Supplemental Digital Content is available in the text.
Keywords: chronic liver disease, cirrhosis, coronavirus, COVID-19, inflammatory markers, liver chemistries, liver function tests, liver test abnormalities, mortality, SARS-CoV-2
Abstract
Background and aims
Liver chemistry abnormalities (LCA) are common in patients with coronavirus disease 2019 (COVID-19), but their causes and clinical impact have not been adequately studied. We assessed the associations between LCA and clinical characteristics, inflammatory serum markers, in-hospital mortality.
Methods
Ten thousand eight hundred fifty-six adult patients with COVID-19 hospitalized in 13 hospitals in New York (1 March to 27 April 2020) were analyzed retrospectively. Abnormalities of liver chemistries [aspartate aminotransferase (AST), alanine aminotransferase, alkaline phosphatase, or total bilirubin] were defined as absent, mild-moderate (at least one value up to four times elevated), or severe.
Results
LCA were mild-moderate in 63.9% and severe in 7.6% at admission. Risk factors for severe LCA were male sex and chronic liver disease. Conversely, hypertension and diabetes mellitus were less likely associated with severe LCA. AST elevation correlated weakly to modestly with inflammatory markers. On adjusted analysis, in-hospital mortality was 1.56 times and 1.87 times increased in patients with mild-to-moderate and severe LCA, respectively. Diabetes, hypertension, male sex, and age greater than 60 years was associated with incremental risk of mortality with increase severity of LCA, especially in the first week of hospitalization. HTN was not associated with increased in-hospital mortality unless LCA was present.
Conclusion
Increasing severity of LCA on hospital admission predicts early in-hospital mortality in COVID-19 patients. Mortality associated with the known risk factors, hypertension, diabetes, male sex, and old age was accentuated in the presence of LCA. AST correlated modestly with inflammatory markers.
Introduction
Liver chemistry abnormalities (LCA) are common in patients hospitalized with coronavirus disease 2019 (COVID-19). A recent study of patients hospitalized with COVID-19 within our healthcare system found an elevated aspartate aminotransferase (AST) level >40 U/L in 58% and an elevated alanine aminotransferase (ALT) level >60 U/L in 39% of the patients [1]. In a study by Guan et al. [2], hyperbilirubinemia was present in 11% of patients on admission. While most patients have mild LCA, significant hepatitis and acute-on-chronic liver failure have been described [3,4]. The significance of early hepatic involvement is incompletely understood. Elevated transaminases were more frequent in patients that developed severe disease [2,5], and a small study of 60 patients noted that AST-predominant transaminase elevation was correlated with disease severity [6]. Another study that focused on ALT as a sole marker found that severe LCA (which the authors defined as ALT above five times their institutional upper limit of normal (ULN) of 50 U/L) were present in 6.4% of 2273 patients and correlated with a more severe clinical course [7]. The latter study also described a correlation of the severity of LCA with inflammatory markers, ferritin, and interleukin 6.
Commonly performed liver chemistry tests include AST, ALT, alkaline phosphatase (ALP), and total bilirubin (TB), and further data are necessary to shed light on the causes of their elevations and their prognostic significance in COVID-19, as the sizes of prior studies were too small to draw firm conclusions. We therefore analyzed the clinical significance of LCA in a large, multicenter cohort of patients hospitalized with COVID-19.
Study population and data collection
We obtained the medical records and compiled data from our electronic medical records on all patients with documented COVID-19 infection who were admitted to 13 hospitals within the Northwell Health system in New York City, and Long Island, New York, from 1 March to 27 April 2020. A confirmed case was defined as a positive reverse-transcriptase PCR on a specimen obtained through nasopharyngeal swabbing. We collected the following demographic information: age, sex, race, ethnicity, insurance status, BMI, heart rate, blood pressure, temperature, oxygen saturation, and the presence of co-morbid conditions. We screened all patients for known CLD by searching our database for ICD-10 diagnostic codes. We followed a systematic approach to confirm whether patients had CLD with or without cirrhosis (described in Supplementary Figure 1, Supplemental digital content 1, http://links.lww.com/EJGH/A652). Any discrepancies were resolved with consensus after re-review by a second transplant hepatologist (N.R.). Patients with a positive hepatitis C antibody and negative hepatitis C RNA – regardless of whether due to false-positive antibody test, prior spontaneous viral clearance, or virologic response to previously received antiviral therapy – were not included in the CLD group unless they had cirrhosis or an etiology of CLD aside from hepatitis C. Race and ethnicity data were collected by self-report in pre-specified fixed categories. Baseline laboratory testing was defined as the first measurement available within 48 h of presentation to the hospital. We excluded patients under 18 years of age and patients with missing baseline values for serum AST, ALT, ALP, or TB. For baseline laboratory parameters, we collected hemoglobin, leukocyte count, platelet, international normalized ratio (INR), creatinine, AST, ALT, ALP, TB, albumin, lactate, ferritin, creatine phosphokinase (CPK), lactate dehydrogenase (LDH), D-dimer, C-reactive protein (CRP), and procalcitonin.
This study was supported by the COVID-19 Research Consortium of Northwell Health and was approved by the Institutional Review Board for the Feinstein Institutes of Medical Research at Northwell Health as minimal-risk research using data collected for routine clinical practice and waived the requirement for informed consent.
Statistical analysis
Liver chemistries, namely, serum levels of AST, ALT, ALP, and TB levels were stratified into three composite groups: (1) no LCA; (2) elevation of at least one parameter above normal but all less than or equal to four times the ULN (mild-to-moderate LCA); and (3) elevation of at least one parameter greater than four times the ULN (severe LCA). As per the American Association for the Study of Liver Disease (AASLD) expert consensus recommendation, we defined the ULN for ALT as 25 U/L for women and 35 U/L for men [8]. ULNs for other liver chemistries were defined based on Northwell laboratories, which were: 40 U/L for AST, 125 U/L for ALP, and 1.2 mg/dl for TB.
We summarized each continuous variable using its median and interquartile range. Categorical variables were summarized using counts and percentages. Comparisons between groups were assessed using Kruskal–Wallis test, chi-squared test, and Fisher’s exact test as appropriate. We performed univariate and multivariate regression analysis to assess predictors of severity of LCA compared to ‘no LCA’ as the composite reference group. We used a stepwise selection approach to select variables to be included in a multivariate logistic regression model.
Pearson’s correlation coefficients (r) with 95% confidence intervals (CIs) and significance levels (P values) were computed to assess the linear associations between AST and ALT with different inflammatory markers. The correlations between ALP and TB with inflammatory markers were not assessed due to relatively small number of patients with elevated levels of these parameters. We defined correlation coefficients as ‘weak’ if r = 0.10 to 0.39, ‘moderate’ if r = 0.4–0.69, ‘strong’ if r = 0.70–0.89, and ‘very strong’ if r > 0.9.
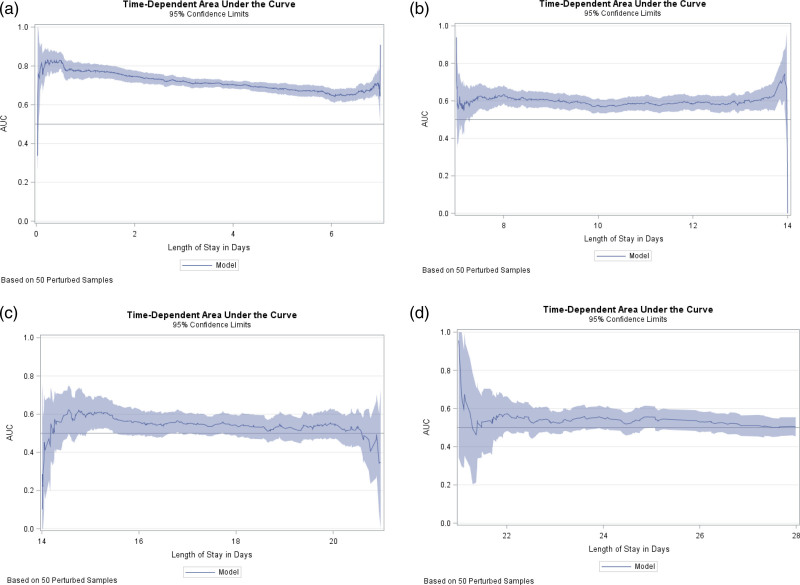
For survival analyses, we censored patients as alive without the event of interest on their date of hospital discharge or at 28 days of follow-up, whichever was earlier. We constructed Kaplan–Meier survival curves with log-rank tests and estimated hazard ratios (HRs) and 95% CIs using both univariate and multivariate Cox proportional hazards models. Multivariate models were adjusted for age (time-varying), sex, race (time-varying), ethnicity, insurance status (time-varying), and presence of significant co-morbid conditions. We checked the proportional hazards assumption for each variable included in our Cox regression models using graphical assessment of the Kaplan–Meier survival curves and log(-log(survival)) vs. log(time) graphs to look for parallel curves and by ensuring that Schoenfeld residuals were independent of time. We also plotted time-dependent area under the curve (AUC) and examined the AUC at all distinct event times by plotting the curve of the AUC of the fitted model including a combination of four-variable LCA model and reported the 95% pointwise confidence limits graphically. We examined the robustness of this model by examining the Time-dependent AUC serially at week one though week 4 [9]. We used two-sided tests with α = 0.05. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina, USA).
Results
There were 11 265 patients with COVID-19 hospitalized in 13 hospitals within the Northwell Health system between 1 March and 27 April 2020. After excluding 106 children who were under 18 years old and 303 patients without baseline measurements of liver chemistries within 48 h of hospital admission, our study population included 10 856 patients. The majority of patients were male (59.0%), age 60 years or older (63.1%), and of non-Hispanic ethnicity (72.0%). The most common preexisting medical conditions were hypertension (58.6%), obesity (38.7%), and diabetes mellitus (35.7%). After a maximum observation period of 28 days, 6846 (65.2%) patients had been discharged from the hospital, 2261 (21.5%) patients had died, 389 (3.71%) patients had been transferred to other facilities, and 1001 (9.5%) patients remained hospitalized. On admission, 3096 (28.5%) patients had normal LCA, 6933 (63.9%) had mild-moderate LCA, and 827 (7.6%) had severe LCA (Table 1).
Table 1.
Baseline characteristics of the study population
| Full cohort (n = 10 856) | No LCA (n = 3096) (28.5%) | Mild to moderate LCA (n = 6933) (63.9%) | Severe LCA (n = 827) (7.6%) | |
|---|---|---|---|---|
| Age (years) | 68 (55–79) | 65(54–76) | 61 (50–72) | |
| <60 | 4004 (36.9%) | 1023 (33.0%) | 2594 (37.4%) | 387 (46.8%) |
| ≥60 | 6852 (63.1%) | 2073 (67.0%) | 4339 (62.6%) | 440 (53.2%) |
| BMI (kg/m2)a | 8525 (78.5%) | 28 (24–33) | 28 (25–33) | 28 (25–33) |
| Vital signs | ||||
| SBP <90 mmHgb | 345 (3.2%) | 97 (3.1%) | 211 (3.0%) | 37 (4.5%) |
| Temperature >38 °Cb | 2993 (27.6%) | 812 (26.3%) | 1945 (28.1%) | 236 (28.6%) |
| Heart rate >90 bpm | 6888 (63.5%) | 1764 (57.1%) | 4533 (65.4%) | 591 (71.5%) |
| Respiratory rate >20 bpm | 4.363 (40.2%) | 966 (31.2%) | 3011 (43.3%) | 386 (46.7%) |
| SpO2 <90% | 2365 (21.8%) | 395 (12.8%) | 1713 (24.7%) | 257 (31.1%) |
| SpO2 <80% | 624 (5.8%) | 81 (2.6%) | 472 (6.8%) | 71 (8.6%) |
| Leukocytes <4 or >12 (×109/L) | 2489 (22.9) | 715 (23.1%) | 1540 (22.2%) | 234 (28.3%) |
| Sex | ||||
| Male | 6404 (59.0%) | 1659 (53.6%) | 4207(60.7%) | 538 (65.1%) |
| Female | 4452 (41.0%) | 1437 (46.4%) | 2726(39.3%) | 289(34.9%) |
| Race | ||||
| White | 4122 (38.0%) | 1395 (45.1%) | 2484 (35.8%) | 243(29.4%) |
| Black | 2301 (21.2%) | 674 (21.8%) | 1443 (20.8%) | 184 (22.3%) |
| Asian | 939 (8.6%) | 215 (6.9%) | 647(9.3%) | 77 (9.3%) |
| Other/multiracial | 3015 (27.8%) | 704 (22.7%) | 2039(29.4%) | 272(32.9%) |
| Unknown | 479 (4.4%) | 108 (3.5%) | 320(4.6%) | 51 (6.2%) |
| Ethnicity | ||||
| Non-Hispanic or Latino | 7822 (72.0%) | 2403 (77.6%) | 4867(70.2%) | 552 (66.8%) |
| Hispanic or Latino | 2330 (21.5%) | 540 (17.4%) | 1582(22.8%) | 208 (25.2%) |
| Other/unknown | 704 (6.5%) | 153 (4.9%) | 484(7.0%) | 67 (8.1%) |
| Insurance | ||||
| Medicare | 5037 (46.4%) | 1679 (54.2%) | 3071 (44.3%) | 287 (34.7%) |
| Medicaid | 2266 (20.9%) | 546 (17.6%) | 1507 (21.7%) | 213 (25.8%) |
| Commercial | 3251 (29.9%) | 816 (26.4%) | 2137 (30.8%) | 298 (36.0%) |
| Self-pay | 179 (1.6%) | 23 (0.8%) | 135 (2.0%) | 21 (2.5%) |
| Others | 123 (1.1%) | 32 (1.0%) | 83 (1.2%) | 8 (1.0%) |
| Presence of co-morbid conditions | ||||
| Hypertension | 6362 (58.6%) | 1970 (63.6%) | 3998 (57.7%) | 394 (47.6%) |
| Diabetes mellitus | 3877 (35.7%) | 1234 (40.0%) | 2432 (35.1%) | 211(25.5%) |
| Coronary artery disease | 1367 (12.6%) | 471 (15.2%) | 820 (11.8%) | 76(9.2%) |
| Heart failure | 859 (7.9%) | 311 (10.1%) | 498 (7.2%) | 50(6.1%) |
| Malignancy | 816 (7.5%) | 291 (9.4%) | 475 (6.9%) | 50(6.0%) |
| Chronic obstructive pulmonary disease | 634 (5.8%) | 254 (8.2%) | 344 (5.0%) | 36 (4.4%) |
| Chronic kidney disease (stage I–IV) | 413 (3.8%) | 159 (5.1%) | 231 (3.3%) | 23 (2.8%) |
| End-stage renal disease | 450 (4.1%) | 186 (6.0%) | 238 (3.4%) | 26 (3.1%) |
| Obesity (BMI ≥ 30)a,b | 3295 (38.7%) | 891 (37.3%) | 2143 (39.0%) | 261(40.6%) |
| Chronic liver disease | 245 (2.3%) | 50 (1.6%) | 162 (2.3%) | 33 (4.0%) |
| Laboratory tests, mean and IQR (within 48 h) | ||||
| AST (IU/L) | 10 856 (100%) | 27 (21–33) | 55 (43–75) | 185 (130–257) |
| ALT (IU/L) | 10 856 (100%) | 19 (14–23) | 41 (29–59) | 151 (109–207) |
| Alkaline phosphatase (IU/L) | 10 856 (100%) | 69 (57–86) | 76 (60–103) | 105 (74–167) |
| Total bilirubin (mg/dl) | 10 856 (100%) | 0.4 (0.3–0.6) | 0.5 (0.4–0.7) | 0.7 (0.5–1.0) |
| INRa | 7017 (65.5%) | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) |
| Albumin (g/dl)a | 10 855 (99.9%) | 3.5 (3.1–3.9) | 3.3 (2.9–3.7) | 3.3 (2.8–3.7) |
| Creatinine (mg/dl)b | 10 856 (100%) | 1.1 (0.8–1.6) | 1.1 (0.8–1.5) | 1.1 (0.8–1.7) |
| Leukocyte (x109/L)a | 10 854 (99.9%) | 6.9 (5.2–9.5) | 7.6 (5.6–10.2) | 8.2 (6.1–11.5) |
| Hemoglobin (g/dL)a | 10 854 (99.9%) | 12.7 (11.3–14.0) | 13.3 (12.0–14.5) | 13.6 (12.1–14.8) |
| Platelet (x109/L)a | 10 839 (99.8%) | 205 (158–269) | 211 (161–273) | 222 (163–300) |
| Lactate (mmol/L)a | 4525 (41.7%) | 1.5 (1.1–2.0) | 1.6 (1.2–2.2) | 1.9 (1.3–3.2) |
| Ferritin (μg/L)a | 8236 (78.6%) | 504 (268–889) | 890 (483–1569) | 1750 (926–3468) |
| LDH (U/L)a | 7020 (64.7%) | 319 (248–400) | 444 (335–585) | 607 (449–807) |
| D-dimer (ng/mL)a | 6756 (62.2%) | 430 (242–840) | 474 (283–993) | 597 (315–1794) |
| CRP (mg/L)a | 8425 (77.6%) | 8.4 (4.2–15.2) | 11.9 (6.5–19.4) | 12.4 (7.2–20.4) |
| Procalcitonin (ng/mL)a | 7840 (72.2%) | 0.2 (0.1–0.4) | 0.2 (0.1–0.6) | 0.4 (0.2–1.1) |
| CPK (mg/dl)a | 4040 (37.2%) | 115 (66–204) | 200 (94–463) | 343 (102–1207) |
Data summarized as n (%) for categorical variables and median (IQR) for continuous variables.
Groups were defined based on AST, ALT, ALP, and TB at admission: ‘no LCA’ if all within normal limits, ‘mild-moderate LCA’ if at least one parameter elevated but all ≤4 times the ULN, and ‘severe LCA’ if at least one parameter elevated >4 times the ULN.
Normal range for laboratory tests: AST 10-40 U/L, ALT 10-35 U/L (men) and 10-35 U/L (women), ALP 40-120 U/L, TB 0.2-1.2 mg/dl, INR 0.88-1.16, albumin 3.3-5.0 g/dL, creatinine 0.5-1.3 mg/dl, leukocytes 3.8-10.5 x109/L, hemoglobin 13-17 g/dL (men) and 11.5-15.5 g/dL (women), platelets 150-400 x109/L, lactate 0.7-2.0 mmol/L, ferritin 30-400 ng/dL (men) and 15-150 ng/dL (women), LDH 50-242 U/L, D-dimer <229 DDU, CRP 0-0.4 mg/dl, procalcitonin ≤0.2 ng/mL, CPK 30-200 U/L (men) and 25-170 U/L (women).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPK, creatine phosphokinase; CRP, C-reactive protein; INR, international normalized ratio; IQR, interquartile range; LCA, liver chemistry abnormalities; LDH, lactate dehydrogenase; SpO2, oxygen saturation; ULN, upper limit of normal.
Missing data, number of patients: BMI and obesity = 2,331, INR = 3,839, albumin = 1, leukocytes and hemoglobin = 2, platelet = 17, lactate = 6,331, ferritin = 2,620, LDH = 3,836, d-dimer = 4,100, CRP = 2,431, procalcitonin = 3,016, CPK = 6,816
P-value is not statistically significant (P > 0.05).
Most patients with LCA had two or more abnormal chemistries (Supplementary Figure 1, Supplemental digital content 1, http://links.lww.com/EJGH/A652). AST was the most frequent elevation among patients with mild-moderate LCA (80.6% with elevated AST vs. 72.9% with elevated ALT), whereas ALT was the most frequent severe elevation among patients with severe LCA (65.8% with AST >4× ULN vs. 71.5% with ALT >4× ULN) (Supplementary Table 1, Supplemental digital content 1, http://links.lww.com/EJGH/A652). Patients with severe LCA were younger (median age: 61 years) than patients with no LCA (median age: 68 years) or mild-moderate LCA (median age: 65 years). With increasing severity of LCA, the proportion of men increased and the proportion of patients self-identifying as white decreased (Table 1). The average BMI was 28 kg/m2 in all three groups and the proportion of obese patients did not differ by severity of LCA. Hypertension, diabetes mellitus, coronary artery disease, and less common comorbidities including heart failure, malignancy, chronic obstructive pulmonary disease, chronic kidney disease, and end-stage renal disease were all less frequent among patients with severe LCA than among those with no or mild-moderate LCA. In contrast, the proportion of patients with underlying chronic liver disease increased with severity of LCA (1.6% of patients with no LCA, 2.3% of patients with mild-moderate LCA, and 4.0% of patients with severe LCA).
Increasing LCA correlated with increased frequency of hypoxia (12.8% vs. 24.7% vs. 31.1%) and tachycardia (57.1% vs. 65.4% vs. 71.5%). Supplementary Figure 2, Supplemental digital content 1, http://links.lww.com/EJGH/A652 show the correlations between inflammatory markers and AST. A moderate correlation was observed for AST and LDH across all severities of LCA. Lactate showed a moderate correlation with AST among patients with severe LCA and a weak correlation with AST among patients with mild-moderate LCA. Ferritin, CRP, procalcitonin, and D-dimer each had weak or no correlation with AST within strata of LCA. No correlation was found for ALT with any inflammatory marker, apart from a weak correlation with ferritin among patients with severe LCA (Supplementary Figure 3, Supplemental digital content 1, http://links.lww.com/EJGH/A652). CPK was moderately correlated with AST among patients with mild-moderate LCA [r = 0.45 (0.42–0.48)] or severe LCA [r = 0.37, (0.27–0.46)] but was not correlated with ALT in any groups (Supplementary Figure 4, Supplemental digital content 1, http://links.lww.com/EJGH/A652]
We used univariate and multivariate logistic regression with stepwise selection to identify predictors of severity of LCA (Table 2). Our final multivariate model included age, sex, race, insurance status, and presence or absence of hypertension, diabetes mellitus, and chronic liver disease. Male sex [odds ratio (OR) 1.27, 95% CI 1.09–1.47, P = 0.002] and chronic liver disease (OR 2.04, 95% CI 1.40–2.97, P < 0.001) were predictive of having severe LCA, whereas the risk of severe LCA was decreased for patients with hypertension (OR 0.78, 95% CI 0.66–0.91, P = 0.002) and patients with diabetes (OR 0.64, 95% CI 0.53–0.75, P < 0.001).
Table 2.
Univariate and multivariate logistic regression analysis for factors associated with severe liver chemistry abnormalities on admission
| Univariate logistic regression | Multivariate logistic regressiona | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age ≥60 years (reference: <60 years) | 0.64 (0.55–0.74) | <0.001 | 0.94 (0.78–1.12) | 0.479 |
| BMI (kg/m2)b | 1.00 (0.99–1.02) | 0.516 | ||
| Sex | ||||
| Male (reference: female) | 1.32 (1.13–1.53) | <0.001 | 1.27 (1.09–1.47) | 0.002 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 1.39 (1.13–1.69) | 0.693 | 1.47 (1.19–1.80) | 0.408 |
| Asian | 1.43 (1.09–1.86) | 0.984 | 1.38 (1.05–1.81) | 0.985 |
| Other/multiracial | 1.58 (1.32–1.90) | 0.125 | 1.42 (1.17–1.74) | 0.636 |
| Unknown | 1.90 (1.38–2.61) | 0.020 | 1.72 (1.23–2.38) | 0.078 |
| Ethnicity | ||||
| Non-Hispanic or Latino | Reference | |||
| Hispanic or Latino | 1.29 (1.09–1.53) | 0.353 | ||
| Other/unknown | 1.39 (1.06–1.81) | 0.142 | ||
| Insurance | ||||
| Medicare | Reference | Reference | ||
| Medicaid | 1.72 (1.42–1.98) | 0.172 | 1.30 (1.03–1.62) | 0.395 |
| Commercial | 1.67 (1.41–1.98) | 0.250 | 1.38 (1.12–1.68) | 0.148 |
| Self-pay | 2.20 (1.37–3.52) | 0.051 | 1.48 (0.90–2.42) | 0.281 |
| Others | 1.15 (0.55–2.38) | 0.389 | 0.89 (0.42–1.85) | 0.329 |
| Comorbidities (reference: absence of the comorbidity) | ||||
| Hypertension | 0.62 (0.53–0.71) | <0.001 | 0.78 (0.66–0.91) | 0.002 |
| Diabetes mellitus | 0.60 (0.50–0.70) | <0.001 | 0.64 (0.53–0.75) | <0.001 |
| Coronary artery disease | 0.69 (0.53–0.87) | 0.002 | ||
| Heart failure | 0.73 (0.54–0.99) | 0.040 | ||
| Malignancy | 0.78 (0.57–1.05) | 0.096 | ||
| Chronic obstructive pulmonary disease | 0.72 (0.50–1.01) | 0.059 | ||
| Chronic kidney disease (stage I–IV) | 0.71 (0.46–1.08) | 0.112 | ||
| End-stage renal disease | 0.74 (0.49–1.10) | 0.135 | ||
| Obesity (BMI ≥30 kg/m2)b | 1.09 (0.92–1.29) | 0.294 | ||
| Chronic liver disease | 1.93 (1.32–2.80) | <0.001 | 2.04 (1.40–2.97) | <0.001 |
CI, confidence interval; LCA, liver chemistry abnormalities; OR, odds ratio.
Variables for inclusion in the multivariate model was selected using a stepwise selection procedure.
Missing data for some patients: BMI and obesity = 2331.
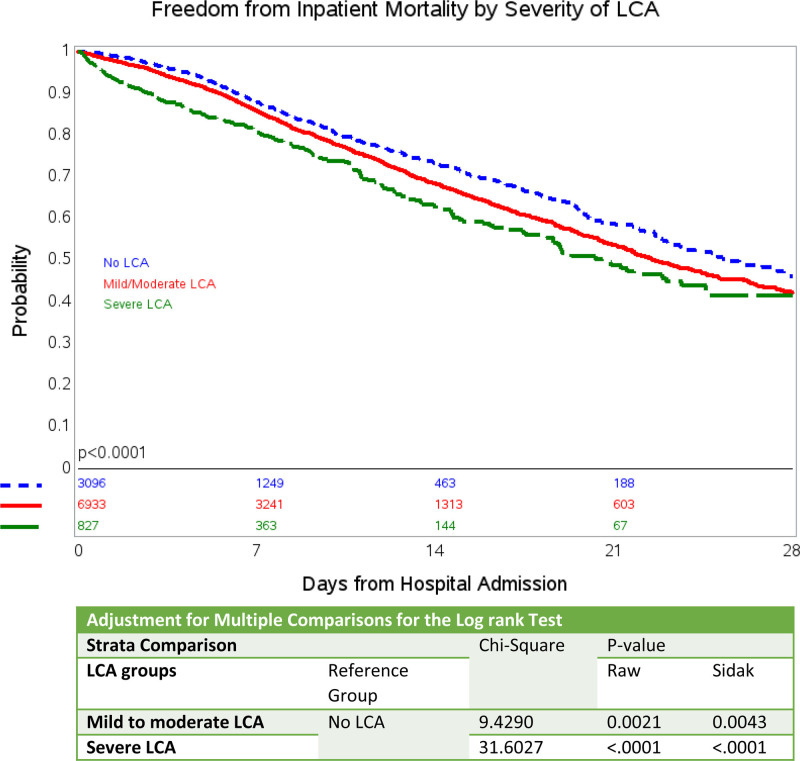
We evaluated the performance of the combination of AST, ALT, ALP, and TB on predicting in-patient mortality at 28 days and compared this model to both individual LCA abnormalities as well various possible combinations of LCA (Supplementary Table 2, Supplemental digital content 1, http://links.lww.com/EJGH/A652) using a logistic regression analysis approach. As noted in Supplementary Table 2, Supplemental digital content 1, http://links.lww.com/EJGH/A652, the model including all four LCA has the most robust AUC of 75% in predicting in-patient mortality at 7 days and have an AUC of 69% at 28 days of hospitalization. We also evaluated model performance throughout the duration of the hospital stay (Fig. 1) using time-dependent AUC in a Cox regression model. As evident in the Fig. 1, mortality was best predicted in the first week of hospitalization (integrated time-dependent area under the curve, 0.73). Further in Kaplan–Meier survival analyses, patients with any degree of LCA had an increased risk of in-hospital mortality (Fig. 2) when compared to patients with no LCA (P < 0.001). Mortality was positively associated with increased severity of LCA in univariate and multivariate Cox proportional hazard models (Table 3). The risk of in-hospital mortalities in unadjusted analysis, compared with patients with no LCA, was 1.21 times (OR 1.21, 95% CI 1.09–1.34, P < 0.001) and 1.57 times (OR 1.57, 95% CI 1.34–1.84, P < 0.001) higher for patients with mild-moderate LCA and severe LCA, respectively. After adjusting for demographic factors and co-morbid conditions and compared to patients with no LCA, the risk of in-hospital mortality was 1.56 times higher for patients with mild-moderate LCA (OR 1.56, 95% CI 1.38–1.76, P < 0.001) and 1.87 times higher for patients with severe LCA (OR 1.87, 95% CI 1.52–2.30, P < 0.001).
Fig. 1.
Time-dependent area under the curve (AUC) with 95% confidence limits for predictive model including four-variable LCA models: (a) week 1 (b) week 2, (c) week 3, (d) week 4. LCA, liver chemistry abnormalities.
Fig. 2.
Kaplan–Meier curve showing freedom from in-hospital mortality by severity of LCA. LCA, liver chemistry abnormalities.
Table 3.
Association between degree of liver chemistry abnormalities and in-hospital mortality among patients hospitalized with confirmed COVID-19 infection
| LCA group | Unadjusted analysis | Adjusted analysisa | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| No LCA | Reference | Reference | ||
| Mild to moderate LCA | 1.21 (1.09–1.34) | <0.001 | 1.56 (1.38–1.76) | <0.001 |
| Severe LCA | 1.57 (1.34–1.84) | <0.001 | 1.87 (1.52–2.30) | <0.001 |
CI, confidence interval; HR, hazard ratio; LCA, liver chemistry abnormalities.
Adjusted for age (time-varying), sex, race (time-varying), ethnicity, insurance status (time-varying), and comorbidities (HTN, DM, CAD, HF, malignancy, COPD, CKD I-IV, ESRD, obesity, and CLD)
Finally, we analyzed the hazard for in-hospital mortality of patients with different degrees of LCA depending on known other risk factors, namely age, sex, and presence of HTN or DM (Table 4). In unadjusted cox-proportional hazard analysis, no difference was observed for men compared to women and diabetics compared to non-diabetics without LCA. However, after adjustment for demographic and co-morbid conditions, both diseases appeared as risk factors for mortality, which increased incrementally with the degree of LCA. LCA had the weakest association with in-hospital mortality in patient below 60, in which mild-to-moderate LCA was not associated with increased mortality compared to those without LCA, however, the combination with severe LCA increased the mortality risk significantly (OR 1.60, 95% CI 1.03–2.47, P = 0.0347). In contrast, age above 60 and LCA conferred the highest mortalities in each severity group of LCAs (OR 3.65, 95% CI 2.60–5.12, P < 0.0001) with severe LCA).
Table 4.
Univariate and multivariate analysis using Cox-proportional hazard model for predictors of in-hospital mortality
| Analysis groups | Univariate Cox regression | Multivariate Cox regressiona | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
| Sex (Ref = female w/ no LCA)a | 1 | 1 | ||
| Female w/ mild to moderate LCA | 1.16 (0.99–1.36) | 0.0577 | 1.41 (1.16–1.70) | 0.0004 |
| Female w/ severe LCA | 1.69 (1.29–2.20) | 0.0001 | 2.03 (1.41–2.92) | 0.0001 |
| Male w/ no LCA | 0.96 (0.80–1.15) | 0.6628 | 1.46 (1.18–1.81) | 0.0005 |
| Male w/ mild to moderate LCA | 1.20 (1.03–1.39) | 0.0150 | 2.44 (2.03–2.91) | <0.0001 |
| Male w/ severe LCA | 1.48 (1.19–1.82) | 0.0003 | 2.73 (2.11–3.54) | <0.0001 |
| Age (Ref < 60 years w/ no LCA)b | 1 | 1 | ||
| <60 years w/ mild to moderate LCA | 1.42 (1.06–1.90) | 0.0171 | 1.25 (0.92–1.71) | 0.1523 |
| <60 years w/ severe LCA | 1.74 (1.16–2.58) | 0.0064 | 1.60 (1.03–2.47) | 0.0347 |
| 360 years w/ no LCA | 3.48 (2.64–4.59) | <0.0001 | 1.91 (1.40–2.60) | <0.0001 |
| 360 years w/ mild to moderate LCA | 4.26 (3.26–5.55) | <0.0001 | 2.84 (2.12–3.79) | <0.0001 |
| 360 years w/ severe LCA | 6.50 (4.82–8.76) | <0.0001 | 3.65 (2.60–5.12) | <0.0001 |
| HTN (Ref = no HTN w/ no LCA)c | 1 | 1 | ||
| No HTN w/ mild to moderate LCA | 1.20 (0.99–1.45) | 0.0617 | 1.57 (1.23–1.98) | 0.0002 |
| No HTN w/ severe LCA | 1.55 (1.19–2.02) | 0.0011 | 1.75 (1.26–2.44) | 0.0009 |
| HTN w/ no LCA | 1.35 (1.10–1.65) | 0.0031 | 1.06 (0.83–1.36) | 0.6277 |
| HTN w/ mild to moderate LCA | 1.69 (1.41–2.03) | <0.0001 | 1.66 (1.31–2.08) | <0.0001 |
| HTN w/ severe LCA | 2.37 (1.85–3.01) | <0.0001 | 2.10 (1.53–2.86) | <0.0001 |
| DM (Ref = no DM w/ no LCA)d | 1 | 1 | ||
| No DM w/ mild to moderate LCA | 1.21 (1.05–1.39) | 0.006 | 1.62 (1.36–1.90) | <0.0001 |
| No DM w/ severe LCA | 1.45 (1.18–1.77) | 0.0003 | 1.96 (1.51–2.53) | <0.0001 |
| DM w/ no LCA | 1.09 (0.91–1.31) | 0.3224 | 1.47 (1.18–1.82) | 0.0004 |
| DM w/ mild to moderate LCA | 1.35 (1.17–1.56) | <0.0001 | 2.20 (1.84–2.63) | <0.0001 |
| DM w/ severe LCA | 2.22 (1.71–2.87) | <0.0001 | 2.60 (1.87–3.60) | <0.0001 |
CAD, coronary artery disease; CI, confidence interval; CKD, chronic liver disease; CLD, chronic liver disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ESRD, end-stage renal disease; HF, heart failure; HTN, hypertension; LCA, liver chemistry abnormalities.
Adjusted for age (time-varying), race (time-varying), ethnicity, insurance status (time-varying), and comorbidities (HTN, DM, CAD, HF, malignancy, COPD, CKD I-IV, ESRD, obesity, and CLD).
Adjusted for sex, race (time-varying), ethnicity, insurance status (time-varying), and comorbidities (HTN, DM, CAD, HF, malignancy, COPD, CKD I-IV, ESRD, obesity, and CLD).
Adjusted for age (time-varying), sex, race (time-varying), ethnicity, insurance status (time-varying), and comorbidities (DM, CAD, HF, malignancy, COPD, CKD I-IV, ESRD, obesity, and CLD).
Adjusted for age (time-varying), sex, race (time-varying), ethnicity, insurance status (time-varying), and comorbidities (HTN, CAD, HF, malignancy, COPD, CKD I-IV, ESRD, obesity, and CLD).
Discussion
In this large series of 10 856 hospitalized patients with COVID-19, we found increasing severity of LCA on hospital admission predicted early in-hospital mortality. Additionally, mortality associated with the known risk factors, hypertension, diabetes, male sex, and old age was accentuated in the presence of LCA in COVID-19 patients.
Although LCA is most often hepatocellular with AST-predominant elevation in patients with COVID-19 infection [2], bilirubin and ALP have also been reported to correlate with the severity of COVID-19 [10,11], we found that a combination of all four LCA (AST, ALT, ALP, and TB) is the best predictor of mortality as opposed to isolated LCA or other combinations. In our study, more than two-thirds of patients had evidence of LCA at presentation to the hospital. Of all the patients, 63.9% had mild-to-moderate LCA and 7.6% had severe LCA. Reported frequencies of LCA have varied from 16 to 53% [12]. While they may depend on the population, different definitions of LCA make a direct comparison difficult.
We found a correlation of inflammatory markers with higher grades of LCA, particularly with AST. However, these inflammatory markers particularly, Ferritin, procalcitonin, CRP, LDH, and D-dimer correlated weakly to modestly with AST; Ferritin and lactate correlated weakly with ALT levels. While these were overall weak to moderate, a contribution of systemic inflammation to LCA is suggested. Further, AST, but not ALT, correlated with increased CPK levels. Since only a small minority of our patients had isolated AST elevation (1293, 11.9%, data not shown), a combination of liver and muscle injury was likely present in patients with elevated CPK.
In our population, men were more likely than women to present with LCA, and LCA was more commonly seen in patients with chronic liver disease than without, which has been observed in other studies as well [13]. Several risk factors of severe COVID-19 did not correlate positively with LCA on admission. Hypertension and diabetes were less frequent among patients with LCA than without. Additionally, age, BMI, CKD, COPD, race, and insurance status were not associated with LCA on multivariate analysis. A recent study from NY similarly reported a inverse association between LCA and DM, HTN and also age in univariate analyses [7]. Multivariate analysis was not performed, likely because the study was too small. While lead-time bias may be a possible explanation for different frequencies of comorbidities upon admission – patients with known hypertension and diabetes could seek medical care sooner than previously healthy patients and be admitted before LCA occurred, such an effect would be expected in patients with other chronic diseases and older people as well. Alternatively, medications such as ACE-inhibitors or ARBs could protect from COVID-19 associated LCA. Interestingly, a recent meta-analysis suggested a reduced risk for severe disease of patients taking these medications [14]. However, since patients with hypertension and diabetes have worse outcomes of COVID-19, further studies are required to investigate these observations.
Irrespective of the prevalence of several comorbidities in patients with LCA, mortality increased incrementally with the severity of LCA, and the highest mortalities were observed in patients with severe LCA and age >60, male sex, diabetes mellitus, or hypertension. Although mild to moderate LCA was not associated with increased mortality risk in younger patients, a significantly increased risk was noted with severe LCAs (Table 4). Interestingly, our study did not show hypertension as a risk factor for increased mortality unless LCA was present. Among patients with LCA, those with hypertension showed a worse outcome than nonhypertensive patients with the same degree of LCA.
Men appeared to have an increased risk of developing severe LCA even after adjusting for baseline confounders. For many viral respiratory tract infections, including but not limited to coronaviruses, the prevalence and severity of infection has been reported to be greater for men [15]. In preclinical studies of mice, males are significantly more susceptible to coronavirus infections, as they are less capable of controlling virus replication, exhibit more post-infection pulmonary damage, and have lower immune responses than females [16].
As was seen in an earlier report of patients in our hospital system that involved 5700 patients, only about 2.3% had known chronic liver disease [1], and an increased disease burden among patients with chronic liver disease, as was expected by CDC and AASLD expert panels[17,18], has not appeared. A recent large propensity-matched study reported that patients with chronic liver disease and COVID-19 have a three-fold higher mortality than patients without liver disease [19]. The presence of underlying chronic liver disease was significantly associated with more severe COVID-19 infections and mortality in another metanalysis [13]. In the present study, the largest to date, we found that patients with chronic liver disease have a two-fold increased risk of severe LCA on admission, and their mortality rises with the level of this LCA.
The mechanisms of hepatic injury in COVID-19 are not well characterized, but several possible causes exist. These include inflammatory/septic injury, hypoxic injury in hypotensive episodes, severe hypoxia, embolic events, or drug-induced liver injury. A direct cytopathic effect is also conceivable [20]. However, hepatocytes, in contrast to biliary epithelial cells, do not express ACE2, which is the cell surface receptor for SARS-CoV-2. Therefore, further research is required to confirm this.
Strengths of our study include the large sample size and long-time follow-up. Furthermore, outcomes of a significant number of hospitals were included. The demographics of our patient population likely represents the general population of the country well. Our analysis included a number of covariates including insurance status that were not assessed in previous studies [7].
Our study has several limitations. The cohort only contains patients that presented to the hospital limiting application to the outpatient setting. The outcomes of the 9.5% of patients who were still hospitalized at the end of the observation period may differ from the other patients and may therefore skew the results. In addition, this study primarily looked at the first laboratory results that were obtained within 48 h of admission and did not evaluate the trend of the LCA. It is also unclear if these patients received any medications prior to presenting to the hospitals that may have contributed to the elevation of the liver chemistries. Survival data for discharged patients were not available, so that only in-hospital mortality could be analyzed. Thus, the cumulative survival rate of about 50% after 3 weeks of hospitalization corresponds to 2334 known deaths (21.5%) of the total study population, which is similar to the rate reported in Richardson et al. Our data stem from the beginning of the crisis in New York, when information about management of hospitalized patients with COVID-19 was sparse. Therefore, mortality rates will likely change in future studies.
Conclusion
In conclusion, the current study delineates the spectrum of LCA with COVID-19 infection. Most of the patients infected with COVID-19 have LCA of mild to moderate severity (63.9%) and a small proportion of patients has severe LCA (7.6%). LCA, particularly AST showed a weak to modest correlation with several proinflammatory markers as well as CPK an indicator of muscle injury. Mortality was accentuated with increasing severity of LCA in presence of older age, male sex, diabetes, and hypertension. Interestingly, hypertension was not associated with increased in-hospital mortality unless LCA was present. Clinicians should monitor the combination of AST, ALT, Bilirubin and ALP as additional prognostic indicator for COVID-19 especially in presence of comorbidities.
Acknowledgements
We would like to acknowledge the contributions of the Northwell Health COVID-19 Research Consortium. We also acknowledge and honor all of our Northwell team members who consistently put themselves in harm’s way during the COVID-19 pandemic; this article is dedicated to them, as their vital contribution to knowledge about COVID-19 and sacrifices on the behalf of patients made it possible.
S.K.S. or C.K. conceptualized the study. S.K.S. performed the statistical analysis and wrote the initial draft. Y.J. supervised the statistical analysis. S.K.S. and J.H. collected the data. Y.J., C.K., and H.Q. interpreted the data, verified analytical methods, contributed significantly by providing major intellectual inputs and revising the initial draft of the manuscript. All authors then participated in additional intellectual input, critical revision, and approval of the manuscript under supervision of S.K.S.
The data that support the findings of this study are available on request from COVID19@northwell.edu. The data are not publicly available due to restrictions as it could compromise the privacy of research participants.
Conflicts of interest
Sanjaya K. Satapathy has served as a speaker for Intercept, Alexion, Dova, as an advisory board member for Gilead, Intercept, Bayer and has received research funding from Gilead, Biotest, Genfit, Conatus, Intercept, Shire, Exact Sciences, Eananta, Dova, Bayer. Sanjaya K. Satapathy is an employee of Northwell Health. The remaining authors declare no conflicts of interest.
Supplementary Material
Footnotes
Sanjaya K. Satapathy and Christian Kuntzen contributed equally to the writing of this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.eurojgh.com.
References
- 1.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al.; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al.; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiu H, Wander P, Bernstein D, Satapathy SK. Acute on chronic liver failure from novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Liver Int 2020; 40:1590–1593. [DOI] [PubMed] [Google Scholar]
- 4.Wander P, Epstein M, Bernstein D. COVID-19 presenting as acute hepatitis. Am J Gastroenterol 2020; 115:941–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovalic AJ, Huang G, Thuluvath PJ, Satapathy SK. Elevated liver biochemistries in hospitalized Chinese patients with severe COVID-19: systematic review and meta-analysis. Hepatology 2020. doi: 10.1002/hep.31472. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, et al. Liver biochemistries in hospitalized patients with COVID-19. Hepatology 2020. doi: 10.1002/hep.31326. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, et al. Acute liver injury in COVID-19: prevalence and association with clinical outcomes in a large U.S. cohort. Hepatology 2020; 72:807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018; 67:1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo C, So Y, Jang W, SAS Institute Inc. Evaluating Predictive Accuracy of Survival Models with PROC PHREG. 2017; 1–16. https://support.sas.com/resources/papers/proceedings17/SAS0462-2017.pdf. [Accessed 4 January 2021]
- 10.Paliogiannis P, Zinellu A. Bilirubin levels in patients with mild and severe Covid-19: a pooled analysis. Liver Int 2020; 40:1787–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology 2020; 72:389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee IC, Huo TI, Huang YH. Gastrointestinal and liver manifestations in patients with COVID-19. J Chin Med Assoc 2020; 83:521–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int 2020; 14:612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barochiner J, Martínez R. Use of inhibitors of the renin-angiotensin system in hypertensive patients and COVID-19 severity: a systematic review and meta-analysis. J Clin Pharm Ther 2020; 45:1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Fang X, Cai Z, Wu X, Gao X, Min J, Wang F. Comorbid chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among COVID-19 patients: a systemic review and meta-analysis. Research (Wash D C) 2020; 2020:2402961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lippi G, de Oliveira MHS, Henry BM. Chronic liver disease is not associated with severity or mortality in Coronavirus disease 2019 (COVID-19): a pooled analysis. Eur J Gastroenterol Hepatol 2021; 33:114–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD expert panel consensus statement. Hepatology 2020; 72:287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. What to Know About Liver Disease and COVID-19. 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/liver-disease.html. [Accessed 4 January 2021]
- 19.Singh S, Khan A. Clinical characteristics and outcomes of Coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology 2020; 159:768–771.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol 2020; 73:807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.