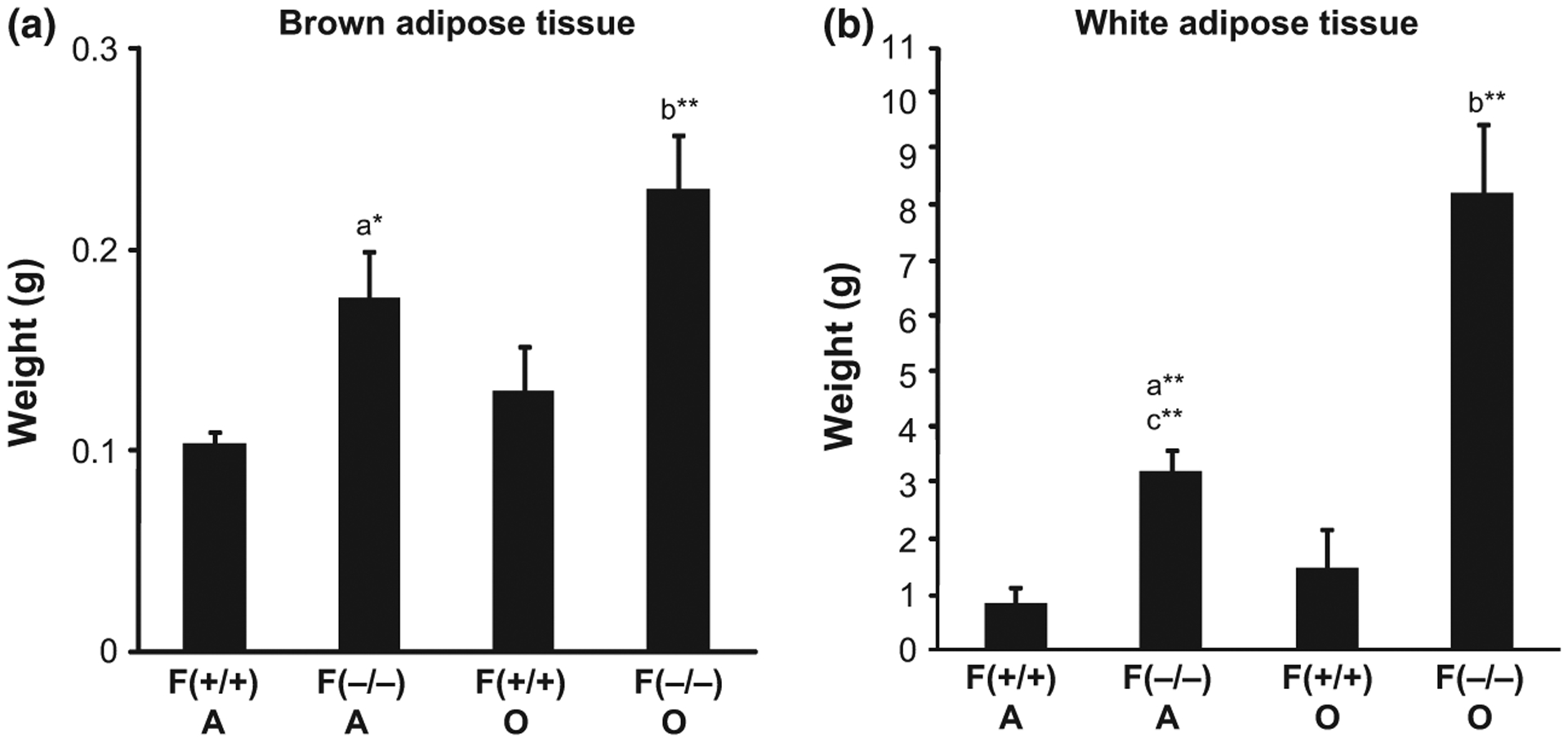
Differences in the weight of brown adipose tissue (a) and white adipose tissue (b) between adult (A = 10 months) and old (O = 20 months) female Hcrt KO and WT mice. Each bar represents the mean value + SEM. a = Significant difference between adult WT (+/+) and KO (−/−) mice, b = significant difference between old WT (+/+) and KO (−/−) mice, c = significant difference between adult and old KO (−/−), at the level of p < 0.05*, p < 0.01**.
Discussion
In the present study, we observed that female Hcrt KO mice were 20% heavier than female WT mice starting as early as 4 months, with the difference increasing with age until 20 months (50–60%), when the animals were sacrificed. This is a longer time interval than previously reported by other investigators. No significant difference in body weight between male KO and male WT mice was noted. Tsuneki et al. (2008) similarly reported that female KO mice became 20% heavier than female WT mice at 9 months (36 weeks), however, no difference in body weight between male KO and male WT mice was observed. Fujiki et al. (2006) also reported that KO mice became heavier than WT mice at just 3.5 months (100 days). This difference was significant only in female mice, although male mice started showing a slight increase in body weight at 18 months.
Besides changes in body weight, we show for the first time, that female Hcrt KO mice, as young as 7–9 months and as old as 18–20 months exhibited significant increases in the major components of the body (fat, muscle and free fluid), compared to age-matched female WT controls. This would account for the larger body weight of female KO mice compared to female WT mice. The difference in the sum total of fat, muscle, and free fluid content (17.14 g) between old female Hcrt KO and WT mice parallels the body weight difference (15.16 g) between them, and is more than double the difference in sum total (8.01 g) and body weight (6.83 g) between old male Hcrt KO and WT mice. The difference in the sum total of the major components of the body (14.83 g) between adult female Hcrt KO and WT mice also closely mimics the difference in their body weight (14.40 g), and closely resembles the changes seen in old female mice. The reason for the greater difference in the sum total of body fat, muscle, and free fluid content between female and male mice is not clear. The increase in body fat in old male Hcrt KO mice, compared to old male WT mice, did not result in increased body weight of these mice. This could be due to the fact that other factors (such as the weight of various internal organs and bone density) not measured in this study, may have counterbalanced the change in body fat. Table 1 shows the changes in the major components of the body, after normalizing for the different body weight. Hcrt KO mice, regardless of age or gender, showed increased body fat percentage and decreased muscle percentage, compared to wild type mice. Free fluid percentage, however, was significantly higher only in old female KO mice compared to old female WT mice.
Table 1.
Fat, muscle, and free fluid percentage in hypocretin knockout and wild type mice
| Animals | % Fat/BW | % Muscle/BW | % FF/BW |
|---|---|---|---|
| F(+/+) A | 15.87 ± 4.17 | 89.16 ± 2.78 | 2.15 ± 0.33 |
| F(−/−) A | 36.82 ± 1.77* | 67.00 ± 1.87* | 1.94 ± 0.23 |
| F(+/+) O | 33.26 ± 5.02 | 71.8 ± 3.82 | 2.15 ± 0.27 |
| F(−/−) O | 44.77 ± 3.52* | 61.48 ± 0.98* | 2.80 ± 0.30* |
| M(+/+) O | 13.71 ± 2.68 | 87.37 ± 2.36 | 2.29 ± 0.48 |
| M(−/−) O | 28.15 ± 2.98* | 75.45 ± 2.74* | 2.04 ± 0.33 |
BW, Body weight; FF, Free Fluid; A, Adult (7–9 months); O, Old (18–20 months); Hcrt KO, hypocretin knockout (−/−); WT, wild type (+/+); F, Female; M, Male.
Indicates significant difference (p < 0.05) between age and gender-matched Hcrt KO and WT mice.
In this study, we report for the first time, that both adult and old female Hcrt KO mice had significantly larger deposits of brown and white adipose tissues, compared to age-matched female WT mice. The mechanism involved in the increased fat content in Hcrt KO mice compared to WT mice remains to be determined. We further showed that KO mice had lower MR compared to WT mice, irrespective of age or gender. The decrease in MR results in increased body fat, seen in all Hcrt KO mice. Sellayah et al. (2011) recently showed that after 6 weeks on a high fat diet, male WT mice gained 15% of their initial body weight, and energy expenditure was increased by 13.5%, compared to male WT mice fed a normal diet. In contrast, male OX (orexin, hypocretin)-null mice on a high fat diet gained 45% body weight, with no change in energy expenditure, compared to male OX-null mice fed a normal diet. Conversely, Funato et al. (2009) reported that both male and female mice over-expressing orexin (CAG/Orexin transgenic mice) did not show any increase in body weight, produced by a high fat diet, as seen in sex-matched WT mice. These CAG/Orexin mice demonstrate ectopic peptide production in several brain regions, all of which have been implicated in various homeostatic, circadian, learned and/or hedonistic aspects of food intake, taste preference or energy homeostasis (Saper et al. 2002). Funato et al. (2009) also showed that male mice over-expressing orexin had higher energy expenditure with effective mass correction, compared to male WT mice, fed a high-fat diet. Taken together, our findings, as well as those of Funato et al. (2009) and Sellayah et al. (2011), indicate that orexin over expression prevents body weight changes, while lack of orexin increases body weight, due to reduction in energy expenditure/MR.
Sellayah et al. (2011) also reported that 6-week-old OX-null male mice showed lower food intake and physical activity, compared to WT male mice, regardless of whether they were on a normal or high-fat diet. Tsuneki et al. (2008) similarly reported that food intake in 1-year-old Hcrt KO mice, fed normal chow, decreased by 27% in male, and 22% in female mice compared to sex-matched WT mice. These authors also showed that locomotor activity decreased by more than 50% in both male and female KO mice, compared to sex-matched WT mice, fed normal chow. We previously reported that male Hcrt KO mice were less motivated to work for a food reward, in the light phase, compared to male WT littermates (McGregor et al. 2011). No performance differences were observed when they were required to bar press to avoid foot shock, in either the light or the dark phase, or when working for a food reward, in the dark phase.
Sex differences and body weight are known to significantly affect serum leptin levels (Frederich et al. 1995; Niskanen et al. 1997). In the current study, we showed that 20-month-old female Hcrt KO mice had significantly higher body weight, body fat, muscle, and free fluid levels, as well as serum leptin levels, compared to age-matched female WT mice. Tsuneki et al. (2008) similarly reported that 9-month-(but not 2 month) old female Hcrt KO mice had larger body weight and higher serum leptin levels, compared to female WT mice of the same age, when maintained on a normal diet. No differences in serum leptin levels or body weight, were seen between male KO and WT mice at any age.
We further observed that both male and female Hcrt KO mice showed higher insulin resistance (HOMA-IR) compared to sex-matched WT mice, at 20 months. This is consistent with the findings of Tsuneki et al. (2008), who reported that insulin sensitivity is decreased (higher insulin resistance), in both male and female KO mice, compared to sex-matched WT mice, at 9 months. This suggests that insulin resistance begins in adult Hcrt KO mice and persists until old age (9–20 months). It is interesting to note that male Hcrt KO mice did not show any significant difference in body weight compared to male WT mice, however, they did show significant increases in body fat content, insulin resistance, and also insulin release. This suggests that body fat, rather than body weight, is a more useful indicator of the increased risk of type-two diabetes.
Other investigators have also reported differences in hypocretin neurotransmission between male and female wild type animals. Higher levels of Hcrt receptors (both type 1 and type 2) in male rats compared to female rats have been reported (Jöhren et al. 2001). Both receptors are located in brown adipose tissue, however, Hcrt-induced adipogenesis and adipose tissue developmental differentiation are dependent on receptor 1 signaling, as indicated by studies in the Hcrt ligand and Hcrt receptor KO mice (Sellayah and Sikder 2012). Funabashi et al. (2009) reported that female, but not male, rats (3 months old) showed increased activation of Hcrt neurons, and increased feeding during rebound, after 48 h fasting. Pirnik et al. (2008) reported a positive correlation between body weight, fat gain, and increased activation of Hcrt neurons in female, but not male mice. Jöhren et al. (2002) reported significantly higher prepro-orexin mRNA levels in the hypothalamus of female rats compared to male rats, although Brownell and Conti (2010) showed that male mice had more Hcrt neurons than female mice (2251 Hcrt neurons in males vs. 1805 in females at 100 days). These authors also showed that the number of Hcrt neurons decreased with age, by the same percentage, in male and female mice (by 800 days, male mice lost 15%, while female mice lost 16% of their Hcrt neurons). They went on to suggest that the decline in the number of Hcrt neurons may help to explain the physiological changes in sleep and energy homeostasis regulation, during aging. We speculate that this decrease in the number of Hcrt neurons may also account for the increased body weight and body fat percentage, observed in aged mice (Sellayah and Sikder 2014).
Unlike our animal studies, human studies indicate that both male and female narcoleptics have increased BMI, compared to patients with idiopathic hypersomnia (Poli et al. 2009), psychiatric controls, including depression, alcohol dependence, schizophrenia or substance abuse patients (Dahmen et al. 2009), patients with neurological disorders (Schuld et al. 2000), as well as to healthy controls (Nishino et al. 2001). A major difference between human narcoleptics and Hcrt KO mice (animal model of narcolepsy) is that, in humans the Hcrt cell is lost in its entity (on average 90% of the Hcrt cell population is lost), as is the case in the Hcrt-ataxin mouse (another animal model of narcolepsy). However, in the Hcrt KO mouse, there is no expression of the Hcrt peptide, due to a mutation of the prepro-orexin gene, but the cells remain intact. Hence, any changes observed in the Hcrt KO mouse compared to the WT mouse, would arise solely from the loss of the Hcrt peptide, and any compensation due to this loss. On the other hand, any changes observed in human narcoleptics compared to control humans, would be due to the loss of any, or all, associated neurotransmitters, including Hcrt, dynorphin, and neuronal activity-regulated pentraxin (NARP) (Blouin et al. 2005; Crocker et al. 2005), resulting from the loss of the Hcrt neurons.
Schuld et al. (2000) reported no differences in BMI between medicated (tricyclic antidepressants and/or psycho-stimulants) and drug naïve narcoleptic patients, suggesting that the higher BMI in narcoleptic patients is a consequence of the disease-related behavior, including reduced locomotor activity and increased amounts of sleep, which may result in lower energy expenditure, rather than the drug treatment. Chabas et al. (2007) observed that narcoleptic patients had lower energy expenditure than controls, however, the two groups were not BMI matched. Fronczek et al. (2008) found no difference in basal MR between male narcoleptic patients and age and BMI-matched controls. Dahmen et al. (2001) studied a mixed group of narcoleptics (2 males and 11 females) and controls (8 males and 22 females), and showed that the basal MR and energy expenditure, of nonobese (BMI < 30) narcoleptics were significantly lower than BMI-matched controls. Lammers et al. (1996) reported decreased daily food intake in narcoleptic humans compared to controls, while Middelkoop et al. (1995) showed that the total intensity of physical activity did not differ between the two groups.
We previously reported that the weight, throughout development (from birth to 12 months), did not differ between genetically mutated narcoleptic Doberman Pinchers and breed-matched controls (John et al. 2004). Whereas narcoleptic mice and humans show a loss of the Hcrt peptide and/or neuron, and thus lower Hcrt levels, narcoleptic canines have normal levels of Hcrt, but a mutation in the Hcrt receptor 2 (John et al. 2004). This suggests that Hcrt signaling through receptor 1, rather than receptor 2, plays a role in obesity. Furthermore, the different neurological changes, as well as species variability, can account for differences in weight regulation between narcoleptic humans, mice, and dogs.
We conclude that absence of the Hcrt peptide has gender-specific effects. Only female Hcrt KO mice show increased body weight, muscle and free fluid content, as well as higher serum leptin levels, compared to female WT mice. On the other hand, both male and female KO mice show greater body fat and higher insulin resistance, compared to sex-matched WT mice. In contrast, prior work has shown that Hcrt-ataxin mice and human narcoleptics show weight gain in both sexes. These subjects lose Hcrt cells, not just the Hcrt peptide, indicating that Hcrt peptide loss produces gender-specific changes.
Acknowledgments and conflict of interest disclosure
Research was supported by National Institutes of Health grants NS14610, MH64109 and the Medical Research Service of the Department of Veterans Affairs. Both authors conceived and designed the experimental procedures. Dr Ramanathan collected and analyzed the data. Both authors wrote the manuscript.
All experiments were conducted in compliance with the ARRIVE guidelines. The authors have no conflicts of interest to declare.
Abbreviations used:
- BMI
body mass index
- Hcrt KO
hypocretin knockout
- HOMA-IR
homeostasis model assessment of insulin resistance
- RQ
respiratory quotient
References
- Beitinger PA, Fulda S, Dalal MA, Wehrle R, Keckeis M, Wetter TC, Han F, Pollmächer T and Schuld A (2012) Glucose tolerance in patients with narcolepsy. Sleep 35, 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin AM, Thannickal TC, Worley PF, Baraban JM, Reti IM and Siegel JM (2005) Narp immunostaining of human hypocretin (orexin) neurons: loss in narcolepsy. Neurology 65, 1189–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell SE and Conti B (2010) Age- and gender-specific changes of hypocretin immunopositive neurons in C57Bl/6 mice. Neurosci. Lett 472, 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabas D, Foulon C, Gonzalez J, Nasr M, Lyon-Caen O, Willer JC, Derenne JP and Arnulf I (2007) Eating disorder and metabolism in narcoleptic patents. Sleep 30, 1267–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM et al. (1999) Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98, 437–451. [DOI] [PubMed] [Google Scholar]
- Crocker A, España RA, Papadopoulou M, Saper CB, Faraco J, Sakurai T, Honda M, Mignot E and Scammell TE (2005) Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology 65, 1184–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmen N, Bierbrauer J and Kasten M (2001) Increased prevalence of obesity in narcoleptic patients and relatives. Eur. Arch. Psychiatry Clin. Neurosci 251, 85–89. [DOI] [PubMed] [Google Scholar]
- Dahmen N, Tonn P, Messroghli L, Ghezel-Ahmadi D and Engel A (2009) Basal metabolic rate in narcoleptic patients. Sleep 32, 962–964. [PMC free article] [PubMed] [Google Scholar]
- Donjacour CE, Pardi D, Aziz NA, Frolich M, Roelfsema F, Overeem S, Pijl H and Lammers GJ (2013) Plasma total ghrelin and leptin levels in human narcolepsy and matched healthy controls: basal concentrations and response to sodium oxybate. J. Clin. Sleep Med 9, 797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB and Flier JS (1995) Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med 1, 1311–1314. [DOI] [PubMed] [Google Scholar]
- Fronczek R, Overeem S, Reijntjes R, Lammers GJ, van Dijk JG and Pijl H (2008) Increased heart rate variability but normal resting metabolic rate in hypocretin/orexin deficient human narcolepsy. J. Clin. Sleep Med 4, 248–254. [PMC free article] [PubMed] [Google Scholar]
- Fujiki N, Yoshida Y, Zhang S, Sakurai T, Yanagisawa M and Nishino S (2006) Sex difference in body weight gain and leptin signaling in hypocretin/orexin deficient mouse models. Peptides 27, 2326–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabashi T, Hagiwara H, Mogi K, Mitsushima D, Shinohara K and Kimura F (2009) Sex differences in the responses of orexin neurons in the lateral hypothalamic area and feeding behavior to fasting. Neurosci. Lett 463, 31–34. [DOI] [PubMed] [Google Scholar]
- Funato H, Tsai AL, Willie JT, Kisanuki Y, Williams SC, Sakurai T and Yanagisawa M (2009) Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 9, 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T et al. (2001) Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30, 345–354. [DOI] [PubMed] [Google Scholar]
- Honda Y, Doi Y, Ninomiya R and Ninomiya C (1986) Increased frequency of non-insulin-dependent diabetes mellitus among narcoleptic patients. Sleep 9, 254–259. [DOI] [PubMed] [Google Scholar]
- John J, Wu MF, Maidment NT, Lam HA, Boehmer LN, Patton M and Siegel JM (2004) Developmental changes in CSF hypocretin-1 (orexin-A) levels in normal and genetically narcoleptic Doberman pinschers. J. Physiol 560, 587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöhren O, Neidert SJ, Kummer M, Dendorfer A and Dominiak P(2001) Prepro-orexin and orexin receptor mRNAs are differentially expressed in peripheral tissues of male and female rats. Endocrinology 142, 3324–3331. [DOI] [PubMed] [Google Scholar]
- Jöhren O, Neidert SJ, Kummer M and Dominiak P (2002) Sexually dimorphic expression of prepro-orexin mRNA in the rat hypothalamus. Peptides 23, 1177–1180. [DOI] [PubMed] [Google Scholar]
- Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M,Komuro I, Fukuda Y and Kuwaki T (2003) Attenuated defense response and low basal blood pressure in orexin knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 285, R581–R593. [DOI] [PubMed] [Google Scholar]
- Kok SW, Overeem S, Visscher TL, Lammers GJ, Seidell JC, Pijl H and Meinders AE (2003) Hypocretin deficiency in narcoleptic humans is associated with abdominal obesity. Obes. Res 11, 1147–1154. [DOI] [PubMed] [Google Scholar]
- Lammers GJ, Pijl H, Iestra J, Langius JA, Buunk G and Meinders AE (1996) Spontaneous food choice in narcolepsy. Sleep 19, 75–76. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC and Aston-Jones G (2012) Multiple roles for orexin/hypocretin in addiction. Prog. Brain Res 198, 79–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D, Hosker JP, Rudenski AS, Naylor BA, Treacher DF and Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419. [DOI] [PubMed] [Google Scholar]
- McGregor R, Wu MF, Barber G, Ramanathan L and Siegel JM(2011) Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement versus operant avoidance and light level. J. Neurosci 31, 15455–15467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelkoop HA, Lammers GJ, Van Hilten BJ, Ruwhof C, Pijl H and Kamphuisen HA (1995) Circadian distribution of motor activity and immobility in narcolepsy: assessment with continuous motor activity monitoring. Psychophysiology 32, 286–291. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T and Scammell TE (2004) Behavioral state instability in orexin knock-out mice. J. Neurosci 24, 6291–6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T, Klerman EB, Sakurai T and Scammell TE (2006) Elevated body temperature during sleep in orexin knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 291, R533–R540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Nevsimalova S, Lammers GJ,Vankova J, Okun M, Rogers W, Brooks S and Mignot E (2001) Low cerebrospinal fluid hypocretin (Orexin) and altered energy homeostasis in human narcolepsy. Ann. Neurol 50, 381–388. [DOI] [PubMed] [Google Scholar]
- Niskanen LK, Haffner S, Karhunen LJ, Turpeinen AK, Miettinen H and Uusitupa MI (1997) Serum leptin in obesity is related to gender and body fat topography but does not predict successful weight loss. Eur. J. Endocrinol 137, 61–67. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W et al. (2000) A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat. Med 6, 991–997. [DOI] [PubMed] [Google Scholar]
- Pirnik Z, Bundzikova J, Mikkelsen JD, Zelezna B, Maletinska L and Kiss A (2008) Fos expression in hypocretinergic neurons in C57B1/6 male and female mice after long-term consumption of high fat diet. Endocr. Regul 42, 137–146. [PubMed] [Google Scholar]
- Poli F, Plazzi G, Di Dalmazi G, Ribichini D, Vicennati V, Pizza F, Mignot E, Montagna P, Pasquali R and Pagotto U (2009) Body mass index-independent metabolic alterations in narcolepsy with cataplexy. Sleep 32, 1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Chou TC and Elmquist JK (2002) The need to feed: homeostatic and hedonic control of eating. Neuron 36, 199–211. [DOI] [PubMed] [Google Scholar]
- Schuld A, Hebebrand J, Geller F and Pollmächer T (2000) Increased body-mass index in patients with narcolepsy. Lancet 355, 1274–1275. [DOI] [PubMed] [Google Scholar]
- Sellayah D and Sikder D (2012) Orexin receptor-1 mediates brown fat developmental differentiation. Adipocyte 1, 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellayah D and Sikder D (2014) Orexin restores aging-related brown adipose tissue dysfunction in male mice. Endocrinology 155, 485–501. [DOI] [PubMed] [Google Scholar]
- Sellayah D, Bharaj P and Sikder D (2011) Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 14, 478–490. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M and Siegel JM (2000) Reduced number of hypocretin neurons in human narcolepsy. Neuron 27, 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuneki H, Murata S, Anzawa Y, Soeda Y, Tokai E, Wada T, Kimura I, Yanagisawa M, Sakurai T and Sasaoka T (2008) Age-related insulin resistance in hypothalamus and peripheral tissues of orexin knockout mice. Diabetologia 51, 657–667. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zeitzer JM, Sakurai T, Nishino S and Mignot E (2007) Sleep/wake fragmentation disrupts metabolism in a mouse model of narcolepsy. J. Physiol 581, 649–663. [DOI] [PMC free article] [PubMed] [Google Scholar]


