Abstract
Background:
‘Voodoo’ is a new substance of abuse that recently spread among youth in Egypt. It has numerous potentially dangerous effects on humans. However, to date the composition of the main constituents of this compound is unknown.
Purpose:
We sought to identify the active components of this unknown substance”voodoo”.
Methods:
Three samples were collected and analysed by high-performance liquid chromatography with photodiode array detector (HPLC-PAD), gas chromatography/mass spectrometry (GC/MS), and ultra-performance liquid chromatography/mass spectrometry (UPLC-MS/MS) using targeted multiple reaction monitoring (MRM).
Results:
HPLC-PAD analysis showed that samples 1 and 2 had some common major peaks, the same retention time, and similar spectra, whereas sample 3 showed different peaks. GC/MS analysis revealed the presence of various putatively identified bioactive compounds, including quinazolines, morphinan alkaloid, cannabinoids, penitrem A, and the well-known synthetic cannabinoid FUB-AMB (methyl(2S)-2-{[1-[(4-fluorophenyl)methyl]indazole-3-carbonyl]amino}−3 methylbutanoate). UPLC-MS/MS analysis revealed the presence of common compounds such as tetrahydrocannabinol (THC), amphetamine, 3,4-methylenedioxyamphetamine, tramadol, and oxazepam.
Conclusion:
We concluded that Voodoo is a mixture of substances of abuse at varying concentrations.
Keywords: Substance abuse, chemical analysis, identification, Voodoo, Egypt
1. Introduction
Substance abuse is a serious public health problem worldwide [1]. It is estimated that 153 to 300 million people between the ages of 15 and 64 years have used an illicit substance at least once in their lives [2].
Globally, estimated 99,000 to 253,000 deaths occur each year as a result of substance abuse. Among people 15 to 64 years of age, 0.5% to 1.3% of all deaths are drug-related [3]. In Egypt, the prevalence of substance abuse has increased sharply, especially over the past few years, due to social and economic instability [4,5]. According to the World Health Organization, substance abuse prevalence in Egypt is 0.8% among individuals aged 15–64 years [6]. Substance abuse causes significant health and social consequences, including increased rates of morbidity and mortality, loss of productivity, and increased healthcare costs [7].
New synthetic designer drugs continue to emerge and attract many users. The United Nations Office on Drugs and Crime (2017) [8] reported the emergence of 739 new substances of abuse belonging to diverse chemical groups from 106 countries worldwide between 2009 and 2016. These substances are marketed in different ways and forms. Some of them emerge and then disappear quickly while others continue to be used, in part because of their availability and cost [9].
During the past few years, the most common emerging substances of abuse have been synthetic cannabinoids. These are potent psychoactive compounds known as ‘Spice’ and ‘K2’, which cause harmful effects in users and present great challenges for forensic laboratories [9,10].
The Egyptian Ministry of Health issued warnings about a new substance of abuse that has captured the attention of many youth in Egypt. Called ‘Voodoo’, this drug mixture is often blended with tobacco and then smoked as cigarettes. It is sold in bags containing a mixture of herbs and is often imported legally as herbal incense or for sedation of animals such as bulls and dogs, with labels that state ‘not for human consumption’ [11].
At the end of 2015, numerous reports surfaced in Egyptian newspapers, social media, and television coverage, warning about the use of Voodoo because of its dangerous effects. Some of these reports suggested that Voodoo is a synthetic cannabinoid and others reported that it contains hallucinogens such as atropine, hyoscine, and hyoscya-mine [11,12]. However, little is actually known about its composition [12]. We decided to analyse samples of the substance known as ‘Voodoo’ and identify its active ingredients.
2. Experimental
2.1. Materials
A variety of ‘Voodoo’ brands are available in Egypt, with street names such as Strox and Dragon. We obtained three herbal packages from users so that we could analyse their contents. Each package contained 3 g of Voodoo.
For our controls, we obtained samples of amphetamine, MDA, methadone, diazepam, oxazepam, tramadol, and tetrahydrocannabinol (THC) from LGC Standards, Egypt [13]. All solvents were of HPLC grade.
2.2. Sample preparation
A 50-mg sample from each bag was dissolved in (3 ×200 μl) of methanol. After soaking for 2 h, the extract was filtered and 10 μl was injected into a high-performance liquid chromatograph and 1 μl was injected into a gas chromatograph/mass spectrometer. This experiment was conducted in triplicate and repeated on three different days. A blank sample has been prepared, by omitting only the street drug sample, using the same extraction method and the same solvent has been carried out and analysed as well.
2.3. Analytic high-performance liquid chromatography with photodiode array detector (HPLC/PAD)
HPLC was performed with a Waters Alliance E2695 HPLC System (XE Separations Module, Austria). Analytic separations were achieved using a SunFire Prep C18 column (5 μm, 10 × 150 mm). The substances were eluted from the column at a flow rate of 1 mL/min with water containing 0.1% (v/v) trifluoroacetic acid. A linear gradient from 5% to 30% acetonitrile over the course of 10 min was applied, followed by 5 min of isocratic elution with 30% acetonitrile. Afterwards, a linear gradient from 30% to 95% acetonitrile was used over the course of 10 min. The percentage of acetonitrile was kept at 95% for an additional 2 min. An injection loop of 10 μL was used. Detection was carried out with a Waters photodiode array detector (PAD) measuring spectra between 200 and 450 nm. The chromatograms presented in this article are based on detection at 214 nm.
2.4. Gas chromatography/mass spectrometry (GC/MS)
2.4.1. GC/MS of samples 1 and 2
GC/MS was performed using a Thermo Scientific, Trace GC Ultra/ISQ Single Quadrupole MS, TG-5MS fused silica capillary column (30 m, 0.25 mm, 0.25-μm film thickness). For GC/MS detection, an ionization system with ionization energy of 70eV was used. Helium was used as the carrier gas at a constant flow rate of 1 mL/min. The injector and MS transfer line temperature was set at 280°C. The oven was programmed at an initial temperature of 40°C (hold for 3 min) to 280 °C as a final temperature at an increasing rate of 5 °C/min (hold for 5 min). All identified compounds were quantified using the per cent relative peak area. Tentative identification was based on a comparison of their relative retention time and mass spectra with the NIST and WILLY library data of the GC/MS system.
2.4.2. GC/MS of sample 3
Chromatographic analysis using GC–MS was performed using Agilent Technologies 7890B GC Systems combined with 5977A Mass Selective Detector. Capillary column was used (HP-5MS Capillary; 30.0 m × 0.25 mm ID × 0.25 μm film) and the carrier gas was helium at a rate of flow of 1.9 ml/min. The sample was analysed with the column held initially for 3 min at 40°C after injection, and then the temperature was increased to 300°C with a 20°C/min heating ramp, with a 3.0-min hold. The injection was carried out in split mode (1:50) at 300°C. MS scan range was (m/z): 40–550 atomic mass units (AMU) under electron impact (EI) ionization (70 eV) and solvent delay 2 min.
2.5. Ultra performance liquid chromatography/mass spectrometry (UPLC-MS/MS) using targeted multiple reaction monitoring (MRM) screening analysis
We used the ACQUITY UPLC I-Class system and the Xevo TQD mass spectrometer. We chose MassLynx (4.1 SCN 714), ChromaLynx, and TargetLynx (Waters) as our system software. Chromatographic separation was performed with an HSS C18 1.8 μm (2.1 × 150 mm) column (Waters) maintained at 50 C, while the sample temperature was maintained at 10 C. Injection volume was 5 μL. Mobile phase A was 5 mM ammonium formate, pH 3.0, and mobile phase B was 0.1% formic acid in acetonitrile. A mobile phase gradient from 87%A and 13%B to 5%A and 95%B was used, with a flow rate of 400 μL/min and a total analytic run time of 15 min. The analyses were run under the following conditions: ESI positive and negative modes (20-ms frequency); capillary voltage, 3.0 kV; cone voltage, 20 V to 95 V in 15-V increments (in-source CID); desolvation temperature, 400 C; desolvation gas flow, 800 L/h; source temperature, 150 C; acquisition range, m/z 80–650; and scan speed, >7000 atomic mass units per second. An in-source CID mass spectral library of 710 (library size at the time of the study) was obtained from Waters. Our criteria for a positive drug finding were retention time within 0.3 min and an average library forward fit ≥650. Alternate criteria were a tentative library fit (450–649) plus identification of the molecular species and two major fragment ions.
MS/MS conditions for UPLC–MS/MS analysis included ESI positive ionization mode; capillary voltage, 3.0 kV; desolvation temperature, 400 C; desolvation gas flow, 800 L/h; source temperature, 150°C; and collision gas (argon) pressure closely maintained at 0.45 Pa. Multiple reaction monitoring (MRM) was performed for the targeted drugs and metabolites using dual-transition ions for all analytes except tramadol, for which only a single detectable transition was produced in the collision cell. Cone voltage and collision energy were optimized for each analyte transition, with a target transition-ion ratio established for use in criteria-based identification of all analytes except tramadol. European Union criteria [14] were used for transition-ion ratio monitoring, including a ratio within 20% of the target ratio for transition ratios ≥0.50, within 25% for transition ratios between 0.20 and <0.50, within 30% for transition ratios between 0.10 and <0.20, and 50% for transition ratios <0.10. Mass calibration was performed prior to each analytic run for both UPLC screening assays [15,16].
The protocol for this study was approved by the institutional review boards at the institutions with which the authors are affiliated.
3. Results
The samples showed significant heterogeneity by all three analytic methods. Although they showed some commonalities, they did not appear to have a specific unifying component.
3.1. HPLC-PAD results
To investigate similarities and differences between the three street drug samples, their extracts were injected into a liquid chromatograph and the major peaks in each chromatogram were compared by retention time and UV spectra. Figure 1 shows the HPLC chromatograms of the three 3 extracts at 214 nm. Samples 1 and 2 had some common major peaks, the same retention time, and similar spectra, whereas sample 3 showed different peaks.
Figure 1.
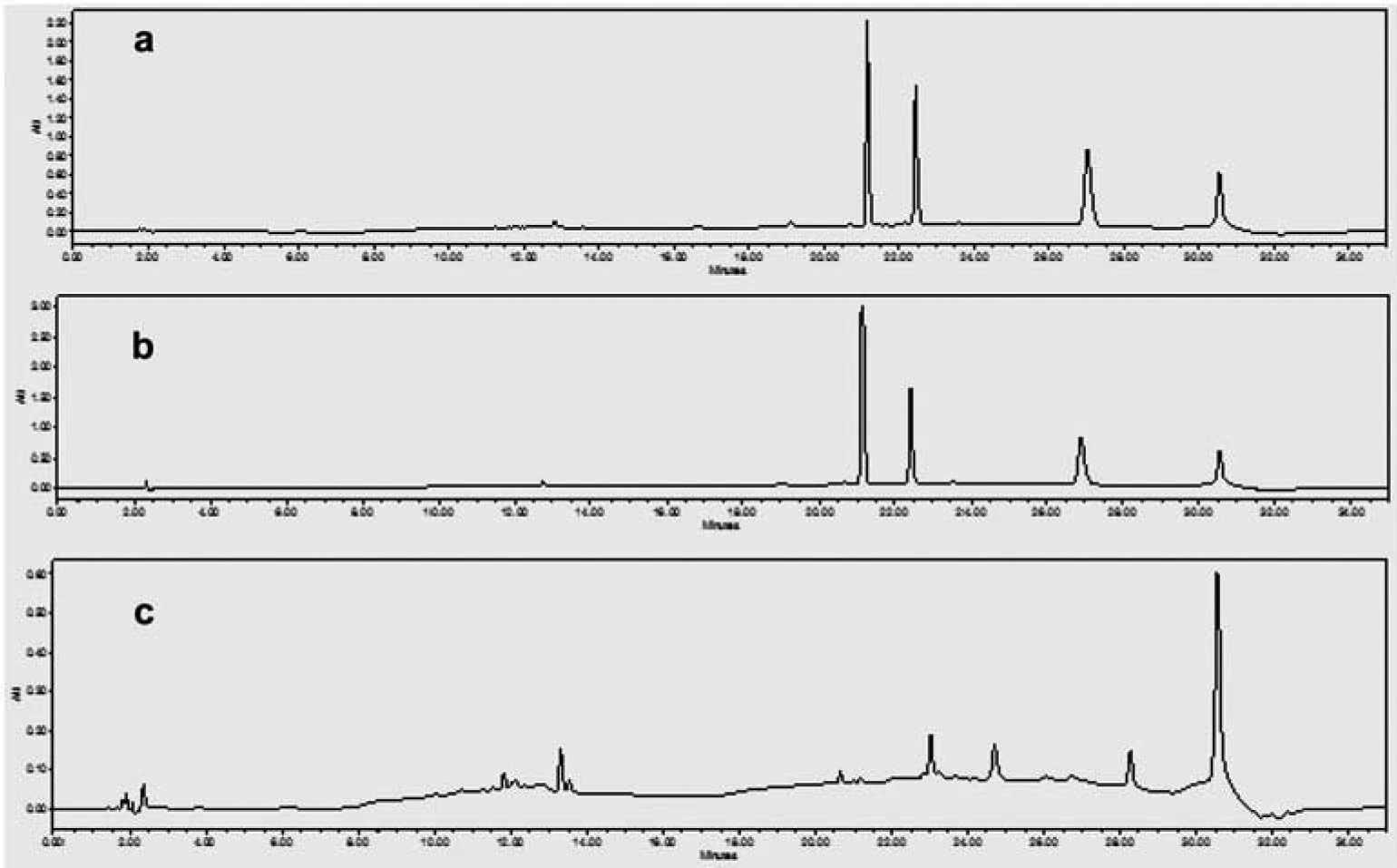
HPLC chromatograms of three street samples of Egyptian ‘Voodoo’. A, sample 1. B, sample 2,. C,sample 3.
3.2. GC/MS analysis
To investigate whether the similar peaks had the same mass spectra and to identify the similarities and differences between various peaks associated with the three samples, a GC/MS analysis was run for each extract. This analysis revealed the presence of known and unknown bioactive compounds. The spectra of the compounds were matched with Wiley 9.0, mainlib, NIST14, and replib libraries. The chromatograms are presented in Figure 2 through 5 as well as supplemental files S1 through S3.
Figure 2.
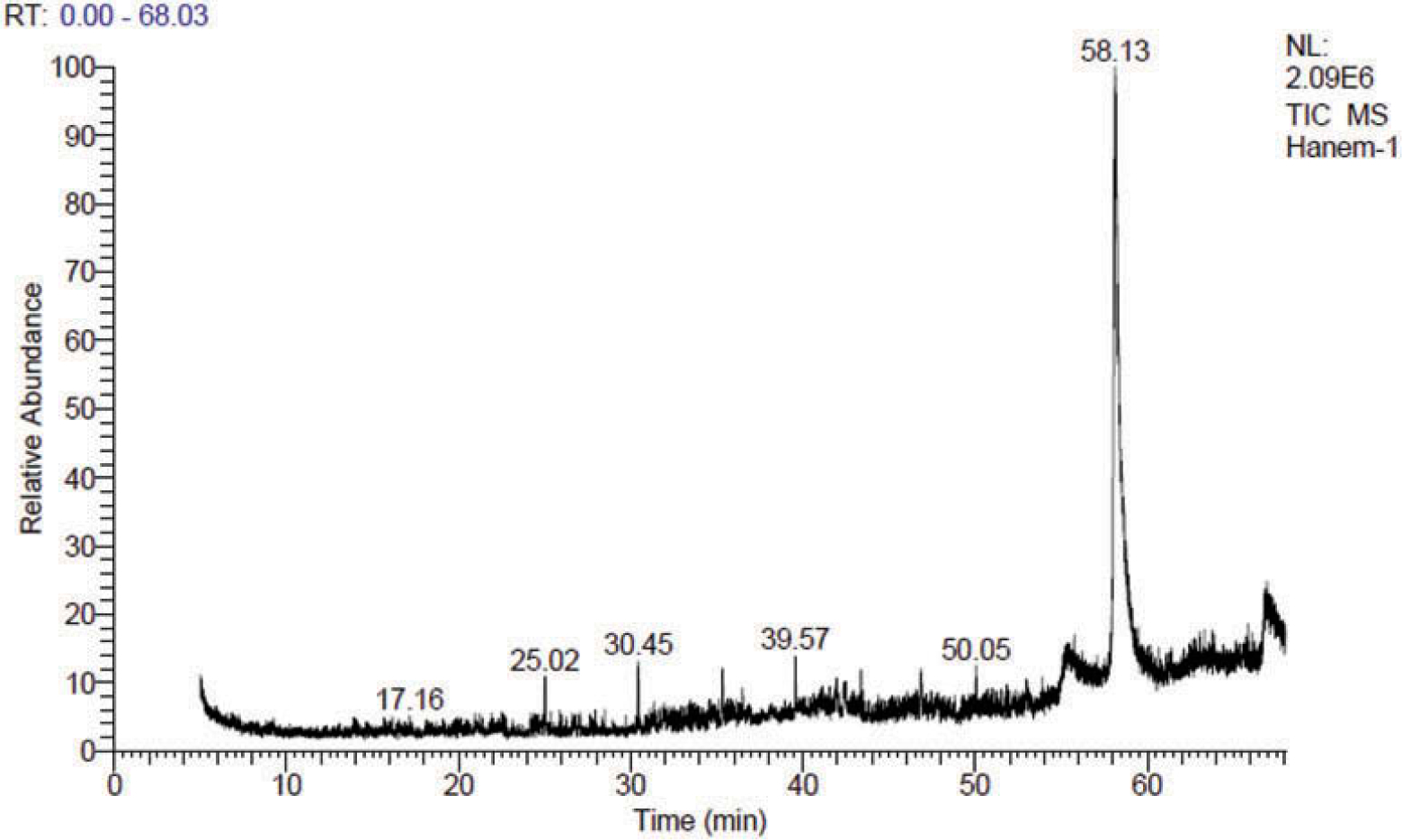
GC/MS TIC chromatogram of a methanolic extract of sample 1.
Figure 5.
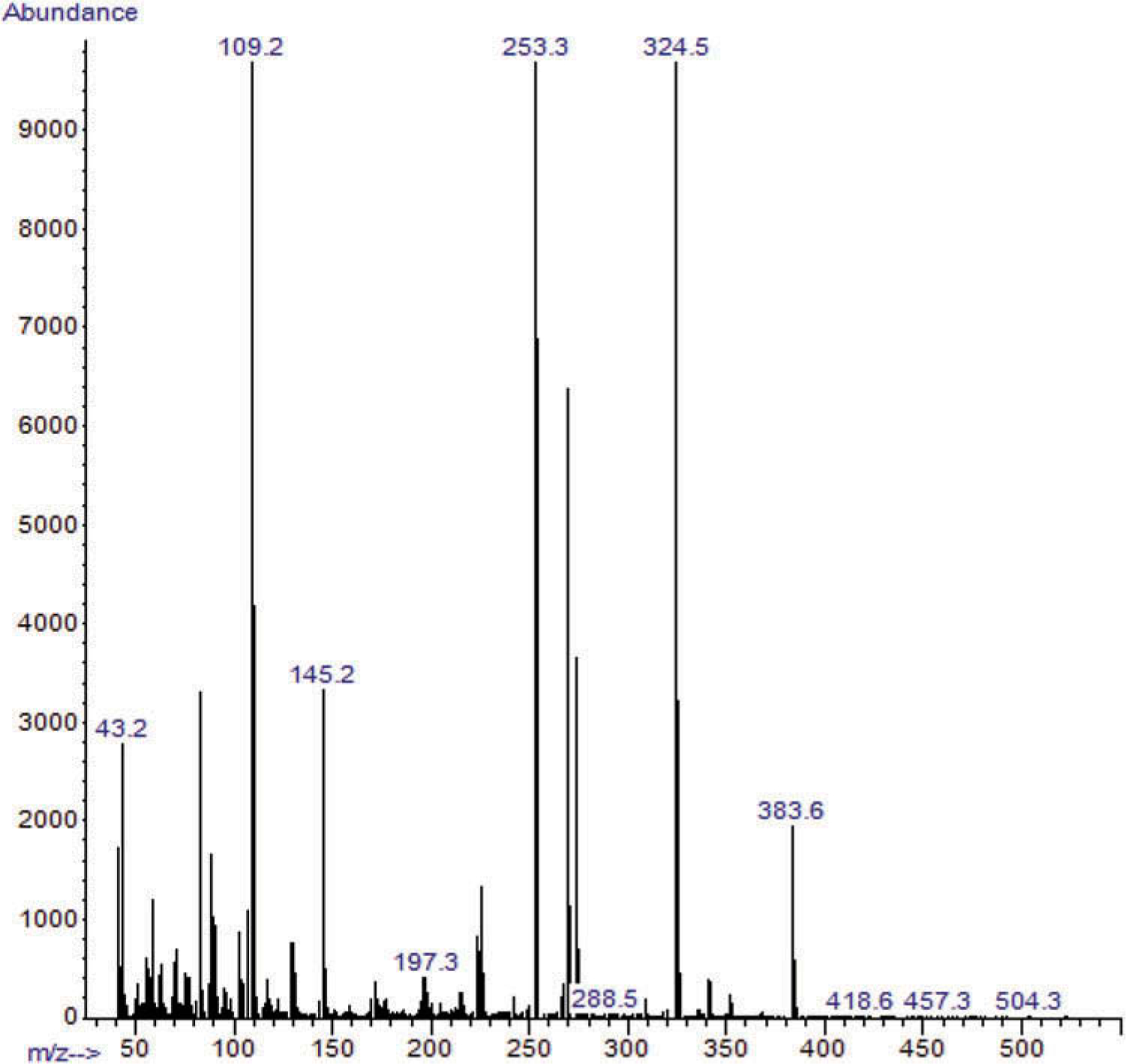
GC/MS spectrum of the AMB-FUB SC putatively identified in sample 3 at 17.055–17.871 min.
Figures 2 and 3 show that samples 1 and 2 clearly shared some putatively identified compounds such as 1,3-bis(4-chlorophenyl)-5,6-dihydrobenzo[f]quinazoline; 3-(5ʹ-chloro-1ʹ, 3-’phenyl pyrazol-4ʹ yl)-1-oxo-(1H)pyrido[2,1b] benzothiazole-2,4-carbonitrile; or 1-phenyl-3-(2”-chlorophenyl)-6-(p-chlorophenyl)-3a,4,4a,7a,8,8a-hexahydro-4,8-epoxy pyrrolo[3,4f] indazole-5,7-(1H,6H)dione; dimethyl N-(3-Bromo-4,5,6,7-tetrahydroisobenzofuroyl)-L-glutamate. These four putatively identified compounds shared the same retention times (Rt = 25.02, 30.45 and 35.33, respectively), and similar spectra in both samples. These results may explain the similarity in the HPLC data, which indicated that Voodoo samples 1 and 2 contain similar compounds.
Figure 3.
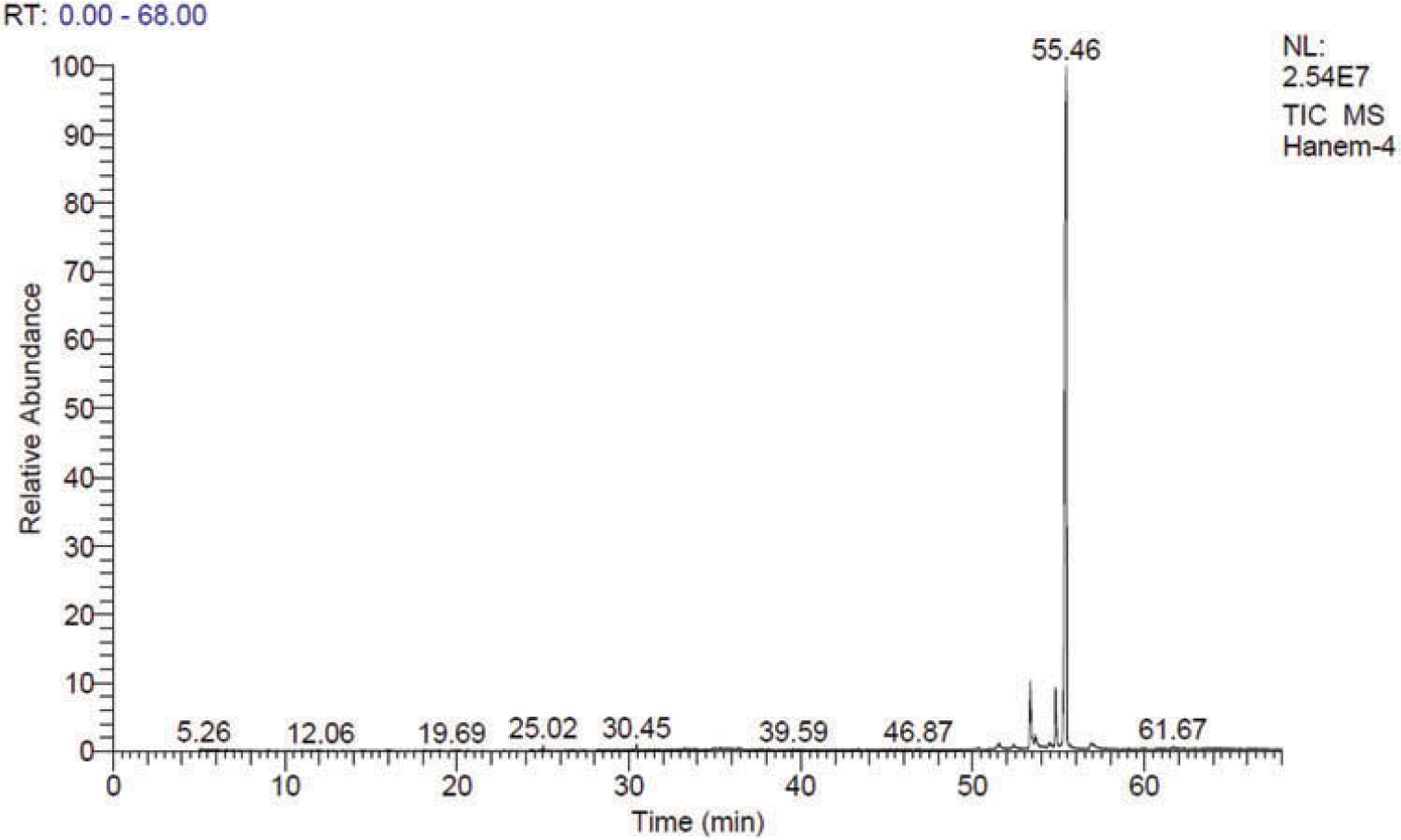
GC/MS TIC chromatogram of a methanolic extract of sample 2.
The GC/MS analysis of sample 1 also revealed the putative identification of the morphinan alkaloid (5à,6à)-4,5-epoxy-6-methoxy-17-propyl-3á-phthalimidomorphinan (Rt = 25.02 min); dodecachloro-3,4-benzophenanthrene (Rt = 33.46 min); penitrem A (Rt = 33.68 min); 2,2-dimesityl-2-silatetracyclo[7.6.0.0(3,8).0(10,15)]hexadecadodecaene (Rt = 39.57 min), methyl-1-{4-Methoxy-3-chloro-6[2-[3-(2ʹ-methoxy-5ʹ-(1,3-dioxan-2-yl) phenyl)-4-methoxyphenyl]ethyl]phenyl}−2-methoxybenzene-4-carboxylate (Rt = 41.95), 4,4ʹ,4”,4ʹ’-tetrabromotetraphenylmethane (Rt = 42.38 min), 3-(5ʹ-chloro-1ʹ,3ʹ-diphenylpyrazol-4ʹ-yl)-1-oxo(1H)-pyrido[2,1-b]benzothiazole-2,4-carbonitrile (Rt = 43.38 min), N(1)-{4ʹ-[3’-oxo-4”-(p-fluorophenyl)-3”,3”a,4”,5”-tetrahydro-2”-methyl(2H)-6”-indazolyl)]phenyl}−5-chloro-2-methoxybenzamide (Rt = 46.86 min), 5,5ʹ-bis[3-bromo-5-(methoxymethoxymethyl)phenyl]-2,2ʹ-bipyridyl (Rt = 47.85 min), 3-(4ʹ-bromophenyl) −5,6-diphenylimidazo[2,1-b]thiazole (Rt = 50.05 min), and 2,2-dimethyl-1-(4-methylphenyl)-1,2,5,10-tetrahydrobenzo[g]quinolone-5,10-dione (Rt = 58.12 min),. The latter was putatively identified as a major compound in the GC/MS chromatogram of sample 1, with peak area percentage of 50.20% (Figure 2). The GC/MS analysis of sample 2 also revealed the putative identification of 2,4-bis(a’-chloroethyl)-6,7-bis[a’-methoxycarbonylethyl]-1,3,5-trimethylporphyrin (Rt = 34.34 min); delta.9-tetrahydrocannabinol (Rt = 51.53 min); 5R/5S)-3,3ʹ,5-trimethyl-4-acetoxy-4ʹ-[(t-butyldimethylsilyl)ox]spiro[2,2ʹ-bis(tetrahy-dropyrane)] (Rt = 52.40 min); cannabidiol (Rt = 53.36 min); 1,6,7-trimethyl-3-phenyl-9H-xanthen-9-one (Rt = 53.65 min); 3-N-pentyl-delta.9-tetrahydrocannabinol (Rt = 54.42 min); delta.8-tetrahydrocannabinol (Rt = 54.84 min); 2-ethylthio-10-hydroxy-9-methoxy-1,4-anthraquinone (Rt = 55.46 min), this compound was putatively identified as the major compound, and 3-hydroxy-N-(p-methoxyphenyl)-4-[(S)-2,2-dimethyl-1,3-dioxolan-4-yl]-3-[3,4-bis(methoxycarbonyl)phenyl]azetidin-2-one (Rt = 62.00 min)(Figure 3).
The GC/MS analysis of sample 3 (Figure 4) revealed the putative identification of various compounds. One had a GC/MS spectrum putatively identified as FUB-AMB (methyl(2S)-2-{[1-[(4-fluorophenyl)methyl]indazole-3-carbonyl]amino}−3-methylbutanoate). This compound was putatively identified as a very big broad peak by matching with the NIST library at retention time from 17.358 min to 17.708 min (Figure 5). These few seconds differences between the first detection of this compound at 17.358 min and the last detection of the same compound at 17.708 could be due to the presence of this compound with relatively high concentration so it took longer time to be eluted on the GC/MS column. Additionally, 4-[[p-(dimethylamino)phenyl]imino]-1-(p-fluorophenyl)-3-methyl-2-pyrazolin-5-one was putatively identified as a major compound in the GC/MS chromatogram of sample 3, with peak area percentage of 54.4% (Figure 4).
Figure 4.
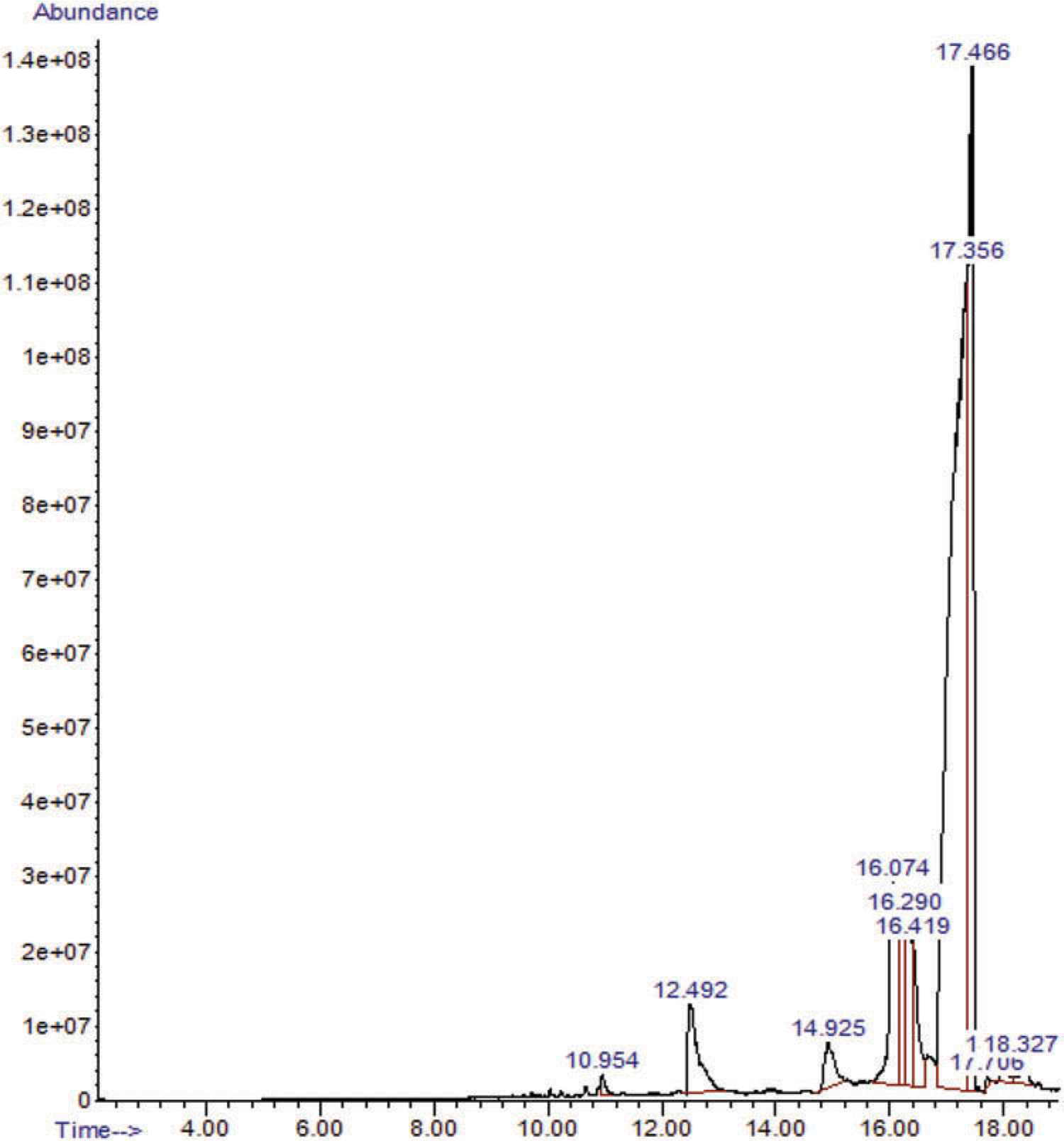
GC/MS TIC chromatogram of a methanolic extract of sample 3.
3.3. UPLC-MS/MS (MRM) analysis of samples extracts
UPLC-MS/MS (MRM) analysis of the three street drug extracts has been carried out using the seven standards for drugs of abuse that were available in our laboratory.
UPLC-MS/MS (MRM) revealed the presence of tetrahydrocannabinol (THC, Figure 6), oxazepam and tramadol in the extracts of samples 1, 2, and 3 (Figures S4–S21). The presence of these compounds was confirmed by matching the MS/MS spectra and both transitions’ retention times with those of the reference standards (Table 1). The three extracts also showed the possibility of the presence of both amphetamine and MDA in all three samples. Amphetamine has been identified by matching the MS/MS spectra and only the first transition retention time with its corresponding standard, because the three samples as well as the reference standard each showed only a peak at the first transition; however, no peaks appeared at the second transition. In the three samples, MDA has been identified by matching the MS/MS spectra. However, in case of reference standard as well as sample 2, only one peak appeared at the first transition, but in case of both samples 1 and 3, two transition peaks have been detected. Methadone has been identified in both samples 1 and 2 by matching the MS/MS spectra and both transitions’ retention times with those of the reference standards and not detected in sample 3. Diazepam has been identified in sample 3 and had not been detected in both samples 1 and 2 by matching the MS/MS spectra and both transitions’ retention times with those of the reference standards (Table 1)
Figure 6.
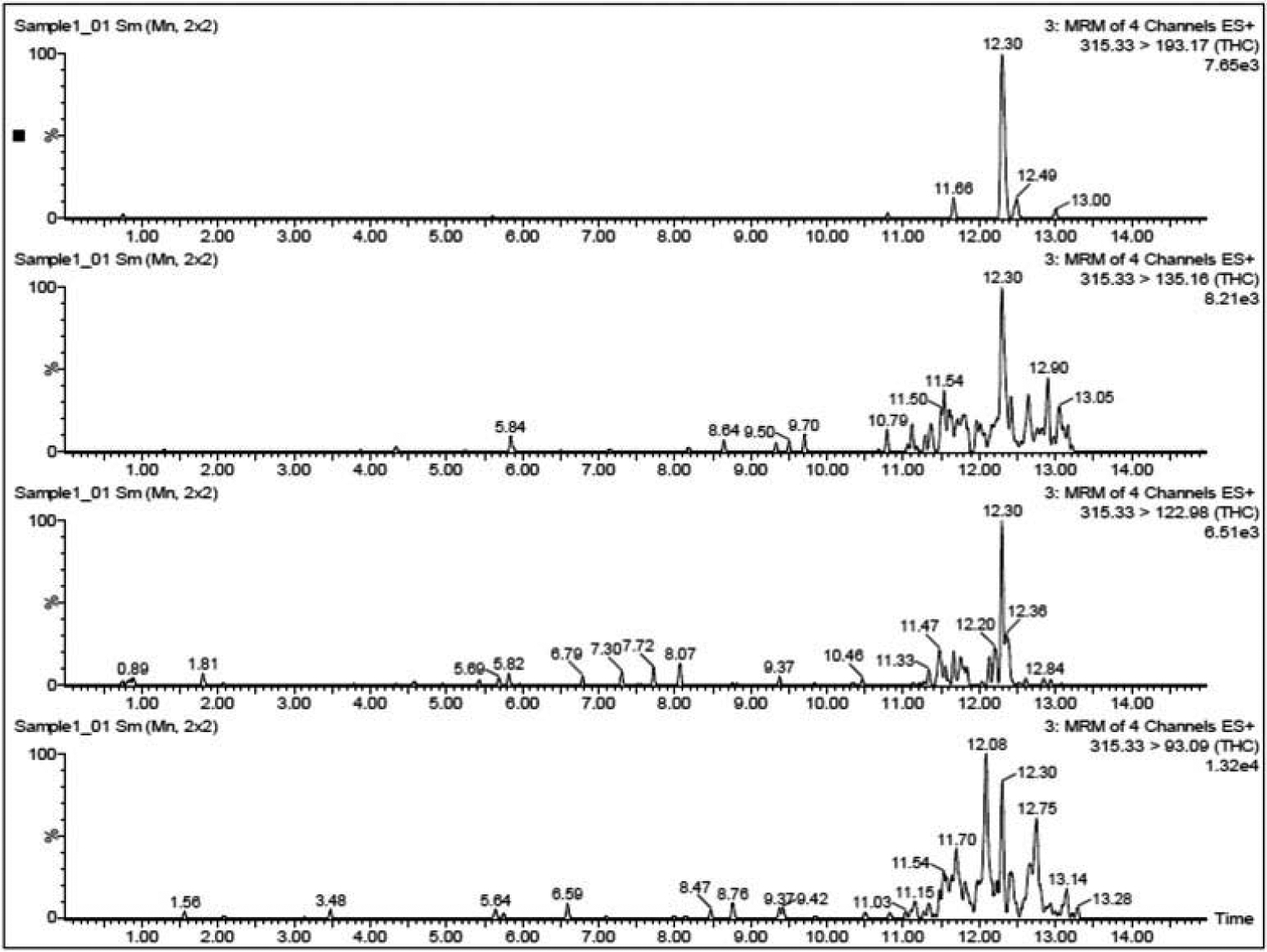
Ultra performance liquid chromatography/mass spectrometry (UPLC-MS/MS) using targeted multiple reaction monitoring (MRM) of THC in sample 1.
Table 1.
LC/MS MRM analysis results of samples 1, 2 and 3 extracts.
| Reference standard | Sample 1 | Sample 2 | Sample 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound Name | Transition | RT | Ratio* | RT | Ratio* | RT* | Ratio* | RT | Ratio* |
| THC | 315.33 > 93.09 | 12.36 | 2.567 | 12.30 | 0.929 | 12.31 | 0.327 | 12.29 | 0.856 |
| 315.33 > 193.17 | 12.28 | 12.30 | 12.17 | 12.29 | |||||
| Amphetamine | 136.1 > 91.1 | 1.25 | - | 1.30 | - | 1.29 | - | 1.30 | - |
| 136.1 > 119.1 | ND | ND | ND | ND | |||||
| MDA | 180.1 > 163.1 | 0.73 | - | 0.73 | 2.052 | 0.73 | - | 0.73 | 104.108 |
| 180.1 > 133.1 | ND | 0.79 | ND | 0.73 | |||||
| Methadone | 310.3 > 91.05 | 12.08 | 2.973 | 12.06 | 3.042 | 12.06 | 3.005 | ND | - |
| 310.3 > 105.1 | 12.13 | 12.06 | 12.06 | ND | |||||
| Tramadol | 264.2 > 58.1 | 3.64 | - | 3.61 | - | 3.61 | - | 3.61 | |
| Oxazepam | 287.1 > 241.2 | 11.65 | 0.486 | 11.61 | 59.189 | 11.62 | 42.675 | 11.49 | 0.404 |
| 287.1 > 269.1 | 11.66 | 11.64 | 11.65 | 11.48 | |||||
| Diazepam | 285.1 > 154.01 | 11.49 | 1.890 | ND | - | ND | - | 11.48 | 3.129 |
| 285.19 > 193.12 | 11.48 | ND | ND | 11.48 | |||||
Ratio* = The peak area detected in the first transition retention time/the peak area detected in the second transition retention time.
ND: not detected.
4. Discussion
The rapid emergence of new substances of abuse is a major problem facing many countries worldwide [17]. They are usually mixtures of substances synthesized in clandestine laboratories, with modifications in their chemical structures, which makes their identification more complicated and sometimes impossible with the usual screens for substances of abuse [18].
The goal of our study was to analyse the composition of some of the new substances that are spreading among youth in Egypt. They carry a variety of brand names, such as Voodoo, Strox, and Dragon. We obtained three packages of these street substances and found substantial chemical variation in their contents.
The GC/MS analysis of samples 1 and 2 revealed the presence of four similar putatively identified compounds in both samples. Most of the putatively identified compounds in the GC/MS spectra in the three samples might be extracted from the herbs used in these street drug samples. The GC/MS data revealed the presence of some psychoactive compounds as well, one morphinan alkaloid (Sample 1); three tetrahydrocannabinols and one cannabidiol (sample 2) and one well-known synthetic cannabinoid FUB-AMB (sample 3). The actions of some of these compounds target the central nervous system (CNS) (18–24). 1,3-bis(4-chlorophenyl)-5,6-Dihydrobenzo[f]quinazoline is a derivative of the natural alkaloids quinazolines, which are heterocyclic compounds with different biological functions. They act as ligands for benzodiazepines and GABA receptors in the CNS and have some calcium channel blocking activity [19,20]. The second compound, a morphinan, is in a large class of psychoactive drugs that includes opioid analgesics, cough suppressants, and hallucinogens. It has less affinity to μ-opioid receptors and higher affinity at the κ receptor than morphine [21]. Penitrem A is a fungal neurotoxin in animals and humans. It induces neurotoxicity in the form of sustained tremors and convulsions via several mechanisms: GABA neurotransmitter dysfunction, blockage of high-conductance Ca2+-activated K+ (BK) channels, and release of the excitatory neurotransmitters glutamate and aspartate [22–25]. Penitrem A also increases the production of reactive oxygen species in human neutrophils, resulting in oxidative stress in neurons [26]. This indole-diterpenoid mycotoxin could be produced by certain species of some fungus which can be found growing on herbal plant used in that street drug sample.
Sample 1 also contained some cannabinoid derivatives, the major psychoactive constituents of cannabis. They act as a partial agonist of the cannabinoid receptor CB1, located mainly in the CNS, and CB2, expressed mainly in immune cells, inducing psychoactive effects [27].
GC/MS analysis of sample 3 revealed a different compound, (methyl (2S)-2-{[1-[(4-fluorophenyl)methyl]indazole-3-carbonyl]amino}−3-methylbutanoate). This is an indole-based synthetic cannabinoid known as FUB-AMB and has a much higher affinity (approximately 380x greater) for cannabinoid receptors (CB1) than traditional cannabis [25]. This substance is marketed as giving a ‘legal high’ but it actually has serious adverse effects such as psychosis, palpitation, agitation, seizures, cardiotoxicity, and death [28]. In addition, it has a strong CNS depressant effect, which causes ‘zombie-like’ behaviour in users [29]. Abass and colleagues [12] also reported that a designer synthetic cannabinoid is a chief component of Voodoo being distributed in Egypt.
The UPLC-MS/MS (MRM) method showed that the three samples shared the psychoactive compounds THC, amphetamine, MDA, oxazepam, and tramadol. Samples 1 and 2 also contained methadone, and sample 3 contained diazepam instead of methadone. These psychoactive substances could be stimulants, hallucinogens, or empathogens. They have a high-abuse potential as well as severe adverse effects, which could lead to fatal intoxication depending on the amount ingested. Users under their influence while driving pose hazards in traffic [17,30,31], and the use of these substances has been associated with psychiatric comorbidities and aggressive behaviour [32].
5. Limitations
We were unable to identify some compounds in the samples by matching them to a spectral library. This lack of connection indicates that analysis of new designer substances, with their chemical modifications, requires an updated mass spectral library.
6. Conclusion
Voodoo, a substance of abuse that is being sold on Egyptian streets, had not been categorized in terms of its chemical content. Based on laboratory analyses, we concluded that substances being sold as ‘Voodoo’ are a heterogeneric mixture of psychoactive substances such as synthetic cannabinoids, amphetamine, tramadol, methadone, MDA, benzodiazepines, morphine derivatives, and penitrem A (a neurotoxin). The chemical constituents and concentrations of these substances vary among Voodoo herbal packages. The adverse effects of these street substances could be fatal for users, as they contain dangerous substances in unknown quantities and of unknown quality.
Supplementary Material
Acknowledgments
The manuscript was copyedited by Linda J. Kesselring, MS, ELS, the technical editor/writer in the Department of Emergency Medicine at the University of Maryland, School of Medicine.
Funding
This work was supported by Fogarty International Center at the National Institutes of Health [2D43TW007296].
Footnotes
Supplemental data for this article can be accessed here.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data described in this article are openly available in the Open Science Framework at DOI: 10.17605/OSF.IO/TPA6U.
References
- [1].El-Sawy H, Abdel Hay M and Badawy A, Egypt J. Neurol. Psychiat. Neurosurg 47, 1273 (2010). [Google Scholar]
- [2].Hamdi E, Gawad T, Khoweiled A, Edward A, Amer D, Mamdouh R, Fathi H and Loza N, Subst. Abuse 34, 97 (2013). doi: 10.1080/08897077.2012.677752. [DOI] [PubMed] [Google Scholar]
- [3].Hsu J, Lin JJ and Tsay WI, J Food Drug Anal 22, 169 (2014). doi: 10.1016/j.jfda.2014.01.019. [DOI] [Google Scholar]
- [4].Sweileh WM, Zyoud SH, Al-Jabi SW and AF S, Subst Abuse Treat Prev Policy 9, 33 (2014). doi: 10.1186/1747-597X-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hamdi E, Sabry N, Sedrak A, Khowailed A and Loza N, J. Addict. Prev 4, 1 (2016). [Google Scholar]
- [6].World Health Organization, Management of Substance Abuse, ATLAS on Substance Use Disorders (ATLAS-SU): Resources for the Prevention and Treatment of Substance Use Disorders. https://www.who.int/substance_abuse/activities/atlas/en/
- [7].INCB report 2013, Chapter 1.,Economic consequences of drug abuse <https://www.incb.org/documents/Publications/AnnualReports/Thematic_chapters/English/AR_2013_E_Chapter_I.pdf>
- [8].United Nations Office on Drugs and Crime, United Nations publication, Sales No. E.17.XI.7. (2017) www.unodc.org/wdr2017/field/Booklet_1_EXSUM.pdf (Accessed on May 20, 2019).
- [9].Fattore L, Psychiatry 79, 539 (2016). doi: 10.1080/00332747.2016.1185892. [DOI] [PubMed] [Google Scholar]
- [10].Liu L, Wheeler SE, Venkataramanan R, Rymer JA, Pizon AF, Lynch MJ and Tamama K, Am. J. Clin. Pathol 149, 105 (2018). doi: 10.1093/ajcp/aqx138. [DOI] [PubMed] [Google Scholar]
- [11].Egypt Drug Regulatory Authority, (2014) http://www.ginad.org/en/info?id=8476 [Google Scholar]
- [12].Abass MA, Hassan MZM, Abd Elhaleem MR, Abd Elaziz HR and AbdAllah RH, Ain Shams J. Forensic Med. Clin. Toxicol 28, 62 (2017). doi: 10.21608/ajfm.2017.18280. [DOI] [Google Scholar]
- [13].LGC Standards, Egypt (2016) <https://www.lgcstandards.com/EG/en>
- [14].European Union Decision, 2002/657/EC 17.8.2002. Off. J. Eur. Commun 8, 221 (2002). [Google Scholar]
- [15].Roberts M, Lee R and Wood M, Waters Application Note, 720002749EN, (2008).
- [16].Rosano TG, Wood M and Swift TA, J. Analyt. Toxicol 35, 411 (2011). doi: 10.1093/anatox/35.7.411. [DOI] [PubMed] [Google Scholar]
- [17].Tkalić RG, Alcohol. Psychiatry Res 54, 5 (2017). doi: 10.20471/may.2018.54.01.01. [DOI] [Google Scholar]
- [18].Martinotti G, Lupi M, Acciavatti T, Cinosi E, Santacroce R, Signorelli MS, Bandini L, Lisi G, Quattrone D, Ciambrone P, Aguglia A, Pinna F, Calò S, Janiri L and Giannantonio M, BioMed. Res. Int. 2014, (2014). DOI: 10.1155/2014/815424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Seo HN, Choi LY, Choe YJ, Kim Y, Rhim H, Lee SH, Kim J, Joo DJ and Lee JY, Med. Chem. Lett 17, 5740 (2007). doi: 10.1016/j.bmcl.2007.08.070. [DOI] [PubMed] [Google Scholar]
- [20].Khan I, Ibrar A, Ahmed W and Saeed A, Eur. J. Med. Chem 90, 124 (2015). doi: 10.1016/j.ejmech.2014.10.084. [DOI] [PubMed] [Google Scholar]
- [21].Vardanyan R and Hruby V, in Synthesis of Best-Seller Drugs, edited by Vardanyan R and Hruby V (Elsevier Science, New York, 2016). [Google Scholar]
- [22].Imlach WL, Finch SC, Dunlop J, Meredith AL, Aldrich RW and Dalziel JE, J. Pharmacol. Exp. Ther 327, 657 (2008). doi: 10.1124/jpet.108.143933. [DOI] [PubMed] [Google Scholar]
- [23].Moldes-Anaya AS, Fonnum F, Eriksen GS, Rundberget T, Walaas SI and Wigestrand MB, Neurochem. Int 59, 1074 (2011). doi: 10.1016/j.neuint.2011.08.014. [DOI] [PubMed] [Google Scholar]
- [24].Talcott P, Peterson ME and Talcott PA, editors, Small Animal Toxicology (Elsevier Inc., 2013). [Google Scholar]
- [25].Banister SD, Longworth M, Kevin R, Sachdev S, Santiago M, Stuart J, Mack JB, Glass M, McGregor IS, Connor M and Kassio M, ACS Chem. Neurosci 7, 1241 (2016). doi: 10.1021/acschemneuro.6b00137. [DOI] [PubMed] [Google Scholar]
- [26].Berntsen HH, Bogen IL, Wigestrand MB, Fonnum F, Walaas SI and Moldes-Anaya A, Toxicology 392, 64 (2017). doi: 10.1016/j.tox.2017.10.008. [DOI] [PubMed] [Google Scholar]
- [27].Hunault CC, Mensinga TT, de Vries I, Kelholt-Dijkman HH, Hoek J, Kruidenier M, Leenders ME and Meulenbelt J, Psychopharmacology 201, 171 (2008). doi: 10.1007/s00213-008-1260-2. [DOI] [PubMed] [Google Scholar]
- [28].Hasegawa K, Wurita A, Minakata K, Gonmori K, Yamagishi I, Nozawa H, Watanabe K and Suzuki O, Forensic Toxicol. 33, 112 (2015). doi: 10.1007/s11419-014-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Adams AJ, Banister SD, Irizarry L, Trecki J, Schwartz M and Gerona R, N. Engl. J. Med 376, 235 (2017). doi: 10.1056/NEJMoa1610300. [DOI] [PubMed] [Google Scholar]
- [30].Liechti M, Swiss Med. Wkly 145, w14043 (2015). [DOI] [PubMed] [Google Scholar]
- [31].Lovrecic B, Lovrecic M, Gabrovec B, Carli M, Pacini M, Maremmani AGI and Maremmani I, Int. J. Environ. Res. Public Health 16, 177 (2019). doi: 10.3390/ijerph16020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bennett KH, Hare HM, Waller RM, Alderson HL and Lawrie S, BMJ Open 7, e015716 (2017). doi: 10.1136/bmjopen-2016-015716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


